Abstract
Hepatitis B virus (HBV) expresses three co-terminal envelope proteins: large (L), middle (M), and small (S), with the S protein driving the secretion of both virions and subviral particles. Virion secretion requires N-linked glycosylation at N146 in the S domain but can be impaired by immune escape mutations. An M133T mutation creating a novel glycosylation site at N131could rescue virion secretion of N146Q mutant (loss of original glycosylation site) and immune escape mutants such as G145R. Here we demonstrate that other novel N-linked glycosylation sites could rescue virion secretion of the G145R and N146Q mutants to variable extents. Both G145R and N146Q mutations impaired virion secretion through the S protein. The M133T mutation restored virion secretion through the S protein, and could work in trans. Impaired virion secretion was not necessarily associated with a similar block in the secretion of subviral particles.
Keywords: hepatitis B surface antigen, hepatitis B virus, immune escape mutant, N-linked glycosylation, subviral particle, vaccine escape, virion secretion
1. Introduction
Hepatitis B virus (HBV) remains widely prevalent with an estimated 240 million chronically infected subjects worldwide (Schweitzer et al., 2015). Each year more than 780,000 deaths can be attributed to complications of chronic hepatitis B including liver cirrhosis and hepatocellular cancer. Unfortunately, interferons are effective only in a small percentage of patients, while nucleoside analogues suppress HBV DNA replication without promoting hepatitis B e antigen (HBeAg) or hepatitis B surface antigen (HBsAg) seroconversion, markers of sustained virological response. The most cost-effective way to reduce the incidence of HBV-related HCC is to prevent infection in the first place. In this regard HBV is an enveloped DNA virus producing three co-terminal envelope proteins: large (L), middle (M), and small (S). The M protein has an extra preS2 domain than the S protein, while the L protein harbors both preS1 and preS2 domains. Besides incorporation onto virion surface, most S protein is secreted alone as the noninfectious subviral particles and detected serologically as HBsAg. Although antibodies against preS1, preS2, and S domains can all neutralize HBV infectivity, the S domain is more conserved among different HBV genotypes. S protein expressed in yeast serves as the current prophylactic HBV vaccine.
The universal vaccination programs in Asian countries have dramatically reduced HBV carrier rate in the younger generation. Still, 5–10% of infants born to HBeAg-positive mothers continue to get infected despite administration of both HBV vaccine and hepatitis B immune globulin (HBIG) immediately after birth. The viral factor responsible for vaccine failure is the immune escape mutants (Carman et al., 1990). The S domain is anchored on the lipid bilayer of viral envelope with residues 101 – 163 exposed on the exterior and termed immunodominant loop. Most anti-S antibodies target the “a” determinant (residues 124 – 147) within the immunodominant loop, where mutations are associated with vaccine failure. The most common vaccine escape mutation, G145R, has been shown to impair the binding of antibodies raised against the wild-type (WT) S protein (Chiou et al., 1997; Cooreman et al., 1999; Waters et al., 1992). The rising prevalence of such immune escape mutants since the inception of the universal vaccination programs in Asian countries (Bian et al., 2013; Hsu et al., 2004) raises serious concerns as to whether they will replace the WT virus and thwart the vaccination programs. Immune escape mutants have also been implicated in reinfection of grafted liver despite passive prophylaxis with HBIG (Carman et al., 1996; Ghany et al., 1998; McMahon et al., 1992; Protzer-Knolle et al., 1998; Shields et al., 1999; Terrault et al., 1998), and can render HBsAg undetectable or poorly detected by immunoassays based on monoclonal antibodies against the S domain of the WT virus, thus contributing to the so-called “occult (HBsAg- negative) HBV infection” (Hollinger and Sood, 2010; Jeantet et al., 2004; Minuk et al., 2005; Raimondo et al., 2007; Shahmoradi et al., 2012; Weinberger et al., 2000; Zaaijer et al., 2008). To accurately estimate the threat posed by the immune escape mutants would require a thorough evaluation of their biological properties, such as virion and HBsAg secretion as well as viral infectivity.
The S domain contains a single N-linked glycosylation site (Asn-X-Ser/Thr, where X is any amino acid except proline) at N146, which is used at about 50% efficiency. Consequently the three envelope proteins exist in two size forms: gp42/p39 (L), gp36/gp33 (M), and gp27/p24 (S). Abolishing N-linked glycosylation in the S domain by either N146Q or N146S mutation prevented the secretion of virions but not subviral particles (HBsAg) (Ito et al., 2010). G145R, the classic immune escape mutation, also severely impaired virion secretion (Ito et al., 2010; Kalinina et al., 2003). Other immune escape mutants display variable reduction in virion secretion efficiency (Huang et al., 2012; Khan et al., 2004; Kwei et al., 2013). Interestingly, immune escape mutants are often accompanied by mutations creating additional N-linked glycosylation sites inside the “a” determinant (Chen et al., 2011; Huang et al., 2012; Ito et al., 2010; Julithe et al., 2014; Wu et al., 2010; Yu et al., 2014). Our previous study revealed that the M133T mutation, which creates a novel glycosylation site at N131 (131NST133), could rescue virion secretion of the N146Q and N146S mutants (Ito et al., 2010). Thus, secretion of the WT HBV requires a glycosylation site, although not necessarily at position 146 inside the S domain. The M133T mutation could also rescue virion secretion of G145R and other immune escape mutants (Ito et al., 2010; Kwei et al., 2013). In the present study, we examined whether other novel N-linked glycosylation sites could rescue virion secretion of the G145R and N146Q mutants. Studies were performed to clarify whether the G145R and N146Q mutations impair virion secretion through the L, M, or S envelope protein, and whether M133T mutation in L, M, or S protein rescues virion secretion of the G145R or N146Q mutant. We also investigated the impact of immune escape and glycosylation mutations on HBsAg secretion.
2. Results
The present study continued to investigate the impact of immune escape mutations and N-linked glycosylation on HBV virion secretion. One question is whether the mutated L, M, or S protein is responsible for the impairment and/or rescue of virion secretion. As detailed in Materials and Methods section, a 1.5mer replication construct was rendered unable to express all the three envelope proteins, while a 0.7mer construct was used to drive expression of L, M, and S proteins. This will avoid complications from amino acid changes in the P protein secondary to immune escape mutations, as the S region is completely overlapped by the P gene and some immune escape mutations alter P gene coding capacity. All the experiments were performed in Huh7 cells, a human hepatoma cell line with high transfection efficiency. Virions were concentrated from culture supernatant by immunoprecipitation with polyclonal anti-preS1 and anti-S antibodies, followed by DNA extraction and quantitative polymerase chain reaction (PCR). Ratio of extracellular virion DNA/intracellular replicative DNA was used as the virion secretion efficiency. Cells transfected with core-minus or P-minus mutant served as negative control for intracellular replicative DNA. They generated 4 logs lower HBV DNA signal from cell lysate (data not shown) suggesting efficient degradation of transfected plasmid DNA by our experimental procedure. Naked core particles were immunoprecipitated from culture supernatant after immunoprecipitation of virions, and HBV DNA inside core particles were also quantified by PCR. To separate the effect of mutation in the L and M proteins from that in the S protein, point mutations were introduced to the 0.7mer construct to render it capable of expressing L + M proteins or S protein only. Finally, to establish the impact of immune escape and glycosylation mutations on subviral particle secretion, HBsAg was measured from both cell lysate and culture supernatant.
2.1. The M133T mutation could improve virion secretion of T114R, T115A, K141E, and D144G mutants.
Consistent with our previous report (Kwei et al., 2013), virion secretion was impaired by the T114R, T115A, K141E, and D144G immune escape mutations introduced into the 0.7mer construct (Fig. 1E & F). Such impairment could be partially rescued by the additional M133T mutation. On the other hand, secretion of naked core particles was independent of the 0.7mer expression construct and unaffected by immune escape mutations (Fig. 1D). The M133T mutation creates a novel N-linked glycosylation site at N131. Thus, while the WT HBV and immune escape mutants such as T114R and T115A produced p24 (nonglycosylated S protein) and gp27 (singly glycosylated S protein), the T114R/M133T and T115A/M133T double mutants produced gp30 (doubly glycosylated S protein) as well (Fig. 1A).
