Abstract
BACKGROUND AND OBJECTIVE
Pulmonary problems are vitally important in newborns. Increased intense and mucoid secretions may lead to atelectasis, pulmonary infections, respiratory distress, prolonged mechanical ventilation or even death. The aim of this study was to evaluate the safety of recombinant human deoxyribonuclease (rhDNase) in the management of persistent atelectasis in term and preterm newborns, unresponsive to the conventional treatment.
DESIGN AND SETTING
Prospective study of patients admitted to a general community setting of a neonatal intensive care unit between December 2007 and December 2009.
PATIENTS AND METHODS
The study included 22 patients (12 premature and 10 term) who were admitted to the neonatal intensive care unit because of respiratory distress and developed atelectasis, and were unresponsive to conventional treatment. Nebulized rhDNase was administered to all patients at a dose of 1 mg/m2 twice daily for 3 days. In patients who did not respond to 3 days of treatment, endotracheal rhDNase was administered at a dose of 1 mg/m2. We assessed the clinical (respiratory rate and oxygen requirement) and radiologic responses (chest radiographic score), recurrence of atelectasis, the need for a repetitive treatment, and mortality rate.
RESULTS
A clinical and radiologic improvement of atelectasis was observed in 18 of 22 patients following 3 days of nebulized rhDNase treatment. Atelectasis relapsed in 4 patients. Following the administration of combined endotracheal and nebulized rhDNase treatment, an improvement of atelectasis was noted in all four recurrent cases. No adverse events were observed in patients because of the rhDNase treatment.
CONCLUSIONS
rhDNase treatment is a safe option and may be used as an effective method for the management of persistent atelectasis in newborns, which is resistant to other conventional treatment methods.
Pulmonary problems are one of the most frequent medical conditions requiring intensive care in preterm and term newborns. Oxygen inhalation, intubation, and mechanical ventilation, as well as respiratory support, may be required in infants treated for respiratory distress in intensive care units. Many factors attenuate the clearance of pulmonary secretions in newborns, and the development of pulmonary problems may lead to life-threatening complications associated with mucus accumulation and mucus plugs in this particular group of patients.1
Treatment alternatives are limited when airway obstruction and pulmonary atelectasis develop secondary to mucus plugs. Respiratory physiotherapy or the treatment with nebulized bronchodilators is not always effective. Recombinant human deoxyribonuclease (rhDNase) is one of the newest treatment alternatives, which may be used in the softening and excretion of mucus plugs.2–4 The aim of this study was to evaluate the safety of rhDNase in the management of persistent atelectasis in term and preterm newborns unresponsive to conventional treatment methods.
PATIENTS AND METHODS
This prospective study included the patients who were admitted to the neonatal intensive care unit (NICU) between December 2007 and December 2009 because of respiratory distress and atelectasis. The patients received rhDNase therapy due to unresponsiveness to conventional treatment measures. The protocol, risks, and benefits were explained to the parents, and an informed consent was obtained from each family.
The gender, birth weight, and gestational age of the patients were recorded. A physical examination was performed, and the respiratory rate and oxygen requirements were monitored. Anteroposterior chest radiographs were obtained prior to and 24 hours after the treatment, and assessed by a radiologist. Chest radiographs were scored using the scoring system recommended by Hendriks et al.5 Each radiograph was evaluated in terms of the presence of atelectasis, hyperinflation, and mediastinal shift. Since a validated scoring system for atelectasis is lacking, a scoring system based on available published studies5–8 and the personal experience of our radiologists was adjusted. Radiographs were scored as 1 in the presence of hyperinflation and/or +1 in the presence of a mediastinal shift, and 0 in the absence of either of these conditions. Atelectasis was scored for each lobe. Partial atelectasis of each pulmonary lobe was scored as 1 and complete atelectasis as 2. The sum of these scores revealed the total radiographic score.
