Abstract
Background and objectives
HDL particles obtained from patients on chronic hemodialysis exhibit lower cholesterol efflux capacity and are enriched in inflammatory proteins compared with those in healthy individuals. Observed alterations in HDL proteins could be due to effects of CKD, but also may be influenced by the hemodialysis procedure, which stimulates proinflammatory and prothrombotic pathways.
Design, setting, participants, & measurements
We compared HDL-associated proteins in 143 participants who initiated hemodialysis within the previous year with those of 110 participants with advanced CKD from the Hemodialysis Fistula Maturation Study. We quantified concentrations of 38 HDL-associated proteins relative to total HDL protein using targeted mass spectrometry assays that included a stable isotope–labeled internal standard. We used linear regression to compare the relative abundances of HDL-associated proteins after adjustment and required a false discovery rate q value ≤10% to control for multiple testing. We further assessed the association between hemodialysis initiation and cholesterol efflux capacity in a subset of 80 participants.
Results
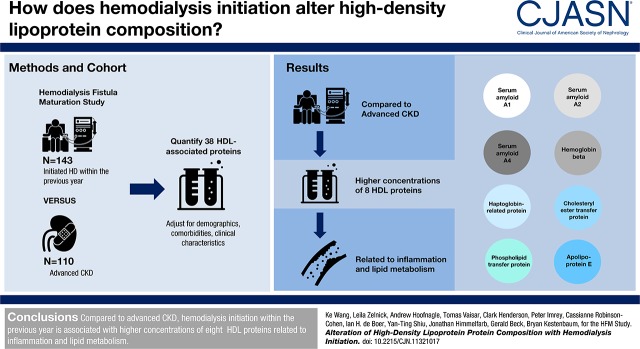
After adjustment for demographics, comorbidities, and other clinical characteristics, eight HDL-associated proteins met the prespecified false discovery threshold for association. Recent hemodialysis initiation was associated with higher HDL-associated concentrations of serum amyloid A1, A2, and A4; hemoglobin-β; haptoglobin-related protein; cholesterylester transfer protein; phospholipid transfer protein; and apo E. The trend for participants recently initiating hemodialysis for lower cholesterol efflux capacity compared with individuals with advanced CKD did not reach statistical significance.
Conclusions
Compared with advanced CKD, hemodialysis initiation within the previous year is associated with higher concentrations of eight HDL proteins related to inflammation and lipid metabolism. Identified associations differ from those recently observed for nondialysis-requiring CKD. Hemodialysis initiation may further impair cholesterol efflux capacity. Further work is needed to clarify the clinical significance of the identified proteins with respect to cardiovascular risk.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2018_07_25_CJASNPodcast_18_8_W.mp3
Keywords: Apolipoproteins; Cardiovascular Diseases; Cholesterol; Comorbidity; Demography; Fistula; Haptoglobins; Hemodialysis Initiation; Hemoglobins; High-density Lipoprotein; Humans; Inflammation; Isotopes; Linear Models; Lipid Metabolism; Lipoproteins, HDL; Mass Spectrometry; Phospholipid Transfer Proteins; Proteomic Analysis; renal dialysis; Renal Insufficiency, Chronic; risk factors
Introduction
Cardiovascular disease is the leading cause of death and disability among patients on chronic dialysis, accounting for approximately one half of all deaths (1). CKD is associated with a unique pattern of dyslipidemia that may contribute to cardiovascular risk. Specific lipid abnormalities observed in patients with CKD include an increase in circulating triglycerides, a reduction in total HDL cholesterol, and a disposition towards dense atherogenic LDL particles (2,3). Hepatic hydroxymethyl glutaryl–CoA reductase inhibitors, which specifically target LDL cholesterol, do not reduce cardiovascular outcomes in clinical trials of patients on chronic dialysis (4,5).
HDL particles are circulating noncovalently bound assemblies of lipids and proteins that mediate reverse cholesterol transport by retrieving excess cholesterol from peripheral tissues. HDL particles exhibit antioxidative and anti-inflammatory functions and inhibit proatherosclerotic processes, such as LDL oxidation, macrophage activation, and platelet adhesion (2,6). Among patients on chronic dialysis, absolute HDL cholesterol concentrations are inconsistently associated with cardiovascular risk, suggesting that functional alterations of HDL particles may be clinically important (7). In this regard, several studies have shown lower cholesterol efflux capacity and a greater abundance of inflammatory proteins in HDL particles obtained from patients on chronic hemodialysis compared with healthy individuals (8–12). However, these findings do not distinguish the potential effects of longstanding kidney failure from those of the hemodialysis procedure itself, which stimulates proinflammatory and prothrombotic pathways. A recent study of HDL composition among patients not on dialysis across a wide range of kidney function showed associations of lower eGFR with higher HDL-associated concentrations of retinol binding protein 4 (RBP4) and APOC3 and lower HDL-associated concentrations of APOL1 and vitronectin (13).
We sought to estimate the effect of the hemodialysis procedure on the HDL proteome. To accomplish this goal, we measured 38 HDL-associated proteins in 143 patients who recently initiated hemodialysis and 110 patients who had advanced CKD and were awaiting dialysis initiation in the Hemodialysis Fistula Maturation (HFM) Study. We hypothesized that dialysis initiation would be associated with structural transformation of the HDL proteome to a more inflammatory phenotype, which in turn, would lead to impaired HDL function.
