Abstract
Methanolic and dichloromethane extracts from the leaves of Congolese Hibiscus species were characterised by chromatographic and spectroscopic techniques and their in vitro biochemical activities against ROS production were evaluated in cellular models and on an enzyme, myeloperoxidase (MPO), involved in inflammation. Hibiscus acetosella has a chemical fingerprint different from Hibiscus cannabinus and Hibiscus sabdariffa both having similar fingerprints. Major compounds were polyphenols, represented mainly by caffeoyl-hydroxycitric acid for H. acetosella and neochlorogenic acid for the two other spiecies. All extracts displayed high cellular antioxidant activity with IC50 values ranging from 0.5 to 3 μg mL−1 using lucigenm on neutrophils. Dichloromethane extracts showed more efficient effects on extracellular ROS production and MPO activity. Antioxidant and anti-inflammatory activities of caffeoyl-hydroxycitric acid were significantly higher than those of neochlorogenic acid. The bioactivities of Hibiscus species were positively correlated with their phytochemical content and could justify the use as local nutraceutical resources and medicines.
Keywords: Caffeoyl-hydroxycitric acid, Hibiscus acetosella, Hibiscus cannabinus, Hibiscus sabdariffa, myeloperoxidase, neochlorogenic acid
Graphical abstract
Phenolic compounds such as quercetin-3-glucoside, kaempferol-rhamnoside, neochlorogenic acid are reported in the leaves from H. canaabinus (Pascoal et al. 2014). By comparison with standards, it was showed that H. acetosella contains chlorogenic acid, quercetin-3-galactoside and other nor-identified flavonoids, in accordance with results of Tsumbu et al. (2012). The two other species contained quercetin-3-rutinoside, quercetin-3-glucoside and kaempferol 3-rutinoside. Caffeic acid is present in all species but at high amounts in H. acetosella. TLC analysis of dichloromethane extracts showed the probable presence of terpenoid compounds (Figure S3). The main phenolic acid detected in H. acetosella was different from this detected H. cannabinus and H. sabdariffa (Figure S2). The isolation of main phenolic compounds was canied out with a Varian HPLC preparative chain. Spectroscopic analysis, including 1D and 2D 1H- and 13C-NMR spectroscopy, combined with a comparison of their NMR data (Figure S4–S9) with those reported in the literature was realised on isolated compounds. MS analysis showed that molecular ions of major phenolic acid appeared at m/z 369.1169 [M–5H]− for the H. acetosella compound and 353.0893 [M–5H]− for the H. cannabinus and H. sabdariffa compound. Our results clearly showed that besides already described flavonoids, the main phenolic acid from H. acetosella is 2-O-trans-caffeoyl-hydroxycitrtc acid, while this from H. cannabinus and H. sabdariffa is neochlorogenic acid (Figure 1). These two species also contain caffeoyl-hydroxycitric acid in low quantities. Thus, these differences in the chromatographic fingerprints may be easily used to distinguish H. acetosella from H. cannabinus or H. sabdariffa.
Figure 1.
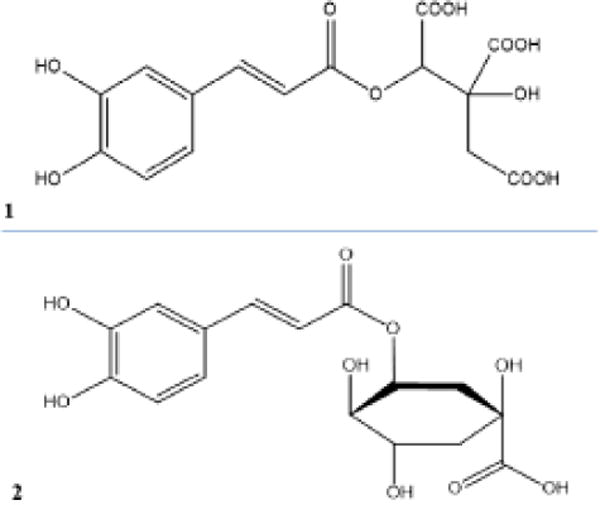
Chemical structures of caffeoyl-hydroxycitric acid (1) and neochlorgenic acid (2).
