Abstract
Background and objectives
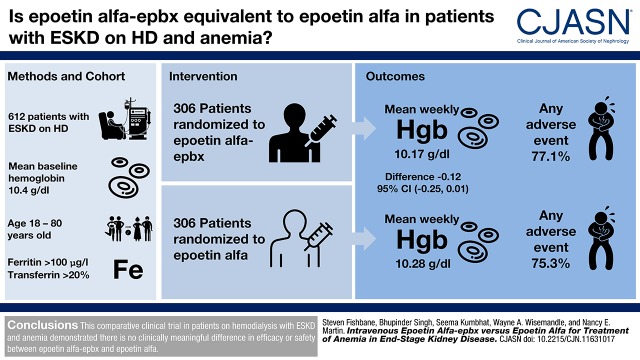
This study was conducted to compare the safety and efficacy of intravenous epoetin alfa-epbx, an epoetin alfa biosimilar, to epoetin alfa in patients on hemodialysis with ESKD and anemia.
Design, setting, participants, & measurements
In this 24-week, multicenter, double-blind comparative efficacy and safety study, 612 patients on hemodialysis with ESKD and anemia who had stable hemoglobin and were receiving stable doses of intravenous epoetin alfa were randomized (1:1) to intravenous epoetin alfa or epoetin alfa-epbx. Dosing was adjusted according to the epoetin alfa prescribing information. The coprimary efficacy end points were the least squares mean difference between the two treatments in mean weekly hemoglobin level and mean weekly epoetin dose per kilogram of body weight during the last 4 weeks of treatment.
Results
The least squares mean difference between epoetin alfa-epbx and epoetin alfa in weekly hemoglobin was −0.12 g/dl and the 95% confidence interval (−0.25 to 0.01) was contained within the prespecified equivalence margin (−0.5 to 0.5 g/dl). The least squares mean difference between epoetin alfa-epbx and epoetin alfa in weekly epoetin dose per kilogram of body weight was 0.37 U/kg per week, and the 95% confidence interval (−10.40 to 11.13) was contained within the prespecified equivalence margin (−45 to 45 U/kg per week). Incidences of adverse events (77.1% versus 75.3%), serious adverse events (24.9% versus 27.0%), and deaths (n=5 versus 6) were similar between the epoetin alfa-epbx and epoetin alfa groups, respectively. Five patients tested positive for anti-recombinant human erythropoietin antibodies at baseline, and two additional patients (n=1 per group) developed anti-recombinant human erythropoietin antibodies while on study treatment. All patients tested negative for neutralizing antibodies, and no patient in either group experienced an event of pure red cell aplasia.
Conclusions
This 24-week, comparative, clinical trial in patients on hemodialysis with ESKD and anemia demonstrated there is no clinically meaningful difference in efficacy or safety between epoetin alfa-epbx and epoetin alfa.
Keywords: chronic kidney disease; Epoetin Alfa; Antibodies, Neutralizing; Biosimilar Pharmaceuticals; Double-Blind Method; Incidence; Confidence Intervals; Least-Squares Analysis; EPO protein, human; erythropoietin; renal dialysis; Kidney Failure, Chronic; anemia; Hemoglobins; Red-Cell Aplasia, Pure; Body Weight
Introduction
Anemia is a common complication of CKD that results from insufficient production of erythropoietin by the kidneys and, therefore, reduced erythropoiesis (1). Anemia often develops early in CKD (2,3) and worsens with disease progression. Epoetin alfa, a genetically engineered recombinant human erythropoietin (rhEPO), is approved for the treatment of anemia in patients with CKD (4,5). Administration of epoetin in this patient population produces clinically significant increases in hemoglobin and reduces the need for transfusion (6).
In 2014, Medicare beneficiaries with CKD represented 10% of the United States Medicare population, yet accounted for 20% of total expenditures (7). In that same year, Medicare spending for patients on hemodialysis with ESKD reached $26 billion (8) and, in 2010, spending for erythropoiesis-stimulating agents (ESAs) in this patient population totaled $1.87 billion (9). Biosimilar epoetins, which have been available in Europe since 2007 (10–14), have the potential to lower the costs of epoetin treatment and may offer a more affordable alternative (15). The Biologics Price Competition and Innovation Act of 2009 (16) created an abbreviated licensure pathway for biosimilars in the United States. This legislation is reflected in US Food and Drug Administration (FDA) guidance that indicates that biosimilarity to a licensed (i.e., reference or originator) biologic product is established on the basis of the totality of the data derived from comparative analytical, nonclinical, and clinical pharmacology and safety and efficacy studies. Furthermore, clinical studies must be conducted in a relevant population to assess clinically meaningful differences between the proposed biosimilar and reference product (16,17).
Epoetin alfa-epbx (Hospira Inc.; Pfizer, Lake Forest, IL) was approved by the US FDA in May 2018 as a biosimilar of epoetin alfa (Amgen Inc., Thousand Oaks, CA) (18). Epoetin alfa-epbx is identical in amino acid sequence and similar in carbohydrate composition to the reference product, epoetin alfa (19). Our study was conducted to demonstrate the equivalence of epoetin alfa-epbx to epoetin alfa when administered intravenously (IV) to patients on hemodialysis with ESKD and anemia, on the basis of maintenance of hemoglobin levels and study drug dose requirements. Comparative safety and tolerability of epoetin alfa-epbx and epoetin alfa were also assessed.
