Abstract
BACKGROUND
Continuous renal replacement therapy (CRRT) has become the preferred mode of dialysis to support critically ill children with acute kidney injury. However, there are limited pediatric data on CRRT use, especially in our region.
OBJECTIVE
Determine the outcome of CRRT among critically ill children.
DESIGN
Retrospective cohort study.
SETTING
Pediatric intensive care unit.
PATIENTS AND METHODS
The study included critically ill children 1–14 years of age who underwent CRRT from July 2009 to June 2015. We report the underlying diagnosis, demographics, indications and modality of CRRT, and associated risk factors. Statistical analyses were used to identify risk factors associated with mortality.
MAIN OUTCOME MEASURES
Mortality and associated risk factors with use of CRRT.
SAMPLE SIZE
96
RESULTS
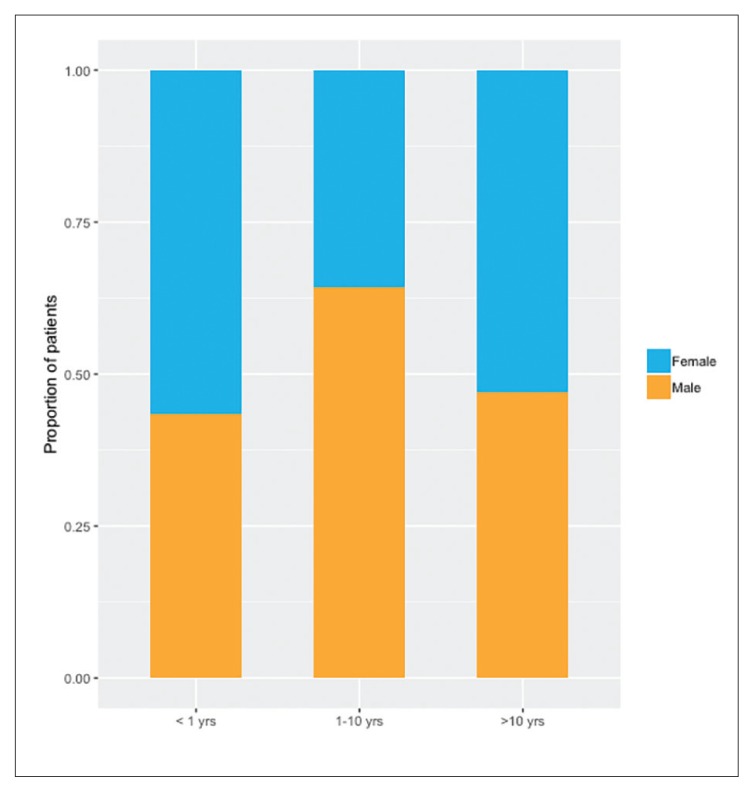
The mean age was 6.0 (standard deviation, 4.4) years, with a male preponderance in the age group from 1–10 years which comprised almost 60% of the study group. The most common primary diagnoses were malignancies [37.5% (36/96)] followed by primary renal diseases [19.8% (19/96)], and immunodeficiency [16.7% (16/96)]. The most common indication for CRRT was fluid overload [67.2% (65/96)] followed by tumor lysis syndrome [18.8%(18/96)], and metabolic encephalopathy [9.4%(9/96)]. The median length of CRRT was 66 hours (IQR, 35.5–161.4), with a median average circuit life of 30.9 hours (IQR, 16.4–45.0). The most common CRRT catheter site was the internal jugular vein [77.1% (74/96)], followed by the femoral vein [18.8%(18/96)] with continuous venovenous hemodiafiltration [82.3%(79/96)] being the most common CRRT modality used. The mortality rate among critically ill children requiring CRRT was 50% (48/96). There was an increased mortality rate among children with hematological diseases (100%, 10/10), immunodeficiency (86.6%, 13/16) and in children who had undergone stem cell transplantation (90.0%, 27/30), with the least mortality in primary renal disease (15.8% (3/19). We identified septic shock and use of inotropic support as being independently associated with mortality in a multivariate analysis.
CONCLUSION
The overall mortality rate among critically ill children who underwent CRRT was 50% with significantly increased mortality among patients with hematological diseases, immunodeficiency, and in children who had undergone stem cell transplantation. Septic shock and use of inotropic support were associated with mortality.
LIMITATIONS
Retrospective and single center data that is not generalizable.