2.2. Antioxidant activity
ABTS and DPPH assays showed that leaf methanolic extracts of the three Hibiscus species have the ability to scavenge free radicals with IC50 values comprised between 43 and 186 μg mL−1 (Table S1). The IC50 values showed that the extract of H. acetosella is the most active followed by H. cannabinus and H. sabdariffa. The dichloromethane extracts showed a weaker capacity to scavenge ABTS+, while this capacity was null with DPPH. Indeed, dichloromethane allowed a better extraction of lipophilic compounds in comparison to methanol and it was shown that lipophilic compounds such as carotenoids did not scavenge the DPPH radicals (Müller, Fröhlich, and Böhm 2011). Carotenoids were also reported in the leaves of hibiscus species (Raju et al. 2007). Beside, some coloured compounds car interfere at specific wavelengths of DPPH, leading to the underestimated antioxidant activity of extracts (Arnao 2001), Previous studies reported that extracts from parts of Hibiscus species such calyx of H. sabdariffa exhibited high scavenging activity (Sarkar et al. 2014) and the leaves of studied Hibiscus have shown a better DPPH scavenging activity (Andabati and Muyonga, 2014; Wang et al. 2014). Isolated caffeoyl-hydraxycitric acid and neochlorogenic acid, exhibited higher scavenging activities than the extracts but lower than gallic acid, used as positive control. Nevertheless, the antiradical activities of these phenolic acids can contribute to the global antioxidant capacity of the Hibiscus extracts.
Beside conventional cell-free antioxidant assays, it can be pertinent to evaluate the antioxidant and anti-catalytic potential of plant extracts in cellular models involved in ROS production and inflammatory responses. In a recent study, Zhen et al. (2016) showed the inhibitory capacity of the H. sabdariffa leaf extract on the nitric oxide synthase activity by LPS stimulated murine macrophage. In the present study, the lucigenin-dependent chemiluminescence (CL) and the intracellular fluorescent probe DCFH-DA were used to evaluate the extra- and intracellular ROS production resulting mainly from NADPH oxidase activity by stimulated neutrophil and HL-60 cells (Derochette et al. 2013). The addition of increasing concentrations of the leaf extracts (0.1–5 μg mL−1), the isolated phenolic acids (10−6 to 5.10−5 M) resulted in a dose-dependent decrease of the neutrophils ROS production in comparison to the control test performed with DMSO, the solvent used to solubilize the extracts and phenolic acids (Figure S10). Our results showed that the cellular antioxidant activity of dichloromethane extracts is significantly higher (p < 0.001) than that of methanolic extracts in the following order: H. acetosella > H. cannabinus > H. sabdariffa. Caffeoyl-hydroxycitric acid is more active than neochlorogenic acid and gallic acid used as positive control.
The addition of increasing concentrations of the leaf Hibiscus extracts (10, 20 and 40 μg mL−1), and the isolated phenolic acids (5.10−6 to 5.10−5 M) and gallic acid (10−5 to 10−4 M) resulted in a dose-dependent decrease of HL-60 ROS production in comparison to the control test performed with DMSO (Figure S11). With this cellular model, our results showed that the activity of methanolic extracts is significantly higher (p < 0.05) than that of dichloromethane extracts. In a previous study, Tsumbu et al. (2012) observed an inhibitory effect of the aqueous extract of H. acetosella on the intracellular ROS production in HL-60 cells suggesting that molecules released by polar extractions were more favourable for this cell model. Dichloromethane extracts were not or few active in this cell model and exhibited a significant pro-oxidant effect at the tested concentrations. In this cell model, neochlorogenic acid appeared less active than caffeoyl-hydroxycitric acid.
