Abstract
Background
Greater psychological resilience may protect against developing depression in a growing geriatric population. Identifying the neural correlates of resilience in geriatric depression could provide neurobiological targets to inform clinical interventions. However, most prior neuroimaging studies have only considered the presence or absence of resilience and have not addressed the multifactorial nature of resilience. The current study aimed to establish the neural correlates of four factors of resilience in the depressed elderly.
Methods
White matter integrity was assessed using diffusion-weighted magnetic resonance imaging (DW-MRI) data collected from 70 older adults with major depressive disorder. We used four resilience factors previously derived in an exploratory factor analysis of the Connor-Davidson Resilience Scale in a large sample of depressed older adults: 1, grit; 2, active coping self-efficacy; 3, accommodative coping self-efficacy, and 4, spirituality.
Results
The Resilience factor “grit” was positively associated with fractional anisotropy in the callosal region connecting prefrontal cortex and fractional anisotropy in cingulum fibers, however, the latter did not survive correction for multiple comparisons.
Conclusion
Structural integrity of major white matter pathways implicated in cognitive control and emotion regulation (i.e., connecting prefrontal cortex) was positively associated with the resilience factor “grit” in our sample of older adults with depression. Prospective studies are needed to determine the utility of the structural integrity of these pathways as a biomarker in predicting risk for depression and treatment response.
Keywords: Resilience, White Matter, Geriatric Depression, major depressive disorder, DTI, MRI, Connor-Davidson Resilience Scale, Grit
The world’s population is rapidly aging, with the number of adults over the age of 65 expected to increase more than 60% in the next 15 years (1). Roughly 7% of adults aged 75 and older suffer from major depression (2), underscoring the importance of developing new and effective interventions for the treatment and prevention of geriatric depression. Psychological resilience, “the ability to maintain or regain mental health, despite experiencing adversity” (3) has been shown to predict wellbeing, longevity, and reduced risk of depression onset in the elderly (4, 5). Furthermore, individuals with greater resilience may be more responsive to psychiatric medications and cognitive behavioral therapy (6). A variety of resilience measures have been developed (4, 5, 7). Among these, the Connor-Davidson Resilience Scale (CD-RISC) (8), the Resilience Scale for Adults (RSA) (9), and the Brief Resilience Scale (BRS) (10) were identified in a recent review as having the strongest psychometric properties (11). However, even among these validated measures, conceptualizations of resilience are variable. While the CD-RISC and RSA focus on psychosocial protective factors (e.g., personal competence, close relationships), the BRS measures physiological reactions to stress (e.g., time required to recover from an acute stressor). The consensus is that resilience is a complex, multifactorial and dynamic system including bidirectional interactions between psychosocial and biological variables (12). Developing a model of resilience that links multiple factors of resilience to their unique neural mechanisms may inform the development of novel diagnostic tools and eventually facilitate the development of more targeted interventions for the treatment and prevention of mood disorders (7, 12–14).
Non-invasive neuroimaging techniques may clarify associations between discrete psychological resilience factors and their corresponding neurobiological mechanisms. However, it is also possible that some psychosocial factors of resilience may not exhibit clear neural underpinnings and may instead rely on diffuse and overlapping functional systems in the brain. Resilience could be examined in terms of integrity of specific neural pathways and structural and functional connectivity between brain regions. While several functional magnetic resonance imaging (fMRI) studies have investigated associations of resilience with regional patterns of gray matter activation (12, 15, 16) and functional connectivity (17), few have addressed the relationship with white matter integrity and structural connectivity (18) using diffusion-weighted MRI (DW-MRI) (19–21). White matter abnormalities are amongst the most commonly identified imaging abnormalities in geriatric depression (21), and in aging more broadly (22). Moreover, DW-MRI measures are very sensitive to cardiovascular risks (23) which are associated with higher vulnerability to geriatric depression (24–26). Together, these findings suggest that white matter integrity is a plausible biomarker of resilience in geriatric depression. However, to our knowledge this hypothesis has not yet been tested.
Several DW-MRI studies have linked white matter connectivity to resilience in non-geriatric samples. For example, one recent study used diffusion tensor imaging (DTI) to examine fractional anisotropy (FA; an index of white matter integrity) in traumatized African American women with and without posttraumatic stress disorder (PTSD) (27). Lower FA in the posterior cingulum connecting the anterior cingulate (ACC) and hippocampus was observed in individuals with PTSD compared to those without. This suggests that regional reductions in white matter FA may serve as a possible marker of risk for PTSD. Similarly, researchers have proposed that the microstructure of the cingulum may indicate resilience vs. vulnerability to depression (19). The radiations of the anterior corpus callosum have further been implicated. For example, one MRI study investigated DTI metrics in the corpus callosum among 123 adolescents with high lifetime exposure to stress (28). They found greater white matter integrity (higher FA) within the anterior corpus callosum in more resilient adolescents (defined as those with fewer behavioral and emotional disturbances). Further, Frodl and colleagues revealed that compared to healthy controls, individuals at high risk for depression (i.e., first-degree unaffected healthy relatives of individuals with major depressive disorder (MDD) and those with greater early life adversity) had greater FA in the body and splenium of the corpus callosum, the inferior fronto-occipital fasciculus, the left superior longitudinal fasciculus, and the right fornix (29). Because these individuals did not develop depression despite their elevated risk, the authors hypothesized that higher indices of white matter integrity might indicate greater resilience.