Continuous renal replacement therapy (CRRT) has become the preferred mode of dialysis to support children with acute kidney injury (AKI) admitted to pediatric intensive care units (PICU).1,2 In the early days of CRRT, blood was passed through extracorporeal tubing and filtering was driven by the patient’s perfusion pressure via an arterial-venous circuit.3 However, with the development of pump-driven volumetric control, CRRT machines with small extracorporeal volumes led to the widespread use of venovenous forms of CRRT.4
The epidemiology of AKI has changed over the last several decades primarily in developed countries where congenital heart disease (corrective/palliative surgeries), acute tubular necrosis, sepsis, and nephrotoxic medications, are considered the most common causes of pediatric AKI.4,5 In a prospective pediatric CRRT (ppCRRT) registry, sepsis (30%) followed by cardiac diseases (19%), and inborn error of metabolism (15%) were the most common primary diagnoses.6 However, in developing countries, primary renal disease (hemolytic uremic syndrome, nephrotic syndrome, and hypovolemic acute tubular necrosis) continues to be the most common cause of AKI.7,8 Also, the spectrum of primary renal diseases was different in the ppCRRT registry, which included autosomal recessive polycystic kidney diseases, cortical necrosis, chronic kidney diseases of undetermined etiology, congenital nephrotic syndrome and bilateral renal agenesis.6 Fluid overload and electrolyte abnormalities were the most common indication for CRRT use and continuous venovenous hemodialysis was the most common mode of CRRT utilized.6
In adults, the mortality rate with severe AKI requiring renal replacement therapy is 50% to 80%.9–11 However, in a pediatric study, the overall mortality rate was 42% in critically ill children requiring CRRT.6 Numerous case reports and case series have described the use of CRRT in children.12,13 Due to recent advances, more awareness, and ease of utilization of CRRT, its use is increasing among different university and teaching hospitals in Saudi Arabia and little used in MOH hospitals because of unavailability. However, there is limited pediatric data on the pattern of CRRT use, its safety, and its effect on ultimate patient survival in PICUs worldwide, especially from our region. Therefore, we aimed to determine the outcomes of CRRT in critically ill children in our PICU.
PATIENTS AND METHODS
All critically ill children admitted to the PICU of King Faisal Specialist Hospital and Research Centre from July 2009 to June 2015, within 1 to 14 years of age, and who underwent CRRT, were included in this study. The study was approved by the hospital ethical review committee (ORA/0602/37). Data was collected on a structured proforma which was then transferred to statistical software for further analysis. Confidentiality of the data was maintained by keeping the proformas in a locker and SPSS files were kept under password. Demographic data included weight, height, age, gender, underlying diagnosis category. Data also included Indications for CRRT, total length of stay in PICU, pRIFLE criteria at the start of CRRT, length of CRRT in hours, and use of inotropes during CRRT. Clinical data included glomerular filtration rate (GFR) at the start of CRRT, urine output in mL/kg/h at the start of CRRT, RIFLE criteria at the start of CRRT, CRRT site, CRRT modality, and final outcome in terms of survival and mortality.
Categorical variables were summarized by count and percentage. For continuous variables, data was summarized by mean and standard deviation or median and interquartile range using SPSS version 20 ( Armonk, NY: IBM Corp). To identify risk factors for increased mortality, the chi-squared test was used for categorical variables and the independent t-test and nonparametric tests were used for continuous variables whether evenly distributed or skewed. A P value <.05 was considered statistically significant. Multivariate regression analysis was performed with significant variables from the univariate analysis to identify independent risk factors for increased mortality.
RESULTS
Of 108 critically ill children who underwent CRRT from July 2009 to June 2015, 12 patients were excluded due to insufficient data, so 96 patients were included in study. The mean age was 6.0 (4.4) years with the majority of the children between 1 to 10 years of age (with a male preponderance [Figure 1]), followed by infants (<1 year old) (Table 1). The most common diagnosis was malignancies followed by primary renal diseases, immunodeficiency, and metabolic diseases (Table 2). Almost half of patients with septic shock required inotropic support, with epinephrine (45.8%) being most commonly used followed by norepinephrine (43.8%), and dopamine (31.3%), with the mean PICU length of stay being approximately 2 weeks. The most common indication for CRRT was fluid overload followed by tumor lysis syndrome, and metabolic encephalopathy (Table 1). The median length of CRRT was 66 hours (IQR, 34.6–164.2), and the median circuit life was 30.9 hours (IQR, 16.4–45.0).
Figure 1.
Gender distribution by age categories.
Table 1.
Characteristics of patients who underwent continuous renal replacement therapy (n=96).
