Abstract
Recognizing the significant prevalence of hepatocellular carcinoma (HCC) in Saudi Arabia, and the difficulties often faced in early and accurate diagnoses, evidence-based management, and the need for appropriate referral of HCC patients, the Saudi Association for the Study of Liver diseases and Transplantation (SASLT) formed a multi-disciplinary task force to evaluate and update the previously published guidelines by the Saudi Gastroenterology Association. These guidelines were later reviewed, adopted and endorsed by the Saudi Oncology Society (SOS) as its official HCC guidelines as well. The committee assigned to revise the Saudi HCC guidelines was composed of hepatologists, oncologists, liver surgeons, transplant surgeons, and interventional radiologists. Two members of the task force served as guidelines editors. A wide based search on all published reports on all aspects of the epidemiology, natural history, risk factors, diagnosis, and management of HCC was performed. All available literature was critically examined and available evidence was then classified according to its strength. The whole document and the recommendations were then discussed in details by members and consensus was obtained. All recommendations in these guidelines were based on the best available evidence, but were tailored to the patients treated in Saudi Arabia. We hope that these guidelines will improve HCC patient care and enhance the multidisciplinary care needed for these patients.
Liver disease is an important health problem in Saudi Arabia. A significant proportion of patients with liver cirrhosis will develop hepatocellular carcinoma (HCC) each year. This has a very significant influence on the patient and places a very high burden on the health care system. Recognizing the significant prevalence of HCC in Saudi Arabia, and the difficulties often faced in early and accurate diagnoses, evidence-based management, and appropriate referral of HCC patients, the Saudi Association for the Study of Liver Diseases and Transplantation (SASLT) formed a multi-disciplinary task force to evaluate and update the previously published guidelines by the Saudi Gastroenterology Association.1 These guidelines were later reviewed, adopted and endorsed by the Saudi Oncology Society (SOS) as its official HCC guidelines as well.
Goals of These Guidelines
To provide a concise evidence-based review of the diagnosis and management of HCC.
To help initiate plans to prevent HCC.
To enhance early and accurate diagnosis of patients with HCC.
To provide an evidence-based approach for the management of HCC patients.
To facilitate a more effective referral system between primary/secondary care physicians and tertiary care centers where advanced treatments are available.
To help adopt a more effective triaging system of patients within tertiary care centers.
To help in standardizing the management of patients with HCC across the country.
Methods
The committee assigned to revise the Saudi HCC guidelines was composed of hepatologists, oncologists, liver surgeons, transplant surgeons, and interventional radiologists. Two members of the task force served as guidelines editors. A widely base search of all published studies on all aspects of the epidemiology, natural history, risk factors, diagnosis, and management of HCC was performed. All available literature was critically examined and available evidence was then classified according to its strength. Members then discussed the whole document and the recommendations in detail, and consensus was obtained. Two international experts in the fields of hepatology and hepatobiliary surgery then reviewed the document. Subsequently, the guidelines were approved and endorsed by SASLT and SOS. All recommendations in these guidelines were based on the best available evidence, but were tailored to patients treated in Saudi Arabia. We hope that these guidelines will improve HCC patient care and enhance the multidisciplinary care needed for these patients.
Grading of Recommendations
| Grade A | Recommendation based on at least one high quality randomized controlled trial or at least one high quality meta-analysis of well-done randomized controlled trials. |
| Grade B | Recommendation based on high quality case-control or cohort studies OR a high quality systematic review. |
| Grade C | Recommendation based on non-analytical studies (case reports or case series). |
| Grade D | Recommendations based on expert opinion only. |
Epidemiology
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver. It represents the sixth most common cancer and the third most common cause of cancer-related death among men and the sixth among women worldwide.2 Annually more than 560 000 people are diagnosed with HCC and approximately the same number die with it.3 It has a variable geographical distribution. The incidence in developing countries is two to three times higher than in Western countries. For example, in Eastern Asia and Middle Africa the age-adjusted incidence rate (AAIR) ranges from 20–28 cases per 105 in men while it is about 1–3 per 105 in Northern Europe, Australia and North America.4 In the United States the incidence of HCC has increased from 1.4 per 100 000 population during the period from 1976–1980 to 2.4 per 100 000 population for the period from 1991–1995.5
In Saudi Arabia, according to the most recent Saudi Cancer Registry in the year 2006, liver cancer accounts for 5.2% (416 diagnosed cases) of all newly diagnosed cancers.6 HCC was the fourth most common cancer affecting Saudi males and the ninth most common cancer affecting females with an overall age standardized rate of 5.3/100 000 population (7.5/100 000 for males and 3.1/100 000 for females). The male to female ratio was 2.3:1. The median age at diagnosis was 67 years for males and 64 years for females. The five most common regions were: Najran 10/100 000, Riyadh 8/100 000, Madinah 6.6/100 000, eastern 5/100 000, and Makkah 3.5/100 000. Compared with the data provided for the year 2000, the age-standardized rate increased from 4.5/100 000 to 5.3/100 000. The age specific incidence rate peaked at age 65 years in both males and females.
In comparison with global trends in age-adjusted incidence rates, the rate of 5.3/100 000 seems intermediate between countries with higher rates (>10/100 000) such as many countries in Asia like Korea, Thailand, and China, and countries with lower rates such as Western countries like the US and UK.
This incidence of HCC in Saudi Arabia is not surprising given the relatively high prevalence of the two major risk factors, namely hepatitis B and hepatitis C infection. In a large epidemiologic study, 7% of Saudi children were found to be positive for HBsAg.7 Not until universal vaccination was applied in Saudi Arabia did this prevalence rate decrease to less than 0.3%.8 Since the initial epidemiologic studies showing a high prevalence of hepatitis B were done on children who are now adults and with an estimation that about 20% of these patients will probably develop cirrhosis with an annual risk of 1% to 4% for HCC, the incidence of HCC is expected to increase dramatically in the next 30 years. Hepatitis C is also common in Saudi Arabia with a prevalence rate of 1% to 3% of the population,9 which further increases the risk of HCC. In addition, Saudi Arabia is known to have a relatively high prevalence of diabetes (20%) and obesity (30%), which are closely associated with non-alcoholic fatty liver disease, adding to the overall risk for the development of cirrhosis and subsequently HCC.10 We can thus expect that HCC will be a significant health problem in Saudi Arabia in the next 30 years.
Three relatively small studies done in the 1980s briefly described the epidemiology of HCC in Saudi Arabia. In the first study by Kingston et al, all cases of liver tumors were studied over a period of 2 years at King Faisal Specialist Hospital in Riyadh.11 A total of 104 cases of HCC were found. These patients were predominantly male (6:1 ratio). In the second study by Ashraf et al, 75 patients with HCC were described from the Gizan area in southern Saudi Arabia.11,12 Eighty percent of these patients were males. In the third study by Atiyeh and Ali, the clinico-pathological features of 54 patients with HCC were described.13 The male-to-female ratio was found to be 10:1 with a peak incidence age between 40 to 60 years.
The Saudi Observatory Liver Disease Registry (SOLID, www.solid-registry.com) reported all registered cases of HCC from 2003 to 2008 collected from two centers (King Khalid University Hospital and Riyadh Military Hospital). Data was available for 366 patients. The mean age of diagnosis was 66 years, and 74% of patients were males. The underlying cause of liver disease was hepatitis C in 48% and hepatitis B in 29%. Most of the patients were diagnosed at advanced stages with 53% of patients having a Cancer of the Liver Italian Program (CLIP) score of 4 to 6 (advanced stages); 55% had large multi-nodular tumors and 16% had vascular invasion or extra-hepatic spread at the time of diagnosis. Unfortunately, BCLC staging was not available in the majority of patients at that stage of the registry. Most of the patients had decompensated cirrhosis at presentation; the Child-Pugh score was A in 30%, B in 44% and C in 26%. Forty-eight percent of the patients died during the study period. Predictors of survival in the univariate analysis were the presence of portal vein thrombosis (P=.03), portal hypertension (P<.0001), presence of ascites (P=.022), hepatic encephalopathy (P<.0001), advanced Child-Pugh class (P<.0001), bilirubin level >22 umol/L (P<.0001) and INR >1.2 (P=.02). Only the presence of portal hypertension, bilirubin >22 umol/L and severe hepatic encephalopathy were significant in the multivariate analysis.14
Risk Factors
The most significant risk factor for the development of HCC is the presence of cirrhosis regardless of its etiology. Some of the important risk factors will be discussed briefly.
Cirrhosis
The development of cirrhosis is a major risk factor for the development of HCC regardless of the underlying cause. The annual incidence of HCC in patients with compensated cirrhosis is about 3%.15 European cohort studies have reported that among patients who died of a liver-related cause, HCC was responsible in 54% to 70% of patients with compensated cirrhosis from all etiologies and in 50% of patients with hepatitis C-related cirrhosis. 16,17 This is thought to be secondary to a potent tumor promoter effect.18 Male sex, age, and duration of cirrhosis are associated with an increased risk of HCC in cirrhotic patients.19 In a recent study on 206 Saudi HCC patients in two centers identified over a 2-year period, all HCC patients had underlying cirrhosis as a risk factor.20 In that cohort 71% were males and hepatitis C accounted for 48% of cases followed by hepatitis B in 31%; the rest were cryptogenic cirrhosis.
Hepatitis B
Hepatitis B is considered the strongest epidemiologic factor associated with HCC in the majority of countries but more importantly in Asia and Africa. Worldwide, chronic hepatitis B infection accounts for more than 50% of HCC cases, but regional variations are common. For example, 70% of HCC in Korea is attributed to hepatitis B compared to 15% in Japan and 3% in the USA and Sweden.21,22
The carrier state of hepatitis B early in life carries a lifetime relative risk of developing HCC of over 100,23 with an annual risk of 0.5%. The annual incidence in cirrhotic hepatitis B patients exceeds 2%24,25 and a lifetime risk of about 10% to 25%.26 Two recent meta-analyses of case-control and cross-sectional studies suggest a lower lifelong risk of HCC of about 15% to 20%.27,28 Many factors are important in determining the risk of HCC in hepatitis B virus-infected patients. These include male gender, older age, longer duration of infection, Asian or African race, family history, exposure to aflatoxin, alcohol consumption, and co-infection with hepatitis C or hepatitis D virus. In addition, recent large studies from Asia have confirmed that HBV DNA levels are also associated with risk of HCC regardless of the HBeAg status or serum alanine aminotransferase (ALT) levels.29 In addition, HBV genotypes have also been shown to have different risks for the development of HCC. HBV genotype C has a higher risk of HCC than genotype B and genotype D has a higher risk than A. The pre-core mutation also seems to have additional risk.30 In a predominantly genotype D-infected population in Saudi Arabia, genotype D did not seem to impart a greater risk of HCC compared to other genotypes.31
Of all the above mentioned factors, the most important factor is the stage of liver disease. In summary, of all the follow-up studies of patients with hepatitis B, it was found that in HBV carriers with persistently normal ALT the annual incidence of HCC was 0%. This incidence increased to 1.2% in patients with histologically active hepatitis, and to 2% to 6% in patients with established cirrhosis.32 On the other hand, studies from Asia suggest that the annual incidence of HCC in HBV carriers is around 0.5%.33,34 Another two related important factors are race and the age at the time of infection. In Caucasian as well as Saudi carriers of hepatitis B virus, HCC occurs most often in the setting of cirrhosis20,24 but in Africa and Asia, HCC may develop more frequently in non-cirrhotic livers.24,25 HBV is thought to be carcinogenic both directly and indirectly17 because HBV DNA is integrated into the cellular DNA of the host; this can be demonstrated in HCC cells in 95% of the cases.35
Hepatitis C
Hepatitis C is considered the most important risk factor for HCC in Western countries and Japan. Hepatitis C was identified as the risk factor for HCC in Saudi patients in 74% of cases.20 In a meta-analysis of 32 case-control studies, the estimated risk for the development of HCC was 17.5-fold greater in hepatitis C virus (HCV) carriers than in non-carriers28 and in a large prospective case-control study from Taiwan it was associated with a 20-fold increased risk of HCC.36 Overall, the rate of HCC development in hepatitis C virus-infected persons ranges from 1% to 3% after 30 years37 and once cirrhosis has been established the annual risk of HCC is 1% to 4%.38 In Japan, the HCC incidence rate was 1.8 per 100 person years in subjects with chronic hepatitis C without cirrhosis and 7.1 in those with compensated cirrhosis.38 This suggests that hepatic parenchymal disease plays a major role in the development of cancer in this disease and it is established that almost all cases occur in patients with cirrhosis in the majority of countries, although rare precirrhotic cases may be seen.39 Whether these rare cases represent sampling error and false negative biopsy results for cirrhosis or whether there is an actual possibility of HCC in non-cirrhotic hepatitis C patients is a matter of debate.
To further clarify the importance of the stage of liver disease on the risk of HCC in hepatitis C virus-infected patients, Colombo summarized follow-up studies of patients with hepatitis C. It was found that the annual risk for development of HCC was 0.4% for unselected HCV carriers with persistently high values of ALT, but it rose to 1.7% in patients with the histological diagnosis of chronic active hepatitis and to 2.5% in those with compensated cirrhosis.32 Although HCV does not integrate into the host genome, there is some evidence that the virus is directly oncogenic.18
Alcohol
The risk of HCC is increased with heavy alcohol consumption defined as ingestion of more than 50 to 70 g/day.40 In a study from Italy, the risk of HCC was found to be 13 times greater in drinkers than in non-drinkers.41 In a recent met-analysis of 3 cohort and 17 case-control studies there was a clear trend towards increased risk of HCC in heavy drinkers.42 Infection with HCV or HBV in drinkers clearly increased this risk, suggesting a synergetic effect.43,44 Data seem to indicate that alcohol does not have a direct carcinogenic effect, but rather causes HCC through the triggering of cirrhosis.
Aflatoxin B1
Aflatoxin B1 derived from some Aspergillus flavus and Aspergillous parasiticus species is an important risk factor for HCC in parts of Africa and Asia.19 These organisms are weedy molds that grow on a large number of substrates, including grains, corn and peanuts, particularly under moist conditions in these parts of the world. Most authorities believe that the effect of aflatoxin is only important in patients who have pre-existing chronic hepatitis B.45 Other studies have shown that the effect of aflatoxin carcinogenesis is likely secondary to a characteristic mutation in the p53 tumor suppression gene that has been found in 30% to 60% of all HCC cases in that area.46
Obesity/Non-alcoholic Fatty Liver Disease
Multiple studies have shown that obesity through fatty liver disease increases the risk of HCC. In two studies from Europe a 2 to 3 fold increased risk for HCC was found in obese people compared to controls.47,48 In a large prospective study from the US, body mass index was clearly associated with higher rates of death of many cancers including liver cancer.49 In addition, it has been shown that obesity also increases the risk of HCC especially in hepatitis C, and less so in hepatitis B patients, especially when associated with diabetes where the risk is increased up to 100 fold from HCV- and HBV-infected patients who are not obese or diabetic.50
Other Risk Factors
In patients with hereditary hemochromatosis, the estimated risk of development of HCC is increased 200 times more than the general population once cirrhosis is established51 with an annual risk of 5%,52 mostly with advanced cirrhosis but also rarely in patients without cirrhosis. HCC develops occasionally in Wilson disease, but usually in association with cirrhosis.53 Other inherited metabolic diseases of the liver such as type 1 glycogen storage diseases and alpha 1 antitrypsin deficiency may all be associated with HCC.