In their seminal review, Feder and colleagues (13) advocate for a translational model of resilience that incorporates both neurobiological and psychosocial resilience factors. The authors identify five psychosocial factors of resilience and propose possible neurobiological underpinnings of each: 1) facing fears and active coping involving the hypothalamus-pituitary-adrenal (HPA) axis, 2) optimism and positive emotions recruiting the mesolimbic dopamine system, 3) cognitive reappraisal, positive reframing and acceptance involving cortico-limbic circuits involved in processes of memory suppression, memory consolidation and cognitive control of emotions, 4) social competence and social support involving brain circuits related to reward value of social attachments and fear responses, and 5) purpose in life, a moral compass, meaning and spirituality, potentially recruiting more diffuse brain areas not currently well-defined.
The current study responds to Feder and colleagues’ challenge by addressing the associations between psychosocial factors of resilience and the regional integrity of white matter microstructure in geriatric depression. Our approach acknowledges the multifactorial nature of resilience by using four resilience factors previously identified in an exploratory factor analysis (EFA) of the CD-RISC in a large sample of older adults with depression (30) (of which the current data are a subset). In that study, each of these factors was found to be negatively correlated with clinical factors such as depression and apathy and positively correlated with quality of life.
We aimed to identify white matter brain correlates of these factors in the context of a combined neuropsychosocial model of resilience. Based on existing evidence, we expected that the higher FA in the frontal cortical projections would be associated with higher grit factor of resilience while higher accommodative coping self-efficacy would be associated with higher FA in pathways of the dopaminergic reward system (more ventral limbic fiber tracts).
Methods
Participants
Data comprised baseline diagnostic interviews and baseline neuroimaging from three clinical trials (one published (31) (NCT00602290) and two ongoing (NCT02460666, NCT01902004)) conducted with depressed adults aged 60 and over at UCLA. Data collection took place between 2015 and 2017. Inclusion criteria were: (1) diagnosis of MDD as defined by Diagnostic and Statistical Manual (DSM)-IV-TR (32) or DSM 5 (33) and (2) normal cognitive functioning as defined by Mini-Mental State Exam (MMSE) score of 24 or greater. Exclusion criteria were: (1) history of any psychiatric disorder (with the exception of a stable comorbid anxiety disorder or stable comorbid insomnia); (2) acute suicidal ideation or suicide attempt within the past year; (3) severe or acute unstable medical illness or neurological disorder; (4) dementia. Participants were required to be free of psychotropic medications for at least two weeks. All study procedures were approved by the UCLA Institutional Review Board. Table 1 provides clinical characteristics of the sample.
Table 1.
Sample Characteristics
| Total Sample (N = 70) |
|
|---|---|
| Sex (%) | |
| Female | 48 (68.6) |
| Male | 22 (31.4) |
| Race (%) | |
| White | 56 (80.0%) |
| Hispanic | 7 (10.0%) |
| Black | 4 (5.7%) |
| Asian | 2 (2.9%) |
| Other | 1 (1.4%) |
| Age (SD) | 70.46 (6.82) |
| Age of onset (SD) | 44.59 (21.69) |
| Years since onset (SD) | 26.76 (21.16) |
| Years of Education (SD) | 16.13 (1.93) |
| MMSE (SD) | 28.60 (1.33) |
| HAM-D (SD) | 17.77 (2.92) |
Note. SD: Standard deviation; HAM-D: Hamilton Depression Rating Scale; CD-RISC: Connor Davidson Resilience Scale.
Measures
Participants completed the CD-RISC (8), a resilience scale validated for use in a variety of clinical populations, including older adults (34). The scale consists of 25 statements. Participants are asked to indicate the extent to which each statement has been true for them over the past month using a 4-point scale: (0) not true at all; (1) rarely true; (2) sometimes true; (3) often true; and (4) true nearly all of the time. Total scores range from 0–100, with higher scores indicating greater resilience, and an average score of about 80 (SD=12.8) reported in the general U.S. population (8). We used factors previously identified in our EFA of the CD-RISC in a large sample (N=337) of older adults (≥60 years) with MDD. Factors (with example items) were: Factor 1, grit (“I have a strong sense of purpose”; “When things look hopeless, I don’t give up”); Factor 2, active coping self-efficacy (“I prefer to take the lead in problem solving”); Factor 3, accommodative coping self-efficacy (“I am able to adapt to change”); and Factor 4, spirituality (“I believe things happen for a reason”) (30). A reliability analysis, using just the items making up each of the factors, yields Cronbach’s α estimates of 0.89, 0.91, 0.90, 0.71 for factors 1 through 4 respectively. Data from the subset of these participants who received neuroimaging with multimodal MRI are reported here; means for each factor score are provided in Table 1.