Figure 1.
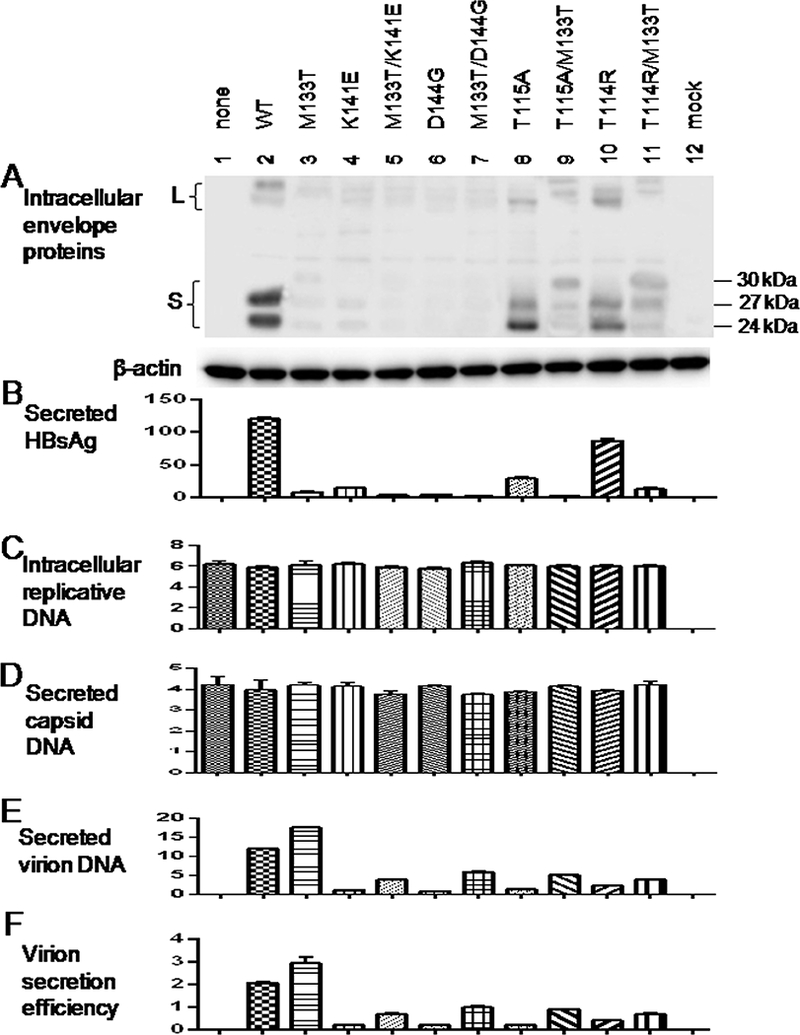
The M133T mutation could rescue virion secretion of several immune escape mutants. Huh7 cells seeded in 6-well plates were transfected with 1.5 μg of the L-/M-/S- 1.5mer replication construct with or without 0.5 μg of the L+/M+/S+ 0.7mer envelope protein expression construct for WT virus or an immune escape mutant. 0.7mer constructs with an additional M133T mutation were studied for comparison. Cells and culture supernatant were harvested at day 5 posttransfection. A: Western blot analysis of HBV envelope proteins in cell lysate using a polyclonal anti-HBs antibody. The 24kDa, 27kDa, and 30kDa species represent nonglycosylated, singly glycosylated, and doubly glycosylated forms of S protein. B: HBsAg in culture supernatant (IU/ml) as quantified by Abbott Architect i2000. C: Quantification of replicative HBV DNA in cell lysate (IU/ml, x107) by real-time PCR. D & E: Quantification of capsid DNA (D) and virion DNA (E) from culture supernatant (IU/ml, x105) by real-time PCR following immunoprecipitation of the two types of particles. F: Virion secretion efficiency (percentile) as the ratio of extracellular virion DNA/intracellular replicative DNA.
2.2. N-linked glycosylation sites other than N131 could rescue virion secretion of the G145R and N146Q mutants.
Besides M133T, immune escape mutations can be accompanied by other mutations creating novel N-linked glycosylation sites elsewhere (Chen et al., 2011; Wu et al., 2010; Yu et al., 2014). To characterize their biological properties, we generated 0.7mer expression construct containing T115N, G130N, 112NG113 (insertion of the NG dipeptide between residues 112 and 113; Fig. 2, top), or 114NT115 mutation (insertion of the NT dipeptide between residues 114 and 115). When co-transfected with the 1.5mer replication construct into Huh7 cells, the glycosylation mutants, especially M133T, supported more efficient virion secretion than the WT envelope proteins (Fig. 2E & F). The amount of secreted HBsAg according to Abbott Architect i2000 quantitative assay was rather reduced, especially for the M133T and 114NT115 mutants (Fig. 2B). Furthermore, we compared ability of the five novel glycosylation mutations to rescue virion secretion of the G145R or N146Q mutant in cis (i.e. with the 0.7mer construct harboring both G145R and T115N mutations, for example). The M133T mutation was most efficient at rescuing virion secretion of the G145R mutant, followed by G115N and G130N (Fig. 3E & F, left panel). The novel glycosylation sites could also rescue virion secretion of the N146Q mutant, with the efficiency being highest for the 114NT115 and M133T mutations (Fig. 3E & F, right panel).
Figure 2.
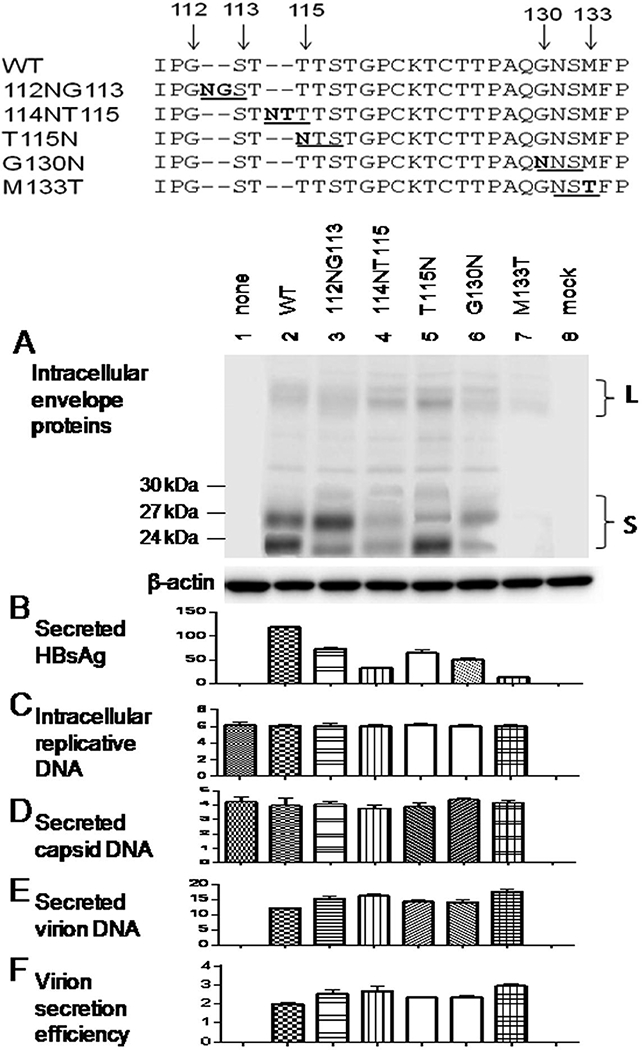
Impact of five novel N-linked glycosylation sites in the “a” determinant on HBsAg and virion secretion from WT HBV. The 112NG113 and 114NT115 insertion mutations as well as T115N, G130N, and M133T substitution mutations were introduced to the 0.7mer expression construct, which was co-transfected with 1.5mer replication construct at 0.5ug/1.5ug ratio into Huh7 cells grown in 6-well plates. Cells and culture supernatant were harvested at day 5 posttransfection. A: Western blot analysis of intracellular envelope proteins. B: Quantification of HBsAg released to culture supernatant. C-E: real-time PCR for intracellular replicative DNA (C), extracellular capsid DNA (D), and virion DNA (E). F: Calculated virion secretion efficiency.