Natural History
The natural history of HCC depends on the stage of the disease, but is poor in the majority of cases. Tumor size at presentation is an important factor in the natural history, but its use as a sole predicting factor is hindered by the fact that tumor doubling time may in fact be very variable. In some patients the tumor growth is slow, doubling in size in 20 months or more, while in others the tumor grows much faster and doubles in less than 1 month.54,55 In symptomatic patients in China and Africa death usually ensues within 4 months,56 while some reports suggest a longer survival and a more indolent course in Western countries.57 Other important factors in the natural history include the stage of the underlying liver disease and the patient’s performance status as discussed in the staging systems below.
RECOMMENDATIONS.
The Saudi Cancer Registry is a good source of data for HCC in Saudi Arabia although it is likely that it underestimates the true prevalence of the disease due to data capturing difficulties. Every effort must be applied to improve registration and utilization (Grade D).
Large epidemiologic studies are needed to further define the epidemiologic features of HCC in Saudi Arabia (Grade D).
Patients with cirrhosis of any etiology, but especially cirrhosis caused by hepatitis B or C, are at high risk for the development of HCC and these patients should be the targets for a screening program (Grade A).
Clinical Features
The classic features of HCC include right upper quadrant pain and weight loss. Weakness, abdominal swelling, non-specific gastrointestinal symptoms, and jaundice are other presenting features. Special clinical scenarios should also raise the suspicion of HCC. These include acute deterioration of liver function in a patient with stable cirrhosis, new onset ascites, and acute intraabdominal bleeding.
Physical findings vary according to the stage of the disease. If the tumor is small, no signs may be found except those related to cirrhosis. In more advanced disease, hepatomegaly is common with a possibility of feeling a mass or a hard irregular liver surface, which may be tender on examination. A bruit may be heard on the liver. Ascites is often found, most commonly as a result of the underlying cirrhosis leading to portal hypertension, but rarely due to tumor spread to the peritoneum. Muscle wasting is common and is usually progressive.
In the three epidemiologic studies done on patients with HCC in Saudi Arabia, presentations were not different from those described above. In the study by Ashraf et al, 91% of the patients presented with hepatic enlargement, 76% with abdominal pain, 33% with splenic enlargement, and 33% with ascites.12 Abnormal liver function tests were found in 97% of the patients. The study by Kingston et al was very similar.11
Diagnosis by Radiological Features
Various imaging modalities, particularly cross-sectional imaging are essential keys in the management of patients with HCC. These powerful techniques allow the detection, characterization, and staging of HCC, as well as planning the appropriate therapy and follow-up post treatment.
Ultrasound
Presently, the main role of ultrasound (US) in the diagnosis of HCC lies in screening. While US has the advantage of being safe, commonly available, and cost-effective, its main disadvantage is its low specificity as HCC can have variable appearances, and is of an operator dependent nature. Newly discovered focal liver masses in patients with liver cirrhosis have a high likelihood of being HCC. US has been reported to have a sensitivity of between 65% and 80% and a specificity of greater than 90% when used as a screening test.58 In a recent systematic review of all available studies Singal et al reported that surveillance US detected the majority of tumors before they presented clinically, with a pooled sensitivity of 94%. However, US was less effective for detecting early HCC with a sensitivity of 63%.59 HCC on US may appear as a hypo-, hyper-, or iso-echoic lesion. When a lesion appears hyperechoic, it may be due to fatty changes, dilated sinusoids or angioma. HCC may present as a solitary mass, a dominant mass with surrounding satellite nodules, multifocal masses, or a diffusely infiltrating mass. In spite of the limitations of US in diagnosing HCC, the low cost, safety, and availability makes it the best first-choice test to be performed when HCC is suspected.
Computerized Tomography
Using multidetector scanners, a triphasic computerized tomography (CT) scan of the liver has proven to be very useful in the diagnosis of HCC. This technique encompasses hepatic arterial (HA), portovenous (PV), and delayed venous phases. Most authorities now require a four-phase CT study to properly document these findings—unenhanced, arterial, venous, and delayed phases.60 HA and PV phases are acquired around 20 and 60 seconds, respectively, from the starting time of injection. This is carried out using a power injector of contrast intravenously at a rate of 4 cc/sec.
This technique utilizes the fact that the blood supply to HCC is predominantly from the hepatic artery resulting in its hypervascular nature. Consequently, HCC appears hyperdense during the HA phase and relatively hypodense during the PV phase due to contrast wash out. This classical pattern of arterial uptake followed by washout is highly specific for HCC.61–63 Large HCC is typically inhomogeneous. Imaging during the arterial phase of the contrast pass is of paramount importance if a small HCC is to be detected and a relatively specific diagnosis of HCC is to be made.15,64 This phase has also replaced conventional angiogram in delineating the hepatic arterial anatomy prior to liver transplantation. In recent studies, CT scanning has been reported to have high sensitivity and specificity rates of 71% to 80% and 80% to 96%, respectively, for contrast-enhanced CT compared to explant histological evaluation.65,66 In addition to its relative accuracy, CT has the advantage of detecting extrahepatic spread and accurately staging HCC. Its local extension and complications, including vascular invasion, biliary obstruction, and peritoneal bleeding due to tumoral rupture are exquisitely demonstrated with CT.
Magnetic Resonance Imaging
This test has become the diagnostic procedure of choice for HCC in many institutions.67 While HCC has variable signals on T1-weighted imaging, it is usually hyperintense on T2. Because of the abundant neovascularity, HCC enhances vividly during the arterial phase of gadolinium-enhanced imaging. In the portovenous phase, HCC is usually isointense. In the delayed phase, HCC will be hypointense because of contrast medium wash out. If the tumor is well-differentiated, it will have a high signal on T1-weighted imaging that is likely attributed to fat deposition, copper or glycoproteins and is therefore isointense on T2-weighted imaging.68
All principal radiology modalities used for the diagnosis of HCC are widely available in most tertiary care centers in Saudi Arabia. Trained abdominal radiologists are available in most tertiary care centers but may not be available in peripheral or private hospitals.
Laboratory Investigations
Serum Alpha-fetoprotein
Serum Alpha-fetoprotein (AFP) is an alpha-1 globulin that is normally present in high concentrations in fetal serum, but only in minute concentrations in adults. The seven major studies reporting on the sensitivity and specificity of AFP in screening for HCC are nicely summarized by Daniele and colleagues and show a sensitivity of 39% to 65%, a specificity of 76% to 94%, and a poor positive predictive value of 9% to 50%.69 In a recent systematic review it was confirmed that AFP has a poor diagnostic ability for detecting HCC at any level of pretest risk.70
Receiver operating curve analyses of AFP used as a diagnostic test suggests that a value of about 20 ng/ mL provides the optimal balance between sensitivity and specificity.71 But at this level the sensitivity is unacceptably low at 60%, while if a higher cutoff is used a progressively smaller proportion of HCC will be detected. This makes the test difficult to use, especially for surveillance purposes. For that reason AFP is no longer recommended as a surveillance test for HCC.60
There is only one study on AFP use in the diagnosis of HCC in Saudi Arabia.20 In a multicenter, case-control study involving 206 cases, 199 cirrhotic and 197 chronic hepatitis controls, the utility of AFP in the diagnosis of HCC was assessed. Sensitivity of AFP at the best cutoff level for HCV, HBV and a non-viral etiology for HCC was 73.7%, 65.6% and 59.5%, respectively. Specificity at this level for HCV, HBV and non-viral etiology was 36.6%, 30.1% and 29.4%, respectively. AFP cutoff levels of 102, 200 and 400 ng/mL showed similar sensitivity (39.8%, 35.9%, 32%, respectively) and specificity (96%, 98.5%, 98.5%, respectively). Positive likelihood ratios for AFP at >11.7, >20, >102, >200, >400 ng/mL were 2.8, 3.3, 9.9, 23.8 and 21.2, respectively. This study concluded that in cirrhotic patients, AFP had a poor screening and diagnostic value for HCC. Underlying viral etiology failed to influence the diagnostic accuracy of this test.
Recent data shows that AFP as a diagnostic test for HCC is less specific than previously thought. It can be increased in patients with intrahepatic cholangiocarcinoma and in some patients with metastatic colon cancer.72,73 This has shed doubt on the utility of AFP in the diagnosis of HCC, especially with the excellent diagnostic yield of radiological tests. For these reasons, AFP is no longer recommended as a diagnostic test in HCC by the American Association for the Study of Liver Diseases (AASLD).60 Other tumor markers are under investigation but none yet is ready for clinical use.
Biopsy
Obtaining a tissue diagnosis for HCC is not considered a mandatory step in the majority of cases of HCC. In the case of nodules larger than 1 cm in diameter the diagnosis of HCC may be established confidently using radiological studies without requiring histology examination. 60,74,75 In patients with cirrhosis the likelihood of lesions being HCC is more than 95%.76,77 Combining this high likelihood of HCC to the advantage of new imaging studies detecting arterial perfusion, the diagnosis of HCC can be made positively in the majority of cases without biopsy. These tests can differentiate HCC from benign liver lesions and from secondary tumors with high degrees of accuracy78–80 and biopsy will only be required if the imaging is atypical. Among 160 patients with 225 focal liver lesions evaluated by means of sequential radiological imaging studies in preparation for planned surgical therapy, the preoperative diagnostic accuracy rate without histological confirmation was reported to be 98.2%, with a sensitivity of 100%, and a specificity of 98.9%.81 In addition, it has been reported that the false negative rate for fine needle aspiration or even true cut biopsy in detecting HCC is as high as 20%.79,82 For lesions smaller than 1 cm a repeat US is recommended in 3 to 6 months. If the lesion is shown to be growing or changing character then it should be further investigated depending on size. If the lesion is stable in size then the imaging modality should be repeated again and if it is stable over 2 years the patient could revert to routine surveillance.
An important although seemingly rare complication of tumor biopsy is needle tract tumor seeding. This complication has been estimated to occur in about 1% to 5% of biopsies and is especially important if the patient is a candidate for liver transplantation or surgical resection74,79,83 In liver lesions without cirrhosis and no clear radiological diagnosis tissue diagnosis is important.60
If histology is required, the highest rate of diagnostic accuracy (97%) is achieved by combined use of fine needle aspiration cytology plus intranodular and extranodular fine needle microhistology.84 Liver biopsy may be useful not just in the diagnosis of HCC but also to evaluate the non-tumorous liver to guide further therapy. If the clinical presentation is doubtful and the stage of cirrhosis needs further clarification then a biopsy of the non-tumorous liver may be helpful.84
A particularly challenging issue for pathologists dealing with HCC is the distinction between high-grade dysplastic nodules from well-differentiated HCC. More recent multiple stain techniques have improved the ability of pathologists to differentiate the two, like heat shock protein (Hsp70), glutamine synthetase, glypican, CD34, and cytokeratin stains and others which are recommended by other guidelines in such cases.60
The Noninvasive Approach in the Diagnosis of HCC
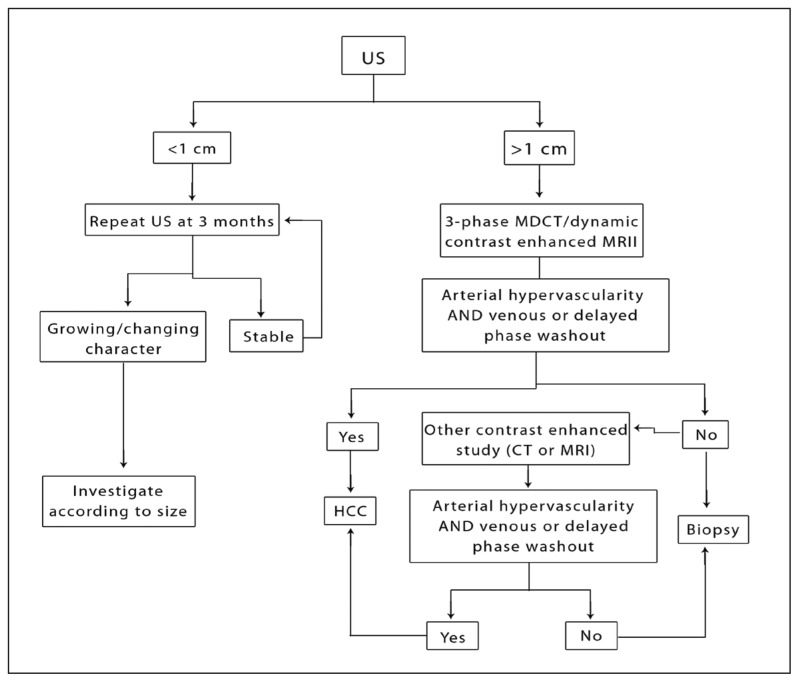
The European Association for the Study of the Liver (EASL) was the first to recommend a non-invasive approach to the diagnosis of HCC (Figure 1).85 In their most recent HCC guidelines the American Association for the Study of the Liver (AASLD) recommends making the diagnosis of HCC based on only one imaging modality (CT or MRI) showing the classical hypervascular appearance and washout pattern described above if the underlying liver is cirrhotic and the nodule exceeds 1 cm.60 Only if the findings are not typical of HCC then a second imaging modality may be performed and if still atypical, the lesion must be biopsied. This applies to lesions larger than 1 cm while all lesions smaller than 1 cm should be followed with repeat imaging in 3 months using the same radiological technique of initial detection (Figure 1).
Figure 1.
Diagnostic algorithm for HCC.
RECOMMENDATIONS.
US should be the initial radiological investigation performed when HCC is suspected (Grade C).
Triphasic or four phasic CT scan or MRI are the radiologic procedures of choice to confirm the diagnosis of HCC (Grade B).
It is extremely important that the CT scan and MRI are done in a standard triphasic or four phasic technique and is read by a trained radiologist (Grade B).
-
The diagnosis of HCC may be positively made if all the following conditions are satisfied (Grade B)
The liver is cirrhotic or the patient has chronic hepatitis B.
The lesion is larger than 1 cm in diameter.
One imaging modality (CT or MRI) confirms early arterial enhancement and venous washout.
If the radiological features are not characteristic or the vascular pattern on imaging is not typical for HCC then another contrast enhanced study should be obtained or a biopsy taken to confirm or rule out the diagnosis (Grade C).
For lesions less than 1 cm it is recommended that a follow up imaging be obtained in 3–6 months using the same modality used for the initial testing. If there is growth in size of the lesion then follow the recommendations of lesions above 1 cm while if there is no growth the lesion must be reimaged in 3–6 months and if no growth is demonstrated over 2 years the patient may revert to the routine surveillance program (Grade B).
-
A histological diagnosis is only recommended in the following circumstances (Grade B):
If the radiological findings are not characteristic or the imaging vascular pattern is not typical for HCC on two contrast-enhanced studies.
If the liver is not cirrhotic and the patient does not have chronic hepatitis B, but the vascular pattern is characteristic of HCC in contrast enhanced imaging studies.
If the biopsy is negative the lesion must be followed with imaging every 3–6 months until the nodule disappears, enlarges or displays diagnostic characteristics of HCC. If the lesion enlarges but is still atypical for HCC on imaging it should be re-biopsied (Grade C).
Because of low sensitivity and specificity, AFP should not be used as a surveillance or diagnostic test for HCC (Grade B).
AFP may be useful in follow-up of patients especially after treatment if it was elevated at diagnosis (Grade B).
This noninvasive diagnostic approach has been validated in a number of studies. For example, Bolondi et al conducted a prospective study in which they compared the presence of arterial hypervasularity on contrast perfusion ultrasonography and CT to the results of biopsy in 72 liver nodules.86 They found that in nodules larger than 2 cm, all lesions demonstrating arterial hypervascularity turned out to be HCC. In lesions less than 2 cm only 71% of these lesions were diagnosed as HCC. On the other hand, 8.3% of proven HCC on biopsy did not show typical hypervascular enhancement on radiological examinations (all less than 3 cm). Similar findings were obtained by other studies validating the noninvasive radiology approach especially the study by Forner et al.63,87,88
It is important to note that in order for these non-invasive guidelines to be accurate, standard techniques and protocols need to be followed while performing the multiphase CT and MRI techniques and experienced pathologists need to be involved in reading HCC biopsy results especially with smaller lesions that can be vastly difficult to interpret. It is also important to note that this method of diagnosis only applies to patients with cirrhosis and probably patients with chronic hepatitis B.