Image Acquisition
Brain scans were acquired at the Ahmanson & Lovelace Brain Mapping Center at UCLA. Thirty-four participants were scanned on a 3T Siemens Trio system with a 32-channel head coil with following parameters: MB factor 3, voxel dimension 1.8 mm3 isotropic, FoV=190 mm, TR=3245ms, TE=84ms, 144 gradient directions and 12 b0’s, 72 slices, flip angle=90°, b-factor= 1000 s/mm2. Total scanning time was 8.39 minutes. Another 36 participants underwent scanning after a hardware upgrade to a Siemens Prisma 3T system at the same Center. Diffusion imaging parameters included: MB factor 4, voxel dimension 1.5 mm3 isotropic, FoV=210 mm, TR=3230ms, TE=89.20ms, 98 gradient directions and 7 b0’s, 92 slices, flip angle=78°, b-factor=1500 s/mm2. Total scanning time was 11:08 minutes including two acquisitions with anterior-posterior and posterior-anterior phase encoding. Type of acquisition protocol was included in the analyses as a covariate.
A high-resolution T1-weighted structural brain scan was also obtained for co-registration with diffusion images for each participant during the same scanning session using a similar multi-echo MPRAGE sequence on both the Siemen’s Trio and Prisma systems: 176 sagittal slices, 1 mm3 isotropic voxel size, TR=2150, TE=1,74, 3.6, 5.46, and 7.32 ms; FoV=256 mm; 256×256 matrix; TI 1260 ms; FA=7°.
DTI Processing
Diffusion imaging data was preprocessed using the Oxford Centre for Functional MRI of the Brain (FMRIB) software library (FSL; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). The data acquired on the Prisma scanner protocol went through topup distortion correction (35); Trio data was registered to the T1 image to correct for anatomical distortions. Preprocessing steps were otherwise the same for both protocols. First, diffusion data was corrected for distortion and motion artifacts induced by eddy currents and head motion using affine registration of each diffusion-weighted image to the first b=0 reference image, and gradient direction vectors (b-vectors) were adjusted accordingly. Then non-brain tissue was removed from the images using the Brain Extraction Tool and the diffusion tensor model was fitted to the data. After tensor fitting, individual fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) maps were created for each participant. Using the regular FSL Tract-Based Spatial Statistics (TBSS) workflow (36), FA individual maps were then registered to the common FA image 1 mm template in MNI space and a group white matter skeleton was created from FA maps and individual diffusion tensor imaging (DTI) metrics were projected onto the skeleton. TBSS workflows have been well-validated and described in detail (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS). One-sample t-tests with the four resilience factors as covariates of interest and age, sex and acquisition protocol as nuisance covariates were performed on skeletonized FA images using nonparametric permutation inference (37). Age, sex and acquisition protocol were added to the model as covariates, because DTI metrics such as FA, AD, RD, MD have been reported as influenced by age and gender (for example, (38, 39)) as well as acquisition protocol (40). The number of permutations was set to 10000 and the results were corrected for multiple comparisons using the threshold-free cluster enhancement method (TFCE) with p<0.05.
Additional ROI Analysis
Based on previous reports showing positive associations between resilience and DTI metrics and the review by Feder and colleagues, we performed an additional hypothesis-driven regions-of-interest (ROI) analysis. ROIs were derived from the ICBM-DTI-81 white-matter labels probabilistic atlas (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). We hypothesized that the grit factor of resilience, which is associated with cognitive control and emotion regulation, may rely on prefrontal structural connectivity within including the genu of corpus callosum (GCC) and cingulum bundle (CB - contains cingulum alongside the cingulate cortex (CGC) and posterior cingulum (CGH)). Our active coping self-efficacy factor is conceptually similar to Feder’s “facing fears and active coping” hypothesized to involve the HPA axis. Since there are no fiber bundles defined in currently available white matter atlases that are directly relevant to the HPA axis (for example, the ansa peduncularis, medial forebrain bundle and other fasciculi with hypothalamic connections), we predicted that these effects might be evident in the voxel-wise whole brain TBSS analysis only. As relevant to the accommodative coping self-efficacy factor, which incorporates optimism, we considered the anterior limb of the internal capsule (ALIC) as a component of the dopaminergic mesolimbic reward system. For all hypothesized pathways, we predicted that positive associations would occur between our resilience factors and FA measurements. Since there is less evidence concerning focal neural correlates of spirituality, we did not generate any specific regional hypotheses for this factor.
