Abstract
Neurotoxicity associated with CAR-T cell therapy can be life-threatening. With rapid development of CAR-T therapies, a systematic method is needed to identify and monitor symptoms of neurotoxicity, elucidate potential etiologies, and compare toxicity across trials. This paper presents a systematic evaluation developed and used to prospectively assess neurotoxicity in our Phase I anti-CD22 CAR-T cell trial and describes the symptoms of neurotoxicity identified using this methodology. Central nervous system (CNS) studies included routine lumbar punctures performed for disease evaluation pre- and post-therapy and a baseline brain MRI. Brief cognitive evaluations, assessing four domains (attention, working memory, cognitive flexibility, and processing speed), were administered pre- and post-infusion. A newly-developed CAR-T-specific neurologic symptom checklist (NSC) was completed by caregivers at three designated time-points. Serial serum cytokine levels were compared with neurotoxicity symptoms and severity. The majority of the first twenty-two consecutively-treated subjects (ages 7-30) demonstrated stable or improved cognitive test scores following therapy and no irreversible neurotoxicity, despite CAR-T related anti-leukemic response, cytokine release syndrome, and trafficking of CAR-T cells to the CSF. The NSC allowed us to document the type and timing of symptoms and explore the etiology of neurotoxicity associated with CD22 CAR-T therapy. Cytokine profiling demonstrated that more concerning symptoms of neurotoxicity, such as hallucination and disorientation, were significantly associated with higher serum cytokine levels, supporting the hypothesis of inflammation-driven neurotoxicity. Systematic assessments of neurotoxicity were feasible in acutely-ill children and young adults and served to characterize and monitor the symptoms associated with CAR-T therapy. We recommend these evaluations be incorporated into future immunotherapy protocols.
Introduction
Neurocognitive deficits and neurologic toxicity are known complications of therapy for acute lymphoblastic leukemia (ALL), and cumulative therapy for those with multiply relapsed or refractory disease may make them particularly vulnerable.1-5 Contributing to this neurotoxicity is the need to treat or prevent central nervous system (CNS) involvement using dose-intensified chemotherapy and radiation therapy. Furthermore, administration of intrathecal chemotherapy may contribute to disruption of the blood-brain barrier, potentially leading to increased acute and chronic neurotoxicity.6 Common acute neurologic toxicities include headaches, peripheral neuropathy, encephalopathy, and seizures.1,7 With increased numbers of patients surviving childhood ALL, there is a greater appreciation for delayed or chronic neurotoxicity, including substantial neurocognitive deficits which may develop over time.4,5,8
Novel immunotherapies, such as small molecule inhibitors, monoclonal antibodies, or chimeric antigen receptor T-cell (CAR-T) therapy, also may be associated with neurotoxicity; however, the mechanism is poorly understood. As seen in early phase studies of blinatumomab, (an FDA approved anti-CD19/CD3 bispecific antibody), and CD19 CAR-T cell therapy, treatment-related neurotoxicity has included tremors, somnolence, encephalopathy, and seizures amongst other manifestations, and range from mild to severe and can be life-threatening.9-11 Given the predilection for neurologic symptoms to develop during cytokine release syndrome (CRS), it is hypothesized that this neurotoxicity may be cytokine-mediated.10-13 It is unclear whether the neurotoxicity is a direct effect (i.e., direct effect of CAR T-cells on the CNS) or is indirectly related to generalized inflammation, potentially from systemic cytokines crossing the blood-brain barrier (BBB) which potentially engage with cytokine receptors in the brain,12-14 or due to an entirely different mechanism. Additional hypotheses include the possibility of neurotoxicity related to CRS-associated pyrexia, off tumor toxicity of CAR-T cells against brain tissue, endothelial activation that could further potentiate disruption of BBB, or even the preparative chemotherapy which frequently includes a fludarabine-based preparative regimen12,13,15. Prior assessments of neurotoxicity have been primarily descriptive, particularly for mild symptoms, and are generally recognized only when severe. A uniform approach to the diagnosis and evaluation of CAR-T-related neurotoxicity is lacking particularly in pediatrics.
While the Common Terminology Criteria for Adverse Events (CTCAE) has standardized criteria for classification and grading of therapy-related adverse events, and includes seizure, encephalopathy, tremor, delirium, and a limited set of other neurologic manifestations, it does not incorporate the full spectrum of symptoms seen with immunotherapy-related neurotoxicity, nor does it have the terminology needed to account for low grade neurotoxicity and capture more subtle signs or potential neurocognitive impairment. To prospectively evaluate the neurotoxicity related to CAR-T therapy, with a goal of early identification of clinical manifestations, we developed a systematic method to assess more subtle changes in children and young adults enrolled on our phase I CD22 CAR-T cell therapy. We report the neurotoxicity outcomes obtained from this evaluation and present a platform that can be incorporated in future CAR-T and immunotherapy protocols to systematically monitor for neurotoxicity and allow for comparison across trials.
Methods
Subjects
All subjects were enrolled on the Phase I dose-escalation study of anti-CD22 CAR-T therapy for children and young adults with relapsed or refractory B cell malignancies. This construct was developed at the NCI and incorporated a 4-1BB co-stimulatory domain16. This study was approved by the Institutional Review Board of the National Cancer Institute, National Institutes of Health. All subjects or guardians provided written informed consent or assent with parental permission as age appropriate. This trial is registered at clinicaltrials.gov (NCT02315612).