Materials and Methods
Study Population
The original study protocol included male and nonpregnant female patients on hemodialysis with ESKD and anemia who, before randomization, were on stable IV epoetin alfa treatment one to three times per week for ≥12 weeks, and had stable hemoglobin (mean 10.0−12.0 g/dl). Patients were aged 18−75 years, on stable, adequate dialysis for ≥12 weeks, and had adequate iron stores (ferritin >100 μg/L and transferrin saturation [TSAT] >20%). Consultation with investigators and kidney dialysis experts indicated that a stable dosing period of 12 weeks would exclude the majority of patients from study and was not necessary to ensure adequate hematopoietic stability for study entry. Therefore, the protocol was amended to require that patients be on stable IV epoetin alfa treatment for ≥4 weeks before randomization. In addition, the target level of hemoglobin was changed from 10.0−12.0 to 9.0−11.0 g/dl to accommodate new US FDA requirements for patients on epoetin alfa. A subsequent protocol amendment extended the upper eligibility age range from 75 to 80 years, on the basis of medical judgment that patients between 75 and 80 years of age, who otherwise meet all protocol eligibility criteria, are representative of the general population intended for this study.
Patients were excluded if they had active, uncontrolled systemic inflammatory or malignant conditions; uncontrolled hypertension; recent myocardial infarction, stroke, major thrombotic event, seizure, or decompensated heart failure; required maintenance doses of epoetin alfa >600 U/kg per week; received long-acting ESAs within 12 weeks before randomization; or had recently donated or lost >475 ml of blood.
Study Design
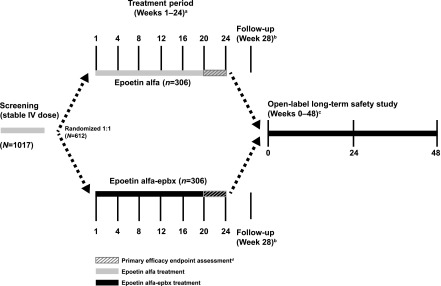
This was a multicenter, randomized, active-controlled, parallel-group, double-blind study conducted to evaluate the comparative efficacy and safety of IV-administered epoetin alfa-epbx or epoetin alfa (one to three times per week) in patients on hemodialysis with ESKD and anemia on stable epoetin alfa treatment before randomization. The study consisted of a screening period (≤4 weeks), a 24-week double-blind treatment period, and a follow-up visit (Figure 1). Patients who fulfilled entry criteria were randomized (1:1) at day 1 of the treatment period to receive either epoetin alfa-epbx or epoetin alfa at the same stable weekly dose of epoetin alfa received during the last week of screening. The randomization was not stratified.
Figure 1.
This comparative clinical trial of IV epoetin alfa-epbx versus epoetin alfa was a randomized, active-controlled, parallel-group, double-blind study including a screening period, 24-week double-blind treatment period, follow-up visit, and 48-week, open-label long-term safety study with epoetin alfa-epbx. aPatients who discontinued early from epoetin alfa-epbx or epoetin alfa received a nonstudy drug standard-of-care ESA until either the follow-up visit or entry into the open-label, long-term safety study. bPatients who completed the treatment period and who did not enter the open-label, long-term safety study underwent a follow-up visit at week 28, 4 weeks after the end of week 24 study activity assessments. cPatients had up to 28 days from completion of the week 24 visit to enter into the open-label, long-term safety study and be treated with IV epoetin alfa-epbx for an additional ≤48 weeks. dCoprimary efficacy end points were assessed during the last 4 weeks of the treatment period.
During the treatment period, dosing for both study drugs was adjusted, in accordance with United States prescribing information for epoetin alfa (20,21), to maintain hemoglobin levels within the target range (9.0−11.0 g/dl). Decisions regarding dose adjustments were made at least weekly by the investigator, on the basis of hemoglobin concentrations over the previous 4 weeks and after proper assessment of iron status. A change of dosage was only recommended in patients with optimal iron status (TSAT >20% and plasma ferritin level ≥100 μg/L). Otherwise, the first step was to optimize IV iron supplementation (Supplemental Table 1). The frequency of dosing during the treatment period was determined at the investigator’s discretion. Patients who discontinued study drug could remain in the study on a nonstudy drug standard-of-care ESA and complete scheduled study assessments for the remainder of the treatment period.
After completing the treatment period, all patients had the opportunity to enter an open-label, long-term, safety study under a separate protocol, and be treated with epoetin alfa-epbx for an additional ≤48 weeks. Patients not entering the long-term safety study had a follow-up visit 4 weeks after the end of the treatment period, at week 28.
The protocol and all amendments were approved by an independent review board (Quorum Review IRB, Seattle, WA) or local institutional review boards at East Carolina University, Washington University in St. Louis, University of Louisville, Texas Tech University Health Sciences Center/Lubbock, AnMed Health, St. Vincent Medical Center, and University of Cincinnati (Clinicaltrials.gov identifier NCT01473407). The study was conducted from December 2011 to February 2014, and in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice Guidelines, and all applicable regulatory requirements. Patients provided written informed consent before randomization.
Study Drug
Epoetin alfa-epbx (Hospira Inc., Lake Forest, IL) was supplied as an aqueous, phosphate-buffered, isotonic solution, containing polysorbate 20. Epoetin alfa (Amgen Inc., Thousand Oaks, CA) was the reference product.
Assessments
At each weekly visit, hemoglobin, study drug dose per kilogram of body weight, vital signs, concomitant medications, adverse events (AEs), and transfusion information were assessed. All AEs were rated by the investigator for severity and relatedness to study drug. Physical examination and electrocardiograms were performed at screening, week 24, and follow-up. Blood was collected and chemistry, hematology, coagulation panels, and iron status were analyzed at screening, before initial dosing, monthly, and at follow-up. Blood was collected before initial dosing, at weeks 12 and 24, and at follow-up for assessment of anti-rhEPO binding antibodies by radioimmunoprecipitation and, if positive, for neutralizing anti-rhEPO antibodies by cell-based assay using current validated methods. All clinical laboratory tests were performed by a central laboratory.