| Characteristic | n (%) or mean (SD) |
|---|---|
|
| |
| Age | 6.0 (4.4) |
| <1 year | 23 (24) |
| 1–10 years | 56 (58.3) |
| >10 years | 17 (17.7) |
| Gender | |
| Male | 54 (56.2) |
| Female | 42 (43.8) |
| Weight (kg) | 19.7 (12.9) |
| Height (cm) | 105.2 (32.4) |
| ICU LOS (days) | 14.9 (18.4) |
| Inotrope use | 48 (50) |
| Epinephrine | 44 (45.8) |
| Norepinephrine | 42 (43.8) |
| Dopamine | 30 (31.3) |
| Dobutamine | 10 (10.4) |
| Milrinone | 1 (1) |
| Length of CRRT (hours), median (IQR) | 66 (34.6–164.2) |
| Circuit life (hours), median (IQR) | 30.9 (16.4–45.0) |
| Patient underwent SCT | 30 (31.3) |
| Patients admitted with sepsis | 43 (44.8) |
| Reason for CRRT | |
| Fluid overload | 65 (67.2) |
| Tumor lysis syndrome | 18 (18.8) |
| Metabolic encephalopathy | 9 (9.4) |
| Increase oxalate | 4 (4.2) |
| Catheter site | |
| Internal jugular | 74 (77.1) |
| Femoral | 18 (18.8) |
| Subclavian | 4 (4.2) |
| CRRT modality | |
| CVVHDF | 79 (82.3) |
| CVVHD | 9 (9.4) |
| CVVH | 6 (6.3) |
| SCUF | 2 (2.1) |
| Heparin use | 23 (24) |
| Parameters before starting CRRT | |
| RIFLE criteria | |
| Risk | 10 (10.4) |
| Injury | 19 (19.8) |
| Failure | 53 (55.2) |
| Loss | 0 (0) |
| ESRD | 14 (14.6) |
| Urine output (mL/kg/hr), median (IQR) | 0.8 (0.4–2.4) |
| GFR (mL/min/1.73m2), median (IQR) | 38.05 (20.5–66.1) |
| Outcome | |
| Discharged | 48 (50) |
| Died | 48 (50) |
Values are n (%) or mean (standard deviation) unless otherwise indicated. ICU LOS: intensive care unit length of stay; CRRT: continuous renal replacement therapy; SCUF: slow continuous ultrafiltration; CVVH: continuous veno-venous hemofiltration; CVVHD: continuous veno-venous hemodialysis; CVVHDF: continuous veno-venous hemodiafiltration; ESRD: end-stage renal disease; GFR: glomerular filtration rate (Schwartz Formula).
Table 2.
Primary diagnosis of critically ill pediatric patients undergone CRRT (N=96).
| Diagnosis | n (%) or mean (SD) |
|---|---|
|
| |
| Malignancies | 36 (37.5) |
| Leukemia | 19 (19.8) |
| T cell acute lymphoblasticleukemia | 9 |
| Acute myelogenous leukemia | 7 |
| B cell acute lymphoblastic leukemia | 3 |
| Lymphoma | 12 (12.5) |
| Burkitt’s lymphoma | 8 |
| Hodgkin’s lymphoma | 2 |
| T cell lymphoma | 1 |
| Diffuse large B cell lymphoma | 1 |
| Solid tumors | 5 (5.2) |
| Neuroblastoma | 2 |
| Ewing sarcoma | 1 |
| Wilms tumor | 1 |
| Medulloblastoma | 1 |
| Renal diseases | 19 (19.8) |
| Hemolytic uremic syndrome | 5 |
| Nephrotic syndrome | 5 |
| Hyperoxaluria | 4 |
| Obstructive uropathy | 2 |
| Cystinosis | 1 |
| Polycystic kidney disease | 1 |
| Dysplastic kidneys | 1 |
| Immunodeficiency diseases | 16 (16.7) |
| Severe combined immunodeficiency | 5 |
| Bare lymphocyte syndrome | 4 |
| Omenn syndrome | 3 |
| Chronic granulomatous disease | 1 |
| Hyper IgE syndrome | 1 |
| X-linked lymphoproliferative disease | 1 |
| Gracile syndrome | 1 |
| Metabolic diseases | 15 (15.6) |
| Maple syrup urine disease | 10 |
| Wilson disease | 3 |
| Propionic acidemia | 1 |
| Metachromatic leukodystrophy | 1 |
| Hematological diseases | 10 (10.4) |
| Hemophagocytic lymphohistiocytosis | 5 |
| Myelodysplastic syndrome | 1 |
| Aplastic anemia | 3 |
| B thalassemia major | 1 |
The most common CRRT catheter site was the internal jugular vein followed by the femoral and subclavian vein. By the RIFLE criteria, renal failure had been the most common reason for CRRT, followed by injury, and end-stage renal disease (ESRD), with GFR of 38.05 mL/min/1.73 m2 (IQR, 20.5–66.1), and urine output of 0.8 mL/kg/h (IQR, 0.4–2.4) before the start of CRRT. The most common CRRT modality was continuous venovenous hemodiafiltration (CVVHDF) followed by continuous venovenous hemodialysis (CVVHD), and continuous venovenous hemofiltration (CVVH), with heparin use in 24% of the patients.