Inclusion criteria for the CD22 CAR-T treatment study included individuals ages 1-30 years with relapsed or refractory CD22+ B cell hematologic malignancies. Subjects who had prior CAR-T therapy or immunotherapy were eligible. Active CNS disease (CNS3 disease defined by the presence of ≥5 white blood cells in the CSF with positive blasts on cytospin, evidence for leptomeningeal enhancement, or a definitive CNS lesion) was exclusionary. This report focuses on neurotoxicity data obtained from the prospective evaluations of the first 22 consecutively treated subjects on study at our institution from December 2014 to September 2016.
Neurotoxicity Assessment Measures
CNS disease evaluation
Subjects underwent standard disease evaluation including CSF analysis pre and post-therapy (day 28 +/− 4 days) and baseline brain MRI. Routine CSF studies included cell count, protein, glucose, and cytospin, along with flow cytometry of the CSF to evaluate for the presence of CAR-T cells and assess for subclinical disease.
Cognitive Assessment
A psychologist or psychology associate administered a brief cognitive assessment to subjects ages 4 years and older prior to CAR-T cell infusion (baseline) and post-infusion (between day 21-28). These assessments, which took approximately 30 minutes to complete, consisted of both computerized and paper-and-pencil measures. All tests yielded standard scores with a mean of 100 and standard deviation of 15, with higher scores indicating better functioning.
The following three tests were administered from the NIH Toolbox, which is a validated computerized battery of tests assessing a range of cognitive functions.17 All instructions for these tests were administered verbally as well as presented visually on a computer or iPad®.
Dimensional Change Card Sort Test (DCCS):
This four-minute test of executive function measures cognitive flexibility and attention. The participant is asked to sort pictures presented with varying dimensions (e.g., color and shape) based on the relevant criterion word “color” or “shape” presented on the screen.
Flanker Inhibitory Control and Attention Test (Flanker):
This three-minute test assesses the ability to inhibit visual attention to irrelevant task dimensions. The participant is asked to focus on a target stimulus while inhibiting attention to the stimuli flanking it.
List Sorting Working Memory Test (List Sort):
This seven-minute working memory test examines a persons’ ability to recall, sort, and sequence information. A series of stimuli are presented sequentially on the screen visually and verbally. The instructions inform the participant to organize and say the stimuli in order of increasing size in the first task and then by both category (e.g., fruits or animals) and increasing size in the second task.
Additionally, two paper-and-pencil tests from the child and adult Wechsler intelligence tests (Wechsler, 2008, 2014)18,19 were administered to assess cognitive processing speed as described below.
Wechsler Processing Speed Index (PSI):
The PSI is a composite standard score based on the participant’s performance on two timed paper and pencil subtests. First, the Symbol Search subtest requires the participant to mark if varying target symbols are present in arrays of several other symbols for two minutes. Second, the Cancellation subtest has two tasks that requires the participant to mark target pictures embedded in a page of other pictures for 45 seconds each. Both tasks assess visual discrimination and processing speed.
Neurologic Symptom Checklist
Based on our prior experience with an institutional CD19 CAR-T therapy10,20 and the global findings regarding CAR-T-associated neurotoxicity, we specifically developed an observer-reported checklist of common neurologic symptoms noted post-CAR to assess for subtle symptoms of neurotoxicity that might not otherwise be documented by current standardized criteria (Figure 1). Our Neurologic Symptom Checklist (NSC) evaluates the severity (mild=1, moderate=2, severe=3) and duration (<24 hours, 24-48 hours, and >48 hours) of symptoms, including fever, visual and auditory hallucinations, responsiveness to commands, disorientation, depressed mood, and pain, in the past seven days as rated by the patient’s primary caregiver. Studies indicate that caregiver report is a valid procedure for assessing current and observable behavior of children21,22. We also established a prospective evaluation schedule to monitor the presence and severity of symptoms throughout therapy. The same caregiver (e.g., parent or spouse) was asked to complete this checklist at three time points: (1) prior to CAR-T infusion (baseline); (2) post-infusion at approximately day 14; and (3) day 21-28. If the caregiver was not present for the baseline or final assessment, the adult subject completed the NSC; importantly, only caregivers filled out the day 14 NSC. Follow-up evaluations were conducted based on subject availability to return for a subsequent visit two to three months later. An average of the NSC severity scores was calculated to obtain a mean total symptom score.
Figure 1. Neurological Symptom Checklist.
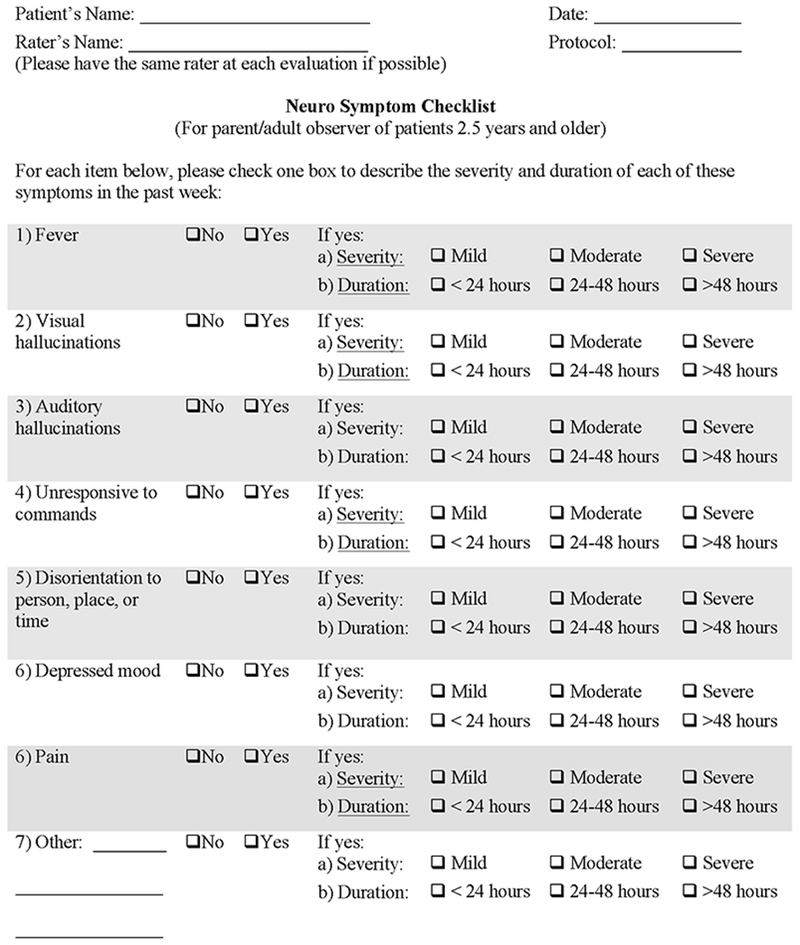
An observer-reported questionnaire, which was developed from our previous CAR-T therapy experience, was completed by the parent or caregiver to assess neurologic toxicities at three time points to assess for changes over time.