Statistical Analyses
Considering a possible 30% attrition rate, 282 patients per group were planned to ensure a minimum of 197 patients per group; this allowed 90% power to determine equivalence for both coprimary end points using two-sided tests of equivalence at a 2.5% significance level. The primary efficacy analysis was conducted using the intent-to-treat (ITT) population: all patients who were randomized into the treatment period. Sensitivity analysis was performed using the per protocol population: a subset of ITT patients who, during the treatment period, had ≥4 weeks of treatment with the study drug, ≥4 weeks of hemoglobin level and dosing data collected while on the study drug, no use of other ESAs during the last 4 weeks of study drug administration, no transfusion during study conduct, and no important protocol deviations. The primary safety analysis was conducted using the safety population: all patients who received one or more dose of study drug treatment.
Demographic and baseline characteristics were summarized descriptively. Coprimary efficacy end points, calculated as the least squares mean difference between treatments in mean weekly hemoglobin level and mean weekly epoetin dose per kilogram of body weight, were derived from hemoglobin level and dose data collected during the last 4 weeks of treatment for the ITT population. For patients who did not have 4 weeks of hemoglobin and/or dose data during the last 4 weeks of the treatment period, the actual number of weeks was used in the calculation of mean weekly hemoglobin level and/or mean weekly epoetin dose per kilogram of body weight for the last 4 weeks of treatment. For patients with no weekly hemoglobin and/or dose data on treatment, baseline value hemoglobin and/or epoetin dose per kilogram of body weight was used as the mean weekly hemoglobin level and/or mean weekly epoetin dose per kilogram of body weight during the last 4 weeks of treatment.
Two-sided 95% confidence intervals (95% CIs) for the two least squares mean differences were calculated using analysis of covariance (containing effect for treatment and baseline value) and compared with prespecified equivalence margins. A hierarchical test strategy was used: if equivalence between the two treatments was concluded for mean hemoglobin level (i.e., two-sided 95% CI for the least squares mean difference resided within the ±0.5 g/dl equivalence margin), the difference in mean weekly epoetin dose per kilogram of body weight was tested; if equivalence was concluded for mean weekly epoetin dose per kilogram of body weight (i.e., two-sided 95% CI for the least squares mean difference resided within the ±45 U/kg per week equivalence margin), the equivalence of epoetin alfa-epbx and epoetin alfa in efficacy was concluded. The test on mean weekly epoetin dose per kilogram of body weight was only performed if equivalence for hemoglobin level was determined, therefore, no adjustment of α-values was required.
Treatment comparison of secondary efficacy end points was on the basis of the distribution type of the respective variables. The proportion of patients with a mean weekly hemoglobin level within the target range (9.0−11.0 g/dl) at weeks 12 and 24 and the proportion of patients receiving blood transfusions were summarized for each treatment group and analyzed by Fisher exact test. Mean weekly epoetin dose per kilogram of body weight was summarized using descriptive statistics and analyzed by t test (or Mann–Whitney–Wilcoxon test).
The safety evaluation included incidence of AEs, serious adverse events (SAEs; including death), and out-of-range hemoglobin levels at any time during the treatment period; clinical laboratories; vital signs; postdialysis weight; 12-lead electrocardiogram; and concomitant medications. The original protocol specified analysis for incidence of hemoglobin levels <9.0 or >13.0 g/dl; however, the incidence of hemoglobin levels <8.0 or >12.0 g/dl was evaluated after a protocol amendment to accommodate new US FDA requirements for patients on epoetin alfa.
Results
Patient Disposition, and Demographic and Baseline Characteristics
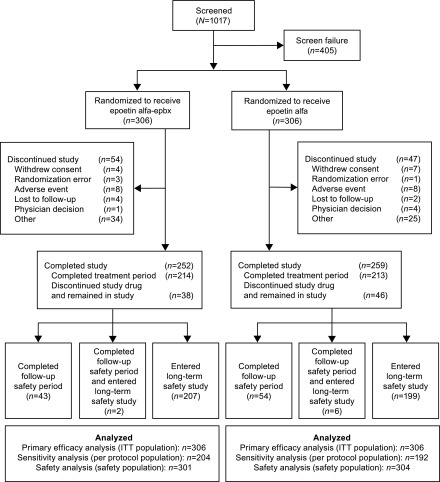
Of the 1017 patients screened, 612 across 95 sites in the United States and Puerto Rico were randomized to receive epoetin alfa-epbx (n=306) or epoetin alfa (n=306) and constituted the ITT population (Figure 2). The safety population consisted of 301 and 304 patients who received epoetin alfa-epbx and epoetin alfa, respectively. For the ITT population, the most common reason for study drug discontinuation was standard-of-care ESA use (epoetin alfa-epbx, n=42 [14%]; epoetin alfa, n=43 [14%]). Thirty-eight (12%) patients in the epoetin alfa-epbx group and 46 (15%) patients in the epoetin alfa group discontinued the study drug before week 24 of the treatment period, but remained in the study receiving a nonstudy drug standard-of-care ESA and completed the study procedures through week 24 (Figure 2). Overall, 72 (24%) patients in each of the epoetin alfa-epbx and epoetin alfa groups received a nonstudy drug standard-of-care ESA for a mean duration of 9.8 and 9.4 weeks, respectively, during the trial.
Figure 2.
This flow diagram shows patient disposition through each phase of the comparative clinical trial of IV epoetin alfa-epbx versus epoetin alfa.
Demographic and baseline characteristics were well matched between groups (Table 1). The primary cause for CKD was either diabetes or hypertension in approximately 80% of patients in both groups; mean baseline hemoglobin was 10.4 g/dl and mean baseline weekly epoetin dose per kilogram of body weight was 107 U/kg per week; mean baseline ferritin and TSAT were 929 ng/ml and 34%, respectively. Patients in both groups had received dialysis treatments for a mean duration of 52 months. Antihypertensive agents were used by 86% and 91% of patients in the epoetin alfa-epbx and epoetin alfa groups, respectively, before entering the study, and by 87% and 92% of patients, respectively, as concomitant therapy. In the epoetin alfa-epbx and epoetin alfa groups, respectively, 205 (67%) and 204 (67%) patients received concomitant iron supplementation at some time during the treatment period. Other common prior and concomitant medications are summarized in Supplemental Table 2. Five patients (epoetin alfa-epbx, n=2; epoetin alfa, n=3) tested positive for anti-rhEPO antibodies at baseline.