Indeed, methanolic extracts were active in the two cell-based assays, but dichloromethane extracts exhibited a higher anti-ROS activity in the lucigenin CL assay. Lucigenin seems to be a more specific probe for the detection of superoxide anions directly produced by the activity of NADPH oxidase (Franck et al. 2013).The high activity of dichloromethane extracts can be attributed to lipophilic compounds such as carotenoids that are known to be powerful scavengers of superoxide anions (Galano et al. 2010). However, the values obtained with dichloromethane extracts on HL-60 cells showed that the extract of H. acetosella had a very weak effect on intracellular ROS production while the other extracts had rather a pro-oxidant effect. These results suggest that the compounds of dichloromethane extracts are not good intracellular ROS scavengers and could even amplify the activation cascade involved in ROS production. Further studies are needed to understand these observations. Nevertheless, Takamatsu et al. (2003) showed that carotenes did not inhibit intracellular DCF production in HL-60 cells, presumably because of a cell intake problem (Takamatsu et al. 2003). Our results suggest that both methanolic and dichloromethane extracts, as well as their major phenolic acids, without having any cytotoxicity are efficient as superoxide anion scavengers while only methanolic extracts appeared more efficient to inhibit intracellular ROS production.
2.3. Anti-inflammatory activity
In some acute and chronic pathologies, the uncontrolled stimulation of neutrophils could contribute to amplify or maintain the inflammatory response with the release of MPO, a pro-oxidant enzyme involved in secondary cell damage an d considered as a marker of inflammation (Franck et a1. 2013). All plant extracts and isolated phenolic acids exhibited a dose-dependent inhibitory effect on MPO activity. Dichloromethane extracts showed a stronger inhibition of MPO in comparison to methanolic extracts in the following order: H. cannabinus> H. acetosella > H. sabdariffa. The dichloromethane allowing a better extraction of lipophilic molecules may allow a better interaction of these molecules with the hydrophobic pocket at the entrance of the active site of MPO (Forbes et al. 2013). Caffeoyl-hydroxycitric acid and neochlorogenic acid are less efficient MFO inhibitors in comparison to gallic acid and they exhibited an inhibition higher than 50% at the highest concentration tested (10−4 M) (Figure 2). The SIEFED method used here to measure MPO activity allowed the detection of compounds that have a direct interaction with the MPO. Compared to gallic acid, caffeoyl-hydroxycitric acid and neochlorogenic acid are larger molecules that cannot enter easily into the active site of MPO and thus inhibit the enzyme (Franck et al. 2013).
Figure 2.
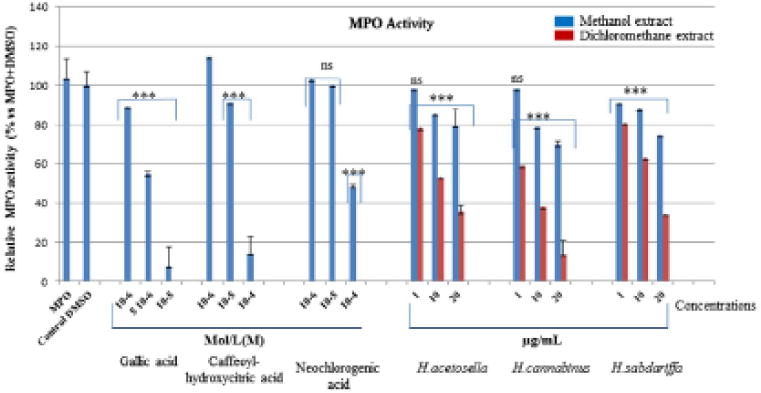
Effect of gallic acid, caffeoyl-hydroxycritric acid, neochlorogenic acid and methanolic and dichloromethane extracts of H. acetosella, H. cannabinus and H. sabdariffa on MPO activity measured by SIEFED.
Notes: The percentage of inhibition was calculated for each sample concentration versus the corresponding control (MPG + DMSO), taken as 100% (mean ± SD. n = 6). Samples vs. Control DM5O:ns: no significance, p-values (****< p0.0001) calculated by two-way ANOVA indicated a significant effect vs. DMSO control set as 100% response. ns = not significant vs. DMSO control. There are statistical differences between dichloromethane and methanolic extracts of all Hibiscus species. Dichloromethane extracts are more active than methanolic extracts, caffeoyl-hydroxycitric and neochlorogenic acids are less active than gallic acid used as positive control. Extract of H. cannabinus are the most active, followed by those of H. acetosella and H. sabdariffa.