Figure 3.
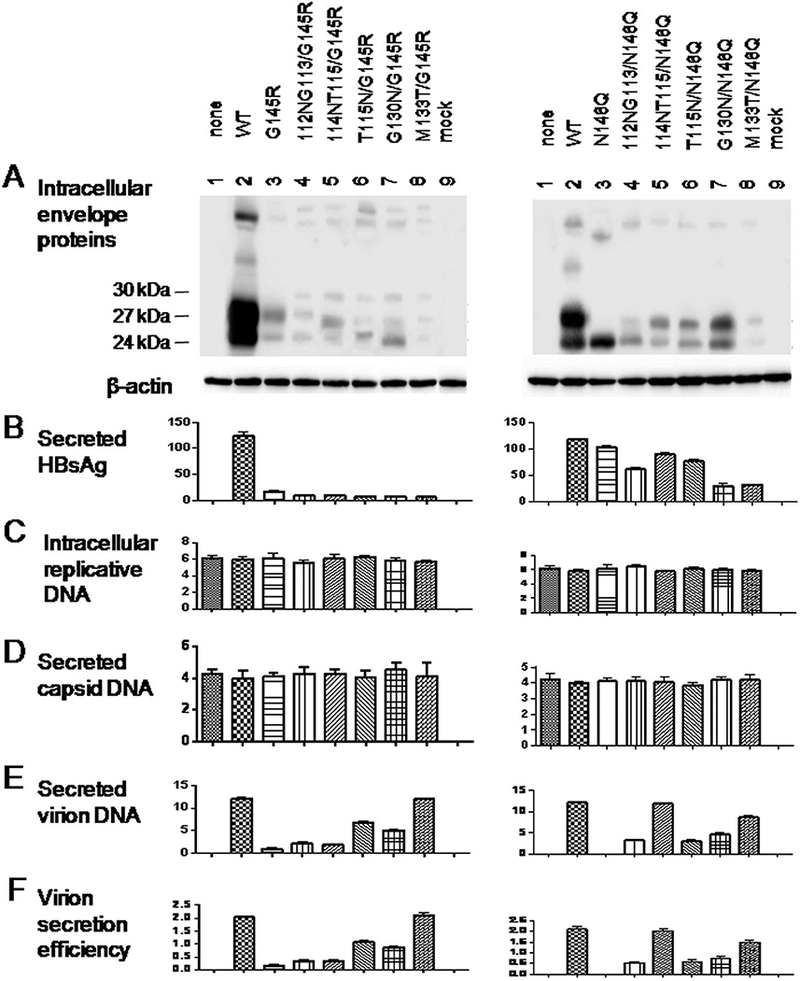
Impact of five N-linked glycosylation sites in the “a” determinant on HBsAg and virion secretion from G145R and N146Q mutants. The 112NG113, 114NT115, T115N, G130N, and M133T mutations were introduced to the 0.7mer construct already harboring the G145R or N146Q mutation. Such envelope protein constructs were co-transfected with the 1.5mer replication construct into Huh7 cells seeded in 6-well plates at 0.5ug/1.5ug ratio Cells and culture supernatant were harvested at day 5 posttransfection followed by analysis of intracellular envelope proteins (A), HBsAg secreted to culture supernatant (B), intracellular replicative DNA (C), extracellular capsid DNA (D) and virion DNA (E). Virion secretion efficiency was calculated (F).
2.3. Both G145R and N146Q mutations impaired virion secretion through the S protein rather than L/M proteins.
L and S proteins are required for virion formation or release (Bruss and Ganem, 1991; Garcia et al., 2009). L+/M+/S- and L-/M-/S+ 0.7mer constructs were generated to establish whether the G145R and N146Q mutations impair virion secretion through L, M, or S protein. Huh7 cells were co-transfected with 1.2 μg of the 1.5mer replication construct, 0.2 μg of the L+/M+/S- construct, and 0.8 μg of the L-/M-/S+ construct. It turned out that the L/M proteins of the G145R or N146Q mutant supported efficient virion secretion if co-expressed with WT S protein (Fig. 4E & F, lane 2, left and right panels). Conversely, the S protein harboring such mutations failed to reconstitute virion secretion in conjunction with WT L/M proteins (lane 3, left and right panels).
Figure 4.
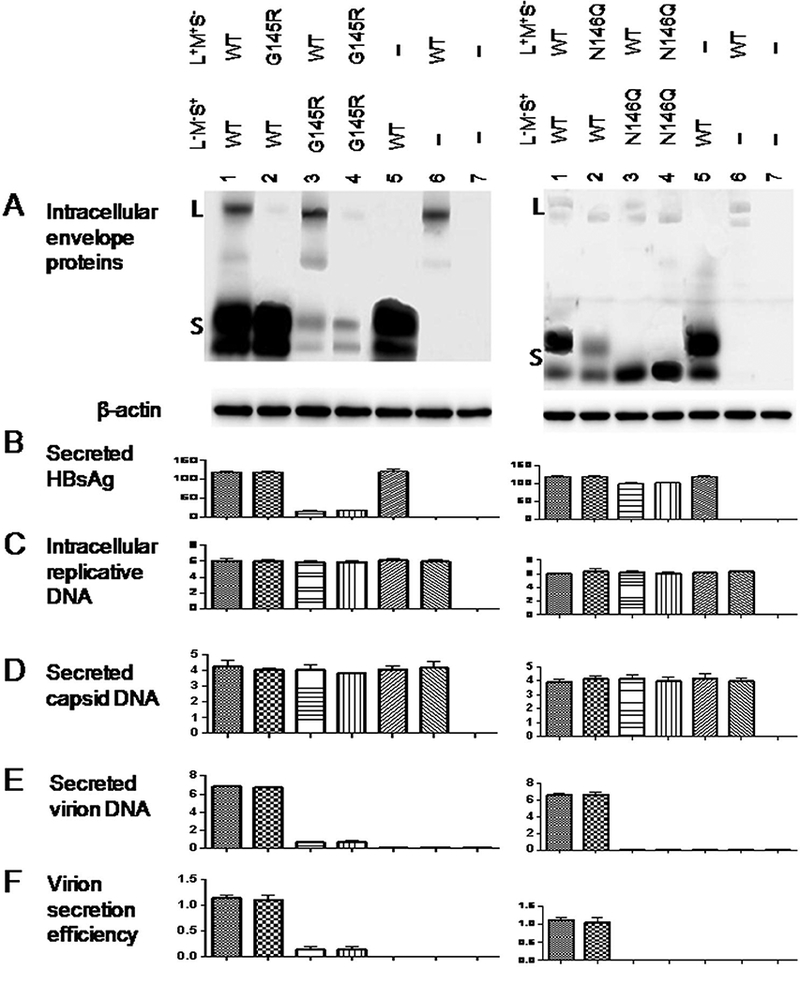
The G145R and N146Q mutations impair virion secretion through the S protein. Huh7 cells seeded in 6-well plates were cotransfected with 1.2 μg of the 1.5mer replication construct, 0.2 μg of the 0.7mer L+/M+/S- construct of either WT virus or the G145R (or N146Q) mutant, and 0.6 pg of the 0.7mer L-/M-/S+ construct of WT virus or the G145R (or N146Q) mutant. Four different combinations were used, and cells receiving just the L+/M+/S- construct or the L-/M-/S+ construct served as negative controls of virion secretion. Cells and culture supernatant were harvested at day 5 posttransfection. A: Western blot analysis of intracellular envelope proteins. B: Quantification of HBsAg released to culture supernatant. C - E: real-time PCR for intracellular replicative DNA (C), extracellular capsid DNA (D) and virion DNA (E). F: Calculated virion secretion efficiency.