Table 1.
Demographic and baseline characteristics of patients with ESKD and anemia enrolled in a comparative clinical trial of epoetin alfa-epbx versus epoetin alfa
| Characteristica | Epoetin alfa-epbx, n=301 | Epoetin alfa, n=304 |
|---|---|---|
| Men, n (%) | 156 (52) | 175 (58) |
| Age, mean (SD), yr | 55 (13) | 57 (11) |
| Race, n (%) | ||
| White | 141 (47) | 150 (49) |
| Black | 146 (49) | 125 (41) |
| Native Hawaiian or other Pacific Islander | 2 (0.7) | 4 (1) |
| Asian | 4 (1) | 13 (4) |
| American Indian or Alaska Native | 0 | 1 (0.3) |
| Other | 8 (3) | 10 (3) |
| Missing | 0 | 1 (0.3) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 94 (31) | 99 (33) |
| Not Hispanic or Latino | 207 (69) | 204 (67) |
| Missing | 0 | 1 (0.3) |
| Weight, mean (SD), kg | 87 (24) | 86 (22)b |
| Time from start of dialysis to randomization, mean (SD), mo | 51 (51) | 54 (51) |
| Hemoglobin level, mean (SD), g/dl | 10.4 (0.8)c | 10.4 (0.7) |
| Weekly epoetin dose by body weight, mean (SD), U/kg per week | 106 (98) | 108 (104)b |
| Dose frequency, per week, n (%) | ||
| 1 | 72 (24) | 74 (24) |
| 2 | 47 (16) | 53 (17) |
| 3 | 182 (60) | 178 (59) |
| Ferritin level, mean (SD), ng/ml | 921 (437) | 937 (419) |
| TSAT, mean (SD), % | 34 (12) | 33 (11) |
| C-reactive protein, mean (SD), mg/dl | 1.0 (1.9) | 1.0 (1.5) |
| Anti-rhEPO antibody status, n (%) | ||
| Negative RIP | 267 (89) | 262 (86) |
| Positive RIP | 2 (0.7) | 3 (0.99) |
| Missingd | 32 (11) | 39 (13) |
| Primary cause of CKD,en (%) | ||
| Diabetes | 145 (47) | 151 (49) |
| Hypertension | 105 (34) | 85 (28) |
| Nephropathies | 36 (12) | 44 (14) |
| Congenital kidney disease | 6 (2) | 10 (3) |
| Other | 10 (3) | 12 (4) |
| Unknown | 3 (0.98) | 3 (0.98) |
TSAT, transferrin saturation; anti-rhEPO, anti-recombinant human erythropoietin antibody; RIP, radioimmunoprecipitation assay.
Analyses for all characteristics except for primary cause of CKD were performed on the safety population. The percentages for race may not add up to 100 because patients could select multiple races and because of rounding. Percentages for other characteristics also may not add up to 100 because of rounding.
Mean (SD) weight on the basis of data for 303 patients treated with epoetin alfa.
Mean (SD) hemoglobin level on the basis of data for 300 patients treated with epoetin alfa-epbx.
Baseline anti-rhEPO antibody samples were missing because of the sample not being drawn or sample handling. Missing samples were to be redrawn at the following visit, but were not considered baseline values.
Analyses for primary cause of CKD were determined using the intent-to-treat population (epoetin alfa-epbx, n=306; epoetin alfa, n=306).
Efficacy End Points
For the primary analysis conducted on the ITT population, the least squares mean difference between epoetin alfa-epbx and epoetin alfa in mean weekly hemoglobin levels was −0.12 g/dl, with a 95% CI of −0.25 to 0.01 g/dl, which was contained within the prespecified equivalence margin of ±0.5 g/dl (Table 2). Similarly, the least squares mean difference between epoetin alfa-epbx and epoetin alfa in mean weekly epoetin dose per kilogram of body weight was 0.37 U/kg per week, with a 95% CI of −10.40 to 11.13 U/kg per week, which was contained within the prespecified equivalence margin of ±45 U/kg per week (Table 2). Sensitivity analysis, conducted on the per protocol population, demonstrated the 95% CIs for the least squares mean differences in mean weekly hemoglobin levels and mean weekly epoetin dose per kilogram of body weight were contained within the respective equivalence margins (Table 2).
Table 2.
Comparative efficacy of epoetin alfa-epbx and epoetin alfa, evaluated by coprimary efficacy end points of mean weekly hemoglobin level and mean weekly epoetin dose by body weight
| Analysis | Epoetin alfa-epbx | Epoetin alfa |
|---|---|---|
| Primary analysis (ITT population) | n=306 | n=306 |
| Mean weekly hemoglobin level during last 4 wk of treatment, g/dl | ||
| Least squares mean (SEM) | 10.17 (0.05) | 10.28 (0.05) |
| Difference (95% CI)a | −0.12 (−0.25 to 0.01) | |
| Mean weekly epoetin dose by body weight during last 4 wk of treatment, U/kg per weekb | ||
| Least squares mean (SEM) | 90.16 (3.87) | 89.79 (3.88) |
| Difference (95% CI)c | 0.37 (−10.40 to 11.13) | |
| Sensitivity analysis (per protocol population) | n=204 | n=192 |
| Mean weekly hemoglobin during last 4 wk of treatment, g/dl | ||
| Least squares mean (SEM) | 10.19 (0.06) | 10.30 (0.06) |
| Difference (95% CI)a | −0.10 (−0.27 to 0.06) | |
| Mean weekly epoetin dose by body weight during last 4 wk of treatment, U/kg per week | ||
| Least squares mean (SEM) | 85.71 (4.73) | 88.12 (4.88) |
| Difference (95% CI)c | −2.41 (−15.77 to 10.95) | |
ITT, intent to treat; 95% CI, 95% confidence interval.