Altogether the results showed that among the extracts tested, the leave extract of H. acetosella, which contains caffeoyl-hydroxycitric acid showed the highest acellular and cellular antioxidant activities but not the highest inhibition on MPO activity. As reported by Franck et al. (2013), the molecules having good antiradical activities are not necessarily appropriate to enter and inhibit the active site of the pro-inflammatory enzyme MPO. Metabolite profiling showed that studied Hibiscus species are rich in bioactive such as phenolic compounds and terpenoids, and they are well known as having high therapeutic interest. Polyphenols have the potential to confer benefit in diverse neurodegenerative disorders associated with oxidative damage (Vauzour, Kerr, and Czank 2013). The antioxidant and anti-inflammatory potentials of these phenolic acids as well as of Congolese Hibiscus leaves could have a beneficial health for Congolese people.
3. Conclusions
Metabolic profiles and biochemical activities of three Congolese Hibiscus species were determined and showed the important content of phenolic acids and terpenoids. The metabolic profile of H. acetosella appears different to those of H. sabdariffa and H. cannabinus having both quite similar profiles. The chromatographic fingerprints may be used to distinguish H. acetosella from H. cannabinus or H. sabdariffa. Caffeoyl-hydroxycitric acid was found to be a major phenolic acid from H. acetosella and neochlorogenic acid the major phenolic acid from H. cannabinus and H. sabdariffa. Our comparative study on Congolese Hibiscus showed that the leaf extract of H. acetosella and its major phenolic acid, caffeoyl-hydroxycitric acid, have the best acellular and cellular antioxidant activities in comparison to the other species. In all species, the methanolic extracts appeared more efficient as radical scavengers and on the inhibition of intracellular ROS production by HL60 cells, while dichloromethane extracts appeared more efficient on the inhibition of the extracellular ROS production by PMN and on the activity of MPO. The antioxidant and anti-inflammatory activities of the leaves of the studied Hibiscus may have potential therapeutic interest and could justify their use in traditional medicine and local nutraceutical resources, but further studies are needed, especially in vivo studies, to demonstrate the benefit of these extracts and phenolic acids on nutrition and health.
Supplementary Material
Acknowledgments
The authors thank Dr. P Stofelen and Mrs. B Mato of the National Botanical Garden of Meise, Belgium, and Mr. A Kikufi of the University of Kinshasa for identification of the plants studied. They also thank JN Wauters, J Romainville and A Niesten for their technical advice.
Funding
This, work was supported by the University of Liège and the Fogarty International Center and NIEHS (U.S A.) under [grant number R01ES019841].
Footnotes
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/14786419.2017.1305382.
Supplementary material
Experimental details, figures and tables, relating to this, article are available online
Disclosure statement
No potential conflict of interest was report-ad by the authors.