Staging
After making the diagnosis of HCC, the next step in the management should be staging. An ideal staging system should be able to separate patients into distinct clinical groups based on survival so that appropriate treatment modalities can be applied. This system should be able to incorporate the tumor characteristics, liver function, and the patient’s overall general functional status. There are now more than 10 staging systems for HCC. They each have specific advantages and disadvantages. The most clinically relevant systems are reviewed.
The TNM Classification System
This system is a cancer staging system that describes the extent of all cancers and is developed and maintained by the International Union against Cancer.89,90 It has the advantage of accurately describing the tumor characteristics and stage, but it does not take into consideration the liver function, which is a major element affecting survival of the patient and the choice of the therapeutic modality. Although this system has undergone multiple revisions it remains unable to accurately prognosticate HCC patients. Similarly, the Japanese classification suffers from similar shortcomings.91
RECOMMENDATIONS.
-
Initial evaluation of patients with HCC should include the following (Grade D):
A complete history.
A full physical examination.
Initial laboratory tests including complete blood count, random serum glucose, serum electrolytes, renal function, alpha-fetoprotein, serum calcium, prothrombin time, liver profile, and investigations for the cause of liver cirrhosis like hepatitis B and C serology.
CT scan of the chest must be performed to rule out metastasis before invasive therapeutic procedures or treatment with curative intent is planned.
For staging purposes, there are many staging systems each with their own advantages and disadvantages. Based on the available literature and associations’ recommendations, the BCLC staging system is the one with the most advantages and has been shown to be most useful in clinical practice (Grade B).
The Okuda System
This system takes into account both the tumor characteristics and the liver function.92 It is based on gross tumor factors and is not very useful clinically. It enjoyed wide acceptance at a time when there were limited therapeutic options for patients with HCC, but has limited clinical utility nowadays.
The Cancer of the Liver Italian Program (CLIP) System
This system has been developed based on a retrospective analysis of 435 Italian patients with HCC using a Cox proportion hazard model.93 This system has the advantage over the Okuda score in that it is more evidence-based and it gives more leeway to assess patients who are not terminal.94 It has been further prospectively validated in two Italian studies95,96 and also retrospectively validated in 662 Japanese patients.97 In two studies, this scoring system was found to be superior to the Okuda system95,96 Some groups have criticized the CLIP score for not being adequately assessed in patients undergoing radical resection. 94 In addition, it does not account for patient symptoms and general status and so has less clinical utility.
The Barcelona Clinic Liver Cancer (BCLC) System
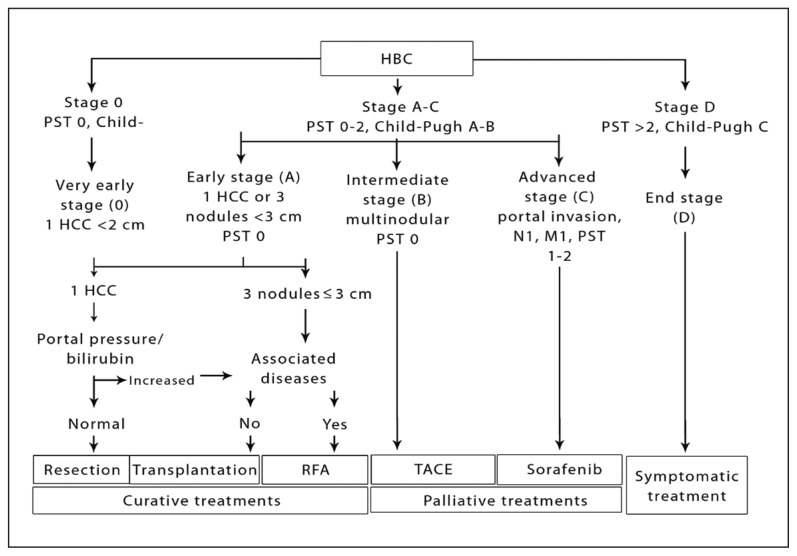
This scoring system is very clinically oriented (Table 1). It takes into account the three major factors that are important in deciding on the treatment options—tumor stage, liver function, and performance status.98 The BCLC staging system differentiates HCC patients to very early, early, intermediate, advanced, and end-stage patients. It also links each stage with specific treatments and prognosis. This staging system has been externally validated.99,100 It clearly separates patients with early disease who should undergo aggressive therapy from end-stage patients. In addition, this staging system is the most frequently used system in recent reported clinical trials addressing HCC therapeutic modalities. It is also recommended by both EASL and AASLD in their most recent guidelines because it remains to be the clinically most relevant staging system (Figure 2).
Table 1.
The Barcelona Clinic Liver Cancer (BCLC) staging system.
| BCLC stage | Performance status | Tumor features | Liver function |
|---|---|---|---|
|
| |||
| A1 | 0 | Single <5 cm | No portal hypertension |
| A2 | 0 | Single <5 cm | Portal hypertension, normal bilirubin |
| A3 | 0 | Single <5 cm | Portal hypertension, abnormal bilirubin |
| A4 | 0 | 3 tumors <3 cm | Not applicable |
| B | 0 | Large multinodular | Child-Pugh A-B |
| C | 1–2 | Vascular invasion or metastases | Child-Pugh A-B |
| D | 3–4 | Any | Child-Pugh C |
Figure 2.
The BCLC staging system and treatment algorithm.
Management
Management Plan
Management of HCC patients must be done using a multidisciplinary approach. Hepatologists, liver surgeons, transplant surgeons, oncologists, diagnostic radiologists, interventional radiologists, palliative care physicians, pathologists, nurses, patient education specialists, and pharmacists should all be active members in the care of HCC patients. A regular liver tumor board meeting is an ideal venue to discuss the management of these patients.
Liver Transplantation
Orthotopic liver transplantation (OLT) is theoretically the best treatment available for HCC because it results in complete excision of the cancer, removes remaining liver tissue at risk for the development of de novo cancer, and restores hepatic function.94 The major practical obstacles to this model of therapy are the extreme shortage of organs and the associated risk of procedure-related mortality, which makes it an impractical option for most patients.
In 1996, Mazzaferro and colleagues reported their experience in transplanting patients with HCC.101 They reported a 75% 4-year survival rate when using the criteria of only transplanting single HCCs that are less than 5 cm, or multiple HCCs that are less than three in number and each less than 3 cm. The excellent survival rate seen in the Mazzaferro series was replicated in multiple published series.102 These criteria (named the Milan criteria) have been accepted worldwide as the standard of care because the survival data is comparable to the majority of patients receiving OLT with and without HCC. In addition, the recurrence rate after transplantation when these criteria are used is extremely low. It is worth mentioning that these criteria were developed in a time when the waiting list for OLT was around 6 months. Currently, most waiting times are much longer resulting in tumor growth and a high percentage of patients dropping off the waiting list. Causes of delisting include extrahepatic spread, increase in the size of the tumor beyond transplantation criteria or vascular invasion. This clinical observation has been shown elegantly by the Barcelona group who compared, in an intention-to-treat analysis, the 2-year survival of patients transplanted in two periods and found a decrease in survival from 84% to 54% as the mean waiting time increased from 62 days to 162 days.93
Many strategies have been suggested to overcome this major limitation. Some centers have suggested expanding the tumor size criteria described by Mazzaferro and colleagues. Yao reported on the survival of 70 consecutive patients undergoing OLT for HCC including 25% with either solitary tumors 5 to 6.5 cm in diameter or less than or equal to three nodules each less than or equal to 4.5 cm with a total tumor diameter less than 8 cm.103 Although these survival results were not totally replicated by other centers, many proposed that the expanded criteria maintained a 5-year survival that exceeds 50%. These results suggest that the Milan criteria can indeed be expanded, which may give patients the chance to stay on current waiting lists.103–106 There are multiple ethical concerns in this approach as the acceptable life expectancy of HCC patients with extended transplant criteria cannot be defined and the influence this has on currently long waiting lists is undefined. This is a particularly difficult issue in Saudi Arabia, which is burdened with a severe organ shortage thereby limiting the availability of transplant opportunities to the majority of patients. In addition to the difficulties above, many studies have shown that the most important predictor of HCC recurrence after transplantation is micro- or macroscopic vascular invasion, parameters that are not available prior to transplantation, making the assessment of recurrence vastly difficult.
Another solution offered to deal with long waiting lists for transplantation in HCC is living-related transplantation. There are now many studies that suggest that this modality is as effective and safe as cadaveric transplantation for patients with HCC.107–112 In addition, the Milan criteria have also been shown to be an effective method to decide on the candidacy of this modality of transplantation. Expansion criteria have also been proposed, but there is very limited data on outcomes of this approach in living-related transplantation. Multiple statistical and mathematical models have also shown that living-related transplantation for HCC may be cost effective and life saving compared to cadaveric transplantation if the waiting time is longer than 7 months.113,114
In many centers, adjuvant therapy is given to delay the progression of HCC while patients are awaiting OLT or to shrink the size of HCC to fit OLT criteria (down staging). For example, radio frequency ablation (RFA) has been tested in a number of small case series. In one study, 50 patients meeting the Milan criteria were treated with RFA and with a mean waiting time of 9.5 months before transplantation there were no dropouts from the waiting list and there was an 83% 3-year survival and a 4% post-transplantation recurrence.115 This approach seems to be effective if the waiting time exceeds 6 months.116
Similarly, chemoembolization has been used in this setting as well. In a study by Graziadei et al, 48 patients satisfying the Milan criteria were treated with chemoembolization while waiting for transplantation. They reported no dropouts from the waiting list and 5-year survival rates of 94% despite a mean waiting time of 178 days.117 In another study, 45 patients were treated in a similar protocol with a 6-month 15% dropout rate due to tumor progression and 25% at 12 months.118 One study compared between patients who received chemoembolization and those who did not, and found that responders to treatment fared better than non-responders with a trend towards improved survival compared with the untreated group.119 Most importantly, complications arising from chemoembolization in these pre-transplantation patients were rare and did not frequently cause dropout from the list in the majority of the studies.102 Results with systemic chemotherapy have been conflicting, but more recent studies have not been favorable.120 It must be mentioned that all adjuvant therapies studies are only useful if the waiting time for transplantation is more than 6 months.
In Saudi Arabia, although liver transplantation is available (with a long and rich experience that has accumulated over the past 15 years), these programs are crippled by the lack of cadaveric organ donation. With current long waiting lists, liver transplantation does not seem to be a practical option for the majority of HCC patients in Saudi Arabia. Living related transplantation is being increasingly performed in the Kingdom and its role in HCC is yet to be defined.
Hepatic Resection
Major advances in the field of hepatobiliary surgery and anesthesia have occurred in the past 20 years making major hepatic resection a less morbid procedure. In most large hepatobiliary centers in the world, operative mortality in well-compensated cirrhotic patients is less than 5%.121 In fact, some leading centers report no mortality after over 100 consecutive cases.122,123
Although tumor resection removes the visible portion of the cancer, it is clearly inferior to transplantation in that it cannot guarantee the removal of non-visible tumor and microscopic satellite lesions. It may leave remaining diseased liver tissue that has the potential to develop other de novo HCC, in addition to the risk of deteriorating hepatic function.
Large series of liver resection for HCC report a 5-year survival of 40% to 55%.60 The population in these studies was heterogeneous with different stages of cirrhosis. In patients with small HCCs and relatively preserved liver function, Bismuth et al have shown excellent long-term survival results of 40% and 26% at 5 and 10 years, respectively.124 Studies that use stringent criteria have reported higher survival rates. These criteria included solitary tumors less than 5 cm in diameter, with no evidence of vascular invasion or extra-hepatic spread, and with either no evidence of cirrhosis or well compensated Child A cirrhosis.
One of the major factors determining the candidacy of patients for radical resection is the stage of the underlying liver disease. Although many surgeons still use the Child-Pugh classification to assess liver function and would perform a liver resection on non-cirrhotic, Child A, and early Child B patients,121 the best evidence suggests that other parameters may be more accurate in determining surgical hepatectomy risk in terms of hepatic decompensation. Two parameters have been shown to be most predictive of decompensation post-liver resection. These are signs of clinically relevant portal hypertension (defined as presence of varices, splenomegaly, platelet count <100 000 or a hepatic vein pressure gradient > 10 mmHg) and elevated bilirubin (more than 1 mg/dL).60 When these specific criteria are used to select patients, the 5-year survival after resection may be as high as 70% and these patients are very unlikely to decompensate after resection. 104
The principles of liver resection in cirrhotic patients are parenchymal preservation, minimal blood loss and a negative resection margin of at least 1 cm. Contraindications to surgical resection of HCC are the presence of extrahepatic metastases, diffuse bilobar disease, and an underlying severe liver dysfunction. Invasion of the biliary confluence or tumor thrombus in the main portal vein, major hepatic veins or inferior vena cava are relative contraindications. Parenchymal preservation is a major challenge given the difficulty in assessing the quality of the liver tissue. Other common difficulties surgeons are faced with when planning HCC resection are related to the extension of the disease that can make liver resection impossible. For this reason, most series show that less than 10% of patients with HCC will be candidates for resection.125 This problem is even worse in Saudi Arabia since effective HCC screening programs are not widely implemented. The other problem is that even in patients who do get resected, the intra-hepatic recurrence rate is high (around 70% in 5 years). The most powerful predictor of recurrence is the presence of microvascular invasion and the presence of satellite lesions besides the primary tumor.60
Based on the above, in patients without cirrhosis or early cirrhosis (indicated by a normal bilirubin and no signs of clinically significant portal hypertension), liver resection should be considered if there is no evidence of extra-hepatic or major vascular spread. This is particularly true if the patient has a single lesion, since most published survival data failed to show similar results in patients with multifocal disease. Long-term survival in patients with multifocal disease is as low as 50% even if the liver is non-cirrhotic.104 Studies on preoperative (neoadjuvant) therapy using local or systemic chemotherapy, and on adjuvant therapy using systemic chemotherapy, immunotherapy, or interferon have not shown improvement in survival.60 Trained hepatobiliary surgeons are available in most tertiary care centers in Saudi Arabia. The main problem faced by all is difficulties with referral in a timely manner of patients who are likely to benefit from surgery.
Ablation
Most patients with HCC are unsuitable for surgical therapies due to the extension of the disease, poor hepatic reserve, or coexistent morbidity. Therefore, non-surgical therapies play a central role in the management of this disease.126 Ablation of HCC has been carried out for many years now. This can be done by either chemical means (absolute alcohol or trichloracetic acid) or by physical means (cryoablation, radiofrequency ablation, microwave coagulation, or injection of hot saline). Percutaneous ethanol injection (PEI) and RFA are described in these guidelines as they are the widely available modalities. In general, percutaneous treatments are best offered to patients with early stage HCC and relatively small size tumors.
Percutaneous ethanol injection (PEI) is a widely accepted minimally invasive method of treating HCC. Its acceptance is based on the ease of treatment, minimal and inexpensive therapeutic equipment required, and good clinical results. It is achieved by injection of 95% absolute ethanol into the tumor under US or CT guidance. Ethanol causes cellular dehydration and subsequent necrosis of the tumor. The goal of this therapy is to achieve complete necrosis of the tumor with extension into the perineoplastic tissues. The amount needed to ablate a given HCC varies with its size. The typical amount given per session is 1 to 8 cc, which can be done two times per week (may be done daily now) and is usually performed as an outpatient procedure under local anesthesia by an interventional radiologist. Small lesions may be ablated at a single session while larger lesions require multiple sessions to avoid excessive toxicity. Alternatively, large-volume PEI can be performed under general anesthesia.127 Post procedure imaging and AFP level should be obtained at 1 month and then every 4 to 6 months to assess tumor response and potential recurrence. Absence of enhancement on CT scan after the procedure is considered evidence of successful tumor necrosis. Common side effects are pain, fever, and a feeling of intoxication. In the largest series to report complications from PEI the mean number of sessions needed to destroy an HCC nodule was 6.7.128 One death (0.09%) and 34 complications (3.2%) were reported, and eight episodes of bleeding and seven cases of tumor seeding occurred.