The 95% CI for the least squares mean of the difference (epoetin alfa-epbx–epoetin alfa) in mean weekly hemoglobin had to reside within the equivalence margin of ±0.5 g/dl for equivalence to be concluded.
One patient in each group did not have dose data reported at baseline. Therefore, primary analysis of mean weekly epoetin dose by body weight during last 4 wk of treatment in the ITT population is on the basis of data for 305 randomized patients in each treatment group.
The 95% CI for the least squares mean of the difference (epoetin alfa-epbx–epoetin alfa) in mean weekly epoetin dose per kilogram of body weight had to reside within the equivalence margin of ±45 U/kg per week for equivalence to be concluded.
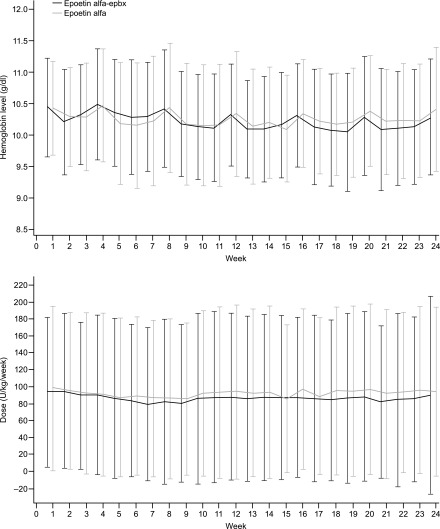
Analysis of secondary efficacy end points revealed the majority of patients in both treatment groups maintained hemoglobin levels within the range of 9.0−11.0 g/dl at week 24 (Table 3). Approximately 6% of patients in each group received transfusions during the study (Table 3). Over the 24-week treatment period, there was no statistically significant difference between epoetin alfa-epbx and epoetin alfa in mean weekly hemoglobin levels (P=0.76) or mean weekly epoetin dose by body weight (P=0.11) (Figure 3).
Table 3.
Comparative efficacy of epoetin alfa-epbx and epoetin alfa, evaluated by secondary efficacy end pointsa
| End Points | Epoetin alfa-epbx, n=306 | Epoetin alfa, n=306 | P Value |
|---|---|---|---|
| Patients with mean weekly hemoglobin level within 9.0−11.0 g/dl at week 24,b n/N (%) | 188/257 (73.2) | 185/259 (71.4) | 0.69 |
| Patients who received a red blood cell transfusion at any time during the 24-wk treatment period,c n/N (%) | 19/303 (6.3) | 18/305 (5.9) | 0.87 |
Secondary efficacy end points were analyzed using the intent-to-treat population.
Percentages were calculated using the number of observations at week 24 within a treatment group as the denominator.
Percentages were calculated using the number of patients participating in the trial at any time during the treatment period within a treatment group as the denominator.
Figure 3.
Mean (SD) weekly hemoglobin level (g/dl) and mean (SD) weekly epoetin dose by body weight (U/kg per week) were similar between epoetin alfa-epbx and epoetin alfa over the duration of the treatment period. ITT population. There was no statistical difference in mean weekly hemoglobin levels (P=0.76) or mean weekly epoetin dose per kilogram of body weight (P=0.11) between the two treatment groups over 24 weeks.
Safety
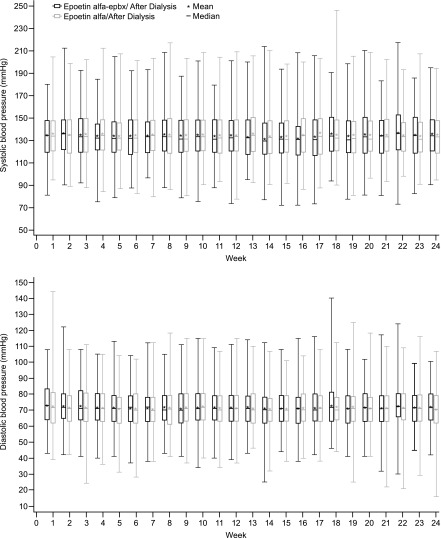
Overall, 76.2% of patients in both treatment groups reported at least one AE. Common AEs, occurring in ≥5% of patients in either group, are presented in Table 4. The incidence of hypertension, identified as an AE, which is common in epoetin-treated patients with ESKD on hemodialysis, was higher in the epoetin alfa-epbx group; however, analysis of BP showed no difference between treatments (Figure 4). Nine (3.0%) patients receiving epoetin alfa-epbx and 11 (3.6%) patients receiving epoetin alfa discontinued the study drug because of AEs.
Table 4.