References
- Andabati B, Muyonga J. Phenolic content and antioxidant activity of selected Ugandan traditional medicinal foods. Afr J Food Sci. 2014;8:427–434. [Google Scholar]
- Arnao MB. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci Technol. 2001;11:419–421. [Google Scholar]
- Da-Gosta-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdoriffa L.—A phytochemical and pharmacological review. Food Chem. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Derochette S, Franck T, Mouithys-Mickalad A, Deby-Dupont G. Intra- and extracellular antioxidant capacities of the new water-soluble form of curcumin (NDS27) on stimulated neutrophils and HL-60 cells. Chem Biol Interact. 2013;201:49–57. doi: 10.1016/j.cbi.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Fernández-Arroyo S, Rodriguez-Medina IC, Beltrán-Debón R, Pasini F, Joven J, Micol V, Segura-Carretero A, Fernández-Gutiérrez A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res Int. 2011;44:1490–1495. [Google Scholar]
- Forbes LV, Sjogren T, Auchere F, Jenkins DW, Thong B, Laughton D, Hemsley P, Pairaudeau G, Turner R, Eriksson H, et al. Potent reversible inhibition of myeloperoxidase by aromatic hydroxamates. J Biol Chem. 2013;288:36636–36647. doi: 10.1074/jbc.M113.507756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck T, Mouithys-Mickalad A, Robert T, Ghitti G, Deby-Dupont G, Neven P, Serteyn D. Differentiation between stoichiometric and anticatalytic antioxidant properties of benzoic acid analogues: a structure/redox potential relationship study. Chem Biol Interact. 2013;206:194–203. doi: 10.1016/j.cbi.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Galano A, Vargas R, Martinez A. Carotenoids can act as antioxidants by oxidizing the superoxideradical anion. Phys Chem Chem Phys. 2010;12:193–200. doi: 10.1039/b917636e. [DOI] [PubMed] [Google Scholar]
- Latham P, ku Mbuta AK. Useful plants of Bas-Congo Province, Democratic Republic of Congo. 2nd. Canterbury: Mystole Publications; 2006. p. 276. [Google Scholar]
- Müller L, Fröhlich K, Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011;129:139–148. [Google Scholar]
- Pascoal A, Fernando AL, Quirantes-Piné R, Segura-Carretero A. Chemical composition and antioxidant activity of kenaf leaves. AAIC Inter Conference Ind Crops Prod. 2014 Available from: http://www.fibrafp7.net/Portals/0/athens/Ana_Luisa_Ferrando_kenaf.pdf.
- Raju M, Varakumar S, Lakshminarayana R, Krishnakantha T. Carotenoid composition and vitamin A activity of medicinally important green leafy vegetables. Food Chem. 2007;101:1598–1605. [Google Scholar]
- Rodriguez-Medina IC, Beltrán-Debón R, Molina VC, Alanso-Villaverde C, Joven J, Menéndez J, Segura-Carretero A, Fernández-Gutiérrez A. Direct characterization of aqueous extract of Hibiscus sabdariffa using HPLC with diode array detection coupled to ESI and ion trap MS. J Sep Sci. 2009;32:3441–3448. doi: 10.1002/jssc.200900298. [DOI] [PubMed] [Google Scholar]
- Sarkar B, Kumar D, Sasmal D, Mukhopadhyay K. Antioxidant and DNA damage protective properties of anthocyanin-rich extracts from Hibiscus and Ocimum: a comparative study. Nat Prod Res. 2014;28:1393–1398. doi: 10.1080/14786419.2014.904309. [DOI] [PubMed] [Google Scholar]
- Ssegawa P, Kasenene JM. Medicinal plant diversity and uses in the Sango bay area, Southern Uganda. Southern Uganda J Ethnopharmacol. 2007;113:521–540. doi: 10.1016/j.jep.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Takamatsu S, Galal AM, Ross S, Ferreira D, ElSohly MA, Ibrahim ARS, El-Feraly FS. Antioxidant effect of flavonoids on DCF production in HL-60 cells. Phyther Res. 2003;17:963–966. doi: 10.1002/ptr.1289. [DOI] [PubMed] [Google Scholar]
- Tsumbu CN, Deby-Dupont G, Tits M, Angenot L, Frederich M, Kohnen S, Mouithys-Mickalad A. Polyphenol content and modulatory activities of some tropical dietary plant extracts on the oxidant activities of neutrophils and myeloperoxidase. Int J Mol Sci. 2012;13:628–650. doi: 10.3390/ijms13010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D, Karr J, Czank C. Plant polyphenols as dietary modulators of brain functions. In: Watson RR, Preedy RV, Zibadi S, editors. Polyphenols inhuman health and disease. London: Academic Press; 2013. pp. 357–370. [Google Scholar]
- Wang J, Cao X, Jiang H, Qi Y, Chin K, Yue Y. Antioxidant activity of leaf extracts from different Hibiscus sabdariffa accessions and simultaneous determination five major antioxidant compounds by LC-Q-TOF-MS. Molecules. 2014;19:21226–21238. doi: 10.3390/molecules191221226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Villani TS, Guo Y, Qi Y, Chin K, Pan M-H, Ho CT, Simon JE, Wu Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016;190:673–680. doi: 10.1016/j.foodchem.2015.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


