Figure 1. Overall architecture of homomeric SWELL1.
(A) Cryo-EM reconstruction of SWELL1 homohexamer viewed from the membrane plane highlighting a dimer pair (top left, red and pink subunits) and an interface between dimers (top right, pink and green subunits), from the extracellular side (bottom left), and from the cytosolic side (bottom right). (B) Detailed view of SWELL1 ‘inner’ protomer. (C) Topology diagram denoting secondary structural elements. Dashed lines indicate unresolved regions on both protomers in a dimer pair, while dashed shape borders indicate regions that are only resolved on one protomer.
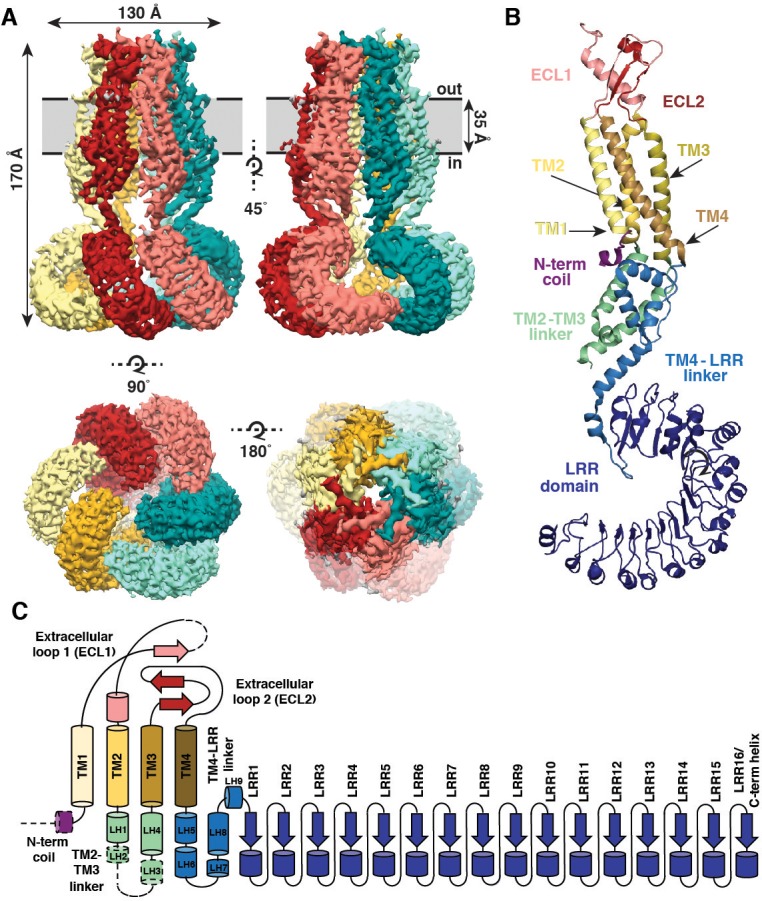
Figure 1—figure supplement 1. SWELL1 overexpression in cells lacking other LRRC8 subunits produces small DCPIB-sensitive swelling-induced whole cell currents.
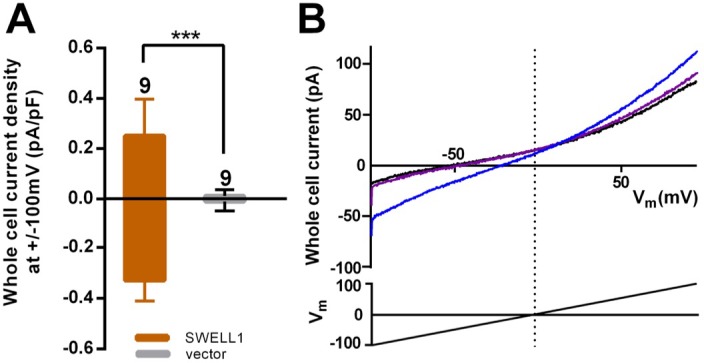
Figure 1—figure supplement 2. Purification of SWELL1-FLAG.