Materials and Methods
Study Population
We analyzed plasma samples collected from the HFM Study, a prospective cohort study of arteriovenous fistula (AVF) maturation that enrolled 602 participants undergoing planned creation of an upper extremity AVF at seven study sites across the United States (14). The institutional review boards of each of these institutions and the Data Coordinating Center approved the study. Each participant provided informed consent before study enrollment. The HFM Study participants were either receiving maintenance dialysis or expected to start dialysis within 3 months of planned AVF surgery. Exclusion criteria were age <18 years old, age ≥80 years old if not receiving maintenance dialysis, or a life expectancy of <9 months. For purposes of this ancillary study, we excluded 349 participants from our analysis (288 without adequate plasma sample for HDL proteome assays, 59 HFM Study participants who had received maintenance dialysis for >1 year, and two participants who were receiving peritoneal dialysis), leaving a final analytic sample of 253 participants. We assessed cholesterol efflux capacity in a subset of 80 study participants; we matched 40 participants who initiated hemodialysis to 40 participants with advanced CKD according to age within one year, race, and sex.
HDL Isolation and Quantification of HDL-Associated Proteins
Blood samples were collected at a baseline preoperative study after overnight fasting. On collection, samples were stored at −80°C, maintained at the National Institute of Diabetes and Digestive and Kidney Diseases Biosample Repository, and then shipped to the University of Washington for measurement of HDL proteins.
The HDL fraction of plasma (d=1.063–1.210 g/ml) was purified using a two-step density gradient ultracentrifugation with potassium bromide (KBr). First, all lipoproteins were floated using a KBr solution of 1.210 g/ml and transferred to a new tube. Second, all lipoproteins less dense than HDL were floated using a KBr solution of 1.063 g/ml. The lipoproteins at the bottom of each sample after this second step were dialyzed and frozen at −80°C before use. Internal standard (15N-labeled APOA1) was added to each sample, and the lipoproteins were denatured using Rapigest (Waters), reduced with dithiothreitol, alkylated using iodoacetamide, and digested with trypsin. The Rapigest, particulates, and phospholipids were removed with a phospholipid removal plate (Phenomenex) before being analyzed by nanoflow-liquid chromatography-tandem mass spectrometry on a Q-Exactive mass spectrometer (Thermo). The same amount of HDL protein (10 μg) was analyzed for each sample.
Data Reduction
The peak area for each endogenous peptide was normalized to the peak area of an internal standard peptide from the stable isotope–labeled internal standard protein to generate a peak area ratio for each peptide in each sample. Peak area ratios for each protein were averaged, and protein peak area ratios were normalized to protein peak area ratios for calibrator samples included in each digestion batch. Because the same amount of HDL protein was used for each sample, changes in relative protein concentrations represent changes in the amount of protein per 10 μg total HDL protein. We quantified 38 proteins that are abundant in the HDL proteome and have been previously assayed using the same protocol (13,15–17).
Cholesterol Efflux Capacity
Serum HDL cholesterol efflux capacity was assessed in J774 macrophages labeled with 3H-cholesterol and stimulated with a cAMP analog (18). Efflux by the ATP binding cassette transporter A1 (ABCA1) pathway was measured with BHK cells expressing mifepristone-inducible human ABCA1 that were radiolabeled with 3H-cholesterol (19). Efflux of 3H-cholesterol was measured after 4 hours of incubation in medium with or without serum depleted of apoB (2.8% vol/vol). The serum HDL cholesterol efflux capacity is calculated as the amount of 3H-cholesterol in the media normalized to the total amount of 3H-cholesterol in the media and the cells.
Ascertainment of Other Study Data
At the baseline study visit, the HFM Study personnel used patient interviews and medical records to obtain patient demographics; medical histories; ESRD history, including modality and number of years undergoing dialysis; social habits; and home medication use. Demographics included age, sex, self-reported race and ethnicity, and smoking status. Comorbid conditions included a prevalent history of cardiovascular disease (angina, myocardial infarction, coronary artery bypass, or percutaneous coronary revascularization) and diabetes. The HFM Study personnel obtained the names of all active oral medications at the time of the study visit; measured height, weight, and three resting BPs in the nonaccess arm; and extracted hemoglobin levels from medical charts. The University of Washington Nutrition Obesity Research Center performed basic chemistries, including creatinine, albumin, C-reactive protein, and uric acid. Serum total cholesterol, HDL cholesterol, and triglyceride were determined using a standard clinical chemistry analyzer at the University of Washington Kidney Research Institute (Beckman Coulter DxC, Indianapolis, IN).
Statistical Analyses
We tabulated baseline characteristics of the HFM Study participants who initiated hemodialysis within the previous year versus those who were awaiting dialysis. We used linear regression to compare differences in plasma concentrations of the 38 HDL-associated proteins between these two groups. The outcome variables were the log-transformed concentrations of each HDL-associated protein, and the independent variable was dialysis status. Exponentiated coefficients from this model can be interpreted as ratios of geometric means of each HDL protein comparing patients who recently initiated hemodialysis with patients with predialysis CKD. We next performed adjusted analyses to control for confounding. The first model included demographic adjustments for age, race (black versus nonblack), and sex. The second model added adjustments for histories of diabetes and prevalent cardiovascular disease, body mass index, smoking status, statin use, and serum total cholesterol, HDL cholesterol, and triglycerides.