Symptoms were attributed to CAR-T therapy only if they were new or worsening from baseline and occurred in the setting of CRS and CAR-T cell expansion. We did not include fever and pain as symptoms of neurotoxicity because they are commonly known symptoms of CRS23.
Serum Cytokines
Serum cytokines were serially obtained during the first month in all subjects and were measured using an ELISA-based assay.
Statistical Analysis
Descriptive statistics were computed to summarize the subject characteristics of the sample. Paired t-tests were used to compare the difference in cognitive test scores and mean total symptoms scores from pre- to post-infusion, which were normally distributed (Shapiro-Wilk statistic range=0.89-0.97; p range=0.06-0.78). Repeated measures Analysis of Variance (within subjects) was used to evaluate changes in the NSC mean total symptom scores completed on subjects over the three time points with post hoc comparisons. In addition, linear and quadratic trends over time were analyzed to assess longitudinal change from the baseline to day 14 and day 21-28 assessments in mean total symptom scores. Mauchly’s Test of Sphericity indicated that the assumption of sphericity had not been violated, χ2(2)=3.2, p=0.21. Subjects’ peak cytokine levels were compared using a Mann-Whitney test of significance according to the following categories: CRS versus no CRS, and on the basis of neurotoxicity presence and severity, with significance set at p<0.05, using a one-tailed p-value.
Results
Subject Characteristics (Table 1)
Table 1. Subject characteristics.
Baseline subject characteristics including age, sex, prior treatment regimens with response, disease status and prior neurotoxicity.
| Demographics | Prior Therapies | Pre-CD22 CAR Disease Status | ||||||
|---|---|---|---|---|---|---|---|---|
| Pt # | Age (yrs) | Sex | Prior HSCT | CD19 Immunotherapy | Prior Neurotoxicity | Marrow | Extramedullary Disease | |
| Agent | Response | |||||||
| 1 | 22 | M | Y | CD19 CAR | CR | M3 | No | |
| 2 | 20 | F | Y (2) | CD19 CAR | CR | M2 | No | |
| 3 | 22 | M | Y | CD19 CAR | CR | M3 | No | |
| 4 | 22 | M | Y | CD19 CAR | PD | M3 | No | |
| 5 | 7 | F | Y | CD19 CAR | CR | M3 | No | |
| 6 | 17 | F | Y | CD19 CAR | CR | M1 | No | |
| 7 | 17 | M | Y (2) | Blinatumomab | CR | M1 | No | |
| 8 | 19 | F | Y | --- | --- | M1 | No | |
| 9 | 21 | F | Y | CD19 CAR | CR | M3 | No | |
| 10 | 26 | M | Y | Blinatumomab | PD | M3 | No | |
| 11 | 7 | M | Y | CD19 CAR | PD | M2 | No | |
| 12 | 15 | M | Y | CD19 CAR | CR | Seizure^& | M1 | Yes |
| 13 | 30 | M | Y | CD19 CAR | CR | Cranial nerve palsy/diplopia^ | M3 | Yes |
| 14 | 14 | M | N | CD19 CAR | PD | Ataxia and aphasia^ | M1* | Yes |
| 15 | 18 | F | Y | --- | --- | M3 | No | |
| 16 | 8 | F | Y | CD19 CAR | CR | M3 | Yes | |
| 17 | 30 | M | Y | Blinatumomab | CR | M3 | No | |
| 18 | 27 | M | Y | CD19 CAR | CR | Seizure, Confusion, memory loss^ | M2 | Yes |
| 19 | 12 | M | N | --- | --- | M3 | No | |
| 20 | 19 | M | Y (2) | CD19 CAR | CR | M3 | No | |
| 21 | 8 | F | Y | CD19 CAR | CR | M3 | No | |
| 22 | 12 | M | N | CD19 CAR | CR | Seizure& | M1 | No |
M=male; F=female; HSCT=allogeneic hematopoietic stem cell transplantation; Y=yes; N=no; CR=complete remission; PD=progressive disease; M1= < 5% marrow involvement; M2=5-< 25% marrow involvement; M3=> 25% marrow involvement.