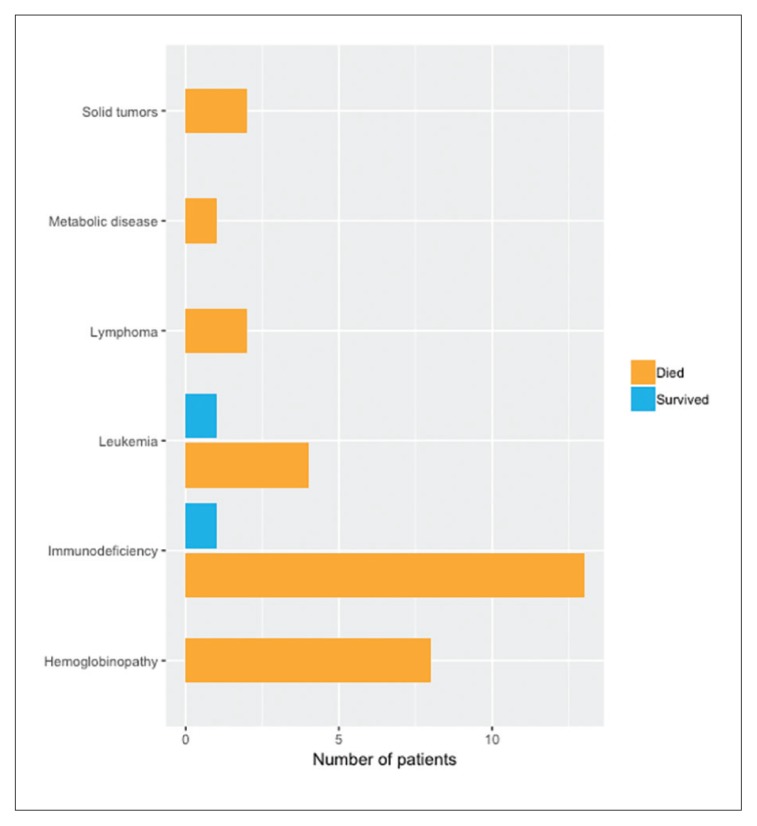
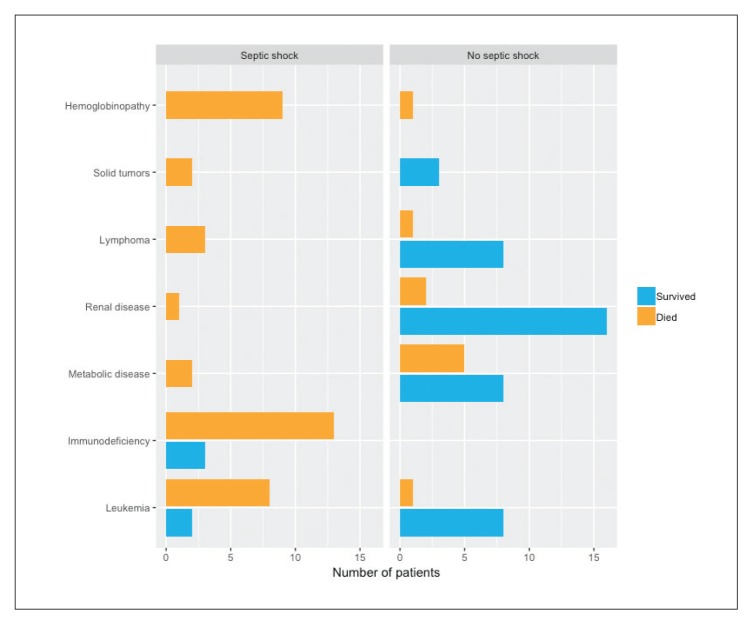
The mortality rate among critically ill children admitted to PICU requiring CRRT was 50% (48/96). An even higher mortality rate occurred in patients with hematological diseases (100%) and in children with immunodeficiency (86.6%) who underwent stem cell transplant and developed septic shock. Of 32 that had undergone a stem cell transplant, 30 died (94%) (Figure 2). Of the 96 patients, 43 presented with septic shock and the mortality was greater in patients with septic shock (88.4% vs 18.9%) (Figure 3). The lowest mortality rate (18.8%) was among critically ill children with primary renal diseases.
Figure 2.
Outcome among patients who had undergone a stem cell transplant (n=30).
Figure 3.
Outcome by diagnosis and presence of septic shock (n=96).
When computing the risk factors for increased mortality, children who presented with septic shock, use of inotropic support, an indication of fluid overload for initiation of CRRT, patient in failure as per RIFLE criteria before CRRT, the presence of oliguric renal failure with urine output <1 mL/kg/h before starting CRRT, were identified as risk factors significantly associated with increased mortality (Table 3). Multivariate regression analysis (using statistically significant variables in the univariate analysis) identified septic shock and use of inotropic support to be independently associated with decreased survival among critically ill children who underwent CRRT (Table 4).
Table 3.