To account for multiple comparisons, we used the Benjamini–Hochberg procedure at a false discovery rate (FDR) q-value threshold of 10% to declare statistical significance (20–22). Under this approach, 10% of those differences in HDL proteins meeting the q-value threshold would be expected to be false positives (23). A protein needed to achieve significance under at least one adjustment model to be explored in further analyses. We implemented the FDR approach using the GenABEL package in R (24). All other statistical analyses were conducted using open source software R version 3.3.0 (https://www.r-project.org) and STATA 11 (StataCorp, College Station, TX).
Results
Description of the Study Population
For this ancillary study, we excluded 288 HFM study participants who had inadequate plasma sample volume for performing the HDL proteome assays, 59 participants who had received maintenance dialysis for longer than 1 year, and two participants who were receiving peritoneal dialysis, leaving a final analytic sample of 253 participants. Characteristics of our 253-person study cohort were generally similar to those of the full 602-person HFM Study population (Supplemental Table 1). Compared with participants who were awaiting dialysis, participants who initiated hemodialysis within the previous year tended to be younger (54±14 versus 58±11 years old); were more likely to be black (51% versus 30%); had lower prevalence rates of diabetes (54% versus 66%) and cardiovascular disease (39% versus 53%); and had lower serum triglycerides and higher serum HDL cholesterol and C-reactive protein concentrations (Table 1).
Table 1.
Baseline participant characteristics by hemodialysis status
| Characteristic | Awaiting Dialysis, n=110 | Recent Hemodialysis,a n=143 |
|---|---|---|
| Age, yr | 58 (11) | 54 (14) |
| Race | ||
| White | 72 (65) | 54 (38) |
| Black | 33 (30) | 73 (51) |
| Other | 5 (5) | 16 (11) |
| Men | 72 (65) | 97 (68) |
| Smoking | ||
| Current | 21 (19) | 24 (17) |
| Former | 48 (44) | 41 (29) |
| Never | 41 (37) | 78 (54) |
| History of diabetes | 73 (66) | 77 (54) |
| Prevalent cardiovascular disease | 58 (53) | 56 (39) |
| Dialysis duration, mo | NA | 4.6 (3) |
| Body mass index, kg/m2 | 32 (7) | 29 (8) |
| Systolic BP, mm Hg | 156 (20) | 152 (25) |
| Statin use | 69 (66) | 64 (46) |
| Niacin use | 2 (2) | 1 (1) |
| Fibrate use | 7 (7) | 3 (2) |
| Insulin use | 49 (47) | 45 (32) |
| eGFR, ml/min per 1.73 m2 | 13 (4) | NA |
| C-reactive protein,b mg/L | 3.4 (1.2–10.7) | 5.6 (2.9–23.8) |
| Uric acid, mg/dl | 7.8 (2.0) | 5.3 (1.7) |
| Albumin, g/dl | 3.6 (0.6) | 3.5 (0.7) |
| Hemoglobin, g/dl | 10.4 (1.6) | 10.3 (1.7) |
| Total cholesterol, mg/dl | 174 (52)c | 172 (42)d |
| HDL-C, mg/dl | 46 (14)c | 51 (17)d |
| Triglycerides, mg/dl | 180 (110)c | 144 (61)d |
Values are expressed as mean (SD) or number (percentage). NA, not applicable; HDL-C, HDL cholesterol.
The Hemodialysis Fistula Maturation Study participants who initiated hemodialysis within the previous year.
Values are expressed as median (interquartile range).
Values are missing for eight participants.
Values are missing for three participants.
Associations of Hemodialysis with HDL-Associated Proteins
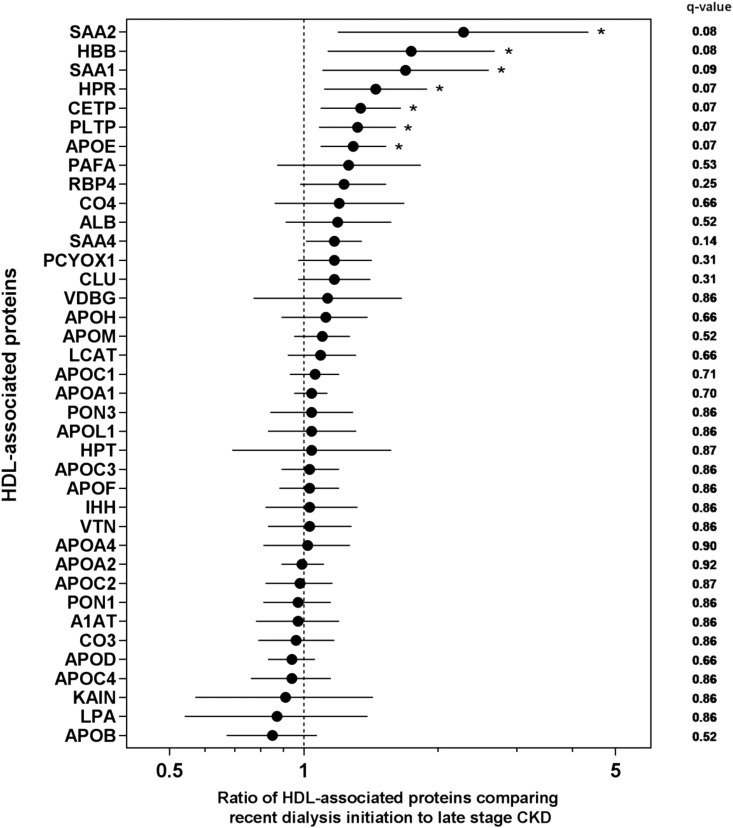
After adjustment for age, race, and sex (model 1), seven of 38 HDL-associated proteins met the specified FDR threshold for statistical significance (Figure 1). Relative concentrations of significant HDL proteins were higher among participants recently initiating hemodialysis compared with those awaiting dialysis. Specifically, recent hemodialysis initiation was associated with higher concentrations of serum amyloid A2 (SAA2), hemoglobin-β, SAA1, haptoglobin-related protein (HPR), cholesterylester transfer protein (CETP), phospholipid transfer protein (PLTP), and APOE. The sizes of these associations after model 1 adjustment ranged from 29% greater concentrations of APOE to 128% greater concentrations of SAA2 (Table 2). After further covariate adjustments under model 2, the association of dialysis initiation with PLTP was no longer statistically significant, whereas the association with SAA4 reached statistical significance. There were no detectable differences in the relative concentrations of APOA1 and APOA2, the two most abundant HDL proteins that account for approximately 90% of total HDL protein mass (25).