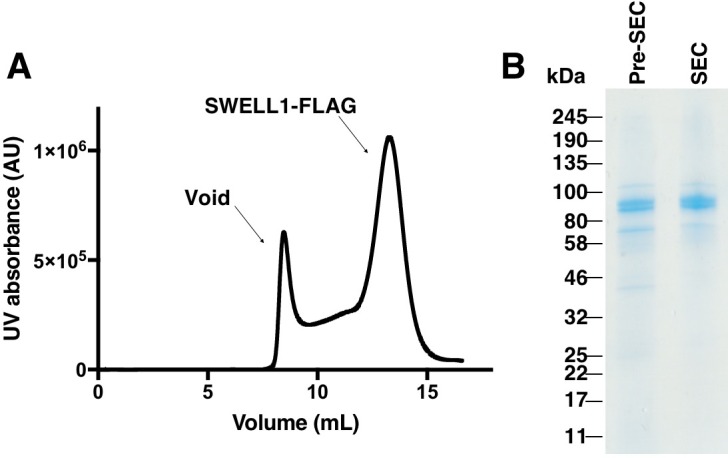
Figure 1—figure supplement 3. Cryo-EM data collection.
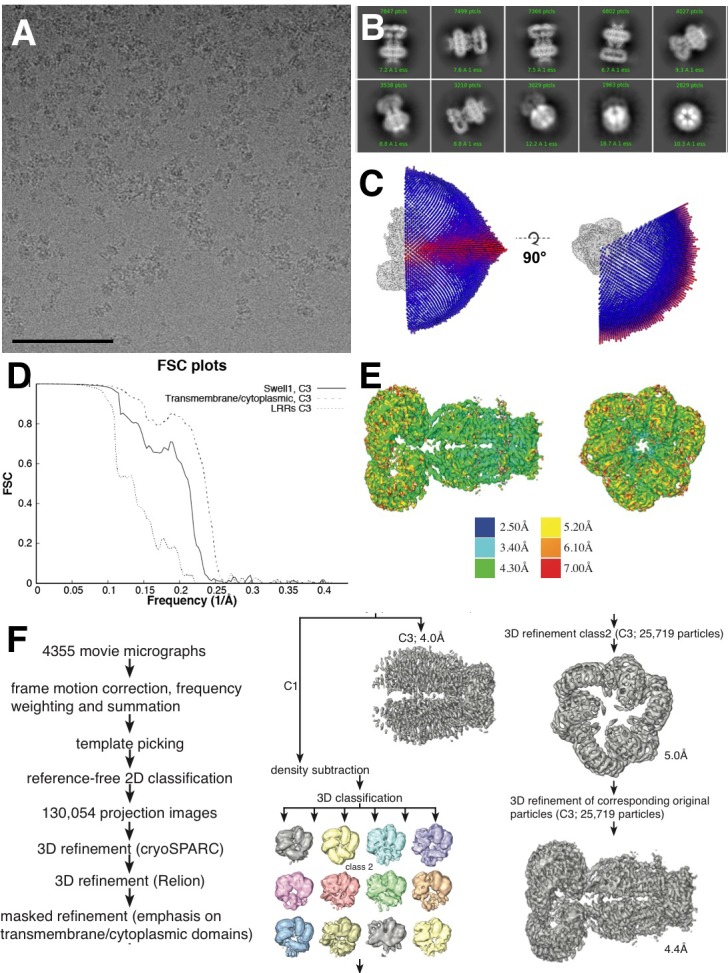
Figure 1—figure supplement 4. Model-to-map fit of electron density.
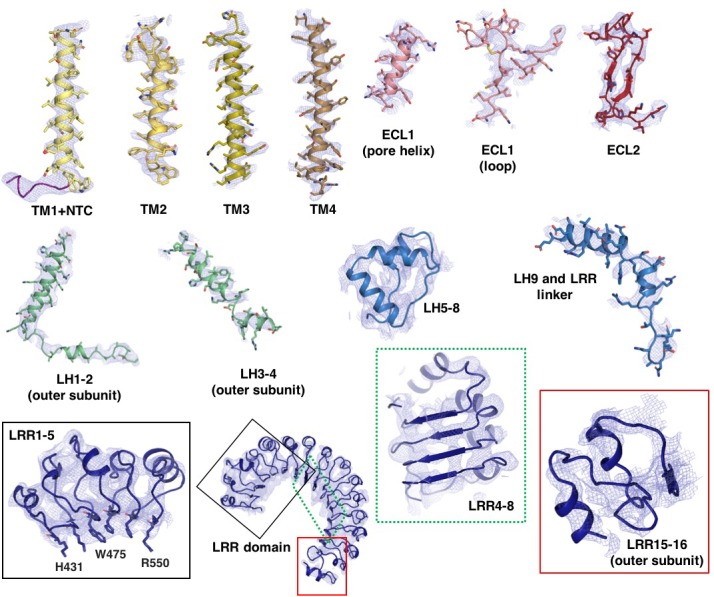
Figure 1—figure supplement 5. SWELL1 subunit pair and structural homology of SWELL1 structure to connexin-26 and innexin-6 structures.