Univariate analysis for increased mortality among patients undergoing continuous renal replacement therapy.
| Characteristics | Alive at discharge n=48 (50%) |
Dead at discharge n=48 (50%) |
P value | OR 95% CI |
|---|---|---|---|---|
|
| ||||
| Age (years) | 6.3 (4.5) | 5.6 (4.2) | .467 | 0.653 (−1.122–2.430) |
| <1 year | 10 (20.8) | 13 (27.1) | .169 | - |
| 1–10 years | 26 (54.2) | 30 (62.5) | ||
| >10 years | 12 (25) | 5 (10.4) | ||
| Gender | ||||
| Male | 31 (64.6) | 23 (47.9) | .149 | 1.892 (0.874–4.495) |
| Female | 17 (35.4) | 25 (52.1) | ||
| Weight (kg) | 20.43 (13.5) | 18.92 (12.5) | .569 | 1.515 (−3.753–6.783) |
| Height (cm) | 107.4 (31.2) | 103.1 (33.7) | .516 | 4.327 (−8.836–17.491) |
| ICU LOS (days) | 9.0 (5.6–14.6) | 11.7 (5.8–17.5) | .500 | - |
| Inotropes use | 7 (14.6) | 41 (85.4) | <.001 | 0.029 (0.009–0.021) |
| Epinephrine | 6 (12.5) | 38 (79.2) | <.001 | 0.038 (0.012–0.113) |
| Norepinephrine | 4 (8.3) | 38 (79.2) | <.001 | 0.024 (0.007–0.083) |
| Dopamine | 2 (4.2) | 28 (58.3) | <.001 | 0.031 (0.007–0.143) |
| Dobutamine | 1 (2.1) | 9 (18.8) | .015 | 0.092 (0.011–0.760) |
| Milrinone | 0 (0) | 1 (2.1) | - | - |
| Length of CRRT (h), median (IQR) | 61.3 (32.3–121.6) | 98.6 (39.3–206.8) | .051 | - |
| Average circuit life (h), median (IQR) | 25 (14.1–44.6) | 35.29 (20.25–45) | .280 | - |
| Reason for CRRT | ||||
| Fluid overload | 21 (43.8) | 44 (91.7) | <.001 | - |
| Metabolic encephalopathy | 7 (14.6) | 2 (4.2) | ||
| Tumor lysis syndrome | 16 (33.3) | 2 (4.2) | ||
| Increase oxalate | 4 (4.2) | 0 (0) | ||
| Catheter site | ||||
| Femoral | 10 (20.8) | 8 (16.7) | .921 | - |
| Internal jugular | 36 (75) | 38 (79.2) | ||
| Subclavian | 2 (4.2) | 2 (4.2) | ||
| CRRT modality | ||||
| SCUF | 2 (4.2) | 0 (0) | .516 | - |
| CVVH | 4 (8.3) | 2 (4.2) | ||
| CVVHD | 4 (8.3) | 5 (10.4) | ||
| CVVHDF | 38 (79.2) | 41 (85.4) | ||
| Heparin use | 15 (31.2) | 8 (16.7) | .150 | 2.273 (0.858–6.020) |
| Parameters before starting CRRT | ||||
| RIFLE criteria | ||||
| Risk | 9 (18.8) | 1 (2.1) | <.001 | - |
| Injury | 15 (31.2) | 4 (8.3) | ||
| Failure | 13 (27.1) | 40 (83.3) | ||
| Loss | 0 (0) | 0 (0) | ||
| ESRD | 11 (22.9) | 3 (6.2) | ||
| Urine output (mL/kg/h), median (IQR) | 1.5 (0.4–3.9) | 0.6 (0.32–1.2) | .035 | - |
| GFR (mL/min/1.73m2), median (IQR) | 32.8 (15.4–78.8) | 38.25 (25.7–55.4) | .631 | - |
Values are n (%) or mean (standard deviation) unless otherwise indicated. ICU LOS: intensive care unit length of stay; CRRT: continuous renal replacement therapy; SCUF: slow continuous ultrafiltration; CVVH: continuous venovenous hemofiltration; CVVHD: continuous venovenous hemodialysis; CVVHDF: continuous venovenous hemodiafiltration; ESRD: end-stage renal disease; GFR: glomerular filtration rate (Schwartz Formula).
Table 4.
Multivariate logistic regression analysis for decreased survival among patients undergoing continuous renal replacement therapy (n=96).