Figure 1.
Ratios of seven HDL-associated proteins are higher in recent hemodialysis compared to late-stage CKD. Forest plot of ratios of HDL-associated protein concentrations comparing participants on hemodialysis for <1 year with participants with late-stage CKD. Exponentiated β-coefficients are plotted along with 95% confidence intervals, with q values, calculated after model 1 adjustment for age, race (black versus nonblack), and sex. The x axis is presented in the logarithmic scale. *Significant HDL-associated proteins after performing false discovery rate correction (q≤10%). ALB, albumin; A1AT, α1-antitrypsin; CETP, cholesteryl ester transfer protein; CLU, clusterin; CO3, complement C3; CO4, complement C4; HBB, hemoglobin-β; HPR, haptoglobin-related protein; HPT, haptoglobin; IHH, Indian hedgehog protein; KAIN, kallistatin; LCAT, phosphatidylcholine-sterol acyltransferase; LPA, lipoprotein(a); PAFA, platelet-activating factor acetylhydrolase; PCYOX1, prenylcysteine oxidase 1; PLTP, phospholipid transfer protein; PON1, paraoxonase 1; PON3, paraoxonase 3; RBP4, retinol binding protein 4; SAA1, serum amyloid A1; SAA2, serum amyloid A2; SAA4, serum amyloid A4; VDBG, vitamin D binding globulin; VTN, vitronectin.
Table 2.
Association of hemodialysis initiation with HDL-associated proteins
| Protein and Model | Ratio of Protein Concentrations Comparing Hemodialysis with Late-Stage CKDa | 95% Confidence Interval | q Value |
|---|---|---|---|
| Serum Amyloid A2 | |||
| Unadjusted | 2.21 | 1.19 to 4.09 | |
| Model 1b | 2.28 | 1.19 to 4.35 | 0.08 |
| Model 2c | 3.21 | 1.60 to 6.43 | 0.04 |
| Serum Amyloid A1 | |||
| Unadjusted | 1.62 | 1.07 to 2.45 | |
| Model 1 | 1.69 | 1.10 to 2.60 | 0.09 |
| Model 2 | 1.99 | 1.26 to 3.16 | 0.04 |
| Hemoglobin-β | |||
| Unadjusted | 1.89 | 1.25 to 2.87 | |
| Model 1 | 1.74 | 1.13 to 2.68 | 0.08 |
| Model 2 | 1.92 | 1.20 to 3.07 | 0.05 |
| Haptoglobin-Related Protein | |||
| Unadjusted | 1.89 | 1.42 to 2.52 | |
| Model 1 | 1.45 | 1.11 to 1.89 | 0.07 |
| Model 2 | 1.44 | 1.09 to 1.89 | 0.06 |
| Cholesterol Ester Transfer Protein | |||
| Unadjusted | 1.36 | 1.11 to 1.65 | |
| Model 1 | 1.34 | 1.09 to 1.65 | 0.07 |
| Model 2 | 1.30 | 1.05 to 1.62 | 0.08 |
| Phospholipid Transfer Proteind | |||
| Unadjusted | 1.34 | 1.10 to 1.63 | |
| Model 1 | 1.32 | 1.08 to 1.61 | 0.07 |
| Model 2 | 1.20 | 0.98 to 1.47 | 0.28 |
| Apolipoprotein E | |||
| Unadjusted | 1.34 | 1.14 to 1.57 | |
| Model 1 | 1.29 | 1.09 to 1.53 | 0.07 |
| Model 2 | 1.31 | 1.09 to 1.56 | 0.04 |
| Serum Amyloid A4e | |||
| Unadjusted | 1.17 | 1.02 to 1.35 | |
| Model 1 | 1.17 | 1.01 to 1.35 | 0.14 |
| Model 2 | 1.25 | 1.07 to 1.46 | 0.04 |
Ratio of geometric means.
Model 1 was adjusted for age, race (black versus nonblack), and sex.
Model 2 adds adjustment for diabetes, body mass index, prevalent cardiovascular disease, smoking, statin use, and serum levels of total cholesterol, HDL-C, and triglycerides.
PLTP was no longer statistically significant after model 2 adjustment.
SAA4 was significant after model 2 adjustment only.