Safety profiles of epoetin alfa-epbx and epoetin alfa: adverse events with ≥5% incidence and serious adverse events occurring in at least four patients in either treatment group
| Preferred Term | Epoetin alfa-epbx, n=301 | Epoetin alfa, n=304 |
|---|---|---|
| Any adverse event, n (%) | 232 (77.1) | 229 (75.3) |
| Nausea | 30 (10.0) | 25 (8.2) |
| Vomiting | 28 (9.3) | 15 (4.9) |
| Muscle spasms | 27 (9.0) | 24 (7.9) |
| Arteriovenous fistula-site complication | 26 (8.6) | 25 (8.2) |
| Headache | 23 (7.6) | 16 (5.3) |
| Dyspnea | 22 (7.3) | 21 (6.9) |
| Diarrhea | 21 (7.0) | 27 (8.9) |
| Dizziness | 20 (6.6) | 15 (4.9) |
| Hypertension | 19 (6.3) | 12 (3.9) |
| Cough | 16 (5.3) | 22 (7.2) |
| Hypotension | 14 (4.7) | 23 (7.6) |
| Back pain | 13 (4.3) | 16 (5.3) |
| Pain in extremity | 10 (3.3) | 17 (5.6) |
| Noncardiac chest pain | 7 (2.3) | 17 (5.6) |
| Any serious adverse event, n (%) | 75 (24.9) | 82 (27.0) |
| Pneumonia | 4 (1.3) | 8 (2.6) |
| Acute respiratory failure | 4 (1.3) | 0 |
| Cardiac failure congestive | 4 (1.3) | 3 (1.0) |
| Dyspnea | 3 (1.0) | 8 (2.6) |
| Noncardiac chest pain | 2 (0.7) | 7 (2.3) |
| Hyperkalemia | 2 (0.7) | 4 (1.3) |
| Angina pectoris | 1 (0.3) | 4 (1.3) |
| Fluid overload | 1 (0.3) | 6 (2.0) |
| Cellulitis | 0 | 6 (2.0) |
Figure 4.
Mean and median systolic and diastolic BPs after dialysis were unchanged over time and similar between epoetin alfa-epbx and epoetin alfa. The box represents the 25th and 75th percentiles and the tails represent the minimum and maximum observed values; the median is indicated by the horizontal line, and the mean by *.
A total of 75 (24.9%) patients in the epoetin alfa-epbx group and 82 (27.0%) patients in the epoetin alfa group reported at least one SAE during the study. The most common SAEs (reported by four or more patients in either group) are presented in Table 4. Five (1.7%) patients in the epoetin alfa-epbx group and 6 (2.0%) patients in the epoetin alfa group died. In the epoetin alfa-epbx group, SAEs resulting in death were azotemia (n=2) and sudden death of unknown cause, sepsis, and metabolic encephalopathy (n=1 each). In the epoetin alfa group, SAEs resulting in death were cardiac arrest (n=3), cardiorespiratory arrest (n=2), and metastatic lung cancer (n=1). All deaths were considered by the investigators as not related or probably not related to the study drug.
At any time during the treatment period, 16 (5.3%) and 33 (10.9%) patients receiving epoetin alfa-epbx and epoetin alfa, respectively, had hemoglobin levels <8.0 g/dl and, 65 (21.6%) and 70 (23.0%) patients, respectively, had hemoglobin levels >12.0 g/dl. Thus, ≥73.1% of patients receiving epoetin alfa-epbx and ≥66.1% receiving epoetin alfa did not have hemoglobin levels <8.0 g/dl or >12.0 g/dl.
Analysis of change from baseline in laboratory parameters, vital signs, and electrocardiograms demonstrated no notable changes within or between groups. Mean changes from baseline to week 24 for plasma ferritin (54.9 and 1.0 ng/ml for epoetin alfa-epbx and epoetin alfa, respectively) and TSAT (−0.9% and 0.7%, respectively) were clinically unremarkable. Throughout the treatment period, iron stores were replete and able to support erythropoiesis stimulated by epoetin for both groups. Furthermore, mean change from baseline to week 24 in C-reactive protein was similar between epoetin alfa-epbx (0.209 mg/dl) and epoetin alfa (0.300 mg/dl), and clinically unremarkable in the setting of patients with ESKD on hemodialysis. Using current validated methods, seven patients (epoetin alfa-epbx, n=3; epoetin alfa, n=4) were confirmed anti-rhEPO antibody-positive, of whom five (epoetin alfa-epbx, n=2; epoetin alfa, n=3) tested positive at baseline, and two (n=1 each per group) developed anti-rhEPO antibodies while on study treatment. For all patients, the titers at any anti-rhEPO antibody-positive time point were between <1:2 and 1:2, and all anti-rhEPO antibody-positive samples were negative for neutralizing anti-erythropoietin antibodies. All patients were clinically stable. Furthermore, no patient in either group developed pure red cell aplasia (PRCA).
Discussion
As part of an overall program to demonstrate the biosimilarity of epoetin alfa-epbx to the reference product epoetin alfa, this study compared efficacy and safety of the two IV treatments in patients with ESKD and anemia who were receiving hemodialysis. The coprimary end points centering on mean weekly hemoglobin levels and mean weekly epoetin dose per kilogram of body weight to maintain hemoglobin levels in the target range were selected as both are clinically relevant and sensitive to evaluate change. Hemoglobin is a well characterized biomarker that is indicative of efficacy of ESAs, and is a well established and clinically relevant end point for ESA product development. Furthermore, epoetin dose routinely requires some adjustment to achieve a particular target hemoglobin range (20,21). Thus, these two variables in tandem are appropriate and relevant for comparison of the efficacy of epoetin alfa-epbx versus epoetin alfa.
The equivalence margin of ±0.5 g/dl for the least squares mean difference in mean weekly hemoglobin levels was on the basis of hemoglobin variability seen in previous studies (22,23). The equivalence margin of ±45 U/kg per week for the least squares mean difference in mean weekly epoetin dose per kilogram of body weight was on the basis of previous studies demonstrating equivalence between originator epoetin (epoetin alfa; Janssen-Cilag Ltd., Buckinghamshire, UK) and a biosimilar epoetin (epoetin zeta; Hospira UK Limited, Hurley, UK) approved for use in the European Union (24–26). Our results indicate that the least squares mean differences between treatment groups for both coprimary end points fell within the prespecified equivalence margins and demonstrated no clinically meaningful differences in efficacy between epoetin alfa-epbx and epoetin alfa. Sensitivity analysis and secondary efficacy end points corroborate this conclusion.