Patients received nebulized rhDNase (Pulmozyme; Roche Genentech Inc., San Francisco, CA, USA) therapy at a dose of 1 mg/m2 (rhDNase was diluted by 1 mL of 0.9% Sodium chloride, NaCl) twice daily (2 hours between the doses) for up to 3 days. Frequent aspiration and physiotherapy were performed following the second rhDNase dose to facilitate mucus drainage. The chest radiograph was repeated 24 hours after the second dose to assess the efficacy of rhDNase. If the atelectasis did not respond to a 3-day-treatment, endotracheal diluted rhDNase (dilution with 1 mL of 0.9% NaCl) was administered at a dose of 1 mg/m2.2
The clinical response was assessed by the respiratory rate, fractional inspired oxygen (FiO2) and partial pressure of carbon dioxide (PCO2) values reflecting the oxygen requirements. The radiologic response was assessed by the chest radiographic scores before and after treatment. Recurrence of atelectasis, treatment duration, adverse events due to rhDNase treatment, and mortality rate were also assessed. Patients were regularly checked and monitored against side effects such as pharyngitis, laryngitis, conjunctivitis, rash, hematologic, and/or coagulation abnormalities and anaphylaxis during the therapy period and also against voice alteration in the long run, although rhDNase is usually well tolerated.
Data were statistically analyzed by Number Cruncher Statistical System 2007 and Power Analysis and Sample Size 2008 Statistical Software (Kaysville, UT, USA) program. In addition to descriptive statistical methods (mean, standard deviation, and frequency), a paired sample test was used for comparison of quantitative variables. P values <.05 were considered statistically significant.
RESULTS
A total of 781 patients were admitted to the NICU because of respiratory distress between December 2007 and December 2009. Atelectasis was noted in 22 patients (2.8%). The demographic and clinical characteristics of the patients are presented on Table 1. Among the newborns; 15 were boys and 7 were girls; 12 were premature and 10 were term neonates. The gestational age ranged between 26 to 40 weeks, and the birth weight ranged between 750 to 3590 g. Ventilation was provided with a mechanical ventilator in 18 patients, an oxygen hood in 3 patients, and oxygen via a nasal cannula in 1 patient. Twelve patients were diagnosed as premature + respiratory distress syndrome, 8 patients were diagnosed with transient tachypnea of the newborn (TTN), and 2 patients were diagnosed with TTN + congenital adrenal hyperplasia. Patent ductus arteriosus (PDA), hydrocephaly, and cardiomyopathy were diagnosed in 6, 1, and 1 premature newborns, respectively. Right partial atelectasis was noted in 13 patients, left partial atelectasis in 3 patients, total atelectasis in 3 patients, left total atelectasis in 1 patient, and right total atelectasis was detected in 2 patients. Atelectasis relapsed in 4 patients.
Table 1.
Demographic and clinical characteristics of the patients.
| Characteristic | Value |
|---|---|
|
| |
| Gender (male/female) | 15/7 |
| Birth weight (g), mean (SD) | 2088.5 (1018.6) |
| Gestational age (week), mean (SD) | 32.8 (5.2) |
| On ventilator/Not on ventilator (n) | 18/4 |
| Age during rhDNase treatment (days), mean (SD) | 11.4 (10.2) |
| Duration of nebulized rhDNase treatment (day), mean (SD) | 3.0 (1.0) |
rhDNase: recombinant human Dnase; SD: standard deviation.
The first patient who received rhDNase was a premature neonate at 27 weeks of gestational age with PDA who was on a mechanical ventilator and developed right total atelectasis. The patient developed atelectasis on the 11th, 18th, 28th, and 44th days of life. The nebulized treatment was administered on the first 3 days and resulted in an improvement of atelectasis. The 2nd, 5th, and last episodes of atelectasis improved in 6, 5, and 2 days, respectively. When atelectasis did not respond to 3 days of nebulized treatment, endotracheal treatment was initiated, which resulted in the improvement of atelectasis. Unfortunately, this patient succumbed to necrotizing enterocolitis (NEC) on the 47th day of life. The second patient who developed recurrent atelectasis was a premature neonate at 28 weeks of gestational age who received mechanical ventilation therapy due to right partial atelectasis. This patient developed atelectasis on the 11th and 21st days of life. Both episodes of atelectasis improved following 3 days of nebulized rhDNase treatment.