Associations of Hemodialysis Duration with HDL-Associated Proteins
In additional analyses restricted to the 143 participants who initiated hemodialysis within the previous year, the duration of dialysis was not associated with differences in any of the candidate HDL proteins (Table 3).
Table 3.
Association of dialysis duration with HDL-associated proteins
| Protein and Model | Ratio of Protein Concentrations per Month Longer on Chronic Dialysisa | 95% Confidence Interval | P Value |
|---|---|---|---|
| Serum Amyloid A2 | |||
| Unadjusted | 1.01 | 0.89 to 1.13 | |
| Model 2b | 1.03 | 0.91 to 1.16 | 0.61 |
| Serum Amyloid A1 | |||
| Unadjusted | 1.05 | 0.97 to 1.14 | |
| Model 2 | 1.05 | 0.97 to 1.15 | 0.19 |
| Hemoglobin-β | |||
| Unadjusted | 0.98 | 0.90 to 1.06 | |
| Model 2 | 0.97 | 0.90 to 1.06 | 0.61 |
| Haptoglobin-Related Protein | |||
| Unadjusted | 1.02 | 0.97 to 1.07 | |
| Model 2 | 1.00 | 0.96 to 1.06 | 0.75 |
| Cholesterol Ester Transfer Protein | |||
| Unadjusted | 0.99 | 0.96 to 1.03 | |
| Model 2 | 0.99 | 0.96 to 1.03 | 0.79 |
| Phospholipid Transfer Protein | |||
| Unadjusted | 1.01 | 0.97 to 1.04 | |
| Model 2 | 1.00 | 0.97 to 1.04 | 0.84 |
| Apolipoprotein E | |||
| Unadjusted | 1.00 | 0.97 to 1.03 | |
| Model 2 | 1.00 | 0.97 to 1.03 | 1.03 |
| Serum Amyloid A4 | |||
| Unadjusted | 1.00 | 0.98 to 1.03 | |
| Model 2 | 1.00 | 0.98 to 1.03 | 0.53 |
Ratio of geometric means.
Model 2 was adjusted for age, race (black versus nonblack), sex, diabetes, body mass index, prevalent cardiovascular disease, smoking, statin use, and serum concentrations of total cholesterol, HDL cholesterol, and triglycerides.
Association of Hemodialysis Initiation with Cholesterol Efflux Capacity
Individuals recently initiated on hemodialysis showed modestly lower total efflux capacity (11.4% versus 11.8%) and ABCA1-mediated efflux capacity (13.6% versus 14.3%) compared with those with advanced CKD; however, this difference was not statistically significant. Adjustment for participant demographics and serum HDL cholesterol level strengthened the association between dialysis initiation and impaired cholesterol efflux capacity (Table 4). Total efflux capacity was highly correlated with ABCA1-mediated efflux capacity (r=0.75). A very weak negative correlation was observed between relative abundance of SAA1- and ABCA1-mediated efflux; however, this was not statistically significant (r=−0.14; P value =0.21) (Supplemental Table 2).
Table 4.
Association between dialysis initiation and cholesterol efflux capacity
| Cholesterol Efflux Capacity | Advanced CKD (%)a | Hemodialysis (%)a | Unadjusted Difference (%; 95% CI) | Adjusted Difference (%; 95% CI)b | Adjusted P Value |
|---|---|---|---|---|---|
| Total | 11.8 (2.7) | 11.4 (2.4) | −0.4 (−1.5 to 0.8) | −0.9 (−2.0 to 0.1) | 0.07 |
| ABCA1 | 14.3 (3.1) | 13.6 (2.2) | −0.6 (−1.8 to 0.6) | −1.0 (−2.2 to 0.1) | 0.08 |
95% CI, 95% confidence interval; ABCA1, ATP binding cassette transporter A1.
n=40.
Adjusted for age, race (black versus nonblack), sex, and HDL-C cholesterol level.
Discussion
We observed associations of recent hemodialysis initiation with a greater relative abundance of eight HDL-associated proteins. The identified HDL constituents in this study are markers or mediators of inflammatory, atherosclerotic, and lipid metabolism pathways. None of these HDL-associated proteins were associated with lower eGFR in a recent study of patients with nondialysis-requiring CKD across a wide range of kidney function (13). We also observed a trend toward lower HDL cholesterol efflux capacity in a subset of study participants. Although the possibility of confounding cannot be excluded, these findings suggest a possible effect of the hemodialysis procedure on the composition and function of the HDL proteome.
The observed associations of recent dialysis initiation with HDL-associated proteins in this study differ from those recently reported for reduced eGFR in patients with nondialysis CKD. Among 509 patients with CKD not receiving dialysis, each 15-ml/min per 1.73 m2 lower eGFR was associated with higher HDL concentrations of RBP4 and APOC3 and lower HDL concentrations of APOL1 and vitronectin (13). None of these proteins were associated with hemodialysis initiation in our study, and conversely, none of the proteins that we identified were associated with reduced GFR among patients in the CKD cohort study. These findings suggest that kidney disease and hemodialysis may each uniquely affect the HDL proteome.