2.4. The M133T mutation rescued virion secretion of the G145R or N146Q mutant in cis through the S protein.
To establish whether M133T rescues virion secretion of the G145R and N146Q mutants through L/M proteins or the S protein, it was introduced to the L+/M+/S- construct or the L-/M-/S+ construct of the G145R (or N146Q) mutant. Presence of the M133T mutation in only the S expressing construct of the G145R mutant restored virion secretion as efficiently as its presence in both the S expressing and L/M expressing constructs (Fig. 5E & F, left panel, compare lanes 4 and 3). In contrast, presence of the M133T mutation in the L/M expressing construct alone failed to rescue virion secretion (lane 5). Similar findings were made for the N146Q mutant (Fig. 5E & F, right panel).
Figure 5.
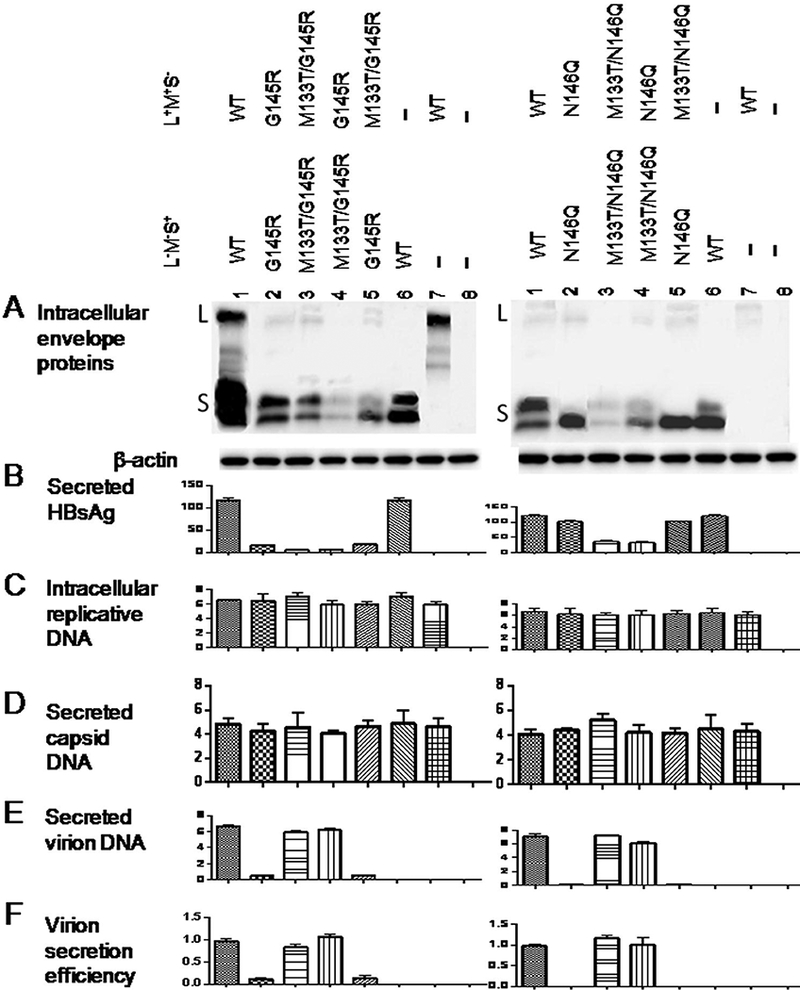
The M133T mutant could rescue virion secretion of the G145R or N146Q mutant in cis through the S protein. Huh7 cells seeded in 6-well plates were cotransfected with 1.2 μg of the 1.5mer replication construct, 0.2 μg of the 0.7mer L+/M+/S- construct, and 0.6 μg of the 0.7mer L-/M-/S+ construct. The M133T mutation accompanied the G145R (or N146Q) mutation either on the L+/M+/S- construct, or the L-/M-/S+ construct. Cells receiving just the L+/M+/S- construct or the L-/M-/S+ construct served as negative controls of virion secretion. A: Western blot analysis of intracellular envelope proteins. B: Quantification of HBsAg released to culture supernatant. C - E: real-time PCR for intracellular replicative DNA (C), extracellular capsid DNA (D) and virion DNA (E). F: Virion secretion efficiency.
2.5. Virion secretion of both G145R and N146Q mutants could be rescued in trans by S protein of WT HBV and especially the M133T mutant.
In another approach, the L+/M+/S+ 0.7mer construct of the G145R or N146Q mutant was co-transfected with the L+/M+/S+, L+/M+/S-, or L-/M-/S+ 0.7mer construct of WT HBV or the M133T mutant. This allowed us to establish whether WT virus or the M133T mutant could rescue virion secretion in trans, and if so whether through L/M proteins or the S protein. As shown in Fig. 6E & F, left panel, virion secretion of the G145R mutant could be rescued by L+/M+/S+ 0.7mer construct of the WT HBV, but more efficiently by the M133T mutant (lanes 3 & 4). Virion secretion could also be rescued by the corresponding L-/M-/S+ construct (lanes 7 & 8), but not the L+/M+/S- construct (lanes 5 & 6). Similarly, the L-/M-/S+ rather than L+/M+/S- 0.7mer construct of the WT HBV and especially the M133T mutant rescued virion secretion of the N146Q mutant (Fig. 6E & F, right panel).
Figure 6.
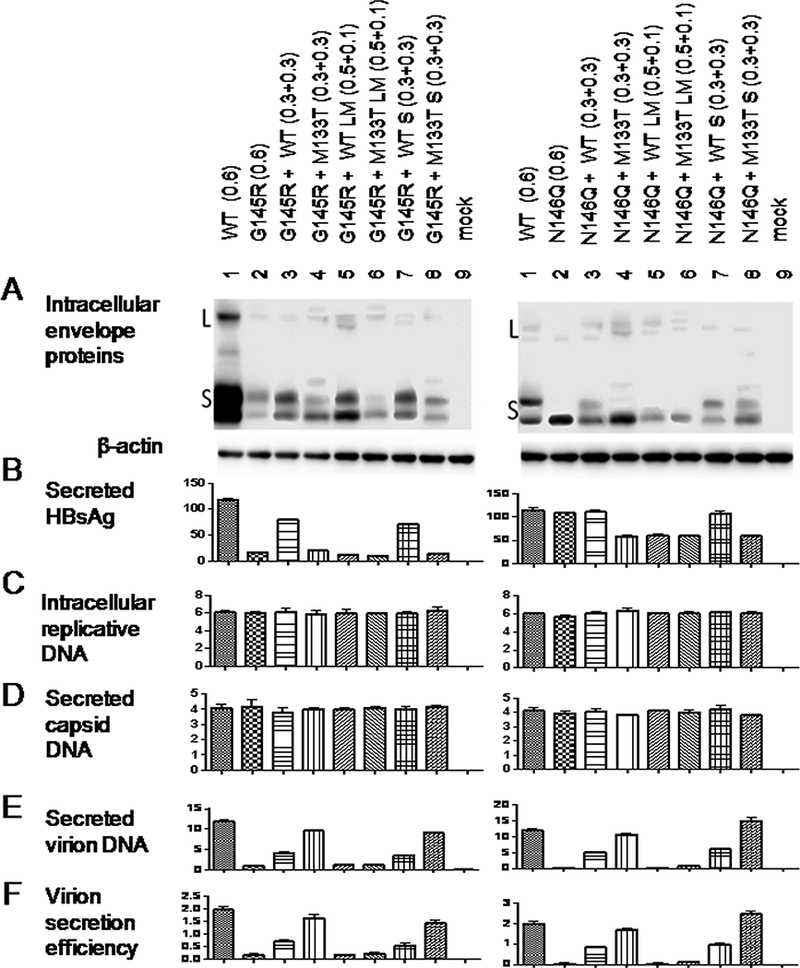
WT HBV and especially the M133T mutant could rescue virion secretion of the G145R or N146Q mutant in trans through the S protein. Huh7 cells were transfected with 1.4 μg of the 1.5mer replication construct together with 1) 0.6ug of the L+/M+/S+ 0.7mer expression construct for the G145R (or N146Q) mutant; 2) 0.3ug of the G145R (or N146Q) mutant together with 0.3ug of the WT virus or the M133T mutant; 3) 0.5ug of the G145R (or N146Q) mutant together with 0. lug of the L+/M+/S- construct of WT virus or the M133T mutant; 4) 0.3ug of the G145R (or N146Q) mutant together with 0.3ug of the L-/M-/S+ construct of WT virus or the M133T mutant. Cells and culture supernatant were harvested at day 5 posttransfection followed by analysis of intracellular envelope proteins (A), HBsAg secreted to culture supernatant (B), intracellular replicative DNA (C), extracellular capsid DNA (D) and virion DNA (E). Virion secretion efficiency was calculated (F).