The third patient was a premature neonate at 28 weeks of gestational age who developed total atelectasis on days 9 and 15, and the last patient was a premature neonate at 29 weeks gestational age who developed atelectasis on the 10th and 30th days. Both patients were on mechanical ventilation therapy. Atelectasis improved after 3 days of nebulized and 3 days of endotracheal rhDNase treatments in these patients. Three of our patients succumbed due to various reasons, and the mortality rate was 13.6%. In 1 patient, mortality occurred due to NEC on day 47. This patient was a premature neonate at 27 weeks gestational age and on a mechanical ventilator with PDA, and developed right total atelectasis. Another patient died due to pulmonary hemorrhage on day 11. He was a premature neonate at 26 weeks gestational age with right partial atelectasis. In the last patient, mortality occurred secondary to hydrocephaly and NEC on day 45. This patient was a premature neonate at 29 weeks gestational age with total atelectasis. The chest radiograph scores, number of breaths per minute, and data related to respiratory function before and after the treatment are summarized in Table 2.
Table 2.
Comparison of the results of radiological and clinical parameters before and after treatment with recombinant human deoxyribonuclease of the neonatal patients included in the study.
| Before treatment | After treatment | P | |
|---|---|---|---|
|
| |||
| Chest x-ray scores | 1.67 (0.79) | 0 | <.001 |
| Respiratory rate (number of breaths/ min) | 62.95 (8.97) | 52.05 (8.21) | .001 |
| FIO2 (%) | 55.62 (12.34) | 43.38 (12.98) | .001 |
| PCO2 (mm Hg) | 60.90 (9.18) | 47.95 (7.98) | .001 |
Values are mean (SD).
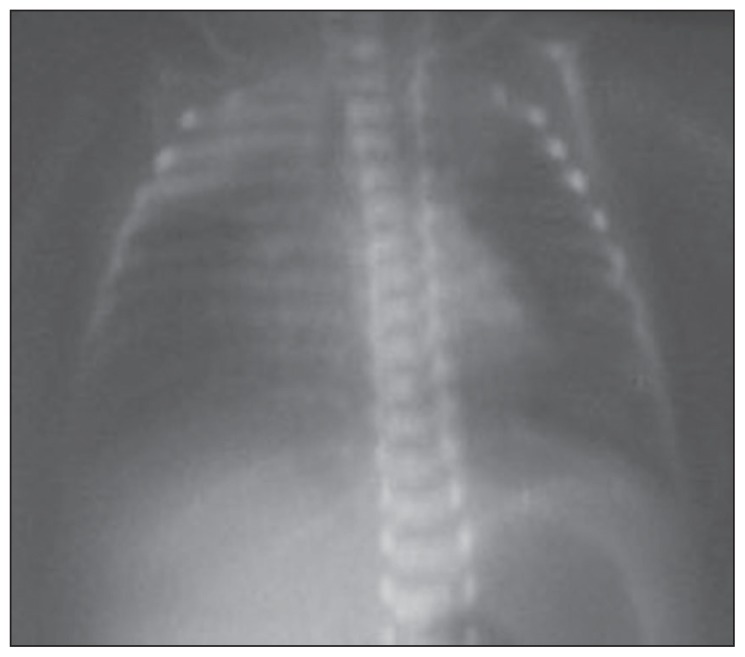
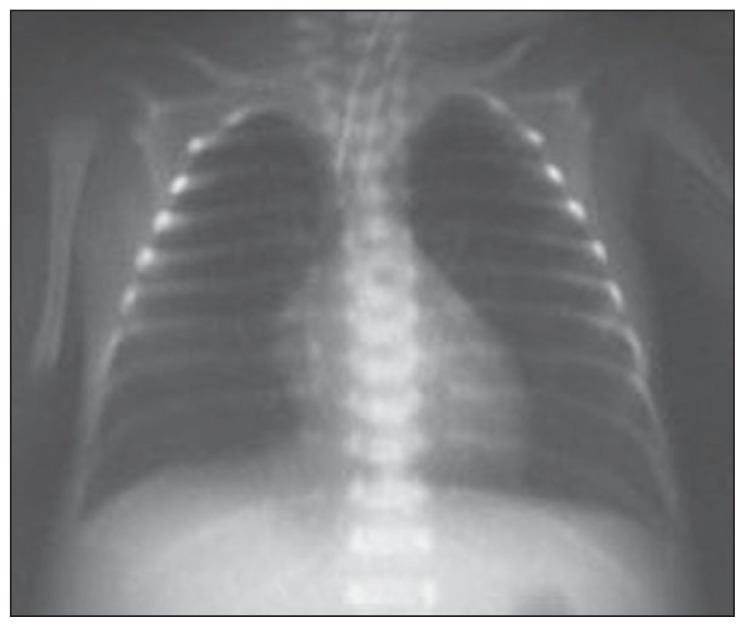
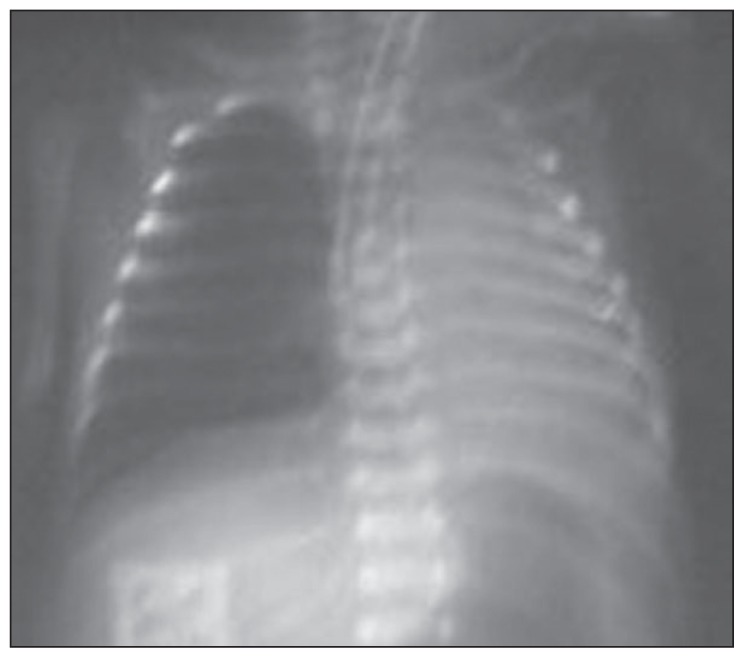
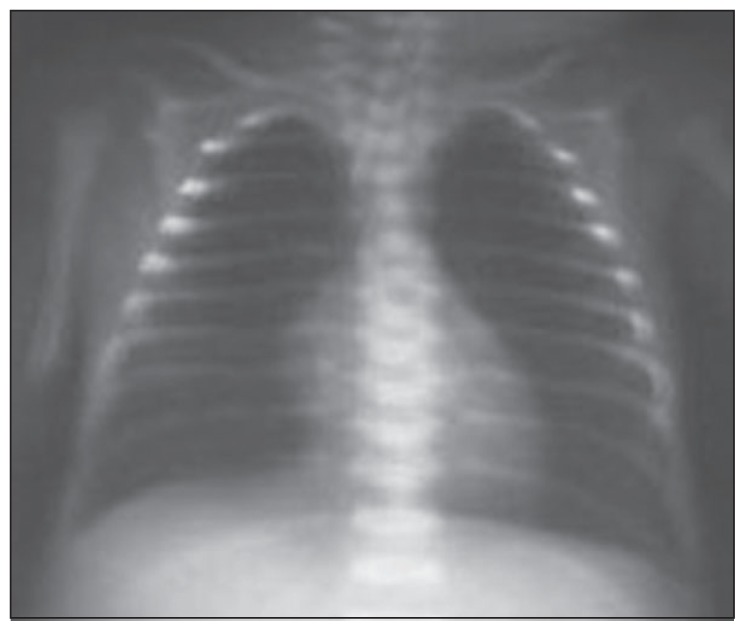
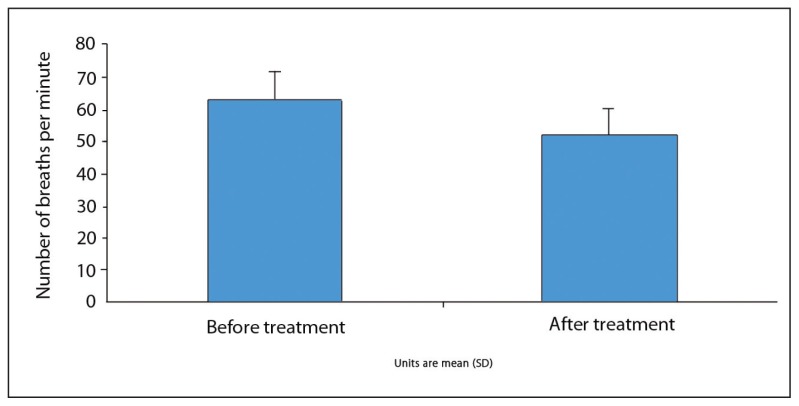
The mean radiographic score before the treatment was 1.67 (0.79), ranging between 1 and 3. While none of our patients had a radiographic score of 0 before the treatment, the score was 0 in all patients after the treatment. A statistically significant improvement was observed in radiographic scores of patients after the treatment (P<.05). The radiographic images of two of our patients before and after the treatment are presented in Figures 1–4, with an improvement in lung atelectasis after the treatment. A statistically significant reduction was noted in the number of breaths per minute after the treatment (P<.05) (Figure 5). Similarly, a statistically significant reduction was also noted in FiO2 and PCO2 values after the treatment (both, P<.05).