Patient with DLBCL had no marrow involvement, was extramedullary disease only. For prior neurotoxicity,
indicates that it developed during CD19 directed immunotherapy, and
indicates it was with prior standard ALL therapy
The mean age was 17.9 years (range 7.3-30.5). Twenty-one subjects had ALL, 1 had diffuse large B-cell lymphoma. Nineteen subjects (86%) underwent prior allogeneic hematopoietic stem cell transplant, of which 18 had received either total body irradiation or whole brain radiation. All had multiply relapsed disease with a median time from initial diagnosis to enrollment of 63 months (range 17-256 months). All had CNS1 status (<5 WBC and no blasts on cytospin) and baseline brain MRI performed in 20 subjects confirmed absence of active CNS disease. Incidentally, MRI showed cerebral atrophy in six subjects and vascular anomalies of unclear significance in two subjects. Of the 18 participants (82%) who had received prior CD19 directed immunotherapy, 4 (18%) experienced CAR-associated neurotoxicity which included seizures (n=2), cerebellar ataxia and expressive aphasia (n=1) and diplopia (n=1). Three subjects (14%) had a prior history of a seizure and 6 (27%) were placed on seizure prophylaxis prior to CAR-T cell infusion, based on either prior CAR-T related neurotoxicity or seizure pre-disposition.
Anti-CD22 CAR-T Therapy Outcomes
The full details regarding response to anti-CD22 CAR can be found in Fry et al. In brief, CRS occurred in 17 of 22 (77%) subjects, and was a maximum Grade 2.23 Twelve subjects (55%) attained a complete remission following CAR-T therapy24. CSF was analyzed in 18 subjects at 1-month post CAR-T infusion and demonstrated trafficking of CD22 CAR T cells to the CSF with a median range of CAR-T positive T cells of 33%, (0-72%). (Table 2) Patients not undergoing CSF analysis at 1 month had clinically significant disease progression (n=3) or active coagulopathy (n=1). No patient received steroids for neurotoxicity. Tocilizumab was given in 1 patient for amelioration of systemic signs of CRS.
Table 2. Post-Infusion Trafficking of CAR Cells into CSF, Maximum Grade CRS and Neurotoxicity Profiles.
All subjects included in this analysis were CNS1 (absence of blasts in cerebral spinal fluid-CSF). Assessment of CSF to evaluate CAR-T % was performed when feasible at day 28 post infusion. Subjects with whom had more severe neurotoxicity were also noted to have higher grade CRS.
| Patient^ | Maximum % CAR Cells in CSF* | CRS Grade | Disease Response | Observer Reported Neurotoxicity& Post-Infusion | Symptoms captured by care team~ | Neurotoxicity Attributable# to CAR Infusion |
|---|---|---|---|---|---|---|
| 1 | NS | none | PD | Mildly unresponsive | No | No |
| 2 | 2.5% | 1 | CR | None | --- | N/A |
| 3 | 32% | 1 | SD | None | --- | N/A |
| 4 | 0% | 2 | SD | Mild disorientation | Yes | Yes |
| 5 | 0% | none | PD | None | --- | N/A |
| 6 | 0% | none | PD | Mildly depressed mood | No | No |
| 7 | 52% | 2 | CR | None | --- | N/A |
| 8 | 26% | 1 | CR | None | --- | N/A |
| 9 | 6% | 2 | CR | Moderate visual hallucinations | No | Yes |
| 10 | NS | 2 | SD | Mild auditory hallucinations | No | Yes |
| 11 | 21% | 1 | CR | None | --- | N/A |
| 12 | 0% | none | SD | None | --- | N/A |
| 13 | 69% | 1 | CR | None | --- | N/A |
| 14 | 33.3% | 2 | SD | Moderately depressed mood | No, but somnolence was noted | Yes |
| 15 | NS | 2 | CR | Moderate visual hallucinations | No | Yes |
| 16 | 61% | 1 | CR | None | --- | N/A |
| 17 | 27% | 1 | CR | None | --- | N/A |
| 18 | 71.6% | 2 | SD | Mild disorientation | No | Yes |
| 19 | NS | none | PD | None | --- | N/A |
| 20 | 45% | 2 | CR | Moderately depressed mood | No, but somnolence and irritability were noted | Yes |
| 21 | 57% | 1 | CR | None | --- | N/A |
| 22 | 41% | 1 | CR | Mildly depressed mood | No | Yes |
All patients were CNS1 status prior to CD22 CAR infusion. CNS1: In cerebral spinal fluid (CSF), absence of blasts on cytospin preparation, regardless of the number of white blood cells.
%CAR Cells as % of total T cells in CSF. NS: no specimen collected. PD: progressive disease. CR: Complete response. SD: Stable Disease. N/A: no observable toxicity reported or attributed to CAR infusion.
Neurotoxicity as defined by symptoms noted on the Neurologic Symptom Checklist that were new or more severe as compared to baseline, that went beyond symptoms such as fever and pain that occur during CRS.
Symptoms noted in the electronic medical record.
Neurotoxic symptoms were attributable to CAR-T cell infusion if they were new or worsening symptoms compared to baseline that occurred in conjunction with CRS and CAR-T cell expansion.
Cognitive Test Results
Eighteen subjects (82%) completed the entire cognitive battery at baseline (three with constitutional Trisomy 21 were not offered the test due to concerns about comprehension and ongoing validation of the NIH Toolbox in individuals with intellectual disability25 and one subject did not complete the baseline test due to nausea (Table 3)). Post-infusion assessments were performed in 15 of the 18 subjects (two refused and one had significant disease progression).
Table 3. Individual change in cognitive test scores from baseline to follow-up (day 21-28).