2.6. Impact of immune escape mutations and N-linked glycosylation on the ratio of extracellular HBsAg/intracellular HBsAg.
Findings described above clearly indicate that both immune escape mutations and N-linked glycosylation modulate virion secretion through the S protein. Considering that the S protein also drives the secretion of subviral particles, one may wonder how secretion of subviral particles is affected by the immune escape mutations and N-linked glycosylation. Also, immune escape mutations may impair detection by anti-S antibodies raised against WT HBV, and extra N-linked glycosylation could mask the antigenic sites. Thus, the K141E and D144G mutants were associated with extremely low HBsAg titer in culture supernatant according to Abbott Architect i2000 assay (Fig. 1B). Similar effect was observed for mutants creating extra glycosylation sites, especially M133T (Fig. 2B). However, ELISA using a commercial kit (KHB, Shanghai, China) revealed less dramatic reduction of HBsAg titer in culture supernatant for these mutants, especially the D144G mutant (Fig. 7B). By simultaneous detection of HBsAg in cell lysate and culture supernatant using this ELISA kit, we found that HBsAg secretion (as defined by ratio of cextracellular HBsAg/intracellular HBsAg) was severely impaired by the C138Y, R169L, R169P, and C149R mutations (Fig. 7C). The K141E, D144G, and G145R mutants showed little or mild reduction in HBsAg secretion despite near loss of virion secretion. Mutations that abolish N-linked glycosylation (N146Q and N146S) or create an extra glycosylation site (M133T) did not markedly affect HBsAg secretion (Fig. 7C).
Figure 7.
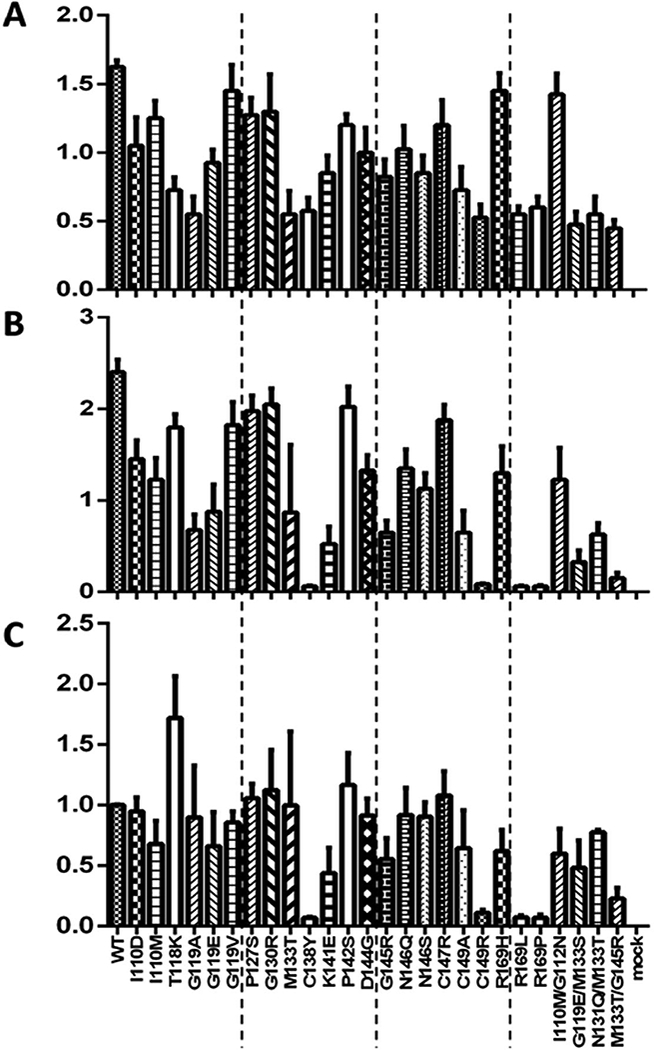
Comparison of HBsAg secretion efficiency among 26 immune escape or glycosylation mutants. Huh7 cells grown in 6-well plates were transfected with 2.0 μg of the 0.7mer L+/M+/S+ construct. Both cells and culture supernatant were harvested 5 days later. HBsAg was measured from 20 ul of 1:1000 diluted cell lysate, and 25 ul of 1:100 diluted culture supernatant, using an ELISA kit from KHB. The experiments were repeated four times. (A) HBsAg (OD450) values in cell lysate. (B) HBsAg (OD450) values in culture supernatant. (C) Ratio of extracellular HBsAg/intracellular HBsAg, with value for the WT virus set arbitrarily at 1.
3. Discussion
All the three HBV envelope proteins can be glycosylated at N146 inside the S domain. The M protein, which is further glycosylated at N4 in the preS2 domain, is dispensable for HBV virion production (Bruss and Ganem, 1991; Garcia et al., 2009). We previously reported that abolishing N-linked glycosylation in HBV envelope proteins by either N146Q or N146S mutation prevented HBV virion secretion (Ito et al., 2010). Introducing the M133T mutation could rescue virion secretion for both N146Q and N146S mutants, suggesting that N-linked glycosylation is essential for HBV virion secretion although the glycans can be attached to a site other than residue 146 (Ito et al., 2010). In the present study we used separate expression constructs for L/M proteins and S protein to demonstrate that N146 glycosylation in S protein, rather than L or M protein, is essential for HBV virion secretion (Fig. 4). M133T mutation in the S rather than L or M protein rescued virion secretion of the N146Q mutant (Fig. 5). Similarly, introduction of the M133T mutation into the S protein construct alone sufficed to rescue virion production of the G145R mutant (Fig. 5). Furthermore, mixed expression of L/M/S proteins of the G145R or N146Q mutant with just S protein of WT virus or the M133T mutant rescued virion secretion (Fig. 6). Taken together, these results strongly suggest that both immune escape mutations and N-linked glycosylation (at position 146 or 131) modulate HBV virion secretion through the S protein. Consistent with these findings, we previously reported that naturally occurring I110M, G119E, and R169P mutations inside the S domain impaired virion secretion through the S rather than L or M protein (Ito et al., 2010). Moreover, the M133T mutation in the S but not L/M proteins rescued virion secretion of the I110M mutant. We further demonstrated that ability of the M133T mutation to rescue virion secretion of the I110M, G119E, and G145R mutants was abrogated by an additional N131Q or N131T mutation indicating that glycosylation at N131, rather than M133T mutation itself, restored virion secretion (Ito et al., 2010).