Mean FA values were subsequently extracted from the individual skeletonized FA maps using tract-based anatomical ROIs CB, GCC and ALIC. The corticospinal tract (CST) was included as a control region, since it was not expected to associate with the resilience factors. To examine associations between regional FA values within each ROI and resilience measures (total, as well as individual factors), we used general linear mixed models with repeated measures for each of the selected tract ROIs (CB, ALIC and CST), with hemisphere (left, right) as the intra-subject classification variable, resilience scores as predictors and age, sex, acquisition protocol as covariates as for the whole brain TBSS analysis described above. For the GCC, which is not a lateralized ROI, a general linear model was used with the same predictor and covariates. All tests were two-tailed and an alpha level of 0.05 was adopted for all inferences. Since our ROI analyses were hypothesis driven with directional predictions, we report statistics for all combinations of resilience scores and FA ROIs to show the specificity of obtained results.
Results
Sample Characteristics
The average age of participants (N=70) was 70 years (range=60–83 years). The majority of participants were White (80%) and female (69%), with an average of 16 years of education (see Table 1).
Diffusion Tensor Imaging
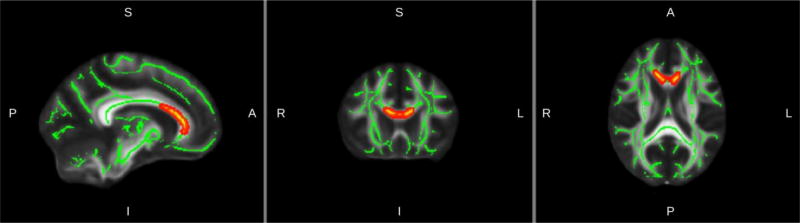
Whole brain TBSS analysis revealed significant positive associations between FA and the grit resilience factor in frontal cortex fibers (forceps minor) traversing the genu of the corpus callosum (Figure 1, Table 2). Other DTI metrics (AD, RD, MD) did not show any significant cluster corrected associations with factors of resilience in the whole brain TBSS analysis.
Figure 1.
Results of TBSS analysis: in red the regions of significant positive association between Grit and FA (p< 0.05, TFCE-corrected; see Table 2 for statistical details). Significant voxels are overlaid on the group mean white matter skeleton (green) and the FA MNI template (Montreal Neurological Institute).
Table 2.
Clusters of Fractional Anisotropy Associated with Grit Factor of Resilience*
| cluster | Number of voxels |
Mean t statistics |
Center of mass MNI coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 1 | 379 | 2.82 | 9.46 | 25.2 | 13.4 |
|
| |||||
| 2 | 335 | 2.9 | −9.02 | 24.9 | 13.6 |
TFCE corrected p-value ≤ 0.05, degrees of freedom (df) = 62
Follow-up ROI analysis revealed associations between grit and FA in GCC in line with whole brain TBSS analysis, as well as in the CB, with higher white matter integrity being associated with greater grit (Table 3; Figure 2). However, these results did not survive correction for multiple comparisons which took into account the number of regions tested. No significant results were obtained for the reward system-related ROI (ALIC), the control ROI (CST) or the other resilience factors (Table 3).
Table 3.
Associations of Fractional Anisotropy with Resilience Factors and Total Resilience
| Brain regions* | ||||
|---|---|---|---|---|
|
| ||||
| Factors | CB | GCC | ALIC | CST |
| Grit | 4.47 (0.04) | 7.54 (0.008) | 1.14 (0.29) | 0.56 (0.46) |
| Active Coping Self-efficacy | 0.44 (0.51) | 0.27 (0.60) | 0.50 (0.48) | 0.63 (0.43) |
| Accommodative Coping Self-efficacy | 1.24 (0.27) | 0.21 (0.65) | 0.10 (0.75) | 0.70 (0.41) |
| Spirituality | 0.99 (0.32) | 0.01 (0.97) | 1.14 (0.29) | 0.02 (0.88) |
| Total CD-RISC | 2.22 (0.14) | 0.87 (0.35) | 0.01 (0.94) | 0.16 (0.69) |
Note. CB: cingulum bundle; GCC: genu of corpus callosum; ALIC: anterior limb of the internal capsule; CST: corticospinal tract.
Numbers presented are F(1,64) statistics (p-values in parentheses) obtained from general linear mixed models with repeated measures for CB, ALIC and CST, with hemisphere (left, right) as the intra-subject classification variable, resilience scores as predictors and age, sex, acquisition protocol as covariates. The observed relations between grit and FA continued to be significant in the absence of covariates. For GCC, which is not a lateralized ROI, a general linear model was used with the same predictor and covariates as above.
Figure 2.
Scatterplots with GLM fits demonstrating associations between Grit and FA in ROIs (statistics are presented in Table 3). CB tract ROI is shown in blue and GCC tract ROI is shown in red. Values for left and right CB were averaged for scatterplot. Effects of age, gender and protocol type were regressed out.