Fifteen subjects were able to complete cognitive tests at baseline and follow-up, with 12/15 demonstrating stable or improved scores on all four tests and two additional subjects showing stable/improved scores on three of four tests. Subject 18 demonstrated decreased test scores in three of four areas; this subject had significant disease burden post-infusion.
| Patient | DCCS Cognitive Flexibility | Flanker Attention/Inhibiton | List Sort Working Memory | Wechsler PSI Processing Speed |
|---|---|---|---|---|
| 1# | N/A | N/A | N/A | N/A |
| 2 | stable | stable | stable | stable |
| 3@ | N/A | N/A | N/A | N/A |
| 4 | stable | stable | stable | stable |
| 5 | stable | stable | stable | stable |
| 6 | stable | stable | stable | increase |
| 7 | increase | stable | stable | stable |
| 8 | stable | increase | stable | increase |
| 9 | stable | stable | stable | stable |
| 10 | stable | stable | stable | decrease |
| 11* | N/A | increase | increase | increase |
| 12^ | N/A | N/A | N/A | N/A |
| 13@ | N/A | N/A | N/A | N/A |
| 14 | stable | stable | stable | increase |
| 15$ | N/A | N/A | N/A | N/A |
| 16 | stable | stable | stable | increase |
| 17 | stable | stable | stable | stable |
| 18 | decrease | decrease | decrease | stable |
| 19^ | N/A | N/A | N/A | N/A |
| 20 | decrease | stable | increase | increase |
| 21 | stable | stable | stable | increase |
| 22^ | N/A | N/A | N/A | N/A |
DCCS = Dimensional Change Card Sort; PSI=Processing Speed Index
Significant increase or decrease in scores was defined as a difference of ≥ .75 SD (11 points) from testing at baseline to Day 28; tests have a normative mean of 100 and SD of 15; higher scores are better
Patient 1 could not complete follow-up cognitive testing due to significant disease progression
Patients 3 and 13 declined to complete follow-up assessments
Patient 11 could not complete one follow-up cognitive flexibility test due to computer problems
Patients 12, 19, and 22 could not complete the cognitive testing due to trisomy 21 due to concerns about comprehending the instructions and ongoing validation of the NIH Toolbox tests in individuals with intellectual disability.18
Patient 15 could not complete the baseline cognitive testing due to significant nausea/illness
Seventeen of 18 subjects completing the baseline assessment scored within the average range on most tests. Examination of the individual data pre- to post-CAR-T therapy revealed that 12/15 demonstrated stable or improved scores on all four tests and two additional subjects had stable/improved scores on three tests with only one test each showing a decrease, but still within the average range (Table 3). Only one subject who had partial response to CAR-T therapy and had significant co-existing disease burden exhibited decreased scores on more than one test. Analysis of the group data indicated that there was no significant change in standard scores from pre- to post-infusion on all three of the NIH Toolbox tests, however there was a significant improvement in the PSI (mean difference=6.06; t=2.15, p=0.048). (Figure 2A-D)
Figure 2. Cognitive Test Scores from Baseline to Follow-up (day 21-28) and Neuro-Symptom Checklist Mean Total Symptom Scores from Baseline to Day 14 and Day 21-28 Follow-up.
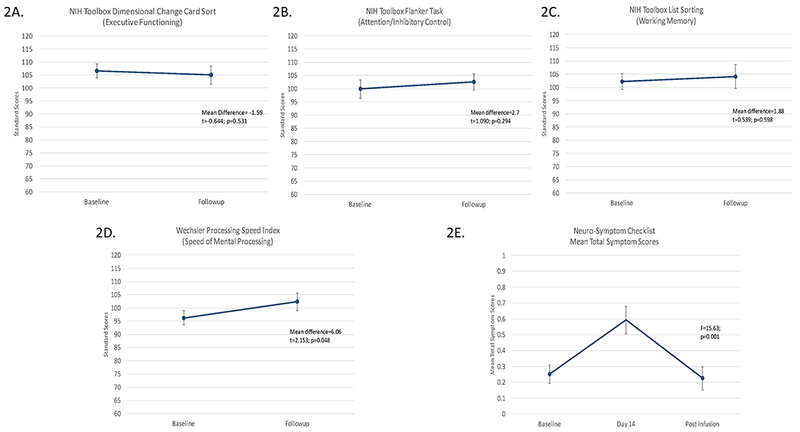
The three NIH Toolbox tests showed no change from pre to post CAR-T infusion (2A, 2B, and 2C) while the Weschler Proccessing Speed Index indicated significant improvement over time (2D). Data collected on the Neuro-Symptom Checklist indicated a significant change in the mean total symptom score over time. (2E)
Neurologic Symptoms
The initial NSC administered at baseline was completed for all 22 subjects. For the follow-up assessments, 21/22 completed the checklist at day 14 (one adult subject refused), and 19/22 completed it at the third time point (one refused, one had died, one was taken off-study due to disease progression).
Using the NSC, new symptoms occurring at any post infusion time point included fever (n=12), pain (n=5), depressed mood (n=3), visual hallucinations (n=2), auditory hallucinations (n=1), unresponsiveness, (n=1) and disorientation (n=2) with worsening of pre-existing pain and fever in 5 subjects each. Neurotoxicity, excluding fever and pain, was reported in 10 of 22 subjects on this study, of which eight were attributed to CD22 CAR-T infusion. Interestingly, only one of 10 symptoms reported by caregivers was captured by the medical team (Table 2). Most symptoms appeared by day 14 post-infusion, but two subjects with extensive disease burden had later CAR-T-cell expansion and delayed CRS, therefore their symptoms of neurotoxicity presented at a typical time after the onset of CRS, each reporting a new symptom at the day 21-28 evaluation. One of the subjects, CD22-10, received the highest dose of CAR T cells, 3 ×10^6 CAR T cells/kg, and required early initiation with steroids for complications of progressive disease and subsequently had delayed CAR expansion. The maximal lymphocyte expansion coincided with observer reported auditory hallucinations. The other patient, CD22-18, who had neurotoxicity symptoms occur at the later timepoint of 21-28 days developed CRS later in his course after the 14 day evaluation. All symptoms demonstrated complete resolution at the post-treatment time point (day 21-28) or were of short duration (<24 hours) except for mild depressed mood reported for one subject; however, this symptom was resolved at the next visit. Follow-up evaluations in six subjects at a subsequent visit two to three months later did not reveal any new or recurrent neurotoxicity.