Previous studies have reported differences in HDL-associated proteins when comparing patients on chronic hemodialysis with healthy controls. Holzer et al. (8) found lower relative concentrations of APOA1 and APOA2 and higher concentrations of SAA1, albumin, and APOC3 in 27 patients on hemodialysis compared with 19 healthy controls. Subsequent studies have reported associations of hemodialysis with lower HDL concentrations of APOA1 and APOA2 (9,11,12) and higher concentrations of SAA (9,11,12) and APOC3 (10–12). Previous studies have also reported associations of chronic hemodialysis with higher HDL concentrations of APOC2 (9–11), lipoprotein-associated phospholipase A2 (8), surfactant protein B (9), α1-microglobulin/bikunin precursor (9,12), α1-acid glycoprotein 2, RBP4, α1-antitrypsin, APOA4, HPR, and transthyretin (12). Most of these identified HDL proteins are acute-phase reactants and important mediators of immune response and thrombosis pathways (12,26). Previous studies of chronic dialysis are limited by small sample size and comparison of patients on dialysis with healthy controls, which conflates potential effects of kidney disease with those of the hemodialysis procedure.
We observed that hemodialysis initiation was associated with a strong trend toward impaired cholesterol efflux capacity from lipid-laden macrophages. Total cholesterol efflux is mediated through several known pathways, including ABCA1, ABCG1, scavenger receptor B1, and aqueous diffusion (27). Both animal and human studies have shown that ABCA1-mediated efflux is the key pathway for HDL reverse cholesterol transport (18,28). In the general population, lower cholesterol efflux capacity is associated with higher risks of cardiovascular events and death (29). Several studies have shown lower cholesterol efflux among patients on dialysis compared with individuals without kidney disease (8,11,30). A recent study showed impaired HDL-mediated cholesterol efflux across varying stages of CKD compared with healthy controls (31). Therefore, our results suggest that hemodialysis may be independently associated with impaired HDL-mediated cholesterol efflux.
Hemodialysis may independently provoke a unique inflammatory microenvironment that could promote atherogenic transformation of the HDL proteome and impair HDL function. We observed higher serum C-reactive protein concentrations among study participants who recently initiated hemodialysis compared with those with late-stage CKD. Prior studies have shown that hemodialysis is associated with increased inflammation independent of kidney function. Patients on hemodialysis exhibit higher serum levels of IL-6 compared with patients who are uremic and are not on dialysis and higher HDL concentrations of inflammatory proteins compared with individuals on peritoneal dialysis (11,32). Serial monitoring of inflammatory markers after dialysis initiation shows that hemodialysis does not attenuate the proinflammatory and pro-oxidative milieu that accompanies kidney disease (33). In fact, hemodialysis may further exacerbate the existing inflammatory milieu of uremia via several plausible mechanisms, such as activation of the immune response through blood interaction with dialysis membrane and back filtration of endotoxins contained within the dialysate (34,35).
HDL proteins associated with hemodialysis in this study are known to be involved in inflammatory, lipid metabolism, and thrombosis pathways. SAA is an activator of the innate immune system and can stimulate thrombosis (36,37). Higher HDL-associated SAA concentrations in healthy individuals and patients on hemodialysis correlate with lower HDL cholesterol efflux capability (8) and are associated with cardiovascular mortality (38). CETP and PLTP mediate HDL cholesterol transport. Although CETP is thought to be proatherogenic in individuals with coronary artery disease (39), evidence suggests that this protein may protect against thrombosis in patients on hemodialysis (40,41). Increased PLTP activity is associated with accelerated atherosclerosis in mice and cardiovascular events in the Framingham Heart Study (42,43). HDL enrichment of APOE can interfere with HDL metabolism and instigate atherogenesis (44,45). The HDL3 fraction isolated from patients with coronary artery disease is enriched with APOE, and higher APOE levels are associated with cardiovascular mortality (46,47). HPR is an acute-phase reactant with a high binding affinity for hemoglobin in the setting of hemolysis. HDL abundances of both HPR and hemoglobin are increased in animal models of atherosclerosis and human subjects with coronary heart disease (48,49).
One strength of our study is the comparison of the HDL proteome in patients on hemodialysis with that of patients with advanced CKD awaiting dialysis from within the same parent study. We used validated, targeted mass spectrometry assays with internal standards to quantify the protein content of isolated HDL particles, and we adjusted for differences in patient characteristics that were measured using uniform data collection procedures in the HFM Study.
The most important limitation of the study is the possibility of residual confounding, in which unmeasured differences in the characteristics of the patients not on dialysis versus patients on dialysis in this study may have distorted the observed associations. For example, patients initiating chronic dialysis may have had more severe uremia, which can lead to transformation of the HDL proteome independent of the dialysis procedure. Ideally, the effect of hemodialysis initiation on HDL-associated proteins would be determined from studies that measure these proteins before and after dialysis initiation within the same individual. Nonetheless, our approach intended to simulate this ideal, because both groups were selected from the same prospective cohort study and accounted for general differences in patient characteristics. A second limitation of our study is that we assessed HDL cholesterol efflux capacity among a relatively small cohort of study participants, therefore limiting the power to detect a statistically significant difference. Additionally, our results cannot elucidate which specific changes in the HDL proteome cause dysfunction.
In conclusion, hemodialysis initiation was associated with higher concentrations of eight HDL-associated proteins. Identified proteins reside in pathways of inflammation, lipid metabolism, and atherosclerosis and differ from those previously associated with reduced kidney function in patients with CKD. Our findings suggest that the hemodialysis procedure may provoke a unique pattern of dysfunctional HDL particles that are less prevalent in late-stage CKD. Further work is needed to confirm these suspected changes and clarify their clinical significance.