PEI can achieve necrosis rates above 90% for HCCs less than 2 cm and above 50% for HCCs 3–5 cm in size.129–131 In a large series of 746 patients, the 5-year survival of patients with well-compensated cirrhosis and a tumor smaller than 5 cm who were treated with PEI was 47%, compared to 29% for patients with more advanced impairment of liver function.132 There are no randomized controlled trials comparing resection versus alcohol injection. In the study by Livraghi including 260 (<5 cm) tumors in Child A cirrhosis, the 3-year survival was 71% for surgery and 79% for PEI compared to 26% for no treatment.128 Similarly, Castells et al reported on 30 patients with HCC treated with alcohol injection compared to 33 patients undergoing surgical resection; survival rates were similar in the two groups.133 Cohort studies suggest that PEI improves the survival of Child A patients with small HCC.133,134 Recurrence after effective percutaneous treatment is as frequent as after surgical resection (about 50% at 3 years and above, 70% in 5 years).130,135 The major disadvantage of PEI is the frequent need for multiple treatment sessions to achieve complete ablation of a lesion. Furthermore, a recent meta-analysis showed that PEI was inferior to RFA, particularly for tumors >2cm.136
In radiofrequency ablation (RFA), thermal destruction is achieved with an electric current that passes to the tumorous tissues via an electrode tip, placed percutaneously under imaging guidance, resulting in heat generation and coagulation necrosis.137 This technique seems to be very effective with low recurrence rates. In a study by Curley where 149 tumors were ablated, all tumors showed initial complete ablation with the local recurrence rate at 19 months of 3.6%.138 In another series of 126 HCCs greater than 3 cm, complete necrosis was produced in about 50% of patients.139
In comparison to PEI, RFA is believed to be more effective and requires fewer ablation sessions, but at an increased cost. In a comparative study between ethanol injection and RFA complete tumor necrosis was seen in 90% of patients with RFA and 80% with ethanol injection. 139 In addition, the number of sessions required to complete the tumor necrosis was less in the RFA group. However, the complication rate was higher in RFA than in the ethanol ablation group in this study. Other randomized studies showed similar findings.140 For example, in a randomized trial involving 232 patients with less than three tumors each smaller than 3 cm, the 4-year survival rate was 75% in the RFA group and 57% in the PEI group with no difference in complications.141
More recently, a large randomized study revealed the superiority of RFA over PEI.142 In this study from Taiwan, 157 patients with HCCs less than 4 cm were randomly assigned to conventional PEI, a higher dose PEI injection, and RFA. The rate of complete tumor necrosis was 88%, 92%, and 96% respectively. Significantly fewer sessions were needed in the RFA arm, and the tumor progression rate was lowest in the RFA arm. Most importantly, the overall survival as well as the cancer-free survival rates were significantly higher in the RFA arm. In a recent Cochrane database systematic review of all the evidence comparing RFA and PEI it was concluded that RFA seems to reach higher recurrence free survival rates.143
RFA is an effective option for small lesions in comparison to surgery. In a prospective randomized trial on 180 patients with a solitary HCC less than or equal to 5 cm, the 1-, 2-, 3-, and 4-year overall survival rates after RFA and surgery were 95.8%, 82.1%, 71.4%, 67.9% and 93.3%, 82.3%, 73.4%, 64.0%, respectively. The corresponding disease-free survival rates were 85.9%, 69.3%, 64.1%, 46.4% and 86.6%, 76.8%, 69%, 51.6%, respectively. Statistically, there was no difference between these two treatments.144
In general, whether for ethanol injection or RFA, a contrast CT at least 4 weeks after the ablation is considered to be the standard imaging modality to assess the effectiveness of the ablation.45,60 RFA is technically difficult and risky when dealing with exophytic lesions, those near the gallbladder or kidney, or lesions high in the dome of the liver. The risk of complications is even higher for tumors in close proximity to the bowel (less than 1 cm), particularly the colon. For such tumors some authors have suggested a laparoscopic RFA approach. In a trial to compare the long-term outcome of percutaneous vs. surgical RFA in dangerous locations, 162 patients were treated with either percutaneous or surgical RFA. No significant difference was observed in the curative rate between the two groups 91.3% vs. 96.8%.145 Possible side effects include bleeding from the needle site, fever, abdominal pain, and transient elevation of serum transaminases. A single report has raised the possibility of a high rate of needle tract tumor seeding (up to 12%),146 but larger series report a rate of only about 3%.60
Keeping in mind these results, it is reasonable to conclude that RFA is more effective than PEI and requires fewer sessions. It may be associated with improved survival when compared with PEI, but probably at the expense of more complications. It is worth mentioning though that for lesions less than 2 cm it is likely that PEI and RFA are equality effective as shown by multiple studies and a meta-analyses.136,139,140
Both PEI and RFA are available in most tertiary care centers in Saudi Arabia but not in many of the more peripheral centers and in private centers. Trained competent interventional radiologists are not readily available in all centers. Again, early and appropriate referral is a major issue.
Transarterial chemoembolization
In this technique, catheterization is performed into the segmental hepatic artery supplying the tumor. Chemotherapeutic agents (commonly doxorubicin or cisplatinum) are mixed with a water-soluble contrast agent or lipiodol (an oily contrast agent that is selectively concentrated in the tumor for many weeks) to form an emulsion, which is then injected into the artery followed by occlusion of the artery using a material to obstruct the flow (e.g. Gelfoam). This allows higher concentration of the drug into the tumor, lower systemic side effects, and induction of necrosis within the tumor. Non-controlled studies have shown that vascular occlusion with particles between 150 and 700 micometers either with gelatin sponge or with polyvinyl alcohol particles provide good response rates. Studies are conflicting in regards to the best chemotherapeutic agent to be used.
Transarterial chemoembolization (TACE) is effective 80% of the time in causing significant necrosis of the tumor. The so called “post-embolization syndrome” consisting of abdominal pain, ileus, and fever may be seen in as many as 60% to 80% of patients. Fever usually resolves with symptomatic therapy within a few days and does not require prophylactic antibiotics.147 Potential serious side effects include liver failure, severe pain, and formation of liver abscess. Death may be seen in as many as 4% of Child A patients and in as many as 10–20% of Child B and C patients.148
TACE should not be carried in the presence of severe liver damage (Child-Pugh C patients), or main portal vein thrombosis due to the high chance of acute liver decompensation secondary to the exaggerated liver necrosis. TACE is also contraindicated in cases of porto-systemic shunts, either surgical or intrahepatic (transjugular intrahepatic portosystemic shunt, TIPS). Measures should be taken to block any existing systemic arterial shunt to the tumor. In patients with partial portal vein thrombosis or thrombosis of an intrahepatic branch of the portal vein, the procedure may probably still be done, but these patients usually carry a poor prognosis to start with and the procedure is likely to be less beneficial. In addition, the complication rates are higher. Treatment response is usually assessed by the reduction in tumor volume or the presence of intratumor necrosis in CT scan 4 weeks after the procedure or the reduction in AFP levels if initially high.
At least six randomized controlled trials have been done to evaluate the efficacy of embolization or TACE compared to conservative management.127 They all showed a significant effect on tumor size but failed to show a survival benefit. More recently, two well-conducted large randomized controlled trials comparing TACE to conservative management revealed the clear effectiveness of TACE with a strong survival benefits. This was confirmed by a recent meta-analysis.149,150 However a recent Cochrane review suggested that the evidence supporting TACE was not enough. This review was weakened by the inclusion of studies of suboptimal value and treatment policy, and also the consideration of studies of patients who should not have been treated with TACE.151
In a large study published by the Barcelona group a clear survival advantage was shown with TACE.152 In this trial, 112 patients with HCC were included and randomized to arterial embolization only, TACE, or control treatment. Survival at 2 years was 62% in the chemoembolization arm, versus 50% in the embolization only arm, and 27% in the untreated arm. In a similar randomized study from Asia, 80 patients were randomized to receive TACE vs. medical management only.153 TACE resulted in a marked tumor response, and the actuarial survival was significantly better in the TACE group (1-year 57%, 2-year 31%, 3-year 26%) than in the control group (1-year 32%, 2-year 11%, 3-year 3%). The likely explanation for the significant effect of TACE in those two trials is the highly selective approach the investigators took in enrollment as compared to the larger tumors and more advanced liver disease seen in the previous trials. In addition, in those two trials each patient received multiple sessions of TACE in a scheduled manner regardless of the response to the first session. Moreover, in a meta-analysis of seven randomized trials by Llovet and Bruix, TACE was again found to significantly reduce the 2-year mortality from 41% in the control group to 27% in the TACE group.154 This effect was only seen in the chemoembolization group and not in non-chemotherapy embolization group. Interestingly, the overall objective response at 1 to 6 months was seen in only 35% of treated patients.
There is only one small study that reported the radiological response rates of TACE in Saudi Arabia.155 In this retrospective study on the initial experience of TACE in King Khalid University Hospital in 2006, 15 patients were studied. Mean age was 63 years and 66% were males. Radiological response was complete in 26%, partial in 13%, and no change in 33%. Lipiodol uptake was estimated to be >75% in 33% of patients, 50–75% in 13%, and <50% in 26%. One patient died and two were lost to follow up.
Because TACE can be thought of as a “medical resection”, it should only be performed in patients with early cirrhosis. Most of the studies include only Child-Pugh A patients (70–90%), Okuda stage 1 (47–90%), with multi-nodular HCC without vascular invasion (overall >95%).154 In spite of that, the trial showing a survival benefit using TACE included patients with Child-Pugh score B.152 If TACE is performed, a protocol similar to the one published by the Barcelona group should be adopted until future evidence proves or disproves the utility of this protocol. In this published protocol in the randomized study mentioned above, TACE was performed at baseline, 2 months, 6 months, and every 6 months thereafter. It is important to note that best results with TACE are obtained when patients with Child A liver disease who are relatively asymptomatic are treated.
More recently, spheres that contain chemotherapy have been developed and have been shown to be associated with less side effects and reduction in systemic effects.156–158 Also, arterial occlusion is more predictable but the overall efficacy is probably similar to the conventional TACE. A recent randomized trial comparing of doxorubicin-drug-eluting-bead (DEB) chemoembolization and doxorubicin/lipiodol embolization showed decreased systemic side effects and decreased rates of liver failure with the DEB treatment.159
TACE is available in large hospitals in main cities in Saudi Arabia. However, like local ablative therapies, there is a shortage of trained interventional radiologists who are able to perform TACE readily in the majority of centers.
Radioembolization
Radioembolization with yttrium-90 (Y90) microspheres is a new concept in radiation therapy for HCC.160 Here, radiolabeled particles are injected through the hepatic artery, become trapped at the precapillary level and emit lethal internal radiation. This method limits exposure to the surrounding normal parenchyma, thus allowing higher dose delivery compared to an external beam. Radioembolization has shown promising outcomes in primary and secondary liver malignancies in several studies. There are currently two types of radioembolization using Y90 microspheres. TheraSphere (MDS Nordion, Ottawa, Ontario, Canada) is made of glass and SIR-Spheres (Sirtex Medical, Sydney, Australia) is made of resin. Treatment response is the same despite differences in physical characteristics. The few studies on the use of TheraSphere in managing HCC have been summarized by Salem and Thurston.161 Kulik et al reported on a group of 21 patients from a large database of 251 patients who had undergone Y90 glass microsphere therapy and subsequently bridged to transplantation.162 The majority of patients experienced toxicities including fatigue. Mean AFP reduced by 33% from pre-treatment levels and 66% of patients had complete necrosis by pathologic exam. The authors concluded that Y90 treatment achieved complete necrosis in the majority of targeted lesions in patients bridged to transplantation, but that recurrence was a possibility despite the radiographic findings of complete necrosis. Subsequently, Kulik et al went on to report the safety of Y90 in a cohort of 108 patient treated with glass microspheres, with subset analyses evaluating differences in patients with and without portal vein thrombosis.163 They concluded that the microembolic effect of Y90 microspheres did not raise the risk of liver adverse events in patients with proven portal vein thrombosis. Glass microspheres did not result in the microembolic effect that is seen with other loco-regional therapies using larger diameter particles.
In a more recent study describing the European experience with this therapy, 108 patients were treated and according to the EASL criteria, 3% had complete response, 37% partial response, and 53% had stable disease. 164 The median overall survival was 16.4 months while the time to progression was 10.0 months. No lung or visceral toxicity was observed. In spite of these very promising results there has been no evidence of a survival benefit from this mode of therapy.
In a recent study published in an abstract form from Saudi Arabia, a retrospective chart review of all HCC patients treated with Y90 microsphere in King Faisal Specialist Hospital and Research Centre in the period from January 2008 to August 2010 was reported.165 Twenty-eight patients (21 males and 7 females) received Y90 therapy. Their ages ranged between 51 and 79 years (mean=66.5). Post treatment follow up duration ranged between 10 and 32 months (mean=21). The procedure was repeated in five patients of whom three had residual tumours while two developed new lesions. The average MELD score was 8.5 and 12 pre- and posttherapy, respectively. The MELD score increased by at least 10 points in five patients within the first 3 months after therapy. Mortality during the follow-up period was 10.7%.
In general, indications for radioembolization are similar to TACE except that it can be done in patients with portal vein thrombosis more safely. Side effects of radioembolization include fatigue, nausea, anorexia, vomiting, fever, abdominal discomfort and cachexia. Severe complications such as ulceration can be caused by the spread of the microspheres to the gastrointestinal tract. Careful mapping of the blood vessels to identify aberrant vasculature from the branches of the hepatic artery that supply the gastrointestinal tract can prevent this. Radiation pneumonitis has been shown to occur when the lung shunt function (LSF) is greater than 13%. Radioembolization has been recently introduced to Saudi Arabia. At least three centers are performing it currently.
Systemic Therapy
Until recently, systemic chemotherapeutic agents have not shown any promising results in HCC. The best single agent was doxorubicin, with response rates of 10% to 15%.166 More aggressive combination therapy showed no improved response.167 In a meta-analysis of the published randomized studies on HCC, neither doxorubicin nor any chemotherapeutic agent has been shown to have any survival benefit for HCC patients.168
More recently, sorafenib has been shown to be effective in improving survival of HCC patients. Sorafenib is a multikinase inhibitor with reported activity against Raf-1, B-Raf, VEGFR2, PDGFR, and c-Kit receptors, among others, and receptor tyrosine kinases and serine threonine kinases.169,170 The basis of therapy with this agent started with a phase II trial in which the observed median survival was 9.2 months and the median time-to-progression was 5.5 months, while the induced partial response was only seen in less than 5% of patients.171 The survival advantage of sorafenib was subsequently proven in a randomized controlled trial (SHARP trial) that included 602 patients with advanced HCC and preserved liver function (Child A).172 This trial observed a 31% decrease in the risk of death with a median survival for the sorafenib arm of 10.7 months vs. 7.9 months for placebo. In addition, sorafenib showed a significant benefit in terms of time-to-progression with a median of 5.5 months compared to 2.8 months for placebo. Common side effects of sorafenib therapy are diarrhea (11% grade 3/4), fatigue, weight loss, and hand-foot syndrome (8% Grade 3/4). The benefit of the treatment was also proven in HBV-related HCC in another randomized controlled trial from Asia.173
Although both large trials recruited mainly patients with Child-Pugh A cirrhosis, some patients recruited were in fact Child B. In addition, the use of sorafenib in patients with Child-Pugh B cirrhosis has also been studied in a phase II trial of 38 patients and serious adverse events were seen in 52% of patients with Child-Pugh A vs. 68% of patients with Child-Pugh B.174 Since the pharmacokinetic profile of sorafenib is similar in Child-Pugh A and B patients and the safety profile seems to be similar, sorafenib can be used in Child B in selected patients although the survival benefit in this subgroup of patients is not well defined.