| B | S.E. | Wald | df | Sig. | Exp (B) | 95% C.I. for Exp (B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
|
| ||||||||
| Sepsis with septic shock | −2.414 | 1.211 | 3.977 | 1 | .046 | .089 | .008 | .959 |
| Bone marrow transplant | .604 | 1.244 | .235 | 1 | .628 | 1.829 | .160 | 20.951 |
| Urine output before CRRT | −.081 | .282 | .082 | 1 | .775 | .922 | .531 | 1.603 |
| Inotropic support use | −2.603 | .763 | 11.643 | 1 | .001 | .074 | .017 | .330 |
| RIFLE criteria | −.148 | .406 | .134 | 1 | .714 | .862 | .389 | 1.909 |
| Length of CRRT | .004 | .003 | 1.291 | 1 | .256 | 1.004 | .997 | 1.010 |
| Constant | 6.836 | 1.937 | 12.451 | 1 | .000 | 931.030 | ||
Omnibus Tests of Model Coefficients: chi-square 66.845, df=6, P<.001; −2 Log likelihood 66.239; Cox & Snell R Square .502
DISCUSSION
Over the last two decades, CRRT has became the modality of choice in critically ill children with AKI and fluid overload.1,2 However, there is still limited pediatric data on the use of CRRT, its safety and its ultimate effect on patient survival in an intensive care setting.1,2,4,14,15 The demographic data in our study was similar to others (age and male preponderance).3,13 Reports from the 1980s highlighted hemolytic uremic syndrome and other primary renal diseases, sepsis and burns as the most common causes of pediatric AKI, but the epidemiology has changed over the last several decades.14,15 There has been an epidemiologic shift, primarily in developed countries, with sepsis, cardiac diseases, inborn errors of metabolism and others now considered the most common etiology for pediatric AKI,4,6 which is reflected in our study with malignancies, sepsis, hematopoietic stem cell transplant being the most common, and with primary renal diseases being the cause in less than 20%. However, in developing countries, primary renal disease (hemolytic uremic syndrome, nephrotic syndrome, and hypovolemic acute tubular necrosis) has continued to be the most common cause of AKI.7,8
Indications for the use of CRRT, as reported in the ppCRRT registry, were fluid overload in 29%, isolated electrolyte abnormalities in 13%, and combined fluid overload and electrolyte abnormalities in 46%; thus over 90% had fluid overload and electrolyte abnormalities related to AKI.6 However, only 4% received CRRT for metabolic diseases, and 2% for intoxication drug overdose,6 which is similar to our study, with fluid overload and tumor lysis syndrome constituting more than 85% of children. In the ppCRRT registry, CVVHD was the most common mode of CRRT followed by CVVHDF, and CVVH,6 while in our case CVVHDF was the most common mode followed by CVVHD and CVVH.
In general, a larger bore of CRRT catheter was associated with higher flow rates and greater CRRT survival.16 Although the use of single lumen 5-French catheters provided viable access in neonates and infants, the data suggested that no circuit survived beyond 20 hours when 5-French 2 single-lumen catheters were used.16 However, 7-French catheters were better tolerated in infants in another study, as in ours.17 In our study, 6-French catheters used in 27% of our patients, mainly infants, and had a better average circuit life.17
The location of the catheter also has as great an impact on CRRT circuit survival as the catheter size.17 Although femoral catheters were used most frequently, nearly three times more frequently than internal jugular (IJ) and subclavian catheters (SC) (69% vs 16% vs 8%, respectively), IJ catheters had significantly greater circuit survival when compared to femoral and SC catheters.16 We also used IJ catheters four times more frequently than femoral and SC catheters, which might be an additional reason for the greater circuit lifespan in our patients. IJ catheters were much better tolerated in infants as well. Many practitioners still prefer the femoral site; with a bedside ultrasound device the femoral site greatly enhances the safety and ease of IJ catheter insertion.17 Also, unless their tip was in the inferior vena cava, femoral catheters were remarkably sensitive to patient movement and usually required the patient to be sedated or even paralyzed for successful use.17 However, nephrologists frequently avoid SC catheters because of SC vein stenosis, which would lead to difficult vascular access for chronic hemodialysis, if the patient failed to regain renal function.17
Previously, lactate based dialysate/replacement solutions were commonly used for CRRT.18 The use of these solutions was commonly associated with lactic acidosis with concomitant cardiac dysfunction and hypotension.18 However, several studies have clearly demonstrated the superiority of bicarbonate based fluids over lactate-based fluids.19,20 We also used bicarbonate-containing fluids, which were considered standard of care in both adults and children.19,20 To prevent clotting and for prolongation of the CRRT circuit span, anticoagulation was commonly employed.17 Only one large study in pediatrics demonstrated that heparin and citrate use were equally efficacious with a similar circuit lifespan, but bleeding complications were more common with heparin.21 However, the study clearly indicated that a markedly reduced circuit lifespan and reduced ability to continue CRRT was associated with no anticoagulant use.21 The use of sodium citrate, first reported by Mehta and Ahmed in the 1990s, improved the ease of administration and had fewer side effects when compared to heparin, but heparin is still in widespread use.22,23 Also systemic hypocalcaemia can be prevented by infusing calcium chloride or calcium gluconate back into the patient at a central site away from the CRRT circuit.17