For the total sample, a repeated measures ANOVA indicated significant changes in the mean total symptom score over time (F=11.03; p=0.0002) with quadratic effects (F=15.63; p=0.001) (Figure 2E). Post hoc analyses revealed a significant increase in the mean total symptom score from baseline to day 14 (F=14.68; p=0.0012) and a significant decrease from day 14 to the day 21-28 (F=12.81; p=0.0021) with no significant change in symptoms when comparing baseline with day 21-28 (F= 0.05; p=0.83).
Cytokine profiles
Serum cytokine levels were captured between days 5 and 21 for all subjects. Cytokines that were significantly higher during CRS included IL-10, IFN-γ, and GM-CSF. When specifically comparing those with neurotoxicity to those without, both IL-10 and IFN-γ remained statistically significantly elevated. When stratifying specifically for subjects with more concerning symptoms of neurotoxicity (hallucinations or disorientation), IL-2, IL-6, IL-8, IL-10, IL-13, IL-15, TNF- α, and GM-CSF were significantly higher in subjects with these symptoms than in those without (all p <.05; Table 4, Figure 3). Importantly all five subjects with hallucinations/disorientation symptoms had higher grade CRS (grade 2).
Table 4. Relationship of cytokine levels to neurotoxicity symptoms.
Cytokine levels were significantly elevated in subjects with symptoms of more concerning neurotoxicity, specifically those with hallucinations and/or disorientation, supporting the hypothesis of inflammation-driven neurotoxicity.
| Cytokines | Association with any Neurotoxicity | Association with Hallucinations/Disorientation |
|---|---|---|
| IL-1B | NS | NS |
| IL-2 | NS | p=0.0250 |
| IL-4 | NS | NS |
| IL-6 | p=0.0445 | p=0.0074 |
| IL-8 | NS | p=0.0010 |
| IL-10 | p=0.0151 | p=0.0250 |
| IL-12p70 | NS | NS |
| IL-13 | NS | p=0.0246 |
| IL-15 | p=0.0223 | p=0.0201 |
| IFN-Y | p=0.0052 | NS |
| TNF-a | NS | p=0.0125 |
| GM-CSF | NS | p=0.0308 |
| MIP-a | p=0.0267 | NS |
Figure 3. Relationship between Cytokine Levels and Hallucinations/Disorientation as Neurotoxicity Symptoms.
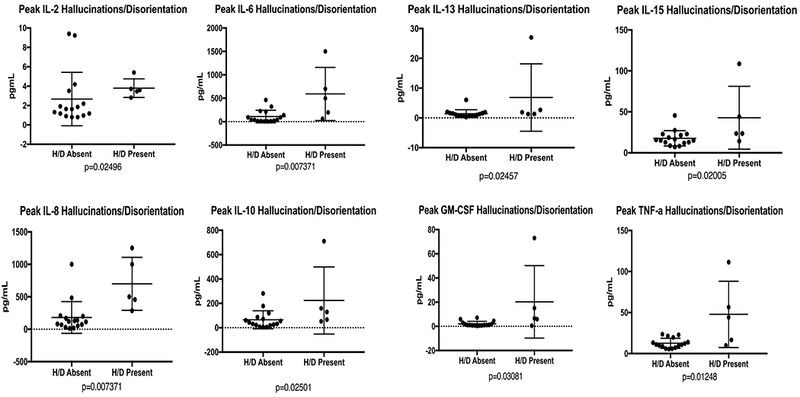
Serum cytokine samples were routinely drawn on subjects from Day 5-21, which corresponded to timing of cytokine release syndrome. In subjects with hallucinations or disorientation several cytokines (IL-2, IL-6, IL-8, IL-10, IL-13, IL-15, TNF- α, and GM-CSF) were significantly higher than in subjects who did not experience hallucinations or disorientation.
Discussion
Neurotoxicity is a major complication associated with ALL therapy, and children and young adults with relapsed disease may be particularly vulnerable due to the cumulative effects of therapy. In the era of novel immunotherapies for the treatment of relapsed/refractory ALL or other malignancies, a systematic assessment to evaluate neurotoxicity is necessary for identifying and characterizing these symptoms, which will allow for comparison across trials. Due to the severe neurotoxicity seen in other CAR-T therapies, particularly that targeting CD19, we sought to prospectively and systematically evaluate for neurotoxicity in this first-in-human trial with anti-CD22 CAR-T therapy based on our groups’ experience with the CD19-28z CAR trial20. Neelapu et al. also devised a multi-disciplinary systematic method to grade and monitor the neurologic changes associated with CAR T-cell therapy, which consisted of parts of the Mini Mental Status Exam (MMSE) and a handwriting sample26. The MMSE primarily is used to assess for delirium or dementia in adults and is not sensitive enough to detect subtle changes in cognition over time27,28. Importantly, Neelapu and colleagues states that alternative tools need to be developed to evaluate children. Our systematic methodology employs a more sensitive assessment, consisting of a brief computerized cognitive test and a short observer-reported checklist, which can be used across a wide age range of patients experiencing a spectrum of neurotoxic effects, enabling comparison across CAR T cell trials. Based on the use of this methodology in the first 22 subjects consecutively treated on our CD22 trial, we describe the neurotoxicity observed in this study, and demonstrate the feasibility of administering prospective cognitive evaluations to our acutely-ill population, which may serve as a model for future immunotherapy studies.