The use of sorafenib as an adjuvant therapy, in combination with other modalities like TACE or RFA, and prior to liver transplantation is a matter of extensive research currently. In addition, multiple trials are under way to investigate the use of sorafenib with other chemotherapeutic agents. A trial comparing sorafenib with doxorubicin versus doxorubicin alone in 96 patients showed an overall survival of 13.8 months for the combination arm vs. 6.5 months for doxorubicin alone indicating the efficacy of sorafenib.175 There is also an ongoing double-blind, phase III trial comparing sorafenib with the combination of sorafenib and erlotinib.176 Sorafenib is available in many tertiary care centers in Saudi Arabia. Because of its high cost there are significant restrictions on its use. Developing clear guidelines for usage will likely help utilizing the drug in patients who are likely to benefit from it.
After the success of sorafenib has been established many other new agents are under extensive evaluation currently. These include other targeted therapies like sunitinib (trial stopped) and bevacizumab, anti-EGFR agents like erlotinib, and other agents that have different mechanisms.177 More recently, FOLFOX (folinic acid, fluorouracil, oxaliplatin) has shown some promising results as systemic therapy for advanced HCC. In an open-label, randomized, multicenter phase III study which was conducted in 371 patients in China, Taiwan, Korea and Thailand who had locally advanced or metastatic HCC and were ineligible for resection, the median overall survival with the FOLFOX4 regimen (n=184) was 6.40 months (95% CI: 5.30, 7.03) vs. 4.97 months (95% CI: 4.23, 6.03) with doxorubicin.178 These results achieved statistical significance only in the post hoc analyses conducted 5 months later. These results are somewhat encouraging and further trials are awaited.
Monitoring Response to Treatment
In general, and for most available therapies, monitoring with a contrast imaging study is the recommended way to assess efficacy of treatment. Both CT and MRI may be used. Special differences between different treatment modalities in terms of assessing response and monitoring has been discussed in their appropriate sections. In patients in whom AFP is high at diagnosis, AFP may be used for monitoring although it should not replace imaging means.
RECOMMENDATIONS.
Patients with HCC who are candidates for active treatment modalities should be managed in centers where expertise is available (Grade D).
The management plan for patients with HCC should be constructed in a multi-disciplinary forum consisting of a hepatologist, oncologist, interventional radiologist, hepatobiliary surgeon, pathologist, and palliative care physician if available (Grade D).
Liver tumor rounds should be held at every center dealing with HCC patients. The goal of this meeting is to discuss new cases of liver tumors and to reach a joint decision on the most appropriate management route for these patients. This would serve to improve recruitment in clinical trials and teaching of residents and fellows (Grade D).
The decision on the best treatment modality should be based on the following factors (Grade B):
1. The status of the underlying liver.
2. The performance status.
3. The number, size, and location of lesions.
4. The status of the portal vein.
Liver transplantation
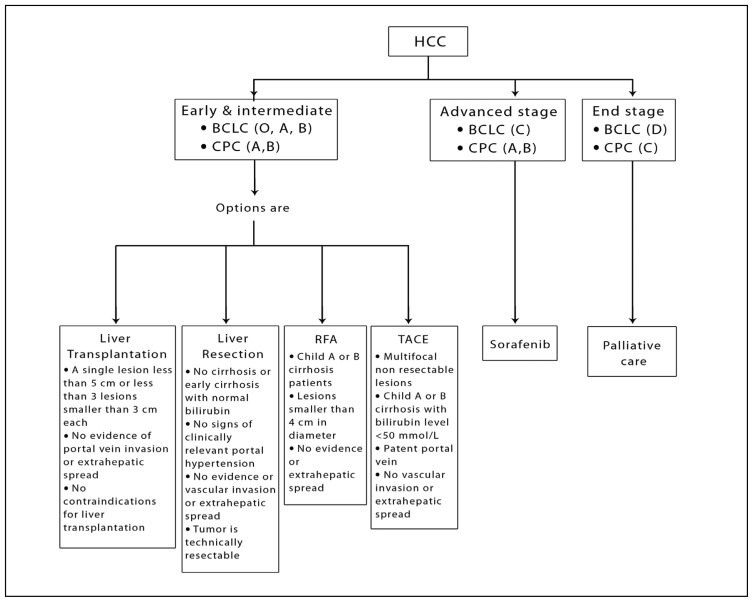
Patients should be considered for liver transplantation if they satisfy all the following indications (Grade B):
1. A single lesion less than 5 cm or less than three lesions smaller than 3 cm each.
2. No evidence of vascular invasion or extrahepatic spread.
3. No contraindications for liver transplantation.
To prevent the patient from outgrowing the above transplantation criteria while waiting on the transplant list, local ablative therapy or chemoembolization may be considered to control tumor growth if the waiting list exceeds 6 months (Grade C).
Living-related transplantation is a valid option for patients with HCC and the same indications for cadaveric transplantation should apply (Grade B).
Liver resection
Patients are optimal candidates for liver resection if they satisfy all the following criteria (Grade B):
1. No cirrhosis or early cirrhosis with normal bilirubin and no signs of clinically relevant portal hypertension (defined as presence of varices, splenomegaly, platelet count <100 000 or a hepatic vein pressure gradient >10 mm Hg).
2. No evidence of major vascular invasion or extrahepatic spread.
3. Tumor is technically resectable.
Best resection results are obtained in small single lesions without vascular invasion; for multifocal or larger lesions, consider other modalities of intervention even if resection is technically feasible (Grade C).
Local ablative therapy
Patients should be considered for local ablative therapies if all the following criteria are satisfied (Grade A):
1. Child A or B cirrhosis patients.
2. Lesions smaller than 4 cm in diameter.
3. No evidence of extrahepatic spread.
The local ablative procedure of choice is RFA. RFA is more effective than alcohol injection especially in larger lesions. Technical consideration in regards to the site of the lesion may favor one method over the other (Grade A). For lesions below 2 cm both PEI and RFA are equivalent (Grade B).
Local ablative therapy is the treatment of choice for lesions less than 2 cm and may be offered as a first line therapy if liver transplantation is not available or while awaiting liver transplantation (Grade B).
To assess response to local ablative therapy a repeat imaging study using the same contrast enhanced study performed for initial diagnosis should be done 1 month after the procedure (Grade C).
Chemoembolization
Patients should be considered for chemoembolization if they satisfy all the following criteria (Grade A):
1. Multifocal non-resectable lesions.
2. Compensated Child A or B cirrhosis with bilirubin level < 50 mmol/L.
3. Patent portal vein.
4. No vascular invasion or extrahepatic spread.
To assess response to TACE a repeat imaging study using the same contrast enhanced study performed for initial diagnosis should be done 1 month after the procedure (Grade B).
The recommended chemotherapeutic agents are doxorubicin or cisplatin (Grade B).
TACE with drug eluting beads may be associated with a better predicted embolization effect and less side effects but with no significant efficacy or survival advantage over conventional TACE (Grade C).
Radioembolization
Radioembolization with yttrium 90-labelled glass beads is effective in inducing necrosis in HCC with a good safety profile but has not been proven to improve survival (Grade B).
Radioembolization may be offered to patients with multifocal non-resectable disease and Child A or B cirrhosis who have either failed TACE or have portal vein thrombosis preventing TACE, and have failed sorafenib therapy (Grade D).
Systemic therapy
Sorafenib is recommended in patients who satisfy all the following criteria (Grade A):
1. Child A cirrhosis.
2. BCLC advanced stage.
3. Not candidates for transplantation, resection, local ablative therapy, or TACE.
Sorafenib may be considered in patients with earlier stages of HCC who have failed or have contraindications for resection, ablation, or chemoembolization (Grade C).
Sorafenib may be considered in selected early Child B patients who have good performance status (Grade B).
Sorafenib should be continued until symptomatic progression occurs or the patient develops unacceptable toxicity (Grade B).
Other therapies
All patients with Child C cirrhosis should be offered palliative care only unless they are candidates for liver transplantation (Grade D).
A specialized palliative care team should preferably be involved in the management of endstage HCC (Grade D).
In general, and for most available therapies, monitoring with a contrast imaging study is the recommended way to assess efficacy of treatment. Both CT and MRI may be used. In patients in whom AFP has been high at diagnosis, it may be used for monitoring although it should not replace imaging means (Grade C).
Prevention
Prevention of Infection
Vaccination is a very powerful measure to reduce the infection rate with hepatitis B and hence reduce the incidence HCC. The nationwide hepatitis B vaccination program launched in Taiwan in 1984 led to a reduction of the hepatitis carrier rate in children from 10% to less than 1% and to a reduction in the incidence of HCC from 0.70 to 0.36 per 100 000 between 1986 and 1994.179 In Saudi Arabia, routine hepatitis B vaccination of children was added as part of the extended program of immunization in 1989. A dramatic reduction was noted in the prevalence of hepatitis B from 6.7% in 1989 to 0.3% in 1997 and 0% in 2008.180 No evidence is available yet on the effect of this reduction on the incidence of HCC but this is expected to manifest with time. No effective vaccine is available for hepatitis C so far. The prevalence of hepatitis C has also reduced in the Kingdom recently, likely secondary to improved living conditions, hygiene, and adequate blood screening measures. This will likely also reflect in a reduction in the incidence of HCC.
Universal precautions for health care workers are effective in reducing the exposure to viral hepatitis. Post-exposure prophylaxis with HBIG and vaccination is important in reducing the risk of chronic hepatitis B. In the case of HCV, HCV-RNA should be measured and patient referred for consideration for early antiviral therapy as recent evidence suggests a very high response rate when patients are treated early.181
RECOMMENDATIONS.
The vaccination of all children in Saudi Arabia against hepatitis B starting at birth should be maintained and further encouraged (Grade B).
Vaccination of people at risk for hepatitis B infection should be encouraged (Grade B).
Post exposure prophylaxis for hepatitis B should be implemented in all hospitals (Grade B).
Post exposure testing for hepatitis C using PCR-based test and early treatment of hepatitis C should be implemented (Grade B).
All patients with viral hepatitis must be properly evaluated by a hepatologist for candidacy for antiviral therapy (Grade B).
All patients with hepatitis B related end-stage liver cirrhosis should be considered for long term antiviral therapy (Grade A).
Surveillance using US should be implemented in all cirrhotic patients every 6 months regardless of the cause of cirrhosis (Grade A).
Surveillance of all patients with chronic hepatitis B without evidence of cirrhosis cannot be recommended at this time but may be offered in certain high risk groups like patients above 40 years of age, patients with a family history of HCC, patients with high viral load and patients with indications of advanced fibrosis by noninvasive fibrosis markers or biopsy (Grade C).
There is no evidence to recommend surveillance of patients with chronic hepatitis C without cirrhosis (Grade C).
Any patient with a positive US should undergo further imaging with a triphasic or four phasic CT scan or an MRI (Grade B).
Treatment of viral hepatitis
If cirrhosis is the most important risk factor for the development of HCC, could the incidence of HCC be reduced by preventing cirrhosis or treating cirrhosis due to viral hepatitis with antiviral therapy? Many studies in hepatitis B and hepatitis C show that treatment of active hepatitis, especially when successful, may lead to a reduction in the incidence of HCC.
Multiple prospective and retrospective studies were performed to assess the effect of treating cirrhotic hepatitis C patients on HCC incidence and risk. In a small Japanese randomized study published in 1995 (and updated in 2001) there was a reduction in the number of HCC cases in patients with cirrhosis caused by HCV treated with interferon (IFN) versus untreated patients.182 In this study, after an average of 8.2 years of follow up, HCC developed in 73% of untreated patients but in only 27% of IFN treated patients. These high rates of HCC shed doubt on this study. Another study from Japan reached similar conclusions.183 Studies from Europe were less clear. Two short-term studies showed no benefit of IFN on the rate of HCC,38,184 while one showed a beneficial effect.185 Two reasons may account for the above discrepancy. First, the response rate of IFN is much higher in Japanese patients than in European patients (due to the difference in hepatitis C genotypes). In addition, the incidence of HCC in Japanese patients is significantly higher than that in Europe. Both of these factors may account for the ability to show a difference in HCC incidence in Japan but not in Europe.186
Since the issue was not resolved by individual studies, three separate meta-analyses187–189 and one systematic review190 were performed on all retrospective and prospective studies on this issue. Most of these reviews indicated that there was a significant but small effect of IFN therapy on the incidence of HCC, especially in patients who achieve a sustained virological response to therapy.
In a recently published extended analysis of the HALT-C trial there was a significantly lower incidence of HCC among patients given pegylated-IFN therapy who had cirrhosis, but not advanced fibrosis, based on an analysis of baseline biopsy samples. After 7 years, the cumulative incidences of HCC in treated and control patients with cirrhosis was significantly reduced to 7.8% and 24.2%, respectively, while in the treated and control patients with advanced fibrosis incidences of HCC was 8.3% and 6.8%, respectively.191 Because this possible benefit was only seen in long-term analyses and not in the actual trial, bias is likely to have influenced this result. In addition, a recent paper from the same HALT-C trial has shown an overall increased mortality in patients treated with interferon in that trial.192 In the Evaluation of PegIntron in Control of Hepatitis C Cirrhosis (EPIC) program there was no effect observed for treatment on incidence of HCC.193 Based on the above trials, IFN cannot be recommended in patients with cirrhosis or advanced fibrosis to reduce HCC incidence.
Furthermore, there is evidence that treatment of hepatitis C patients who are not cirrhotic may reduce the incidence of HCC. In a large retrospective study from Japan a reduction in HCC was seen in all patients receiving IFN (1.1%) compared to untreated patients (3.1%) after a median of 4.3 years of follow up.194 This effect was seen more in patients who achieved a sustained virologic response. These results are supported by two other large Japanese studies.195,196 From the above data, it seems reasonable to conclude that effective and early treatment of chronic hepatitis C patients with the intent to eradicate the virus and prevent the development of cirrhosis may reduce the incidence of HCC.
There is reasonable but non-conclusive evidence to suggest that the risk of HCC in patients with chronic hepatitis B is related to the level of viral replication.197 One randomized study reported a reduction in the rate of HCC among IFN-treated patients with chronic hepatitis B from 12% to 1.5% after 1–10 years of follow up.198 In addition, there are a number of non-randomized studies suggesting the same effect, but all were unable to provide conclusive evidence because of the small number of patients. At least seven non-randomized studies investigated the effect of IFN therapy on the rate of HCC development in patients with hepatitis B related cirrhosis which were summarized in a recent meta-analysis.188 This analysis suggests that indeed there is significant reduction in the incidence of HCC with IFN treatment.
Much more impressive results are seen with lamivudine. In a trial involving 651 patients with advanced cirrhosis secondary to hepatitis B who were randomized to receive lamivudine or placebo for 5 years, the study required early termination because of a marked reduction in mortality and achievement of end points in the lamivudine arm compared to the placebo arm.199 The Child-Pugh score increased in 3.9% in the lamivudine arm vs. 7.4% in the placebo arm. The incidence of HCC was 7.4% in the placebo arm vs. 3.9% in the lamivudine arm, which was statistically significant. Studies on the other oral agents are underway but none has been able to show a reduction in HCC incidence so far.