The M133T mutation could efficiently rescue virion secretion of the I110M, T118K, G119E, P127S, G145R, and R169H mutants to nearly WT level, and moderately improve virion secretion of the G130R, C138Y, C147R, and C149R mutants (Ito et al., 2010; Kwei et al., 2013). In the present study, we demonstrated its ability to moderately rescue virion secretion of the T114R, T115A, K141E, and D144G mutants as well (Fig. 1). Thus, M133T is a versatile compensatory mutation for virion secretion of a large number of immune escape mutants. In the current work we also performed limited study on four additional mutants creating novel N-linked glycosylation sites: T115N, G130N, 112NG113, and 114NT115. As shown in Fig. 3, the 114NT115 insertion was very effective at rescuing virion secretion of the N146Q mutant, but none of the mutations rescued virion secretion of the G145R mutant as efficiently as M133T. In our previous study, a G112N mutation (creating a glycosylation site at residue 112) partially rescued virion secretion of the G119E mutant but failed to rescue virion secretion of the G145R mutant (Ito et al., 2010). Thus, while different N-linked glycosylation sites were selected in chronic carriers of HBV as described in a Chinese cohort (Yu et al., 2014), their ability to rescue virion secretion of different immune escape mutants appears highly variable. Whether the rescue depends on the efficiency of glycosylation at the new site and/or its location relative to the immune escape mutation warrants further investigation.
Immune escape mutations such as G145R reduce the affinity of the mutant envelope proteins for anti-HBs antibody (Chiou et al., 1997; Cooreman et al., 1999; Waters et al., 1992), thus leading to diagnostic failure, occult HBV infection, vaccine failure and breakthrough infection despite presence of neutralizing antibodies against WT S domain. Since in the present study polyclonal or monoclonal antibodies against WT virus were used for Western blot detection of S protein, ELISA and Abbott Architect i2000 quantification of HBsAg, and immunoprecpitation of virions, caution should be exercised in data interpretation for the immune escape mutants and N-linked glycosylation mutants. Indeed, with the Abbott Architect i2000 assay, the K141E, D144G, and G145R escape mutants as well as M133T glycosylation mutant were associated with very little HBsAg in culture supernatant (Fig. 1B, Fig. 3B). Similarly, Western blot analysis revealed very little intracellular S protein for the K141E, D144G, G145R, and M133T mutants (panel A in Figs. 1–6). Very likely antibody binding was severely hampered by the immune escape mutations or by the extra glycans, although reduced S protein stability cannot be excluded. Since a combination of polyclonal anti-S and anti-preS1 antibodies were used to immunoprecipitate virions prior to quantitative PCR analysis of virion DNA, differential binding to the anti-S antibodies might lead to underestimation of the virion secretion efficiency of some immune escape mutants. The problem could be more severe in HBsAg detection by ELISA, in which monoclonal rather than polyclonal antibodies were used. We therefore calculated the ratio of extracellular HBsAg/intracellular HBsAg as a marker of subviral particle secretion (Fig. 7).
Since the S protein drives secretion of both virions and subviral particles, immune escape (and glycosylation) mutations probably impair the secretion of both types of particles at a shared step in particle morphogenesis or egress. However, HBV virions are secreted through the ESCRT/MVB (endosomal sorting complex required for transport/multivesicular bodies) machinery, in contrast to the constitutive secretory pathway implicated in release of subviral particles (Lambert et al., 2007; Patient et al., 2009; Patient et al., 2007; Watanabe et al., 2007). For the glycosylation mutants, both N146Q and N146S continued to secrete HBsAg efficiently despite blocked virion release (Fig. 3B, Fig. 7B) (Ito et al., 2010). We found that HBsAg secretion (ratio of extracellular HBsAg/intracellular HBsAg) was severely impaired by C138Y, C149R, R169L, and R169P mutants (Fig. 7C). These mutants are also completely defective in virion secretion (Ito et al., 2010; Kwei et al., 2013). Of the other immune escape mutants with severely impaired virion secretion, I110M, G145R, and K141E mutants showed mild to moderate reduction in HBsAg secretion, while G119V, G130R, and C147R mutants had more or less WT efficiency of HBsAg secretion (Fig. 7C). The T118K mutant actually had increased HBsAg release. While virion secretion was restored in the M133T/G145R double mutant in comparison with the G145R mutant, HBsAg secretion was rather reduced in the double mutant than G145R single mutant (Fig. 7C). Therefore, except for the C138Y, C149R, R169L, and R169P mutants, virion secretion phenotype of the immune escape mutants and glycosylation mutants did not correlate with the phenotype of subviral particle release.
The M133T/G145R double mutant did not display higher HBsAg titer in either cell lysate or culture supernatant than either G145R or M133T mutant (Fig. 7A and 7B). Therefore, adding the M133T mutation most likely does not alter the immune escape property of the G145R mutant. This remains to be verified through neutralization experiments using cell culture or small animal models of HBV infection. The serious concern is: will vaccine escape mutants such as G145R with restored virion secretion capacity through M133T mutation gradually replace the WT virus and eventually make the current HBV vaccine obsolete (Shouval and Locarnini, 2012; Teo and Locarnini, 2010)?
4. Materials and methods
4.1. HBV DNA constructs and site-directed mutants.
As previously described, co-transfection between a 1.5mer replication construct and a 0.7mer expression construct for envelope proteins was used to restore virion secretion (Ito et al., 2010; Kwei et al., 2013). The 1.5-mer HBV construct has a 4.8-kb HBV DNA fragment (nucleotides 1044 – 3221/1 – 2600) cloned to the pBluescript vector. It was rendered unable to express envelope proteins by mutation of the S gene start codon and conversion of codon 36 into a stop codon. Consequently the L-/M-/S- 1.5mer construct could drive HBV genome replication but not virion formation. The 0.7mer construct has a 2.3-kb fragment covering nucleotides 2721 – 3221/1 – 1770 followed by the SV40 poly A signal cloned into the pBluescript vector. It could express all the three envelope proteins (L+/M+/S+) under endogenous promoters and enhancers, thus driving HBsAg production. For some experiments, we rendered the 0.7mer construct unable to express S protein (L+/M+/S-) by mutating its start codon into GCG, or unable to express L and M proteins (L-/M-/S+) by converting the 43rd codon of the pre-S2 region into TAG. Mutations creating novel N-linked glycosylation, such as 112NG113, 114NT115, T115N, and G130N, were introduced to the 0.7mer construct by ClonExpress MultiS kit (Vazyme, China). DNA constructs were purified by the High-speed Plasmid Midi Kit (Macherey-Nagel, Duren, Germany), followed by phenol and chloroform extraction.
4.2. Transient co-transfection and measurement of secreted HBsAg.
The human hepatoma cell line Huh7 was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum. Transient transfection was performed on cells seeded in 6-well plates using Lipofectamine 3000 reagent (Invitrogen). In most experiments cells were cotransfected with 1.5 μg of the 1.5mer replication construct and 0.5 μg of the 0.7mer construct. Cells and culture supernatant were harvested at day 5 posttransfection. HBsAg in culture supernatant were quantified on Abbott Architect i2000 by customer service at Adicon Clinical Laboratories, Inc., China.
4.3. Western blot analysis of intracellular envelope proteins
Cells from one well of 6-well dishes were lysed in 80μ1 of lysis buffer (10 mM Hepes pH7.5, 100 mM NaCl, 1 mM EDTA, and 1% NP40), and l/8th of the cell lysate was subjected to Western blot analysis. Proteins were separated by electrophoresis in SDS-12% polyacrylamide gel. Following transfer, the blot was incubated at 4°C overnight with a 1:4000 dilution of rabbit polyclonal anti-HBs antibody (Novus) (Zhang et al., 2017). After further incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody at 1:10,000 dilution, signals were revealed by enhanced chemiluminescence (PerkinElmer) and visualized by chemiluminescent imaging system (Tanon). The antibodies were removed by the stripping buffer (CWBio), and the blot was incubated sequentially with mouse anti-actin antibody (Proteintech) (1:3000 dilution) and HRP-conjugated goat antimouse antibody (1:10,000 dilution).