The AEs, including SAEs, observed in this study are consistent with those expected for an ESKD population receiving epoetin, were generally similar between the two groups, and concordant with the type and incidences of AEs described for epoetin alfa (20,21). SAEs resulting in deaths occurred in five and six patients in the epoetin alfa-epbx and epoetin alfa groups, respectively. The adjusted mortality rate for patients with ESKD on hemodialysis is 8.8–12.0 times higher than for the general Medicare population aged 65–74 years, and 3.8–4.0 times higher at age ≥75 years (8). Furthermore, cardiovascular disease is the leading cause of mortality in this patient population, accounting for 54% of the deaths with known causes (8). Therefore, the deaths from cardiovascular events that occurred in the epoetin alfa group in this trial are not unexpected.
All protein therapeutic drugs have the potential for immunogenicity (27), and neutralizing antibodies to epoetin can result in PRCA or severe anemia (20,21). The presence of both binding and neutralizing anti-rhEPO antibodies was investigated throughout the study. Using current validated methods, seven patients were confirmed anti-rhEPO antibody-positive, including five who tested positive at baseline. However, no patient in either group developed neutralizing antibodies during the study and all patients were clinically stable. There were no reported events of PRCA or hypersensitivity consistent with immunogenic responses to epoetin in either treatment group.
Patients with ESKD and anemia who were receiving hemodialysis were selected for this comparative efficacy and safety study because this population is the most erythropoietin-deficient and least immunocompromised compared with other anemic patient populations for which epoetin is indicated. The patient population in this study is representative of the general population of patients with ESKD on hemodialysis. Therefore, the results of this study are generalizable to the broader population of patients with ESKD on hemodialysis.
Using an equivalence design, similarity was established between epoetin alfa-epbx and epoetin alfa. One limitation of the study is that patients were permitted to switch from the study drug to a nonstudy drug standard-of-care ESA and remain in the trial. However, it is unlikely that nonstudy drug standard-of-care ESA exposure influenced the efficacy results, as the primary analysis of the coprimary efficacy end points, using data from the last 4 weeks of treatment, was corroborated using the per protocol population, which excluded patients receiving nonstudy drug standard-of-care ESA during the last 4 weeks of treatment. The 24-week duration of the study also limited the duration of evaluation of epoetin alfa-epbx treatment. Another limitation of the study is that it did not evaluate correlates of ESA hyporesponsiveness, such as elevated parathyroid hormone levels (28), or characterize ESA resistance. However, parathyroid hormone levels may not be a strong predictor of ESA response (29), and randomization of patients to the study drug would be expected to generate comparable treatment groups with similar incidence of secondary hyperparathyroidism and/or other risk factors of ESA resistance. Furthermore, baseline hemoglobin and ESA dose data are consistent with those reported for patients in the United States with ESKD on hemodialysis at the time of the study (8) and do not indicate ESA resistance.
Strengths of this trial include a large sample size, its double-blind nature, use of the ITT population as the primary analysis population, dosing of study drug according to established standard-of-care guidelines (20,21), rigor associated with coprimary end points required to demonstrate equivalence in efficacy, and robustness added by the sensitivity analysis and secondary end points. Long-term safety and efficacy of epoetin alfa-epbx have been evaluated in a 48-week, open-label, extension trial.
Approximately 80% of patients in the United States with prevalent ESKD are covered by Medicare, which reimburses dialysis providers at a bundled rate that includes the cost of ESAs such as epoetin (8). However, Medicare reimbursement under the bundled payment system may be insufficient to cover the costs of services, placing financial strain on dialysis facilities that could limit their operation or services, affect the quality of patient care, or lead to closure (30,31). Availability of lower-cost epoetin biosimilars could generate savings for dialysis providers and help to ensure patient access to high-quality care. The results of this study demonstrating no clinically meaningful differences in efficacy and safety between epoetin alfa-epbx and epoetin alfa support the opportunity to expand the benefits of erythropoietin therapy to more patients at a lower overall cost.
Disclosures
W.A.W. is an employee of and owns stock or options in Hospira Inc., a Pfizer company. N.E.M. was an employee of and held stock or options in Hospira Inc., a Pfizer company, at the time of the study. S.F. has served on advisory boards for Hospira Inc., Keryx Inc., AstraZeneca Inc., and Akebia Inc. B.S. is currently employed by AstraZeneca, previously held stock options for ZS Pharma, and declares research grant funding to a previous institution (Southwest Kidney Institute) from Hospira Inc. S.K. was an employee of Hospira Inc. at the time of the study.
Supplementary Material
Acknowledgments
Medical writing support was provided by Dr. Laurel Mengle-Gaw and Dr. Benjamin Schwartz of the Camden Group and was funded by Hospira Inc., which was acquired by Pfizer Inc. in September 2015. Medical writing/editorial support was provided by Elyse Smith, PhD, of Engage Scientific Solutions and was funded by Pfizer Inc.
This study was funded by Hospira Inc., which was acquired by Pfizer Inc. in September 2015.
The study sponsor monitored patient data collected by the investigators for completeness and acceptability throughout the course of the study; generated and validated statistical analyses; provided funding for medical writing and editorial support; and reviewed the final, author-approved version for intellectual property protection.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11631017/-/DCSupplemental.