Is Surveillance Cost-Effective?
Surveillance for HCC meets a few but not most of the standard criteria for assessing the feasibility of screening programs. First, the disease is common and is associated with high morbidity and mortality rates. Second, surveillance has been shown to improve survival. Although many modeling data exist suggesting that surveillance can reduce HCC-related disease specific mortality in a cost-effective manner,200,201 there is only one randomized trial showing a survival benefit while many other studies did not. In the study showing benefit from China, 18 816 patients screened with 6-monthly AFP and US showed a reduced mortality rate by 37% in the screened arm even though the adherence to the surveillance was only around 60%.202 The screened population in this study was patients with current or previous exposure to hepatitis B.
Some small studies have also shown that tumors detected by screening are usually smaller and more amenable to potentially curative therapies. These studies were summarized and tabulated by Collier and Sherman.200 For example, in a study from Japan, 81% of 391 HCC detected by surveillance were considered suitable for curative resection compared with 46% of 1251 symptomatic HCC.203 When all the available studies were considered, of tumors detected by surveillance, 50% to 75% were unifocal and 3 cm or less in size and thus potentially curable, but in the majority of studies only 29% to 54% were actually resected due to the presence of other contraindications.200 All the studies in this area are subject to lead time bias resulting in a false impression of an extended survival which in fact is secondary only to longer detection and not to a real prolongation of life compared to unscreened patients. In their own screening program, Sherman et al screened 1069 HBV carriers for periods of 6 months to 5 years.204 Over this period, 14 tumors were detected, of which six were resected. Two tumors recurred after resection and one patient died while only three patients survived more than 2 years from diagnosis. Five other screening programs results were summarized by Collier and Sherman and all showed that a large number of patients are needed to be able to detect early tumors.200 In another study from Spain, Velazquez et al screened 463 cirrhotic patients every 3 to 6 months using AFP and US205 and were able to diagnose 38 patients with HCC during a mean of 33 months follow up. Thirdly, HCC surveillance seems to be cost effective. Many studies have been performed to assess the cost-effectiveness of screening for HCC.206,207 Allowing for limitations, all studies showed that the cost of these screening programs compared to the number of lives saved is within what would be considered a cost-effective screening tool.
Target population for surveillance
Although it is quite clear that the target population for screening for HCC should be patients with cirrhosis especially secondary to hepatitis B or C, the majority of these patients may remain asymptomatic for a long time.208 In addition, 20% to 56% of patients presenting with HCC may have previously undiagnosed cirrhosis. Authorities suggest that best candidates for screening are Child class A cirrhotic patients (as they may be candidates for resection or local ablative therapies), and Child B and C cirrhotic patients who are candidates for liver transplantation.60 Because potentially curative treatment options cannot be used in all patients with HCC, some suggest that only patients who are candidates for these therapies should undergo screening.
Recommendations are not clear regarding the need to screen chronic hepatitis B carriers. Studies from Asia suggest that the annual incidence of HCC in hepatitis B carriers is around 0.5%.33,34 On the other hand studies in North America are conflicting. Although the incidence of HCC in hepatitis B carriers is low, it is estimated to be at least 100 times more common that the general population.34 The annual incidence of HCC in male hepatitis B carriers from Asia only starts to exceed 0.2% at about 40 years irrespective of the presence of cirrhosis while it is much more related to the presence of underlying cirrhosis in western populations. For these reasons, if a patient is above 40 years of age or has a family history of HCC then screening may be offered as recommended by other guidelines.60 This particularly applies to male patients while surveillance in Asian women is recommended only after the age of 50. It is also known that HCC may develop at a younger age in Africans, but there is no clear recommendation in that regard. It is not clear how Saudi patients behave in this regard, but it would be safer to use recommendations for Asian patients recommended by the AASLD for Saudi patients due to infection at younger age and higher levels of endemicity compared to Western countries. Additionally, since HBV genotype D is the most common genotype in Saudi Arabia (a more carcinogenic genotype compared to genotype A) this also supports earlier screening. According to the Saudi Cancer Registry, HCC starts to peak at about the age of 45 years and so screening of patients after that age would be reasonable.6 Otherwise the risk of development of HCC in non-cirrhotic, inactive hepatitis B carriers is low and there is no evidence that they need to be screened.
As discussed before, the risk of HCC in cirrhotic HCV patients is about 2% to 8%. For that reason, all cirrhotic HCV-infected patients must undergo surveillance. In the HALT-C study the lifelong risk of HCC in HCV non-cirrhotic (but with advanced fibrosis) patients was 4.8%200 and most authorities do not recommend surveillance for this low risk. Cirrhotic patients who have cleared the virus have a reduced risk of HCC as discussed before, but still have a significant risk and must be included in surveillance programs like other cirrhotic patients.
Surveillance Methods
After identifying the target population, there needs to be a safe and effective screening tool. The two screening tools available are AFP and US. AFP has a low sensitivity (about 40%–60%), a reasonable specificity (80%–90%), but a poor positive predictive value of 9% to 32%, which makes it a poor screening tool.200,209 This poor utility of AFP has been confirmed by the HALT-C trial. In this study, designed to analyze the effect of maintenance IFN and ribavirin therapy in cirrhotic patients, both AFP and des-gamma carboxyprothrombin (DCP) were inadequate screening tools for HCC.210 The AASLD and EASL guidelines have concluded that AFP is an inadequate screening test for HCC.60 As a screening tool for HCC, US has a sensitivity of about 70% (depending on the size of the lesion and the operator) and a specificity of about 90%, but like AFP has a poor positive predictive value of 14%.211 Better results are reported in a study on patients who are on a waiting list for liver transplantation where sensitivity was 58%, specificity 94%, and the positive predictive value 69%.212
Table 2.
Surveillance recommendations.
| Strongly Recommended | Probably recommended |
|---|---|
|
| |
| HBV cirrhosis | HBV non cirrhotic above 45 years |
| HCV cirrhosis | HBV non-cirrhotic with family history of HCC |
| Cirrhosis secondary to other causes | HBV non-cirrhotic with high viral load |
| HBV non-cirrhotic with indications of advanced fibrosis | |
Surveillance Interval
Most authorities suggest screening interval of 6 months based on data suggesting that the time from an undetectable lesion to a 2 cm lesion is about 4 to 12 months.60 In addition the only randomized controlled trial that has shown a survival benefit used 6-month intervals.202 Studies are conflicting regarding the difference between a 6-month interval policy and a 12-month interval policy. Some studies, especially in hepatitis C patients, have shown that there are no survival benefits between these two intervals.71 This has been shown by other studies as well.213 On the other hand, studies in hepatitis B patients showed the superiority of a 6-month surveillance compared to 12 months.214 In a more recent study by Santi et al on more than 600 patients, it was found that semiannual surveillance increases the detection rate of very early HCCs and reduces the number of advanced tumors as compared to the annual program. This translates into a greater applicability of effective treatments and into a better prognosis.215 Once a lesion has been found by any of these tests then a confirmatory test must be done immediately (refer to the diagnosis section).
Figure 4.
Treatment algorithm for HCC.
REFERENCES
- 1.Abdo AA, Karim HA, Al Fuhaid T, Sanai FM, Kabbani M, Al Jumah A, et al. Saudi Gastroenterology Association guidelines for the diagnosis and management of hepatocellular carcinoma: summary of recommendations. Ann Saudi Med. 2006 Jul-Aug;26(4):261–5. doi: 10.5144/0256-4947.2006.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010 Dec 15;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Stewart B, Kleihues P, editors. World Cancer Report. Lyon: IARC Press; 2003. [Google Scholar]
- 4.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19(3):271–85. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999 Mar 11;340(10):745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 6.Kingdom of Saudi Arabia Ministry of Health. Saudi Cancer Registry. 2006 [Google Scholar]
- 7.al-Faleh FZ, Ayoola EA, Arif M, Ramia S, al-Rashed R, al-Jeffry M, et al. Seroepidemiology of hepatitis B virus infection in Saudi Arabian children: a baseline survey for mass vaccination against hepatitis B. J Infect. 1992 Mar;24(2):197–206. doi: 10.1016/0163-4453(92)93006-c. [DOI] [PubMed] [Google Scholar]
- 8.Al-Faleh FZ, Al-Jeffri M, Ramia S, Al-Rashed R, Arif M, Rezeig M, et al. Seroepidemiology of hepatitis B virus infection in Saudi children 8 years after a mass hepatitis B vaccination programme. J Infect. 1999 May;38(3):167–70. doi: 10.1016/s0163-4453(99)90245-1. [DOI] [PubMed] [Google Scholar]
- 9.Madani TA. Hepatitis C virus infections reported in Saudi Arabia over 11 years of surveillance. Ann Saudi Med. 2007 May-Jun;27(3):191–4. doi: 10.5144/0256-4947.2007.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingdom of Saudi Arabia Ministry of Health Statistics. 2009. [Google Scholar]
- 11.Kingston M, Ali MA, Lewall D. Hepatic tumors in Saudi Arabia. A practical approach to diagnosis. Cancer. 1985 Apr 1;55(7):1579–85. doi: 10.1002/1097-0142(19850401)55:7<1579::aid-cncr2820550728>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Ashraf SJ, Arya SC, el-Sayed M, Sahay R, Parande CM, Tajuddin MR, et al. A profile of primary hepatocellular carcinoma patients in the Gizan Area of Saudi Arabia. Cancer. 1986 Nov 1;58(9):2163–8. doi: 10.1002/1097-0142(19861101)58:9<2163::aid-cncr2820580934>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Atiyeh M, Ali MA. Primary hepatocelluar carcinoma in Saudi Arabia. A clinicopathological study of 54 cases. Am J Gastroenterol. 1980 Jul;74(1):25–9. [PubMed] [Google Scholar]
- 14.The Saudi Observatory Liver Disease Registry [database on the Internet] 2009. [cited 20 April 2010]. Available from: http://www.solid-registry.com/home.html.
- 15.Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991 Sep 5;325(10):675–80. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 16.Benvegnu L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004 May;53(5):744–9. doi: 10.1136/gut.2003.020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002 Nov;97(11):2886–95. doi: 10.1111/j.1572-0241.2002.07057.x. [DOI] [PubMed] [Google Scholar]
- 18.Arbuthnot P, Kew M. Hepatitis B virus and hepatocellular carcinoma. Int J Exp Pathol. 2001 Apr;82(2):77–100. doi: 10.1111/j.1365-2613.2001.iep0082-0077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kew M. Hepatic tumors and cysts. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 2002 [Google Scholar]
- 20.Sanai FM, Sobki S, Bzeizi KI, Shaikh SA, Alswat K, Al-Hamoudi W, et al. Assessment of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma in Middle Eastern patients. Dig Dis Sci. 2010 Dec;55(12):3568–75. doi: 10.1007/s10620-010-1201-x. [DOI] [PubMed] [Google Scholar]
- 21.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006 Jun 15;118(12):3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 22.Kim SR, Kudo M, Hino O, Han KH, Chung YH, Lee HS. Epidemiology of hepatocellular carcinoma in Japan and Korea. A review. Oncology. 2008;75(Suppl 1):13–6. doi: 10.1159/000173419. [DOI] [PubMed] [Google Scholar]
- 23.Beasley RP, Hwang LY. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis. 1984 May;4(2):113–21. doi: 10.1055/s-2008-1040651. [DOI] [PubMed] [Google Scholar]
- 24.Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995 Jan;21(1):77–82. doi: 10.1002/hep.1840210114. [DOI] [PubMed] [Google Scholar]
- 25.Liaw YF, Tai DI, Chu CM, Lin DY, Sheen IS, Chen TJ, et al. Early detection of hepatocellular carcinoma in patients with chronic type B hepatitis. A prospective study. Gastroenterology. 1986 Feb;90(2):263–7. doi: 10.1016/0016-5085(86)90919-4. [DOI] [PubMed] [Google Scholar]
- 26.Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000 Mar;64(1):51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, Zhu L, Liu S, Xie WF. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005 Feb 14;92(3):607–12. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998 Jan 30;75(3):347–54. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006 Jan 4;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Tapias JM, Costa J, Mas A, Bruguera M, Rodes J. Influence of hepatitis B virus genotype on the long-term outcome of chronic hepatitis B in western patients. Gastroenterology. 2002 Dec;123(6):1848–56. doi: 10.1053/gast.2002.37041. [DOI] [PubMed] [Google Scholar]
- 31.Abdo AA, Al-Jarallah BM, Sanai FM, Hersi AS, Al-Swat K, Azzam NA, et al. Hepatitis B genotypes: relation to clinical outcome in patients with chronic hepatitis B in Saudi Arabia. World J Gastroenterol. 2006 Nov 21;12(43):7019–24. doi: 10.3748/wjg.v12.i43.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colombo M. Malignant neoplasms of the liver. Schiff’s Diseases of the Liver. 2003 [Google Scholar]
- 33.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–33. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 34.Sakuma K, Saitoh N, Kasai M, Jitsukawa H, Yoshino I, Yamaguchi M, et al. Relative risks of death due to liver disease among Japanese male adults having various statuses for hepatitis B s and e antigen/antibody in serum: a prospective study. Hepatology. 1988 Nov-Dec;8(6):1642–6. doi: 10.1002/hep.1840080628. [DOI] [PubMed] [Google Scholar]
- 35.Kew MC. Clinical, pathologic, and etiologic heterogeneity in hepatocellular carcinoma: evidence from southern Africa. Hepatology. 1981 Jul-Aug;1(4):366–9. doi: 10.1002/hep.1840010415. [DOI] [PubMed] [Google Scholar]
- 36.Lu SN, Wang JH, Liu SL, Hung CH, Chen CH, Tung HD, et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer. 2006 Nov 1;107(9):2212–22. doi: 10.1002/cncr.22242. [DOI] [PubMed] [Google Scholar]
- 37.Hassan MM, Frome A, Patt YZ, El-Serag HB. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2002 Sep;35(3):266–9. doi: 10.1097/00004836-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997 Feb;112(2):463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 39.Sherman M. Hepatocellular carcinoma: New and emerging risks. Dig Liver Dis. 2010 Jul;42(Suppl 3):S215–22. doi: 10.1016/S1590-8658(10)60508-7. [DOI] [PubMed] [Google Scholar]
- 40.Yu H, Harris RE, Kabat GC, Wynder EL. Cigarette smoking, alcohol consumption and primary liver cancer: a case-control study in the USA. Int J Cancer. 1988 Sep 15;42(3):325–8. doi: 10.1002/ijc.2910420304. [DOI] [PubMed] [Google Scholar]
- 41.Cottone M, Turri M, Caltagirone M, Parisi P, Orlando A, Fiorentino G, et al. Screening for hepatocellular carcinoma in patients with Child’s A cirrhosis: an 8-year prospective study by ultrasound and alphafetoprotein. J Hepatol. 1994 Dec;21(6):1029–34. doi: 10.1016/s0168-8278(05)80613-0. [DOI] [PubMed] [Google Scholar]
- 42.Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001 Nov 30;85(11):1700–5. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohnishi K, Iida S, Iwama S, Goto N, Nomura F, Takashi M, et al. The effect of chronic habitual alcohol intake on the development of liver cirrhosis and hepatocellular carcinoma: relation to hepatitis B surface antigen carriage. Cancer. 1982 Feb 15;49(4):672–7. doi: 10.1002/1097-0142(19820215)49:4<672::aid-cncr2820490415>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998 Jun;28(6):930–8. doi: 10.1016/s0168-8278(98)80339-5. [DOI] [PubMed] [Google Scholar]