To determine whether the observed associations differ between older and younger adults, we also examined the interaction of age with resilience scores. With FA of GCC or CB as the outcome measures, the interaction terms were not significant (GCC: F(1,63)=2.2, p=.2; CB: F(1,63)=3.3, p=.08).
Discussion
Although previous research has investigated associations between global resilience and white matter microstructural correlates (19, 27, 28), recent studies suggest that resilience is comprised of multiple factors, each of which may have distinct underlying biological substrates. The current study expanded upon existing literature by examining the relationship between brain white matter integrity and four factors of resilience previously identified in a large sample of older adults with depression (30). Whole brain TBSS analysis revealed significant positive associations between FA and grit in frontal cortex fibers traversing the GCC. This finding provides support for the notion that resilience is comprised of multiple psychosocial factors, each of which may have distinct underlying neurobiology. Consistent with our regional and directional hypotheses, ROI analyses found that grit was associated with FA in GCC and CB. ROI results should be interpreted with caution, as they did not survive correction for multiple comparisons. Nevertheless, we believe they offer preliminary evidence that grit (that is, “perseverance and passion for long-term goals”, or the ability to remain goal-oriented in the face of obstacles (41)) may involve anterior corpus callosum (GCC) projections including connections between prefrontal executive control regions (42, 43). These areas have been previously identified as contributing to impulse control (44) and emotion regulation via reappraisal (45), each constructs which have been shown to be closely related to grit (46). Previous research indicates that decreased white matter integrity of the corpus callosum is associated with poorer impulse control in individuals with substance use and gambling disorders (47, 48) as well as higher suicidal behavior in individuals with bipolar and borderline disorders (49, 50). Our results are aligned with previous findings suggesting the contribution of interhemispheric anterior corpus callosum structural integrity in resilience via emotion regulation and impulse control.
Our ROI results also suggest a possible association between grit and the cingulum. The cingulum connects frontal cortex and limbic structures of the brain and is thought to underlie processes of cognitive control (51) and emotion regulation (52). These results are consistent with previous research on individuals at risk of psychiatric disorders which has indicated the cingulum as a possible anatomical substrate of resilience (19, 27). Although this was an a priori hypothesis, this result should nevertheless be interpreted with caution, as it did not survive Bonferroni correction.
Contrary to expectations, no significant associations were observed between white matter integrity parameters and any of the other three resilience factors. The null effects for the two coping self-efficacy factors cannot be explained by differences in alpha reliability because reliability was equally high for the active and accommodative coping self-efficacy factors (0.91 and 0.90, respectively) as it was for grit (α=.89). The active coping self-efficacy factor is conceptually similar to the “facing fears and active coping” construct hypothesized by Feder and colleagues to involve the HPA axis (13). Since smaller or less myelinated white matter pathways such as the ansa peduncularis, medial forebrain bundle, and other fasciculi with hypothalamic connections are less amenable to investigation with standard diffusion imaging acquisition and metrics, future studies may benefit from the use of additional biomarkers of HPA axis such as cortisol (53).
The accommodative coping self-efficacy factor contains elements of optimism which has been shown to be associated with activity in the dopaminergic mesolimbic reward system (54, 55). However, we found no significant associations between accommodative coping self-efficacy and FA in portions of the mesolimbic reward system pathways (for example, ALIC in ROI analyses). This negative result may be a consequence of the specificity of the sample or limitations in extracting pathways of this functional network. Dopamine level declines significantly with both age and depression (56, 57). In older depressed adults the decrease of functionality of the dopamine system may render optimism more related to a learned behavioral strategy than a biologically determined personal trait. If this hypothesis is correct, the accommodative coping self-efficacy factor of resilience may be a more appropriate target for psychotherapy in geriatric depression, because it is related to depression severity in this population (30). Investigation of the association between optimism and the integrity of the dopaminergic mesolimbic reward system in depressed and control subjects across the adult lifespan may help clarify this question.
Despite the relevance of spirituality in depressed populations (58) we found no associations between indicators of white matter integrity and spirituality in our sample. It is possible that the reduced alpha reliability observed in the spirituality factor (α=.71) may partially account for this null finding. Several fMRI and cortical thickness studies (58, 59) have shown brain correlates of several aspects of spiritual beliefs, suggesting that methods targeting gray matter may be more sensitive to neural correlates of spirituality. Future studies may benefit from using multimodal neuroimaging techniques to investigate neural correlates of spirituality.