Figure 1.
Chest x-ray of the newborn before treatment (right partial atelectasis).
Figure 2.
Chest x-ray of the newborn after treatment (right partial atelectasis improved with treatment).
Figure 3.
Chest x-ray of the 28-week premature newborn before treatment (left total atelectasis was improved with treatment).
Figure 4.
Chest x-ray of the 28-week premature newborn after treatment. (left total atelectasis was improved with treatment).
Figure 5.
Comparison of number of breaths per minute before and after treatment.
In summary, a clinical and radiological improvement of atelectasis was noted following 3 days of nebulized rhDNase treatment in 18 of 22 patients. Atelectasis relapsed in 4 patients, and 4 times in 1 patient. Combined endotracheal and nebulized treatments were administered in the patients with recurrent atelectasis, and atelectasis improved in all 4 patients. No rhDNase-related adverse effects were observed in any of the patients.
DISCUSSION
Excessive, intense, and mucoid pulmonary secretions lead to atelectasis, pulmonary infections, and prolonged mechanical ventilation in newborns with respiratory distress.2,4,9 A high deoxyribonucleic acid (DNA) content is reported in the mucus plugs, which occurs as a result of tissue destruction caused by inflammatory cells. DNA is a polyanion compound, which is responsible for the viscosity and adhesiveness of the pulmonary secretions. rhDNase hydrolyzes extracellular DNA within the mucus and transforms it from an adhesive gel into a liquid form of fluid through dilution within minutes.2,9–11 Nebulized rhDNase is considered as an effective treatment for the liquefaction of viscous mucus in cystic fibrosis.3,12,13 There have also been reports of successful use of DNase treatment in patients suffering from respiratory distress due to conditions other than cystic fibrosis, including bronchiolitis secondary to respiratory syncytial virus, mycoplasma pneumoniae, and after cardiac surgery.9,14,15 Safe and effective treatment protocols are needed to treat or prevent pulmonary atelectasis, which may be a lethal condition in certain patient populations and with few treatment alternatives. The use of rhDNase in the management of neonatal atelectasis is a rather recent indication. A limited number of case reports exist regarding the use of rhDNase in atelectasis of the newborn resistant to the conventional treatment in published studies.