- 45.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001 Sep;35(3):421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 46.Zhang YJ, Chen Y, Ahsan H, Lunn RM, Chen SY, Lee PH, et al. Silencing of glutathione S-transferase P1 by promoter hypermethylation and its relationship to environmental chemical carcinogens in hepatocellular carcinoma. Cancer Lett. 2005 Apr 28;221(2):135–43. doi: 10.1016/j.canlet.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 47.Moller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A(3):344–50. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 48.Wolk A, Gridley G, Svensson M, Nyren O, McLaughlin JK, Fraumeni JF, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001 Jan;12(1):13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 49.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 50.Wang CS, Yao WJ, Chang TT, Wang ST, Chou P. The impact of type 2 diabetes on the development of hepatocellular carcinoma in different viral hepatitis statuses. Cancer Epidemiol Biomarkers Prev. 2009 Jul;18(7):2054–60. doi: 10.1158/1055-9965.EPI-08-1131. [DOI] [PubMed] [Google Scholar]
- 51.Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985 Nov 14;313(20):1256–62. doi: 10.1056/NEJM198511143132004. [DOI] [PubMed] [Google Scholar]
- 52.Fargion S, Fracanzani AL, Piperno A, Braga M, D’Alba R, Ronchi G, et al. Prognostic factors for hepatocellular carcinoma in genetic hemochromatosis. Hepatology. 1994 Dec;20(6):1426–31. doi: 10.1002/hep.1840200608. [DOI] [PubMed] [Google Scholar]
- 53.Polio J, Enriquez RE, Chow A, Wood WM, Atterbury CE. Hepatocellular carcinoma in Wilson’s disease. Case report and review of the literature. J Clin Gastroenterol. 1989 Apr;11(2):220–4. doi: 10.1097/00004836-198904000-00022. [DOI] [PubMed] [Google Scholar]
- 54.Okazaki N, Yoshino M, Yoshida T, Suzuki M, Moriyama N, Takayasu K, et al. Evaluation of the prognosis for small hepatocellular carcinoma based on tumor volume doubling time. A preliminary report. Cancer. 1989 Jun 1;63(11):2207–10. doi: 10.1002/1097-0142(19890601)63:11<2207::aid-cncr2820631124>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 55.Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992 Jul;16(1):132–7. doi: 10.1002/hep.1840160122. [DOI] [PubMed] [Google Scholar]
- 56.Kew MC, Geddes EW. Hepatocellular carcinoma in rural southern African blacks. Medicine (Baltimore) 1982 Mar;61(2):98–108. doi: 10.1097/00005792-198203000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Kew MC, Dos Santos HA, Sherlock S. Diagnosis of primary cancer of the liver. Br Med J. 1971 Nov 13;4(5784):408–11. doi: 10.1136/bmj.4.5784.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001 Feb;48(2):251–9. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009 Jul;30(1):37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011 Mar;53(3):1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003 Oct;38(4):1034–42. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 62.Yu JS, Kim KW, Kim EK, Lee JT, Yoo HS. Contrast enhancement of small hepatocellular carcinoma: usefulness of three successive early image acquisitions during multiphase dynamic MR imaging. AJR Am J Roentgenol. 1999 Sep;173(3):597–604. doi: 10.2214/ajr.173.3.10470886. [DOI] [PubMed] [Google Scholar]
- 63.Forner A, Vilana R, Ayuso C, Bianchi L, Sole M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008 Jan;47(1):97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 64.Okuda K. Early recognition of hepatocellular carcinoma. Hepatology. 1986 Jul-Aug;6(4):729–38. doi: 10.1002/hep.1840060432. [DOI] [PubMed] [Google Scholar]
- 65.Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003 Feb;226(2):533–42. doi: 10.1148/radiol.2262011980. [DOI] [PubMed] [Google Scholar]
- 66.Chalasani N, Horlander JC, Sr, Said A, Hoen H, Kopecky KK, Stockberger SM, Jr, et al. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999 Oct;94(10):2988–93. doi: 10.1111/j.1572-0241.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 67.Yamashita Y, Takahashi M, Baba Y, Kanazawa S, Charnsangavej C, Yang D, et al. Hepatocellular carcinoma with or without cirrhosis: a comparison of CT and angiographic presentations in the United States and Japan. Abdom Imaging. 1993;18(2):168–75. doi: 10.1007/BF00198057. [DOI] [PubMed] [Google Scholar]
- 68.Mitsuzaki K, Yamashita Y, Ogata I, Nishiharu T, Urata J, Takahashi M. Multiple-phase helical CT of the liver for detecting small hepatomas in patients with liver cirrhosis: contrast-injection protocol and optimal timing. AJR Am J Roentgenol. 1996 Sep;167(3):753–7. doi: 10.2214/ajr.167.3.8751695. [DOI] [PubMed] [Google Scholar]
- 69.Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004 Nov;127(5 Suppl 1):S108–12. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 70.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003 Jul 1;139(1):46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 71.Trevisani F, De NS, Rapaccini G, Farinati F, Benvegnu L, Zoli M, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience) Am J Gastroenterol. 2002 Mar;97(3):734–44. doi: 10.1111/j.1572-0241.2002.05557.x. [DOI] [PubMed] [Google Scholar]
- 72.Sato Y, Sekine T, Ohwada S. Alpha-fetoproteinproducing rectal cancer: calculated tumor marker doubling time. J Surg Oncol. 1994 Apr;55(4):265–8. doi: 10.1002/jso.2930550414. [DOI] [PubMed] [Google Scholar]
- 73.Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65(2):95–101. doi: 10.1159/000072332. [DOI] [PubMed] [Google Scholar]
- 74.Durand F, Regimbeau JM, Belghiti J, Sauvanet A, Vilgrain V, Terris B, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol. 2001 Aug;35(2):254–8. doi: 10.1016/s0168-8278(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 75.Torzilli G, Minagawa M, Takayama T, Inoue K, Hui A-M, Kubota K, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30(4):889–93. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 76.Kanematsu T, Sonoda T, Takenaka K, Matsumata T, Sugimachi K, Inokuchi K. The value of ultrasound in the diagnosis and treatment of small hepatocellular carcinoma. Br J Surg. 1985 Jan;72(1):23–5. doi: 10.1002/bjs.1800720111. [DOI] [PubMed] [Google Scholar]
- 77.Frazer C. Imaging of hepatocellular carcinoma. J Gastroenterol Hepatol. 1999 Aug;14(8):750–6. doi: 10.1046/j.1440-1746.1999.01946.x. [DOI] [PubMed] [Google Scholar]
- 78.Bizollon T, Rode A, Bancel B, Gueripel V, Ducerf C, Baulieux J, et al. Diagnostic value and tolerance of Lipiodol-computed tomography for the detection of small hepatocellular carcinoma: correlation with pathologic examination of explanted livers. J Hepatol. 1998 Mar;28(3):491–6. doi: 10.1016/s0168-8278(98)80324-3. [DOI] [PubMed] [Google Scholar]
- 79.Huang GT, Sheu JC, Yang PM, Lee HS, Wang TH, Chen DS. Ultrasound-guided cutting biopsy for the diagnosis of hepatocellular carcinoma--a study based on 420 patients. J Hepatol. 1996 Sep;25(3):334–8. doi: 10.1016/s0168-8278(96)80120-6. [DOI] [PubMed] [Google Scholar]
- 80.Horigome H, Nomura T, Saso K, Itoh M, Joh T, Ohara H. Limitations of imaging diagnosis for small hepatocellular carcinoma: comparison with histological findings. J Gastroenterol Hepatol. 1999 Jun;14(6):559–65. doi: 10.1046/j.1440-1746.1999.01915.x. [DOI] [PubMed] [Google Scholar]
- 81.Torzilli G. Japanese approach to hepatocellular carcinoma. Dig Liver Dis. 2001 Mar;33(2):118–20. doi: 10.1016/s1590-8658(01)80063-3. [DOI] [PubMed] [Google Scholar]
- 82.Longchampt E, Patriarche C, Fabre M. Accuracy of cytology vs. microbiopsy for the diagnosis of well-differentiated hepatocellular carcinoma and macroregenerative nodule. Definition of standardized criteria from a study of 100 cases. Acta Cytol. 2000 Jul-Aug;44(4):515–23. doi: 10.1159/000328523. [DOI] [PubMed] [Google Scholar]
- 83.Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl. 2000 Jan;6(1):67–72. doi: 10.1002/lt.500060103. [DOI] [PubMed] [Google Scholar]
- 84.Borzio M, Borzio F, Macchi R, Croce AM, Bruno S, Ferrari A, et al. The evaluation of fine-needle procedures for the diagnosis of focal liver lesions in cirrhosis. J Hepatol. 1994 Jan;20(1):117–21. doi: 10.1016/s0168-8278(05)80477-5. [DOI] [PubMed] [Google Scholar]
- 85.Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003 May;52(Suppl 3):iii1–8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005 Jul;42(1):27–34. doi: 10.1002/hep.20728. [DOI] [PubMed] [Google Scholar]
- 87.Kim SE, Lee HC, Shim JH, Park HJ, Kim KM, Kim PN, et al. Noninvasive diagnostic criteria for hepatocellular carcinoma in hepatic masses >2 cm in a hepatitis B virus-endemic area. Liver International. 2011 doi: 10.1111/j.1478-3231.2011.02529.x. no-no. [DOI] [PubMed] [Google Scholar]
- 88.Leoni S, Piscaglia F, Golfieri R, Camaggi V, Vidili G, Pini P, et al. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am J Gastroenterol. 2010 Mar;105(3):599–609. doi: 10.1038/ajg.2009.654. [DOI] [PubMed] [Google Scholar]
- 89.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002 Mar 15;20(6):1527–36. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 90.Fleming ID. AJCC/TNM cancer staging, present and future. J Surg Oncol. 2001 Aug;77(4):233–6. doi: 10.1002/jso.1101. [DOI] [PubMed] [Google Scholar]
- 91.Ikai I, Takayasu K, Omata M, Okita K, Nakanuma Y, Matsuyama Y, et al. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol. 2006 Sep;41(9):884–92. doi: 10.1007/s00535-006-1878-y. [DOI] [PubMed] [Google Scholar]
- 92.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985 Aug 15;56(4):918–28. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 93.CLIP Investigators. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology. 1998;28(3):751–5. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 94.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002 May;122(6):1609–19. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 95.Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer. 2000 Dec 1;89(11):2266–73. [PubMed] [Google Scholar]
- 96.Prospective validation of the CLIP score: A new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;31(4):840–5. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 97.Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001 Sep;34(3):529–34. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- 98.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 99.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005 Apr;41(4):707–16. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 100.Grieco A, Pompili M, Caminiti G, Miele L, Covino M, Alfei B, et al. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005 Mar;54(3):411–8. doi: 10.1136/gut.2004.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996 Mar 14;334(11):693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 102.Befeler AS, Hayashi PH, Di Bisceglie AM. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2005 May;128(6):1752–64. doi: 10.1053/j.gastro.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 103.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001 Jun;33(6):1394–403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 104.Llovet JM, Fuster J, Bruix J. Intention- to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999 Dec;30(6):1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 105.Marsh JW, Dvorchik I, Bonham CA, Iwatsuki S. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000 Feb 1;88(3):538–43. doi: 10.1002/(sici)1097-0142(20000201)88:3<538::aid-cncr7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 106.Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002 Apr;235(4):533–9. doi: 10.1097/00000658-200204000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steinmuller T, Pascher A, Sauer I, Theruvath T, Muller A, Settmacher U, et al. Living-donation liver transplantation for hepatocellular carcinoma: time to drop the limitations? Transplant Proc. 2002 Sep;34(6):2263–4. doi: 10.1016/s0041-1345(02)03228-1. [DOI] [PubMed] [Google Scholar]
- 108.Kawasaki S. Living-donor liver transplantation for hepatocellular carcinoma. Hepatogastroenterology. 2002 Jan-Feb;49(43):53–5. [PubMed] [Google Scholar]
- 109.Gondolesi GE, Roayaie S, Munoz L, Kim-Schluger L, Schiano T, Fishbein TM, et al. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004 Feb;239(2):142–9. doi: 10.1097/01.sla.0000109022.32391.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Todo S, Furukawa H. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004 Sep;240(3):451–9. doi: 10.1097/01.sla.0000137129.98894.42. discussion 9–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008 Jul;14(7):935–45. doi: 10.1002/lt.21445. [DOI] [PubMed] [Google Scholar]
- 112.Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25(4):310–2. doi: 10.1159/000106910. [DOI] [PubMed] [Google Scholar]
- 113.Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: A life-expectancy and cost-effectiveness perspective. Hepatology. 2001 May;33(5):1073–9. doi: 10.1053/jhep.2001.23311. [DOI] [PubMed] [Google Scholar]
- 114.Cheng SJ, Pratt DS, Freeman RB, Jr, Kaplan MM, Wong JB. Living-donor versus cadaveric liver transplantation for non-resectable small hepatocellular carcinoma and compensated cirrhosis: a decision analysis. Transplantation. 2001 Sep 15;72(5):861–8. doi: 10.1097/00007890-200109150-00021. [DOI] [PubMed] [Google Scholar]
- 115.Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004 Nov;240(5):900–9. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002 Jan;50(1):123–8. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003 Jun;9(6):557–63. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 118.Maddala YK, Stadheim L, Andrews JC, Burgart LJ, Rosen CB, Kremers WK, et al. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl. 2004 Mar;10(3):449–55. doi: 10.1002/lt.20099. [DOI] [PubMed] [Google Scholar]
- 119.Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997 Dec;226(6):688–701. doi: 10.1097/00000658-199712000-00006. discussion-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pokorny H, Gnant M, Rasoul-Rockenschaub S, Gollackner B, Steiner B, Steger G, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant. 2005 Apr;5(4 Pt 1):788–94. doi: 10.1111/j.1600-6143.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 121.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004 Nov;127(5 Suppl 1):S248–60. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 122.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001 Jul;234(1):63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg. 1995 Mar;221(3):291–8. doi: 10.1097/00000658-199503000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bismuth H, Chiche L, Castaing D. Surgical treatment of hepatocellular carcinomas in noncirrhotic liver: experience with 68 liver resections. World J Surg. 1995 Jan-Feb;19(1):35–41. doi: 10.1007/BF00316977. [DOI] [PubMed] [Google Scholar]
- 125.Hassoun Z, Gores GJ. Treatment of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2003 Jan;1(1):10–8. doi: 10.1053/jcgh.2003.50003. [DOI] [PubMed] [Google Scholar]
- 126.Rilling WS, Drooz A. Multidisciplinary management of hepatocellular carcinoma. J Vasc Interv Radiol. 2002 Sep;13(9 Pt 2):S259–63. doi: 10.1016/s1051-0443(07)61794-1. [DOI] [PubMed] [Google Scholar]
- 127.Dodd GD, 3rd, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000 Jan-Feb;20(1):9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- 128.Livraghi T, Bolondi L, Buscarini L, Cottone M, Mazziotti A, Morabito A, et al. No treatment, resection and ethanol injection in hepatocellular carcinoma: a retrospective analysis of survival in 391 patients with cirrhosis. Italian Cooperative HCC Study Group. J Hepatol. 1995 May;22(5):522–6. doi: 10.1016/0168-8278(95)80445-5. [DOI] [PubMed] [Google Scholar]
- 129.Okada S. Local ablation therapy for hepatocellular carcinoma. Semin Liver Dis. 1999;19(3):323–8. doi: 10.1055/s-2007-1007121. [DOI] [PubMed] [Google Scholar]
- 130.Ishii H, Okada S, Nose H, Okusaka T, Yoshimori M, Takayama T, et al. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1996 May 1;77(9):1792–6. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1792::AID-CNCR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 131.Livraghi T, Bolondi L, Lazzaroni S, Marin G, Morabito A, Rapaccini GL, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer. 1992 Feb 15;69(4):925–9. doi: 10.1002/1097-0142(19920215)69:4<925::aid-cncr2820690415>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 132.Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995 Oct;197(1):101–8. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- 133.Castells A, Bruix J, Bru C, Fuster J, Vilana R, Navasa M, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993 Nov;18(5):1121–6. [PubMed] [Google Scholar]