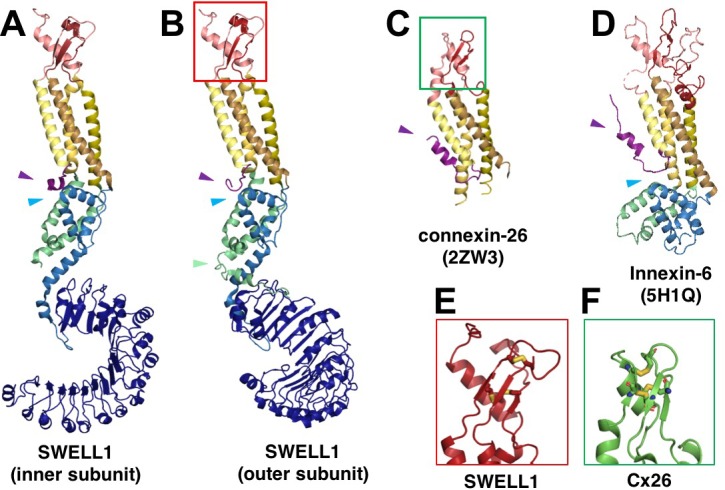
Figure 1—figure supplement 6. Alignment of LRRC8 subunits (res 1–435).
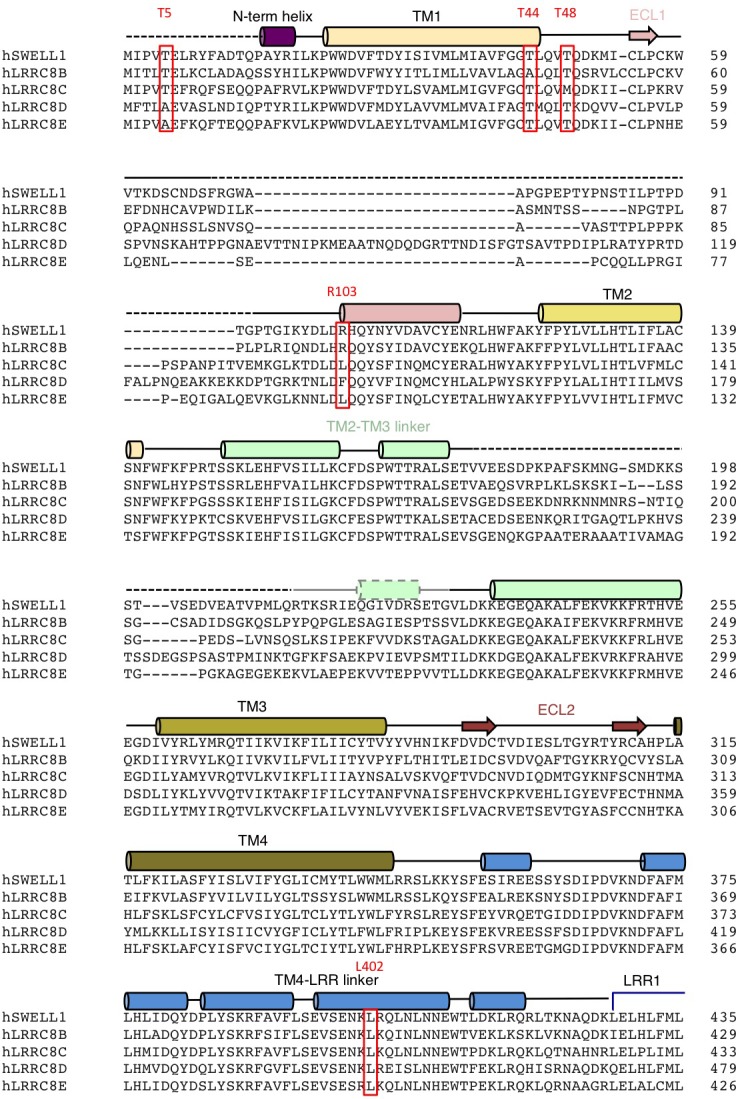
Figure 1—figure supplement 7. Alignment of LRRC8 subunits (res 436–810).