El Hassan et al2 reported the first case series involving three neonate patients with a successful DNase treatment on air trapping and atelectasis and receiving mechanical ventilator therapy preterm. The authors obtained successful results following administration of endotracheal DNase in one patient at a dose of 1 mg/m2, and one half of the standard pediatric doses in two patients (2 hours of nebulized DNase 1.25 mg).2 They reported that endotracheal DNase is effective as a rescue treatment to maintain airway stability in cases of atelectasis unresponsive to the conventional treatment.2,4 Kupeli et al3 reported that they have not observed any adverse events with the use of rhDNase for the treatment of atelectasis in a premature newborn at 29 weeks gestational age; however, they failed to achieve a successful outcome. They concluded that further studies supporting the use of rhDNase as an effective treatment in preterm newborns are needed.3
Our study, which is currently the largest series in the published studies involving the treatment of atelectasis in premature newborns with rhDNase therapy, was based on the study by Erdeve et al,16 in which 1.25 mg of nebulized rhDNase was administered twice daily for 3 days in 12 newborns (10 of which were preterm newborns). A rapid clinical and radiological improvement was noted in 10 of the 12 patients (83%) in this study.16 An improvement was observed by an endotracheal treatment in the other 2 newborns unresponsive to 3 days of nebulized treatment.16 In our study, it has been shown that the use of rhDNase in newborns who are unresponsive to the conventional treatment has clinically reduced the number of breaths per minute and the oxygen requirement, and has resulted in a significant improvement of the chest radiographic score and arterial blood gases. Different clinical studies in the published studies indicate the safety of rhDNase inhalation therapy without any serious side effects.2–4,13,15,17–19 Recombinant human DNase is often well tolerated. The most frequent adverse events are associated with the respiratory tract, and as they have been reported with a similar frequency in the placebo groups, it is difficult to differentiate whether they are associated with the underlying disease.11 The most common side effects that have been reported include voice alteration, laryngitis, and rash.11,18,19 Pharyngitis, chest pain, and conjunctivitis are among the other known side effects, and there have been no reports of hematologic or coagulation abnormalities or anaphylaxis during rhDNase treatment.11 The need for treatment cessation due to side effects occurs in approximately 3% of patients, which is similar to the rate in placebo-treated patients.11 In our series, no adverse events were observed in patients due to the rhDNase treatment.
We have administered 1 mg/m2 rhDNase twice daily for 3 days in 22 patients with atelectasis and repeated an endotracheal dose in those who did not respond to this treatment. Three patients succumbed due to other causes not related with the use of rhDNase. Both clinical and radiological improvement of atelectasis was noted in all patients, which was statistically significant. The radiographic scores were 0 in all patients after the treatment. A significant reduction in respiratory rate, FiO2, and PCO2 levels was also observed. The current study includes the largest series involving the treatment of premature newborns with atelectasis by endotracheal rhDNase therapy in published studies. Successful outcomes without any adverse effects suggest that rhDNase can also be used in preterm newborns for alleviating mucus plugs formed in the pulmonary case of atelectasis, and other pulmonary conditions leading to respiratory distress.
The major limitation of the study may be regarded as the lack of a control group. The protocol was used as a salvage treatment only in patients in whom other methods could not be successful, and as a last option rhDNase therapy was suggested. Another limitation may be the lack of a validated scoring system for atelectasis, but this was overcome by referencing to published studies and relying on a blinded radiologist’s experience. Since we aimed to present our experience with rhDNase in the management of persistent atelectasis in term and preterm newborns unresponsive to the conventional treatment, a homogenous group could not be adjusted and the patient population included 12 preterm infants; among them 6 patients had PDA, which might have exacerbated the diagnosis of atelectasis. Moreover, 13 patients had partial atelectasis, which may raise the bias level of this study.
In conclusion, recombinant human DNase was shown to be a safe option and may be used as an effective method for the management of persistent atelectasis in newborns, which is resistant to other conventional treatment methods. Future studies, including greater number of subjects, will provide further information on the efficacy and safety of rhDNase treatment and aid in creation of a treatment protocol in this challenging and labile patient population.