There are a few limitations to the current study. Our sample size was relatively small, thus limiting statistical power. Our lack of non-depressed control group prevents conclusions regarding the specificity vs. generalizability of our findings to depression. Similarly, because all of our participants were above age 60, we cannot rule out the possibility that a similar relation between grit and white matter integrity would be observed in younger adults with depression. Furthermore, if the results are specific to geriatric depression, the cross-sectional nature of the study means that it is impossible to determine the directionality of effects (i.e., whether those with higher FA are more able to maintain resilience with age or whether resilience leads to a perseveration of FA). Finally, as noted above, standard diffusion imaging methods may not be sufficiently sensitive to isolate alterations in smaller, more complex pathways. Future research may benefit from the use of multiple imaging modalities and biomarkers of stress responsivity to identify additional neurobiological correlates of resilience.
The current study directly addresses Feder and colleagues’ call for a merged model of resilience combining neurobiological and psychosocial factors to enhance understanding of the underlying circuits and mechanisms of resilience (13). Although previous research has strongly implicated white matter abnormalities in the pathogenesis of geriatric vascular depression (24–26), this study is the first to investigate the relation between white matter microstructure and resilience in geriatric depression. Our novel approach of using resilience factors previously identified in an EFA conducted in a large sample of older adults with depression allowed the identification of white matter pathways uniquely associated with grit. To the best of our knowledge, this is the first study to directly test for neural underpinnings of distinct resilience factors and is thus an important step in improving our understanding of the neurobiology of resilience.
Conclusion
Our findings provide further support for the conceptualization of resilience as a complex system comprised of multiple factors by showing distinct links with pathway-specific brain white matter integrity. The association between “grit” with FA in the GCC and CB is consistent with prior research indicating the involvement of these regions in emotion regulation and cognitive control. However, the association between “grit” and FA in the CB did not survive correction for multiple comparisons and should therefore be interpreted with caution. Future studies will benefit from larger samples of both depressed and non-depressed individuals, the use of multimodal brain imaging, and comprehensive assessment of stress responsivity to further our understanding of the neurobiological mechanisms of resilience and eventually facilitate the development of more personalized treatments for geriatric depression.
Highlights.
White matter integrity in the Genu of the corpus callosum and cingulum was associated with the Grit factor of resilience in geriatric depression.
No white matter integrity correlates were found for the other resilience factors (Active Coping Self-efficacy, Accommodative Coping Self-efficacy, and Spirituality).
These results provide further support for the conceptualization of resilience as a complex dynamic system comprised of multiple factors with distinct underlying neurobiology.
Acknowledgments
This work was supported by NIH grants AT008383, AT009198, MH097892, and Alzheimer’s Research and Prevention Foundation grants to Dr. Lavretsky.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Lavretsky received research support from Allergan/ Forest Laboratories. The remaining authors declare no conflicts of interest.
References
- 1.He W, Goodkind D, Kowal P. An Aging World 2015 [updated March 2016. United States Census Bureau website.] Available from: https://www.census.gov/library/publications/2016/demo/P95-16-1.html.
- 2.Luppa M, Sikorski C, Luck T, Ehreke L, Konnopka A, Wiese B, et al. Age- and gender-specific prevalence of depression in latest-life--systematic review and meta-analysis. Journal of affective disorders. 2012;136:212–21. doi: 10.1016/j.jad.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 3.Wald J, Taylor S, Asmundson GJG, Jang L, Stapleton J, editors. Literature review of concepts: psychological resiliency. Toronto (ON): Defence R&D Canada: 2006. [Google Scholar]
- 4.MacLeod S, Musich S, Hawkins K, Alsgaard K, Wicker ER. The impact of resilience among older adults. Geriatric nursing (New York, NY) 2016;37:266–72. doi: 10.1016/j.gerinurse.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Zeng Y, Shen K. Resilience significantly contributes to exceptional longevity. Current Gerontology and Geriatrics Research. 2010;2010:1–9. doi: 10.1155/2010/525693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson JRT, Payne VM, Connor KM, Foa EB, Rothbaum BO, Hertzberg MA, et al. Trauma, resilience and saliostasis: effects of treatment in post-traumatic stress disorder. International clinical psychopharmacology. 2005;20(1):43–8. doi: 10.1097/00004850-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Horn SR, Charney DS, Feder A. Understanding resilience: New approaches for preventing and treating PTSD. Experimental neurology. 2016;284:119–32. doi: 10.1016/j.expneurol.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Connor KM, Davidson JRT. Development of a new resilience scale: the Connor- Davidson Resilience Scale (CD-RISC) Depression and anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 9.Friborg O, Hjemdal O, Rosenvinge JH, Martinussen M. A new rating scale for adult resilience: what are the central protective resources behind healthy adjustment? International journal of methods in psychiatric research. 2003;12:65–76. doi: 10.1002/mpr.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. International journal of behavioral medicine. 2008;15:194–200. doi: 10.1080/10705500802222972. [DOI] [PubMed] [Google Scholar]