Our prospective approach incorporated baseline analysis of CNS disease, CNS-directed imaging, serial serum cytokine measurements, repeated cognitive testing, including the computerized NIH Toolbox, and a newly-developed brief neurologic symptom checklist. Interestingly, despite remission rates comparable to the experiences with anti-CD19 CAR-T and evidence of CAR trafficking to the CNS, both the degree of CRS (maximum grade 2) and the severity of neurotoxicity was limited—specifically, no subject experienced seizure, ataxia, or dysphasia10,11,29. Corresponding cytokine analysis showed that higher cytokine values, in particular TNF- α, IL-6, IL-8 and IL-15, was associated with worse neurotoxicity, corresponding to similar findings;10,13,30 yet no patient required specific treatment for neurotoxicity. While it is possible that the use of seizure prophylaxis prevented seizures, other neurotoxicity symptoms were not absent but limited. As this is the first evaluation of CAR-related neurotoxicity related to targeting of an antigen other than CD19, the relatively lower degree of CRS and neurotoxicity suggests the possibility of different toxicity profiles based on the antigen targeted, further supporting the need for a systematic evaluation that can be used in the growing field of immunotherapy across a broad range of CAR trials, full spectrum of toxicities, and wide age range of patients.
With incorporation of these measures, we were able to identify a range of neurotoxicity symptoms in patients that otherwise may not have been appreciated. Symptoms included pain, depressed mood, visual and auditory hallucinations, unresponsiveness, and disorientation at the time of CRS that subsequently resolved. Furthermore, we demonstrated the feasibility of using these state-of-the-art techniques to assess our acutely-ill subjects in a systematic fashion that allowed for the identification of mild to severe symptoms of neurotoxicity and provided a consistent method for monitoring these symptoms for progression or resolution.
The ability to monitor children and young adults over time is particularly important in this cohort who have had prior neurotoxic therapies and may have residual neurocognitive impairments to help determine whether these new therapies will lead to further decline or stable to improved functioning. The repeated cognitive assessments can be administered in approximately 30 minutes and are feasible and acceptable in the majority of subjects. Additional experience using this battery with future CAR T cell trials will determine which tests are most sensitive to neurotoxicity and which, if any, may be omitted. Furthermore, incorporating an observer-reported outcome measure allowed the monitoring of changes in subjects, even in those who may be too sick to actively participate in any testing. From this brief assessment, cognitive tests results documented the stability of executive function, attention, and working memory, and improvement in cognitive processing speed after CD22 CAR-T therapy. The NSC data showed a significant increase in symptoms around the time of CRS and CAR expansion and then a significant decrease in symptoms at the final or subsequent evaluation, indicating reversibility of CAR-T related neurotoxicity. Additionally, the NSC was superior in identifying subtle symptoms that the medical team evaluation did not detect.
One potential concern about using this prospective cognitive assessment in other CAR-T studies is whether the NIH Toolbox can be easily administered in other hospital settings. However, the NIH Toolbox is commercially available, and the tests are brief and administered on an iPad® (www.nihtoolbox.org or www.healthmeasures.net). The battery was designed so that a variety of researchers and clinicians can administer the test, although we recommend having a psychologist available to train and supervise the examiners and assist with the interpretation of the data. We are continuing to explore the use of other computerized tests, which are developed specifically for clinical trials, are more easily set up, and have alternate forms, which can decrease the risk of practice effects with repeated testing, although the data from this study did not suggest the occurrence of practice effects. We also are slightly adapting the NSC to include additional symptoms that were identified from this study including drowsiness/sleepiness, blurred vision, and difficulty speaking, and we added definitions of mild, moderate, and severe to help standardize these categories. We plan to add one additional post-infusion administration of the NSC between day 35-42 to assess for late-developing or resolving neurotoxicity. Future plans at our center include incorporating a standardized neurological examination performed by the neurology consultation service at baseline, at the beginning of CRS, and after resolution of CRS, which will capture additional observations from health professionals with expertise in neurologic evaluations.
A limitation to our study is that the feasibility of our neurotoxicity assessments was evaluated in subjects who only experienced relatively mild CRS (maximum grade 2). Given the association of higher grade CRS with more neurotoxicity, further study of this assessment is needed in individuals with more severe CRS, including those who are intubated where neurologic assessments will be more difficult. In addition, the reliability and validity of these tools also need to be examined.
Ideally, use of a systematic analysis for neurotoxicity would provide a parallel assessment for when treatment of neurotoxicity would be indicated. Although intuitively the onset of low-grade symptoms could be a prelude to more severe toxicity, without having more severe toxicity in this cohort, we were not able to test or prove that hypothesis and that work is ongoing.
Current management of neurotoxicity associated with immunotherapy has primarily been symptomatic treatment, such as anti-pyretics, anti-seizure medications, incorporation of corticosteroids, or direct antibodies targeting specific cytokines to reduce the effects of inflammation on the CNS. It is unclear which, if any therapy may be the most efficacious and least detrimental to the efficacy of CAR-T and further investigation is warranted. In this regard, future plans will incorporate the possibility of measuring CSF cytokines in those with severe neurotoxicity to better guide treatment strategy for amelioration of symptoms.