4.4. Extraction of replicative HBV DNA from cell lysate and virion DNA from culture supernatant
Established protocol was followed to extract replicative DNA associated with core particles (Ito et al., 2010; Kwei et al., 2013; Qin et al., 2011). Half of the lysate from 6-well plates was supplemented with 10 mM CaCl2, 12 mM MgCl2, and digested at 37°C for 20 min with DNase I (3U) and mung bean nuclease (7.5U) to degrade transfected plasmid DNA. Core particles were precipitated with polyethylene glycol (PEG)/sucrose solution (1.2M NaCl, 60mM EDTA, 26% PEG, 30% sucrose), resuspended in 50 μl of solution containing 10 mM Tris (pH 7.5), 6 mM MgCl2, 8 mM CaCl2, and digested with 3 U of DNase I and 3 U of mung bean nuclease at 37°C for 20 min to further degrade transfected DNA. After the addition of 135 μl of protease K buffer (25 mM Tris [pH 7.5], 10 mM EDTA, 100 mM NaCl, 0.5% SDS), samples were digested at 37°C for 2 h with 0.5mg/ml of proteinase K. DNA was extracted with phenol, precipitated with ethanol, and dissolved in water for real-time PCR.
Virions were immunoprecipitated from 1.3 ml of precleared culture supernatant by a mixture of custom-made polyclonal rabbit anti-preS1 antibody (Genscript, Nanjing, China) (3 μl) and rabbit anti-HBs antibody (Novus) (1 μL) precojugated to 10 μl of protein G-agarose beads (BioVision, USA). The preS1 antibody targets residues 12 – 46 (MGTNLSVPNPLGFFPDHQLDPAFGANSNNPDWDFN) (Chen et al., 2016; Li et al., 2016). After wash with PBS, the precipitate was dissolved in 50 ul of 10 mM Tris (pH 7.5)- 6 mM MgCl2-8 mM CaCl2 solution for nuclease digestion followed by proteinase K digestion as described above for cell lysate. DNA was extracted with phenol and precipitated with ethanol. Naked core particles were immunoprecipitated from culture supernatant with a rabbit anti-HBc antibody from Dako. The subsequent steps were similar to those for virions.
4.5. Quantification of replicative HBV DNA and virion DNA
DNA associated with extracellular virions, extracellular naked core particles, and intracellular replicative DNA were quantified by TaqMan real-time PCR (Applied Bio Systems, 7500) at Adicon Clinical Laboratories, Inc. The PCR primers were sense (position 218–240) 5’-TTG ACA AGA ATC CTC ACA ATA CC-3’ and antisense (position 309– 328) 5’-GGA GGT TGG GGA CTG CGA AT-3’. HBV DNA was expressed as international units (IU)/ml.
4.6. Impact of immune escape and glycosylation mutations on HBsAg secretion
Huh7 cells seeded in 6-well plates were transfected with 2 μg of 0.7mer L+/M+/S+ expression construct using Lipofectamine 3000 reagent. Cells and culture supernatant were harvested 5 days post-transfection. Cells were lysed in 80 ul of lysis buffer, and 20 ul of 1: 1000 diluted sample was used for measurement of HBsAg by an enzyme-linked immunosorbent assay (ELISA) using a commercial kit (KHB, Shanghai, China). HBsAg was also measured from 25 ul of 1:100 diluted culture supernatant. The ratio of extracellular HBsAg/intracellular HBsAg was calculated to serve as an indicator for the efficiency of HBsAg secretion. The ratio shown in Fig. 7 originated from four independent transfection experiments.
4.7. Statistical analysis
Each experiment was repeated at least 3 times and the mean value was calculated. Quantitative variables are expressed as the mean ± standard deviation. Statistical analysi was performed by GraphPad Prism 6. One-way ANOVA was chosen for comparing the means. P values <0.05 were considered as statistically significant.
Highlights.
Novel glycosylation sites rescue virion secretion of G145R and N146Q mutants.
G145R, N146Q, and M133T mutations modulate virion secretion through S protein.
Many immune escape mutants are not impaired in secretion of subviral particles.
Acknowledgement
This study was supported by NIH grant AI116639, and also by grants 81371822, 31370195, and 81672064 from National Science Foundation of China.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bian T, Yan H, Shen L, Wang F, Zhang S, Cao Y, Zhang S, Zhang Y, Bi S, 2013. Change in hepatitis B virus large surface antigen variant prevalence 13 years after implementation of a universal vaccination program in China. J. Virol 87, 12196–12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss V, Ganem D, 1991. The role of envelope proteins in hepatitis B virus assembly. Proc. Natl. Acad. Sci. USA 88, 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman WF, Trautwein C, van Deursen J, Colman K, Dornan E, McIntyre G, Waters J, Kliem V, Müller R, Thomas HC, 1996. Hepatitis B virus envelope variation after transplantation with and without hepatitis B immune globulin prophylaxis. Hepatology 24, 489–493. [DOI] [PubMed] [Google Scholar]
- Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman AJ, Thomas HC, 1990. Vaccine-induced escape mutant of hepatitis B virus. Lancet 336, 325–329. [DOI] [PubMed] [Google Scholar]
- Chen C, Jia H, Zhang F, Qin Y, Zong L, Yuan Q, Wang Y, Xia N, Li J, Wen Y, Tong S, 2016. Functional characterization of hepatitis B virus core promoter mutants revealed transcriptional interference among co-terminal viral mRNAs. J. Gen. Virol 97, 2668–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Qian F, Yuan Q, Li X, Wu W, Guo X, Li L, 2011. Mutations in hepatitis B virus DNA from patients with coexisting HBsAg and anti-HBs. J. Clin. Virol 52, 198–203. [DOI] [PubMed] [Google Scholar]
- Chiou H-L, Lee T-S, Kuo J, Mau Y-C, Ho M-S, 1997. Altered antigenicity of a’determinant variants of hepatitis B virus. J. Gen. Virol 78, 2639–2645. [DOI] [PubMed] [Google Scholar]
- Cooreman MP, van Roosmalen MH, te Morsche R, Sünnen CM, de Ven EM, Jansen JB, Tytgat GN, de Wit PL, Paulij WP, 1999. Characterization of the reactivity pattern of murine monoclonal antibodies against wild-type hepatitis B surface antigen to g145r and other naturally occurring “a” loop escape mutations. Hepatology 30, 1287–1292. [DOI] [PubMed] [Google Scholar]
- Garcia T, Li J, Sureau C, Ito K, Qin Y, Wands J, Tong S, 2009. Drastic reduction in the production of subviral particles does not impair hepatitis B virus virion secretion. J. Virol 83, 11152–11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghany MG, Ayola B, Villamil FG, Gish RG, Rojter S, Vierling JM, Lok AS, 1998. Hepatitis B virus S mutants in liver transplant recipients who were reinfected despite hepatitis B immune globulin prophylaxis. Hepatology 27, 213–222. [DOI] [PubMed] [Google Scholar]
- Hollinger F, Sood G, 2010. Occult hepatitis B virus infection: a covert operation. J. Viral Hepat 17, 1–15. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Chang MH, Ni YH, Chen HL, 2004. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut 53, 1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Yuan Q, Chen PJ, Zhang YL, Chen CR, Zheng QB, Yeh SH, Yu H, Xue Y, Chen YX, Liu PG, Ge SX, Zhang J, Xia N, 2012. Influence of mutations in hepatitis B virus surface protein on viral antigenicity and phenotype in occult HBV strains from blood donors. J. Hepatol 57, 720–729. [DOI] [PubMed] [Google Scholar]