References
- 1.Zadrazil J, Horak P: Pathophysiology of anemia in chronic kidney diseases: A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 159: 197–202, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Kausz AT, Khan SS, Abichandani R, Kazmi WH, Obrador GT, Ruthazer R, Pereira BJ: Management of patients with chronic renal insufficiency in the Northeastern United States. J Am Soc Nephrol 12: 1501–1507, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Kazmi WH, Kausz AT, Khan S, Abichandani R, Ruthazer R, Obrador GT, Pereira BJ: Anemia: An early complication of chronic renal insufficiency. Am J Kidney Dis 38: 803–812, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Janssen Products LP: Procrit (Epoetin Alfa) US Prescribing Information. Available at: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/PROCRIT-pi.pdf. Accessed February 6, 2018
- 5.Amgen Inc: Epogen (Epoetin Alfa) US Prescribing Information. Available at: https://pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/epogen/epogen_pi_hcp_english.ashx. Accessed April 17, 2017
- 6.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW: Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med 316: 73–78, 1987 [DOI] [PubMed] [Google Scholar]
- 7.United States Renal Data System: 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 1: Chronic Kidney Disease in the United States. Available at: https://www.usrds.org/2016/download/v1_CKD_16.pdf. Accessed April 18, 2017
- 8.United States Renal Data System: 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 2: End-Stage Renal Disease in the United States. Available at: https://www.usrds.org/2016/download/v2_c11_CostsofESRD_16.pdf. Accessed April 27, 2017
- 9.United States Renal Data System: USRDS: 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Volume 2: Atlas ESRD. Available at: https://www.usrds.org/2012/pdf/v2_ch11_12.pdf. Accessed April 17, 2017
- 10.European Medicines Agency: Retacrit (Epoetin Zeta): European Public Assessment Report. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000872/human_med_001031.jsp&mid=WC0b01ac058001d125. Accessed April 17, 2017
- 11.European Medicines Agency: Abseamed (Epoetin Alfa): European Public Assessment Report. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000727/human_med_000621.jsp&mid=WC0b01ac058001d124. Accessed April 17, 2017
- 12.European Medicines Agency: Binocrit (Epoetin Alfa): European Public Assessment Report. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000725/human_med_000675.jsp&mid=WC0b01ac058001d124. Accessed April 17, 2017
- 13.European Medicines Agency: Epoetin Alfa Hexal (Epoetin Alfa): European Public Assessment Report. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000726/human_med_000768.jsp&mid=WC0b01ac058001d124. Accessed April 17, 2017
- 14.European Medicines Agency: Silapo (Epotin Zeta): European Public Assessment Report. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000760/human_med_001051.jsp&mid=WC0b01ac058001d124. Accessed April 17, 2017
- 15.Fishbane S, Shah HH: The emerging role of biosimilar epoetins in nephrology in the United States. Am J Kidney Dis 65: 537–542, 2015 [DOI] [PubMed] [Google Scholar]
- 16.US Congress: An Act Entitled The Patient Protection And Affordable Care Act. Pub. L. 111–148 (H.R. 3590). 124 Stat. 119. Available at: https://www.gpo.gov/fdsys/pkg/STATUTE-124/pdf/STATUTE-124-Pg119.pdf. Accessed April 19, 2017
- 17.US Food and Drug Administration: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed February 24, 2016
- 18.Hospira Inc: Retacrit (Epoetin Alfa-epbx) US prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125545s000lbl.pdf. Accessed May 30, 2018
- 19.Pfizer Inc: Epoetin Hospira, a Proposed Biosimilar to Epogen/Procrit (Epoetin Alfa): Briefing Document for the Oncologic Drugs Advisory Committee. Available at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM559968.pdf. Accessed February 6, 2018
- 20.Amgen Inc: Epogen (Epoetin Alfa) US Prescribing Information, 2011. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103234Orig1s5166_103234Orig1s5266lbl.pdf. Accessed February 6, 2018
- 21.Amgen Inc: Epogen (Epoetin Alfa) US Prescribing Information, 2013. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103234s5323lbl.pdf. Accessed February 6, 2018
- 22.Berns JS, Elzein H, Lynn RI, Fishbane S, Meisels IS, Deoreo PB: Hemoglobin variability in epoetin-treated hemodialysis patients. Kidney Int 64: 1514–1521, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Lacson E Jr ., Ofsthun N, Lazarus JM: Effect of variability in anemia management on hemoglobin outcomes in ESRD. Am J Kidney Dis 41: 111–124, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Krivoshiev S, Todorov VV, Manitius J, Czekalski S, Scigalla P, Koytchev R; Epoetin Zeta Study Group : Comparison of the therapeutic effects of epoetin zeta and epoetin alpha in the correction of renal anaemia. Curr Med Res Opin 24: 1407–1415, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Krivoshiev S, Wizemann V, Czekalski S, Schiller A, Pljesa S, Wolf-Pflugmann M, Siebert-Weigel M, Koytchev R, Bronn A; Epoetin Zeta Study Group : Therapeutic equivalence of epoetin zeta and alfa, administered subcutaneously, for maintenance treatment of renal anemia. Adv Ther 27: 105–117, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Wizemann V, Rutkowski B, Baldamus C, Scigalla P, Koytchev R; Epoetin Zeta Study Group : Comparison of the therapeutic effects of epoetin zeta to epoetin alfa in the maintenance phase of renal anaemia treatment. Curr Med Res Opin 24: 625–637, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Büttel I, Völler K, Schneider C; European Medicines Agency : Immunogenicity and its impact on benefit/risk considerations in the authorisation of biopharmaceuticals. Curr Drug Saf 5: 287–292, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Bamgbola OF: Pattern of resistance to erythropoietin-stimulating agents in chronic kidney disease. Kidney Int 80: 464–474, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Gillespie IA, Macdougall IC, Richards S, Jones V, Marcelli D, Froissart M, Eckardt KU; ARO Steering Committee : Factors precipitating erythropoiesis-stimulating agent responsiveness in a European haemodialysis cohort: Case-crossover study. Pharmacoepidemiol Drug Saf 24: 414–426, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borelli M, Paul DP 3rd, Skiba M: Renal dialysis and its financing. Hosp Top 94: 33–38, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Wish D, Johnson D, Wish J: Rebasing the Medicare payment for dialysis: Rationale, challenges, and opportunities. Clin J Am Soc Nephrol 9: 2195–2202, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.