In conclusion, we provide the first systematic assessment designed specifically for the evaluation of CAR-T-related neurotoxicity in children and adults. Such prospective studies will allow for detecting and monitoring subclinical neurotoxicity, which may precede worsening symptoms, allowing for a window of opportunity to provide earlier intervention that may prevent more severe, life-threatening problems. Additionally, it could assist in the development of prophylactic measures to enhance the safety of cellular therapy. Given the brief time commitment required to administer this assessment, good feasibility of incorporating such an approach in this patient population, and useful information obtained from the evaluation, we suggest that other CAR-T programs adopt similar strategies. Consistent use of these evaluations will allow the field to collectively evaluate and define neurotoxicity related to CAR-T therapy, which can be used to guide early intervention strategies, monitor symptoms over time, and compare symptoms across trials.
Acknowledgements
This work was supported by the Intramural Research Program of the National Cancer Institute. We gratefully acknowledge the study participants and their families, referring medical care teams, the faculty and staff of the NIH, and the data managers involved with this work.
DWL reports grants from St. Baldrick’s Foundation during the conduct of the study. CLM is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. C.L.M is one of the inventors on a patent for the CD22 CAR.
Funding: NCI Intramural Research Program
Footnotes
Contributions
HS, PLW, NNS drafted the manuscript. Additionally, SM, MAT, MCR, KS, CLM, DWL, TJF helped design the research study and supervised study testing. BY,CD provided criticial review of the manuscript and provided data collection for important intellectual content. EK performed data analysis. All authors take responsibility for the accuracy and completeness of the data and for the analyses.
Conflict of Interest Statements
The other authors have no conflicts of interest to disclose.
References
- 1.Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity--focus on newer treatments. Nat Rev Clin Oncol 2016; 13(2): 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole PD, Kamen BA. Delayed neurotoxicity associated with therapy for children with acute lymphoblastic leukemia. Ment Retard Dev Disabil Res Rev 2006; 12(3): 174–83. [DOI] [PubMed] [Google Scholar]
- 3.Winick N Neurocognitive outcome in survivors of pediatric cancer. Curr Opin Pediatr 2011; 23(1): 27–33. [DOI] [PubMed] [Google Scholar]
- 4.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol 2013; 31(35): 4407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy KK, Embry L, Kairalla JA, et al. Neurocognitive Functioning of Children Treated for High-Risk B-Acute Lymphoblastic Leukemia Randomly Assigned to Different Methotrexate and Corticosteroid Treatment Strategies: A Report From the Children’s Oncology Group. J Clin Oncol 2017; 35(23): 2700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soffietti R, Trevisan E, Ruda R. Neurologic complications of chemotherapy and other newer and experimental approaches. Handb Clin Neurol 2014; 121: 1199–218. [DOI] [PubMed] [Google Scholar]
- 7.Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs 2003; 63(15): 1549–63. [DOI] [PubMed] [Google Scholar]
- 8.Schiff D, Kesari S, Wen PY. Cancer neurology in clinical practice neurologic complications of cancer and its treatment Current clinical oncology. 2nd ed. Totowa, NJ: Humana Press,; 2008. p. 1 online resource (xvi, 636 p., 5 p. of plates). [Google Scholar]
- 9.Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015; 16(1): 57–66. [DOI] [PubMed] [Google Scholar]
- 10.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385(9967): 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371(16): 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014; 123(17): 2625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gust J, Hay KA, Hanafi LA, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tasian SK, Gardner RA. CD19-redirected chimeric antigen receptor-modified T cells: a promising immunotherapy for children and adults with B-cell acute lymphoblastic leukemia (ALL). Ther Adv Hematol 2015; 6(5): 228–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J Clin Oncol 2017; 35(16): 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 2013; 121(7): 1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology 2013; 80(11 Suppl 3): S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wechsler. Wechsler Adult Intelligence Scale-IV. Administration and Scoring Manual.; 2008.
- 19.Wechsler. Wechsler Intelligence Scale for Children-V. Administration and Scoring Manual. ; 2014.
- 20.Martin SW P; Shah NN; Delbrook C; Yates B; Fry TJ; Mackall CL; Lee DW No consistent neurocognitive declines are observed with CD19 CAR T cell therapy for children with acute lymphocytic leukemia: Preliminary findings. Presented at 2015 Conference on Immunotherapy in Pediatric Oncology 2015. [Google Scholar]
- 21.Youngstrom EA, Findling RL, Danielson CK, Calabrese JR. Discriminative validity of parent report of hypomanic and depressive symptoms on the General Behavior Inventory. Psychol Assess 2001; 13(2): 267–76. [PubMed] [Google Scholar]
- 22.Byrne JM, Backman JE, Smith IM. Developmental Assessment - the Clinical Use and Validity of Parental Report. J Pediatr Psychol 1986; 11(4): 549–59. [DOI] [PubMed] [Google Scholar]
- 23.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124(2): 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hessl D, Sansone SM, Berry-Kravis E, et al. The NIH Toolbox Cognitive Battery for intellectual disabilities: three preliminary studies and future directions. J Neurodev Disord 2016; 8(1): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018; 15(1): 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman MA, Tremont-Lukats I, Meyers CA, et al. Neurocognitive and functional assessment of patients with brain metastases: a pilot study. Am J Clin Oncol 2003; 26(3): 273–9. [DOI] [PubMed] [Google Scholar]
- 28.Meyers CA, Wefel JS. The use of the mini-mental state examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol 2003; 21(19): 3557–8. [DOI] [PubMed] [Google Scholar]
- 29.Davila ML, Bouhassira DC, Park JH, et al. Chimeric antigen receptors for the adoptive T cell therapy of hematologic malignancies. Int J Hematol 2014; 99(4): 361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015; 33(6): 540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