- Ito K, Qin Y, Guarnieri M, Garcia T, Kwei K, Mizokami M, Zhang J, Li J, Wands JR, Tong S, 2010. Impairment of hepatitis B virus virion secretion by single-amino-acid substitutions in the small envelope protein and rescue by a novel glycosylation site. J. Virol 84, 12850–12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeantet D, Chemin I, Mandrand B, Tran A, Zoulim F, Merle P, Trepo C, Kay A, 2004. Cloning and expression of surface antigens from occult chronic hepatitis B virus infections and their recognition by commercial detection assays. J. Med. Virol 73, 508–515. [DOI] [PubMed] [Google Scholar]
- Julithe R, Aboujaoude G, Sureau C, 2014. Modification of the hepatitis B virus envelope protein glycosylation pattern interferes with secretion of viral particles, infectivity, and susceptibility to neutralizing antibodies. J. Virol 88, 9049–9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina T, Iwanski A, Will H, Sterneck M, 2003. Deficiency in virion secretion and decreased stability of the hepatitis B virus immune escape mutant G145R. Hepatology 38, 1274–1281. [DOI] [PubMed] [Google Scholar]
- Khan N, Guarnieri M, Ahn SH, Li J, Zhou Y, Bang G, Kim K-H, Wands JR, Tong S, 2004. Modulation of hepatitis B virus secretion by naturally occurring mutations in the S gene. J. Virol 78, 3262–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwei K, Tang X, Lok AS, Sureau C, Garcia T, Li J, Wands J, Tong S, 2013. Impaired virion secretion by hepatitis B virus immune escape mutants and its rescue by wild-type envelope proteins or a second-site mutation. J. Virol 87, 2352–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Döring T, Prange R, 2007. Hepatitis B virus maturation is sensitive to functional inhibition of ESCRT-III, Vps4, and γ2-Adaptin. J. Virol 81, 9050–9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li Z, Sureau C, Barker L, Wands JR, Tong S, 2016. Unusual features of sodium taurocholate cotransporting polypeptide as a hepatitis B virus receptor. J. Virol 90, 8302–8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G, Ehrlich PH, Moustafa ZA, McCarthy LA, Dottavio D, Tolpin MD, Nadler PI, Ostberg L, 1992. Genetic alterations in the gene encoding the major HBsAg: DNA and immunological analysis of recurrent HBsAg derived from monoclonal antibody-treated liver transplant patients. Hepatology 15, 757–766. [DOI] [PubMed] [Google Scholar]
- Minuk GY, Sun D. f., Uhanova J, Zhang M, Caouette S, Nicolle LE, Gutkin A, Doucette K, Martin B, Giulivi A, 2005. Occult hepatitis B virus infection in a North American community-based population. J. Hepatol 42, 480–485. [DOI] [PubMed] [Google Scholar]
- Patient R, Hourioux C, Roingeard P, 2009. Morphogenesis of hepatitis B virus and its subviral envelope particles. Cell. Microbiol 11, 1561–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient R, Hourioux C, Sizaret PY, Trassard S, Sureau C, Roingeard P, 2007. Hepatitis B virus subviral envelope particle morphogenesis and intracellular trafficking. J. Virol 81, 3842–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzer- Knolle U, Naumann U, Bartenschlager R, Berg T, Hopf U, Meyer zum Büschenfelde KH, Neuhaus P, Gerken G, 1998. Hepatitis B virus with antigenically altered hepatitis B surface antigen is selected by high- dose hepatitis B immune globulin after liver transplantation. Hepatology 27, 254–263. [DOI] [PubMed] [Google Scholar]
- Qin Y, Tang X, Garcia T, Hussain M, Zhang J, Lok A, Wands J, Li J, Tong S, 2011. Hepatitis B virus genotype C isolates with wild-type core promoter sequence replicate less efficiently than genotype B isolates but possess higher virion secretion capacity. J. Virol 85, 10167–10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo G, Pollicino T, Cacciola I, Squadrito G, 2007. Occult hepatitis B virus infection. J. Hepatol 46, 160–170. [DOI] [PubMed] [Google Scholar]
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ, 2015. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 386, 1546–1555. [DOI] [PubMed] [Google Scholar]
- Shahmoradi S, Yahyapour Y, Mahmoodi M, Alavian SM, Fazeli Z, Jazayeri SM, 2012. High prevalence of occult hepatitis B virus infection in children born to HBsAg- positive mothers despite prophylaxis with hepatitis B vaccination and HBIG. J. Hepatol 57, 515–521. [DOI] [PubMed] [Google Scholar]
- Shields P, Owsianka A, Carman W, Boxall E, Hubscher S, Shaw J, O’Donnell K, Elias E, Mutimer D, 1999. Selection of hepatitis B surface “escape” mutants during passive immune prophylaxis following liver transplantation: potential impact of genetic changes on polymerase protein function. Gut 45, 306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval D, Locarnini S, 2012. Increased prevalence of HBV envelope mutants in Taiwan: an emerging public health risk or a false alarm? Gastroenterology 143, 290–293. [DOI] [PubMed] [Google Scholar]
- Teo CG, Locarnini SA, 2010. Potential threat of drug-resistant and vaccine-escape HBV mutants to public health. Antivir. Ther 15, 445–449. [DOI] [PubMed] [Google Scholar]
- Terrault NA, Zhou S, McCory RW, Pruett TL, Lake JR, Roberts JP, Ascher NL, Wright TL, 1998. Incidence and clinical consequences of surface and polymerase gene mutations in liver transplant recipients on hepatitis B immunoglobulin. Hepatology 28, 555–561. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Sorensen EM, Naito A, Schott M, Kim S, Ahlquist P, 2007. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc Natl Acad Sci U S A 104, 10205–10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Kennedy M, Voet P, Hauser P, Petre J, Carman W, Thomas H, 1992. Loss of the common” a” determinant of hepatitis B surface antigen by a vaccine- induced escape mutant. J. Clin. Invest 90, 2543–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger KM, Bauer T, Bohm S, Jilg W, 2000. High genetic variability of the group-specific a-determinant of hepatitis B virus surface antigen (HBsAg) and the corresponding fragment of the viral polymerase in chronic virus carriers lacking detectable HBsAg in serum. J. Gen. Virol 81, 1165–1174. [DOI] [PubMed] [Google Scholar]
- Wu C, Zhang X, Tian Y, Song J, Yang D, Roggendorf M, Lu M, Chen X, 2010. Biological significance of amino acid substitutions in hepatitis B surface antigen (HBsAg) for glycosylation, secretion, antigenicity and immunogenicity of HBsAg and hepatitis B virus replication. J. Gen. Virol 91, 483–492. [DOI] [PubMed] [Google Scholar]
- Yu D-M, Li X-H, Mom V, Lu Z-H, Liao X-W, Han Y, Pichoud C, Gong Q-M, Zhang DH, Zhang Y, Deny P, Zoulim F, Zhang XX, 2014. N-glycosylation mutations within hepatitis B virus surface major hydrophilic region contribute mostly to immune escape. J. Hepatol 60, 515–522. [DOI] [PubMed] [Google Scholar]
- Zaaijer H, Torres P, Ontanon A, Ponte LG, Koppelman M, Lelie P, Van Hemert F, Boot H, 2008. Multiple surface antigen mutations in five blood donors with occult hepatitis B virus infection. J. Med. Virol 80, 1344–1349. [DOI] [PubMed] [Google Scholar]
- Zhang F, Tang X, Garcia T, Lok AS, Wang Y, Jia H, Qin Y, Chen C, Wen Y, Li J, 2017. Characterization of contrasting features between hepatitis B virus genotype A and genotype D in small envelope protein expression and surface antigen secretion. Virology 503, 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