Large studies in adults have suggested a mortality rate of 50% to 80% can be expected with severe AKI requiring renal replacement therapy.9–11 Data from adults has shown that vasopressor support, mechanical ventilation, sepsis, severity of illness at presentation, multiorgan dysfunction syndrome (MODS), involving in addition to the kidney, the heart, liver, intestines, brain and lungs, and a more positive fluid balance to be significantly associated with increased mortality.11,24,25 In the ppCRRT registry, overall mortality rate was 42%,13 while another study reported an overall mortality rate of 39%,3 both of which were lower than in our study, which had an overall mortality rate of 50%. The increased mortality in our study may be due to more patients with hematological disorders, immunodeficiency in stem cell transplant patients, as well as septic shock at presentation. In addition, these patients were more fluid overloaded and oliguric before the start of CRRT. Higher mortality rates were described in the ppCRRT registry in patients with liver failure or liver transplant, pulmonary disease or lung transplant, and stem cell transplant (69%, 55%, 55% respectively) with the least mortality in patients with primary renal disease.13,17,26,27
In one prospective review of 76 pediatric CRRT patients, overall mortality was 44.7% with sepsis, MODS, and greater fluid overload being more common among patients who died.28 Fluid overload had been shown to be associated with increased mortality among critically ill children and adults.13,17,27 Even the most recent retrospective ppCRRT analysis identified greater fluid overload at initiation of CRRT to be independently associated with increased mortality even after controlling for severity of illness, suggesting a 3% increase in mortality risk with each 1% increase in fluid overload, with patients having a greater than 20% fluid overload at initiation of CRRT being 8.5 times more likely to die than those with <20% fluid overload.27 Therefore, available data suggest that initiation of CRRT earlier in the course of illness has a superior outcome rather than later initiation with a greater degree of fluid overload.24 Also a large single center study determined that a higher Pediatric Risk of Mortality (PRISM) score, lower blood pressure at presentation and greater vasopressor support to be associated with increased mortality.29 We also identified risk factors for increased mortality that included having undergone stem cell transplantation, presentation with septic shock, use of vasopressor support, fluid overload at the initiation of CRRT, renal failure as in pRIFLE criteria before CRRT, and oliguric renal failure before CRRT with UOP <1 mL/kg/h. Among these risk factors we identified presentation with septic shock and use of inotropic support to be independently associated in a multivariate analysis with decreased survival rate.
Our study is the first on the usage of CRRT among critically ill children in Saudi Arabia. Further study is needed, however, because there were limitations. First, it was a retrospective and thus observational, which could lead to recall and interpretation bias, and incomplete data. Second, we were unable to include the details of CRRT (i.e. priming of CRRT with blood vs albumin vs saline) and information like blood flow rate, dose of heparin, daily effluent volume and type of filter/membrane and others. Third, we also did not include any history of blood transfusion (pack RBCs, platelets, and fresh frozen plasma) before or during CRRT. Fourth, our study population was a small and highly heterogeneous in a single center, so results are not generalizable to other centers even within Saudi Arabia. Currently, there is little evidence to guide dialysis prescription in very small children. Therefore most clinicians use approaches based on adult experience or larger children.13 Safety, efficacy and different clinical approaches for priming at initiation of CRRT also needs to be further explored. Also, the long-term clinical and survival benefits of CRRT as compared to peritoneal dialysis and conventional intermittent hemodialysis among critically ill pediatric AKI patients, has yet to be determined.
In conclusion, the overall mortality among critically ill children who underwent CRRT was 50% with significantly increased mortality among patients with hematological diseases, immunodeficiency and in post-stem cell transplant patients. In addition, sepsis and septic shock and use of inotropic support were independently associated with decreased survival.
Footnotes
Funding: None.
CONFLICT OF INTEREST: None.