REFERENCES
- 1.Guglani L, Lakshminrusimha S, Ryan RM. Transient tachypnea of the newborn. Pediatr Rev. 2008;29:e59–65. doi: 10.1542/pir.29-11-e59. [DOI] [PubMed] [Google Scholar]
- 2.El Hassan NO, Chess PR, Huysman MW, Merkus PJ, de Jongste JC. Rescue use of DNase in critical lung atelectasis and mucus retention in premature neonates. Pediatrics. 2001;108:468–70. doi: 10.1542/peds.108.2.468. [DOI] [PubMed] [Google Scholar]
- 3.Küpeli S, Teksam O, Dogru D, Yurdakök M. Use of recombinant human DNase in a premature infant with recurrent atelectasis. Pediatr Int. 2003;45:584–6. doi: 10.1046/j.1442-200x.2003.01791.x. [DOI] [PubMed] [Google Scholar]
- 4.Durward A, Forte V, Shemie SD. Resolution of mucus plugging and atelectasis after intratracheal rhDNase therapy in a mechanically ventilated child with refractory status asthmaticus. Crit Care Med. 2000;28:560–2. doi: 10.1097/00003246-200002000-00045. [DOI] [PubMed] [Google Scholar]
- 5.Hendriks T, de Hoog M, Lequin MH, Devos AS, Merkus PJ. DNase and atelectasis in non-cystic fibrosis pediatric patients. Crit Care. 2005;9:R351–6. doi: 10.1186/cc3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friis B, Eiken M, Hornsleth A, Jensen A. Chest X-ray appearances in pneumonia and bronchiolitis. Correlation to virological diagnosis and secretory bacterial findings. Acta Paediatr Scand. 1990;79:219–25. doi: 10.1111/j.1651-2227.1990.tb11442.x. [DOI] [PubMed] [Google Scholar]
- 7.Babcook CJ, Norman GR, Coblentz CL. Effect of clinical history on the interpretation of chest radiographs in childhood bronchiolitis. Invest Radiol. 1993;28:214–7. doi: 10.1097/00004424-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Nasr SZ, Kuhns LR, Brown RW, Hurwitz ME, Sanders GM, Strouse PJ. Use of computerized tomography and chest x-rays in evaluating efficacy of aerosolized recombinant human DNase in cystic fibrosis patients younger than age 5 years: A preliminary study. Pediatr Pulmonol. 2001;31:377–82. doi: 10.1002/ppul.1061. [DOI] [PubMed] [Google Scholar]
- 9.Erdeve O, Akin O, Caner I, Hepyanar V, Sarici SU. rhDNase rescue treatment in early neonatal atelectasis: Case report. J Ankara Univ Fac Med. 2008;61:96–9. [Google Scholar]
- 10.Merkus PJ, de Hoog M, van Gent R, de Jongste JC. DNase treatment for atelectasis in infants with severe respiratory syncytial virus bronchiolitis. Eur Respir J. 2001;18:734–7. [PubMed] [Google Scholar]
- 11.Fitzgerald DA, Hilton J, Jepson B, Smith L. A crossover, randomized, controlled trial of dornase alfa before versus after physiotherapy in cystic fibrosis. Pediatrics. 2005;116:e549–54. doi: 10.1542/peds.2005-0308. [DOI] [PubMed] [Google Scholar]
- 12.Jones AP, Wallis CE. Recombinant human deoxyribonuclease for cystic fibrosis. Cochrane Database Syst Rev. 2003;3:CD001127. doi: 10.1002/14651858.CD001127. [DOI] [PubMed] [Google Scholar]
- 13.Suri R. The use of human deoxyribonuclease (rhDNase) in the management of cystic fibrosis. BioDrugs. 2005;19:135–44. doi: 10.2165/00063030-200519030-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kamin W, Klär-Hlawatsch B, Truebel H. Easy removal of a large mucus plug with a flexible paediatric bronchoscope after administration of rhDNase (Pulmozyme) Klin Padiatr. 2006;218:88–91. doi: 10.1055/s-2005-836608. [DOI] [PubMed] [Google Scholar]
- 15.Riethmueller J, Kumpf M, Borth-Bruhns T, Brehm W, Wiskirchen J, Sieverding L, et al. Clinical and in vitro effect of dornase alfa in mechanically ventilated pediatric non-cystic fibrosis patients with atelectases. Cell Physiol Biochem. 2009;23:205–10. doi: 10.1159/000204109. [DOI] [PubMed] [Google Scholar]
- 16.Erdeve O, Uras N, Atasay B, Arsan S. Efficacy and safety of nebulized recombinant human DNase as rescue treatment for persistent atelectasis in newborns: case-series. Croat Med J. 2007;48:234–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Nasr SZ, Strouse PJ, Soskolne E, Maxvold NJ, Garver KA, Rubin BK, et al. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest. 2001;120:203–8. doi: 10.1378/chest.120.1.203. [DOI] [PubMed] [Google Scholar]
- 18.Bryson HM, Sorkin EM. Dornase alfa. A review of its pharmacological properties and therapeutic potential in cystic fibrosis. Drugs. 1994;48:894–906. doi: 10.2165/00003495-199448060-00006. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331:637–42. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]