- 11.Windle G, Bennett KM, Noyes J. A methodological review of resilience measurement scales. Health and quality of life outcomes. 2011;9(1):8. doi: 10.1186/1477-7525-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Werff SJA, van den Berg SM, Pannekoek JN, Elzinga BM, van der Wee NJA. Neuroimaging resilience to stress: a review. Frontiers in behavioral neuroscience. 2013;7:39. doi: 10.3389/fnbeh.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature reviews Neuroscience. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osorio C, Probert T, Jones E, Young AH, Robbins I. Adapting to Stress: Understanding the Neurobiology of Resilience. Behavioral medicine (Washington, DC) 2016:1–16. doi: 10.1080/08964289.2016.1170661. [DOI] [PubMed] [Google Scholar]
- 15.Peres JFP, Foerster B, Santana LG, Fereira MD, Nasello AG, Savoia M, et al. Police officers under attack: resilience implications of an fMRI study. Journal of psychiatric research. 2011;45:727–34. doi: 10.1016/j.jpsychires.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto T, Toki S, Siegle GJ, Takamura M, Takaishi Y, Yoshimura S, et al. Increased amygdala reactivity following early life stress: a potential resilience enhancer role. BMC psychiatry. 2017;17:27. doi: 10.1186/s12888-017-1201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Workman CI, Lythe KE, McKie S, Moll J, Gethin JA, Deakin JFW, et al. A novel resting-state functional magnetic resonance imaging signature of resilience to recurrent depression. Psychological medicine. 2017;47:597–607. doi: 10.1017/S0033291716002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffens DC, Wang L, Manning KJ, Pearlson GD. Negative Affectivity, Aging, and Depression: Results From the Neurobiology of Late-Life Depression (NBOLD) Study. The American Journal of Geriatric Psychiatry. 2017 doi: 10.1016/j.jagp.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bracht T, Linden D, Keedwell P. A review of white matter microstructure alterations of pathways of the reward circuit in depression. Journal of affective disorders. 2015;187:45–53. doi: 10.1016/j.jad.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 20.Gatt JM, Korgaonkar MS, Schofield PR, Harris A, Clark CR, Oakley KL, et al. The TWINE project in emotional wellbeing: study protocol and preliminary heritability results across four MRI and DTI measures. Twin research and human genetics. 2012;15(3):419–41. doi: 10.1017/thg.2012.12. [DOI] [PubMed] [Google Scholar]
- 21.Houenou J, Emsell L. DTI in Psychiatry. In: Hecke VW, Emsell Louise, Sunaert S, editors. Diffusion Tensor Imaging. Springer; 2016. pp. 359–72. [Google Scholar]
- 22.Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P, et al. White matter tract integrity in aging and Alzheimer's disease. Human brain mapping. 2009;30(4):1051–9. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power MC, Tingle JV, Reid RI, Huang J, Sharrett AR, Coresh J, et al. Midlife and Late-Life Vascular Risk Factors and White Matter Microstructural Integrity: The Atherosclerosis Risk in Communities Neurocognitive Study. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease. 2017;6(5):e005608. doi: 10.1161/JAHA.117.005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. 'Vascular depression' hypothesis. Archives of general psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. The American journal of psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 26.Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 1999;7:309–16. [PubMed] [Google Scholar]
- 27.Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, et al. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2740–6. doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galinowski A, Miranda R, Lemaitre H, Paillere Martinot M-L, Artiges E, Vulser H, et al. Resilience and corpus callosum microstructure in adolescence. Psychological medicine. 2015;45:2285–94. doi: 10.1017/S0033291715000239. [DOI] [PubMed] [Google Scholar]
- 29.Frodl T, Carballedo A, Fagan AJ, Lisiecka D, Ferguson Y, Meaney JF. Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. Journal of psychiatry & neuroscience : JPN. 2012;37:37–45. doi: 10.1503/jpn.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laird KT, Lavretsky H, Paholpak P, Vlasova RM, Roman M, St Cyr N, et al. Clinical correlates of resilience factors in geriatric depression. Int Psychogeriatr. 2018:1–10. doi: 10.1017/S1041610217002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavretsky H, Reinlieb M, St Cyr N, Siddarth P, Ercoli LM, Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(6):561–9. doi: 10.1176/appi.ajp.2014.14070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Psychiatric A, American Psychiatric A, Task Force on D-I. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 33.American Psychiatric A. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- 34.Cosco TD, Kaushal A, Richards M, Kuh D, Stafford M. Resilience measurement in later life: a systematic review and psychometric analysis. Health and quality of life outcomes. 2016;14:16. doi: 10.1186/s12955-016-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003;20:870–88. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 36.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage. 2014;92:381–97. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu JL, Leemans A, Bai CH, Lee CH, Tsai YF, Chiu HC, et al. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage. 2008;39(2):566–77. doi: 10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Inano S, Takao H, Hayashi N, Abe O, Ohtomo K. Effects of Age and Gender on White Matter Integrity. American Journal of Neuroradiology. 2011;32(11):2103. doi: 10.3174/ajnr.A2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrio-Arranz G, de Luis-García R, Tristán-Vega A, Martín-Fernández M, Aja-Fernández S. Impact of MR Acquisition Parameters on DTI Scalar Indexes: A Tractography Based Approach. PLoS ONE. 2015;10(10):e0137905. doi: 10.1371/journal.pone.0137905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duckworth AL, Quinn PD. Development and validation of the Short Grit Scale (GRIT–S) Journal of personality assessment. 2009;91(2):166–74. doi: 10.1080/00223890802634290. [DOI] [PubMed] [Google Scholar]
- 42.Yasuno F, Kudo T, Matsuoka K, Yamamoto A, Takahashi M, Nakagawara J, et al. Interhemispheric functional disconnection because of abnormal corpus callosum integrity in bipolar disorder type II. British Journal of Psychiatry Open. 2016;2 doi: 10.1192/bjpo.bp.116.002683. 335 LP - 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Ji B, Hu J, Zhou C, Li L, Li Z, et al. Aberrant interhemispheric homotopic functional and structural connectivity in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery & Psychiatry. 2016 doi: 10.1136/jnnp-2016-314567. [DOI] [PubMed] [Google Scholar]
- 44.Kim S, Lee D. Prefrontal Cortex and Impulsive Decision Making. Biological psychiatry. 2011;69:1140–6. doi: 10.1016/j.biopsych.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, et al. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PloS one. 2012;7:e48107. doi: 10.1371/journal.pone.0048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duckworth A, Gross JJ. Self-control and grit: Related but separable determinants of success. Current Directions in Psychological Science. 2014;23(5):319–25. doi: 10.1177/0963721414541462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:610–7. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- 48.Yip SW, Lacadie C, Xu J, Worhunsky PD, Fulbright RK, Constable RT, et al. Reduced genual corpus callosal white matter integrity in pathological gambling and its relationship to alcohol abuse or dependence. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2013;14:129–38. doi: 10.3109/15622975.2011.568068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cyprien F, Courtet P, Malafosse A, Maller J, Meslin C, Bonafe A, et al. Suicidal behavior is associated with reduced corpus callosum area. Biological psychiatry. 2011;70:320–6. doi: 10.1016/j.biopsych.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 50.Lischke A, Domin M, Freyberger HJ, Grabe HJ, Mentel R, Bernheim D, et al. Structural Alterations in the Corpus Callosum Are Associated with Suicidal Behavior in Women with Borderline Personality Disorder. Frontiers in human neuroscience. 2017;11:196. doi: 10.3389/fnhum.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metzler-Baddeley C, Jones DK, Steventon J, Westacott L, Aggleton JP, O'Sullivan MJ. Cingulum Microstructure Predicts Cognitive Control in Older Age and Mild Cognitive Impairment. The Journal of Neuroscience. 2012;32 doi: 10.1523/JNEUROSCI.3299-12.2012. 17612 LP - 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keedwell PA, Doidge AN, Meyer M, Lawrence N, Lawrence AD, Jones DK. Subgenual Cingulum Microstructure Supports Control of Emotional Conflict. Cerebral Cortex (New York, NY) 2016;26:2850–62. doi: 10.1093/cercor/bhw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaffey AE, Bergeman CS, Clark LA, Wirth MM. Aging and the HPA axis: Stress and resilience in older adults. Neuroscience & Biobehavioral Reviews. 2016;68:928–45. doi: 10.1016/j.neubiorev.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drozd R, Cieslak PE, Rychlik M, Rodriguez Parkitna J, Rygula R. Cognitive Judgment Bias Interacts with Risk Based Decision Making and Sensitivity to Dopaminergic Challenge in Male Rats. Frontiers in behavioral neuroscience. 2016;10:163. doi: 10.3389/fnbeh.2016.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolpe N, Nombela C, Rowe JB. Dopaminergic modulation of positive expectations for goal-directed action: evidence from Parkinson's disease. Front Psychol. 2015;6:1514. doi: 10.3389/fpsyg.2015.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dreher J-C, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proceedings of the National Academy of Sciences. 2008;105(39):15106. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gunning FM, Smith GS. FUNCTIONAL NEUROIMAGING IN GERIATRIC DEPRESSION. The Psychiatric clinics of North America. 2011;34(2) doi: 10.1016/j.psc.2011.02.010. 403-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller L, Bansal R, Wickramaratne P, Hao X, Tenke CE, Weissman MM, et al. Neuroanatomical correlates of religiosity and spirituality: a study in adults at high and low familial risk for depression. JAMA psychiatry. 2014;71:128–35. doi: 10.1001/jamapsychiatry.2013.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kapogiannis D, Barbey AK, Su M, Zamboni G, Krueger F, Grafman J. Cognitive and neural foundations of religious belief. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4876–81. doi: 10.1073/pnas.0811717106. [DOI] [PMC free article] [PubMed] [Google Scholar]