Disclosures
B.K. has received consulting fees from Sanofi Inc. The laboratory of A.N.H. receives grant funding from Waters, a mass spectrometry manufacturer. The other authors indicate no disclosures.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the technical assistance of Jennifer Wallace in the preparation of the HDL particles.
The Hemodialysis Fistula Maturation Study was funded by grants U01DK082218, U01DK082222, U01DK082232, U01DK082236, U01DK082240, U01DK082179, U01DK082189 from the National Institute of Diabetes and Digestive and Kidney Diseases. This study was funded by University of Washington Nutrition Obesity Research Center grants P30DK035816, T32DK00746733 (to K.W.), R01HL111375 (to A.N.H.), T32HL007028 (to C.M.H.), and R01DK094891 (to B.K.).
Because I.H.d.B. is a Deputy Editor of the Clinical Journal of the American Society of Nephrology, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript. Rajnish Mehrotra, the Editor-in-Chief, is at the same institution as some of the authors, including the Deputy Editor, and therefore, was also not involved in the peer review process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11321017/-/DCSupplemental.
Contributor Information
Collaborators: KestenbaumBryan12on behalf of the HFM Study
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Vaziri ND: Dyslipidemia of chronic renal failure: The nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol 290: F262–F272, 2006 [DOI] [PubMed] [Google Scholar]
- 3.de Boer IH, Astor BC, Kramer H, Palmas W, Seliger SL, Shlipak MG, Siscovick DS, Tsai MY, Kestenbaum B: Lipoprotein abnormalities associated with mild impairment of kidney function in the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol 3: 125–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F; AURORA Study Group : Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M: In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int 76: 437–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, Pfahler K, Seiler S, Heine GH, März W, Silbernagel G, Fliser D: HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol 25: 1073–1082, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G: Uremia alters HDL composition and function. J Am Soc Nephrol 22: 1631–1641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weichhart T, Kopecky C, Kubicek M, Haidinger M, Döller D, Katholnig K, Suarna C, Eller P, Tölle M, Gerner C, Zlabinger GJ, van der Giet M, Hörl WH, Stocker R, Säemann MD: Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol 23: 934–947, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangé A, Goux A, Badiou S, Patrier L, Canaud B, Maudelonde T, Cristol JP, Solassol J: HDL proteome in hemodialysis patients: A quantitative nanoflow liquid chromatography-tandem mass spectrometry approach. PLoS One 7: e34107, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzer M, Schilcher G, Curcic S, Trieb M, Ljubojevic S, Stojakovic T, Scharnagl H, Kopecky CM, Rosenkranz AR, Heinemann A, Marsche G: Dialysis modalities and HDL composition and function. J Am Soc Nephrol 26: 2267–2276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao B, de Boer I, Tang C, Mayer PS, Zelnick L, Afkarian M, Heinecke JW, Himmelfarb J: A cluster of proteins implicated in kidney disease is increased in high-density lipoprotein isolated from hemodialysis subjects. J Proteome Res 14: 2792–2806, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubinow KB, Henderson CM, Robinson-Cohen C, Himmelfarb J, de Boer IH, Vaisar T, Kestenbaum B, Hoofnagle AN: Kidney function is associated with an altered protein composition of high-density lipoprotein. Kidney Int 92: 1526–1535, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dember LM, Imrey PB, Beck GJ, Cheung AK, Himmelfarb J, Huber TS, Kusek JW, Roy-Chaudhury P, Vazquez MA, Alpers CE, Robbin ML, Vita JA, Greene T, Gassman JJ, Feldman HI; Hemodialysis Fistula Maturation Study Group : Objectives and design of the hemodialysis fistula maturation study. Am J Kidney Dis 63: 104–112, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson CM, Vaisar T, Hoofnagle AN: Isolating and quantifying plasma HDL proteins by sequential density gradient ultracentrifugation and targeted proteomics. Methods Mol Biol 1410: 105–120, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoofnagle AN, Becker JO, Oda MN, Cavigiolio G, Mayer P, Vaisar T: Multiple-reaction monitoring-mass spectrometric assays can accurately measure the relative protein abundance in complex mixtures. Clin Chem 58: 777–781, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsillach J, Becker JO, Vaisar T, Hahn BH, Brunzell JD, Furlong CE, de Boer IH, McMahon MA, Hoofnagle AN; DCCT/EDIC Research Group : Paraoxonase-3 is depleted from the high-density lipoproteins of autoimmune disease patients with subclinical atherosclerosis. J Proteome Res 14: 2046–2054, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH: The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol 30: 796–801, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughan AM, Oram JF: ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res 47: 2433–2443, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and power approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300, 1995 [Google Scholar]