REFERENCES
- 1.Warady BA, Bunchman T. Dialysis therapy for children with acute renal failure: survey results. Pediatr Nephrol. 2000;15(1–2):11–13. doi: 10.1007/s004670000420. [DOI] [PubMed] [Google Scholar]
- 2.Belsha CW, Kohaut EC, Warady BA. Dialytic management of childhood acute renal failure: a survey of North American pediatric nephrologists. Pediatr Nephrol. 1995;9(3):361–363. doi: 10.1007/BF02254215. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen O, Jepsen SB, Toft P. Continuous renal replacement therapy for critically ill infants and children. Danish Med J. 2012;59(2):A4385. [PubMed] [Google Scholar]
- 4.Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxvold NJ, Brophy PD. Pediatric acute renal failure: outcome by modality and disease. Pediatr Nephrol. 2001;16(12):1067–1071. doi: 10.1007/s004670100029. [DOI] [PubMed] [Google Scholar]
- 5.Hui-Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005;45(1):96–101. doi: 10.1053/j.ajkd.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(4):732–738. doi: 10.2215/CJN.03200906. [DOI] [PubMed] [Google Scholar]
- 7.Vachvanichsanong P, Dissaneewate P, Lim A, McNeil E. Childhood acute renal failure: 22-year experience in a university hospital in southern Thailand. Pediatrics. 2006;118(3):e786–791. doi: 10.1542/peds.2006-0557. [DOI] [PubMed] [Google Scholar]
- 8.Van Biljon G. Causes, prognostic factors and treatment results of acute renal failure in children treated in a tertiary hospital in South Africa. J Tropical Ped. 2008;54(4):233–237. doi: 10.1093/tropej/fmm079. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. Intensity of continuous renal-replacement therapy in critically ill patients. NEJM. 2009;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 10.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. Intensity of renal support in critically ill patients with acute kidney injury. NEJM. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 12.Symons JM, Brophy PD, Gregory MJ, McAfee N, Somers MJ, Bunchman TE, et al. Continuous renal replacement therapy in children up to 10 kg. Am J Kidney Dis. 2003;41(5):984–989. doi: 10.1016/s0272-6386(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 13.Askenazi DJ, Goldstein SL, Koralkar R, Fortenberry J, Baum M, Hackbarth R, et al. Continuous renal replacement therapy for children </=10 kg: a report from the prospective pediatric continuous renal replacement therapy registry. J Pediatr. 2013;162(3):587–592 e583. doi: 10.1016/j.jpeds.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lattouf OM, Ricketts RR. Peritoneal dialysis in infants and children. Am Surg. 1986;52(2):66–69. [PubMed] [Google Scholar]
- 15.Williams DM, Sreedhar SS, Mickell JJ, Chan JC. Acute kidney failure: a pediatric experience over 20 years. Arch Pediatr Adolesc Med. 2002;156(9):893–900. doi: 10.1001/archpedi.156.9.893. [DOI] [PubMed] [Google Scholar]
- 16.Hackbarth R, Bunchman TE, Chua AN, Somers MJ, Baum M, Symons JM, et al. The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs. 2007;30(12):1116–1121. doi: 10.1177/039139880703001212. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland SM, Alexander SR. Continuous renal replacement therapy in children. Pediatr Nephrol. 2012;27(11):2007–2016. doi: 10.1007/s00467-011-2080-x. [DOI] [PubMed] [Google Scholar]
- 18.Davenport A, Will EJ, Davison AM. Hyperlactataemia and metabolic acidosis during haemofiltration using lactate-buffered fluids. Nephron. 1991;59(3):461–465. doi: 10.1159/000186609. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman D, Cotman P, Ting R, Karanicolas S, Tobe SW. Continuous veno-venous haemodialysis with a novel bicarbonate dialysis solution: prospective cross-over comparison with a lactate buffered solution. Nephrol Dial Transplant. 1999;14(10):2387–2391. doi: 10.1093/ndt/14.10.2387. [DOI] [PubMed] [Google Scholar]
- 20.Barenbrock M, Hausberg M, Matzkies F, de la Motte S, Schaefer RM. Effects of bicarbonate- and lactate-buffered replacement fluids on cardiovascular outcome in CVVH patients. Kidney Int. 2000;58(4):1751–1757. doi: 10.1046/j.1523-1755.2000.00336.x. [DOI] [PubMed] [Google Scholar]
- 21.Brophy PD, Somers MJ, Baum MA, Symons JM, McAfee N, Fortenberry JD, et al. Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT) Nephrol Dial Transplant. 2005;20(7):1416–1421. doi: 10.1093/ndt/gfh817. [DOI] [PubMed] [Google Scholar]
- 22.Mehta RL, McDonald BR, Aguilar MM, Ward DM. Regional citrate anticoagulation for continuous arteriovenous hemodialysis in critically ill patients. Kidney Int. 1990;38(5):976–981. doi: 10.1038/ki.1990.300. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad S, Yeo KT, Jensen WM, Landicho D, Gregory B, Moritz JL, et al. Citrate anticoagulation during in vivo simulation of slow hemofiltration. Blood Purif. 1990;8(4):177–182. doi: 10.1159/000169964. [DOI] [PubMed] [Google Scholar]
- 24.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostermann M, Chang RW. Correlation between parameters at initiation of renal replacement therapy and outcome in patients with acute kidney injury. Crit Care. 2009;13(6):R175. doi: 10.1186/cc8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein SL. Continuous renal replacement therapy: mechanism of clearance, fluid removal, indications and outcomes. Curr Opin Pediatr. 2011;23(2):181–185. doi: 10.1097/MOP.0b013e328342fe67. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 28.Hayes LW, Oster RA, Tofil NM, Tolwani AJ. Outcomes of critically ill children requiring continuous renal replacement therapy. Crit Care. 2009;24(3):394–400. doi: 10.1016/j.jcrc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez C, Lopez-Herce J, Flores JC, Galaviz D, Ruperez M, Brandstrup KB, et al. Prognosis in critically ill children requiring continuous renal replacement therapy. Pediatr Nephrol. 2005;20(10):1473–1477. doi: 10.1007/s00467-005-1907-8. [DOI] [PubMed] [Google Scholar]