- 134.Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993 May;160(5):1023–8. doi: 10.2214/ajr.160.5.7682378. [DOI] [PubMed] [Google Scholar]
- 135.Castellano L, Calandra M, Del Vecchio Blanco C, de Sio I. Predictive factors of survival and intrahepatic recurrence of hepatocellular carcinoma in cirrhosis after percutaneous ethanol injection: analysis of 71 patients. J Hepatol. 1997 Nov;27(5):862–70. doi: 10.1016/s0168-8278(97)80324-8. [DOI] [PubMed] [Google Scholar]
- 136.Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgrò G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: A meta-analysis. Journal of hepatology. 2010;52(3):380–8. doi: 10.1016/j.jhep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 137.Choti MA. Surgical management of hepatocellular carcinoma: resection and ablation. J Vasc Interv Radiol. 2002 Sep;13(9 Pt 2):S197–203. doi: 10.1016/s1051-0443(07)61787-4. [DOI] [PubMed] [Google Scholar]
- 138.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000 Sep;232(3):381–91. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999 Mar;210(3):655–61. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 140.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003 Jul;228(1):235–40. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 141.Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009 Feb;104(2):514–24. doi: 10.1038/ajg.2008.80. [DOI] [PubMed] [Google Scholar]
- 142.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004 Dec;127(6):1714–23. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 143.Galandi D, Antes G. Radiofrequency thermal ablation versus other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2004;(2):CD003046. doi: 10.1002/14651858.CD003046.pub2. [DOI] [PubMed] [Google Scholar]
- 144.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006 Mar;243(3):321–8. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang JW, Hernandez-Alejandro R, Croome KP, Yan LN, Wu H, Chen ZY, et al. Surgical vs percutaneous radiofrequency ablation for hepatocellular carcinoma in dangerous locations. World J Gastroenterol. 2011 Jan 7;17(1):123–9. doi: 10.3748/wjg.v17.i1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Llovet JM, Vilana R, Bru C, Bianchi L, Salmeron JM, Boix L, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001 May;33(5):1124–9. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 147.Castells A, Bruix J, Ayuso C, Bru C, Montanya X, Boix L, et al. Transarterial embolization for hepatocellular carcinoma. Antibiotic prophylaxis and clinical meaning of postembolization fever. J Hepatol. 1995 Apr;22(4):410–5. doi: 10.1016/0168-8278(95)80103-0. [DOI] [PubMed] [Google Scholar]
- 148.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004 Nov;127(5 Suppl 1):S179–88. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 149.Hayashi K, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, et al. Usefulness of measurement of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein as a marker of prognosis and recurrence of small hepatocellular carcinoma. Am J Gastroenterol. 1999 Oct;94(10):3028–33. doi: 10.1111/j.1572-0241.1999.01378.x. [DOI] [PubMed] [Google Scholar]
- 150.Yamashita F, Tanaka M, Satomura S, Tanikawa K. Monitoring of lectin-reactive alpha-fetoproteins in patients with hepatocellular carcinoma treated using transcatheter arterial embolization. Eur J Gastroenterol Hepatol. 1995 Jul;7(7):627–33. [PubMed] [Google Scholar]
- 151.Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;3:CD004787. doi: 10.1002/14651858.CD004787.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002 May 18;359(9319):1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 153.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002 May;35(5):1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 154.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003 Feb;37(2):429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 155.Al Fuhaid T, Al Madi M, Al Abdul Kareem H, Al Dukhayil M, Abdo AA. Radiological response in Saudi patients undergoing transarterial chemoembolization for hepatocellular carcinoma. Saudi J Gastroenterol. 2007 Jan-Mar;13(1):21–4. doi: 10.4103/1319-3767.30461. [DOI] [PubMed] [Google Scholar]
- 156.Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, et al. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008 Mar-Apr;31(2):269–80. doi: 10.1007/s00270-007-9226-z. [DOI] [PubMed] [Google Scholar]
- 157.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007 Mar;46(3):474–81. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 158.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007 Sep;5(9):1100–8. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 159.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010 Feb;33(1):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ali SM. Radioembolization for hepatocellular carcinoma using TheraSphere(R) Saudi J Gastroenterol. 2011 May-Jun;17(3):215–7. doi: 10.4103/1319-3767.80388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol. 2006 Oct;17(10):1571–93. doi: 10.1097/01.RVI.0000236744.34720.73. [DOI] [PubMed] [Google Scholar]
- 162.Kulik LM, Atassi B, van Holsbeeck L, Souman T, Lewandowski RJ, Mulcahy MF, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006 Dec 1;94(7):572–86. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 163.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008 Jan;47(1):71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 164.Hilgard P, Hamami M, Fouly AE, Scherag A, Muller S, Ertle J, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010 Nov;52(5):1741–9. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 165.Abaalkhail F, Al-Hamoudi W, Al Sebayel M, Alsuhaibani H, Kamel Y, Khalaf H, et al. Safety and Efficacy of Yttrium-90 Microspheres for the Treatment of Hepatocellular Carcinoma: A Single Center Experience. Journal of hepatology. 2011;54:S252. [Google Scholar]
- 166.Halm U, Etzrodt G, Schiefke I, Schmidt F, Witzigmann H, Mossner J, et al. A phase II study of pegylated liposomal doxorubicin for treatment of advanced hepatocellular carcinoma. Ann Oncol. 2000 Jan;11(1):113–4. doi: 10.1023/a:1008386822906. [DOI] [PubMed] [Google Scholar]
- 167.Gebbia V, Maiello E, Serravezza G, Giotta F, Testa A, Borsellino N, et al. 5-Fluorouracil plus high dose levofolinic acid and oral hydroxyurea for the treatment of primary hepatocellular carcinomas: results of a phase II multicenter study of the Southern Italy Oncology Group (G.O.I.M.) Anticancer Res. 1999 Mar-Apr;19(2B):1407–10. [PubMed] [Google Scholar]
- 168.Mathurin P, Rixe O, Carbonell N, Bernard B, Cluzel P, Bellin MF, et al. Review article: Overview of medical treatments in unresectable hepatocellular carcinoma--an impossible meta-analysis? Aliment Pharmacol Ther. 1998 Feb;12(2):111–26. doi: 10.1046/j.1365-2036.1998.00286.x. [DOI] [PubMed] [Google Scholar]
- 169.Ben Mousa A. Sorafenib in the treatment of advanced hepatocellular carcinoma. Saudi J Gastroenterol. 2008 Jan;14(1):40–2. doi: 10.4103/1319-3767.37808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006 Oct;5(10):835–44. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 171.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006 Sep 10;24(26):4293–300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 172.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359(4):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 173.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009 Jan;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 174.Abou-Alfa G, Amadori D, Santoro A, Figer A, De Greve J, Lathia C, et al. Is sorafenib (S) safe and effective in patients (pts) with hepatocellular carcinoma (HCC) and Child-Pugh B (CPB) cirrhosis? J Clin Oncol [Abstract] 2008 May 20;26(suppl):4518. [Google Scholar]
- 175.Abou-Alfa G, Johnson P, Knox J, Davidenko I, Lacava J, Leung T, et al. Final results from a phase II (PhII), randomized, double-blind study of sorafenib plus doxorubicin (S+D) versus placebo plus doxorubicin (P+D) in patients (pts) with advanced hepatocellular carcinoma (AHCC). Gastrointestinal Cancers Symposium; Orlando, FL. 2008. p. 128. [Google Scholar]
- 176.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008 May 21;100(10):698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 177.Rimassa L, Santoro A. The present and the future landscape of treatment of advanced hepatocellular carcinoma. Dig Liver Dis. 2010 Jul;42(Suppl 3):S273–80. doi: 10.1016/S1590-8658(10)60516-6. [DOI] [PubMed] [Google Scholar]
- 178.Thongprasert S, Qin S, Lim H, Bhudhisawasdi V, Yin X, Gang W, et al. Efficacy of oxaliplatin plus 5-fluorouracil/leucovorin (FOLFOX4) versus doxorubicin in advanced HCC: Updates on the EACH study. J Clin Oncol. 2011;29(suppl 4) abstr 160. [Google Scholar]
- 179.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997 Jun 26;336(26):1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 180.AlFaleh F, AlShehri S, AlAnsari S, AlJeffri M, AlMazrou Y, Shaffi A, et al. Long-term protection of hepatitis B vaccine 18 years after vaccination. Journal of Infection. 2008;57(5):404–9. doi: 10.1016/j.jinf.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 181.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009 Apr;49(4):1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995 Oct 21;346(8982):1051–5. doi: 10.1016/s0140-6736(95)91739-x. [DOI] [PubMed] [Google Scholar]
- 183.Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999 Apr;29(4):1124–30. doi: 10.1002/hep.510290439. [DOI] [PubMed] [Google Scholar]
- 184.Valla DC, Chevallier M, Marcellin P, Payen JL, Trepo C, Fonck M, et al. Treatment of hepatitis C virus-related cirrhosis: a randomized, controlled trial of interferon alfa-2b versus no treatment. Hepatology. 1999 Jun;29(6):1870–5. doi: 10.1002/hep.510290616. [DOI] [PubMed] [Google Scholar]
- 185.Serfaty L, Aumaitre H, Chazouilleres O, Bonnand AM, Rosmorduc O, Poupon RE, et al. Determinants of outcome of compensated hepatitis C virus-related cirrhosis. Hepatology. 1998 May;27(5):1435–40. doi: 10.1002/hep.510270535. [DOI] [PubMed] [Google Scholar]
- 186.Esteban R. Can interferon prolong life? Hepatology. 2003 Aug;38(2):292–4. doi: 10.1053/jhep.2003.50337. [DOI] [PubMed] [Google Scholar]
- 187.Baffis V, Shrier I, Sherker AH, Szilagyi A. Use of interferon for prevention of hepatocellular carcinoma in cirrhotic patients with hepatitis B or hepatitis C virus infection. Ann Intern Med. 1999 Nov 2;131(9):696–701. doi: 10.7326/0003-4819-131-9-199911020-00011. [DOI] [PubMed] [Google Scholar]
- 188.Camma C, Giunta M, Andreone P, Craxi A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J Hepatol. 2001 Apr;34(4):593–602. doi: 10.1016/s0168-8278(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 189.Papatheodoridis GV, Papadimitropoulos VC, Hadziyannis SJ. Effect of interferon therapy on the development of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a meta-analysis. Aliment Pharmacol Ther. 2001 May;15(5):689–98. doi: 10.1046/j.1365-2036.2001.00979.x. [DOI] [PubMed] [Google Scholar]
- 190.Chou R, Clark EC, Helfand M. Screening for hepatitis C virus infection: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004 Mar 16;140(6):465–79. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 191.Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim HY, Sterling RK, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011 Mar;140(3):840–9. doi: 10.1053/j.gastro.2010.11.050. quiz e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Di Bisceglie AM, Stoddard AM, Dienstag JL, Shiffman ML, Seeff LB, Bonkovsky HL, et al. Excess mortality in patients with advanced chronic hepatitis C treated with long-term peginterferon. Hepatology. 2011 Apr;53(4):1100–8. doi: 10.1002/hep.24169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bruix J, Poynard T, Colombo M, Schiff E, Burak K, Heathcote EJ, et al. Maintenance Therapy With Peginterferon Alfa-2b Does Not Prevent Hepatocellular Carcinoma in Cirrhotic Patients With Chronic Hepatitis C. Gastroenterology. 2011 Mar;:17. doi: 10.1053/j.gastro.2011.03.010. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 194.Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999 Aug 3;131(3):174–81. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 195.Imai Y, Kawata S, Tamura S, Yabuuchi I, Noda S, Inada M, et al. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Osaka Hepatocellular Carcinoma Prevention Study Group. Ann Intern Med. 1998 Jul 15;129(2):94–9. doi: 10.7326/0003-4819-129-2-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 196.Okanoue T, Itoh Y, Minami M, Sakamoto S, Yasui K, Sakamoto M, et al. Interferon therapy lowers the rate of progression to hepatocellular carcinoma in chronic hepatitis C but not significantly in an advanced stage: a retrospective study in 1148 patients. Viral Hepatitis Therapy Study Group. J Hepatol. 1999 Apr;30(4):653–9. doi: 10.1016/s0168-8278(99)80196-2. [DOI] [PubMed] [Google Scholar]
- 197.Lok AS. Prevention of hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2004 Nov;127(5 Suppl 1):S303–9. doi: 10.1053/j.gastro.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 198.Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology. 1999 Mar;29(3):971–5. doi: 10.1002/hep.510290312. [DOI] [PubMed] [Google Scholar]
- 199.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004 Oct 7;351(15):1521–31. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 200.Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology. 1998 Jan;27(1):273–8. doi: 10.1002/hep.510270140. [DOI] [PubMed] [Google Scholar]
- 201.Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996 Oct;101(4):422–34. doi: 10.1016/S0002-9343(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 202.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004 Jul;130(7):417–22. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tang ZY, Yu YQ, Zhou XD, Yang BH, Ma ZC, Lin ZY. Subclinical hepatocellular carcinoma: an analysis of 391 patients. J Surg Oncol Suppl. 1993;3:55–8. doi: 10.1002/jso.2930530516. [DOI] [PubMed] [Google Scholar]
- 204.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995 Aug;22(2):432–8. [PubMed] [Google Scholar]
- 205.Velazquez RF, Rodriguez M, Navascues CA, Linares A, Perez R, Sotorrios NG, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003 Mar;37(3):520–7. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 206.Arguedas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003 Mar;98(3):679–90. doi: 10.1111/j.1572-0241.2003.07327.x. [DOI] [PubMed] [Google Scholar]
- 207.Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, et al. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis. Br J Cancer. 2008;98(7):1166–75. doi: 10.1038/sj.bjc.6604301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zaman SN, Johnson PJ, Williams R. Silent cirrhosis in patients with hepatocellular carcinoma. Implications for screening in high-incidence and low-incidence areas. Cancer. 1990 Apr 1;65(7):1607–10. doi: 10.1002/1097-0142(19900401)65:7<1607::aid-cncr2820650726>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 209.Sherman M. Alphafetoprotein: an obituary. Journal of hepatology. 2001;34(4):603–5. doi: 10.1016/s0168-8278(01)00025-3. [DOI] [PubMed] [Google Scholar]
- 210.Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, et al. Des-gammacarboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010 Feb;138(2):493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Di Bisceglie AM. Issues in screening and surveillance for hepatocellular carcinoma. Gastroenterology. 2004 Nov;127(5 Suppl 1):S104–7. doi: 10.1053/j.gastro.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 212.Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol. 2000 Jun;95(6):1535–8. doi: 10.1111/j.1572-0241.2000.02091.x. [DOI] [PubMed] [Google Scholar]
- 213.Santagostino E, Colombo M, Rivi M, Rumi MG, Rocino A, Linari S, et al. A 6-month versus a 12-month surveillance for hepatocellular carcinoma in 559 hemophiliacs infected with the hepatitis C virus. Blood. 2003 Jul 1;102(1):78–82. doi: 10.1182/blood-2002-10-3310. [DOI] [PubMed] [Google Scholar]
- 214.Kim D, Han K, Sang H, et al. Semiannual surveillance for hepatocellular carcinoma improved patient survival compared to annual surveillance (Korean experience) Hepatology. 2007;46:403A. [Google Scholar]
- 215.Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010 Aug;53(2):291–7. doi: 10.1016/j.jhep.2010.03.010. [DOI] [PubMed] [Google Scholar]