- 21.Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, Wang J, Imhann F, Brandsma E, Jankipersadsing SA, Joossens M, Cenit MC, Deelen P, Swertz MA, Weersma RK, Feskens EJ, Netea MG, Gevers D, Jonkers D, Franke L, Aulchenko YS, Huttenhower C, Raes J, Hofker MH, Xavier RJ, Wijmenga C, Fu J; LifeLines cohort study : Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352: 565–569, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu W, Browning SR, Browning BL, Akey JM: Robust inference of identity by descent from exome-sequencing data. Am J Hum Genet 99: 1106–1116, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storey JD, Tibshirani R: Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100: 9440–9445, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aulchenko YS, Ripke S, Isaacs A, van Duijn CM: GenABEL: An R library for genome-wide association analysis. Bioinformatics 23: 1294–1296, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Pamir N, Hutchins P, Ronsein G, Vaisar T, Reardon CA, Getz GS, Lusis AJ, Heinecke JW: Proteomic analysis of HDL from inbred mouse strains implicates APOE associated with HDL in reduced cholesterol efflux capacity via the ABCA1 pathway. J Lipid Res 57: 246–257, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AS, Tan L, Long JL, Davidson WS: Proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J Lipid Res 54: 2575–2585, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ: Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldán A, Pei L, Lee R, Tarr P, Tangirala RK, Weinstein MM, Frank J, Li AC, Tontonoz P, Edwards PA: Impaired development of atherosclerosis in hyperlipidemic Ldlr-/- and ApoE-/- mice transplanted with Abcg1-/- bone marrow. Arterioscler Thromb Vasc Biol 26: 2301–2307, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Rohatgi A, de Lemos JA, Shaul PW: HDL cholesterol efflux capacity and cardiovascular events. N Engl J Med 372: 1871–1872, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, Fazio S, Kon V: Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol 60: 2372–2379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaseda R, Tsuchida Y, Yang HC, Yancey PG, Zhong J, Tao H, Bian A, Fogo AB, Linton MRF, Fazio S, Ikizler TA, Kon V: Chronic kidney disease alters lipid trafficking and inflammatory responses in macrophages: Effects of liver X receptor agonism. BMC Nephrol 19: 17, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Memoli B, Libetta C, Rampino T, Dal Canton A, Conte G, Scala G, Ruocco MR, Andreucci VE: Hemodialysis related induction of interleukin-6 production by peripheral blood mononuclear cells. Kidney Int 42: 320–326, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Pupim LB, Himmelfarb J, McMonagle E, Shyr Y, Ikizler TA: Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int 65: 2371–2379, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Chenoweth DE, Cheung AK, Henderson LW: Anaphylatoxin formation during hemodialysis: Effects of different dialyzer membranes. Kidney Int 24: 764–769, 1983 [DOI] [PubMed] [Google Scholar]
- 35.Hakim RM, Fearon DT, Lazarus JM: Biocompatibility of dialysis membranes: Effects of chronic complement activation. Kidney Int 26: 194–200, 1984 [DOI] [PubMed] [Google Scholar]
- 36.He R, Sang H, Ye RD: Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood 101: 1572–1581, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Lee HY, Kim MK, Park KS, Shin EH, Jo SH, Kim SD, Jo EJ, Lee YN, Lee C, Baek SH, Bae YS: Serum amyloid A induces contrary immune responses via formyl peptide receptor-like 1 in human monocytes. Mol Pharmacol 70: 241–248, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Kopecky C, Genser B, Drechsler C, Krane V, Kaltenecker CC, Hengstschläger M, März W, Wanner C, Säemann MD, Weichhart T: Quantification of HDL proteins, cardiac events, and mortality in patients with type 2 diabetes on hemodialysis. Clin J Am Soc Nephrol 10: 224–231, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuivenhoven JA, Jukema JW, Zwinderman AH, de Knijff P, McPherson R, Bruschke AV, Lie KI, Kastelein JJ; The Regression Growth Evaluation Statin Study Group : The role of a common variant of the cholesteryl ester transfer protein gene in the progression of coronary atherosclerosis. N Engl J Med 338: 86–93, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Kimura H, Gejyo F, Yamaguchi T, Suzuki S, Imura T, Miyazaki R, Arakawa M: A cholesteryl ester transfer protein gene mutation and vascular disease in dialysis patients. J Am Soc Nephrol 10: 294–299, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Kimura H, Miyazaki R, Suzuki S, Gejyo F, Yoshida H: Cholesteryl ester transfer protein as a protective factor against vascular disease in hemodialysis patients. Am J Kidney Dis 38: 70–76, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Lie J, de Crom R, van Gent T, van Haperen R, Scheek L, Sadeghi-Niaraki F, van Tol A: Elevation of plasma phospholipid transfer protein increases the risk of atherosclerosis despite lower apolipoprotein B-containing lipoproteins. J Lipid Res 45: 805–811, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Robins SJ, Lyass A, Brocia RW, Massaro JM, Vasan RS: Plasma lipid transfer proteins and cardiovascular disease. The Framingham Heart Study. Atherosclerosis 228: 230–236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaziri ND: HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol 12: 37–47, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Kwan BC, Kronenberg F, Beddhu S, Cheung AK: Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol 18: 1246–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW: Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 117: 746–756, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mooijaart SP, Berbée JF, van Heemst D, Havekes LM, de Craen AJ, Slagboom PE, Rensen PC, Westendorp RG: ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Med 3: e176, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe J, Chou KJ, Liao JC, Miao Y, Meng HH, Ge H, Grijalva V, Hama S, Kozak K, Buga G, Whitelegge JP, Lee TD, Farias-Eisner R, Navab M, Fogelman AM, Reddy ST: Differential association of hemoglobin with proinflammatory high density lipoproteins in atherogenic/hyperlipidemic mice. A novel biomarker of atherosclerosis. J Biol Chem 282: 23698–23707, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Watanabe J, Grijalva V, Hama S, Barbour K, Berger FG, Navab M, Fogelman AM, Reddy ST: Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J Biol Chem 284: 18292–18301, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.