Abstract
Hypertension affects nearly one third of the adult US population and is a significant risk factor for chronic kidney disease (CKD). An expanding body of recent studies indicates that gut microbiome has crucial roles in regulating physiological processes through, among other mechanisms, one mode of short chain fatty acids (SCFA) and their target receptors. In addition, these SCFA receptors are potential targets of regulation by host miRNAs, however, the mechanisms through which this occurs is not clearly defined. Hydrogen sulfide (H2S) is an important gasotransmitter involved in multiple physiological processes and is known to alleviate adverse effects of hypertension such as reducing inflammation in the kidney. To determine the role of host microRNAs in regulating short chain fatty acid receptors in the kidney as well as the gut, C57BL/6J wild-type mice were treated with or without Ang-II and H2S donor GYY4137 (GYY) for 4 weeks to assess whether GYY would normalize adverse effects observed in hypertensive mice and whether this was in part due to altered gut microbiome composition. We observed several changes of SCFA receptors, including Olfr78, Gpr41/43 and predicted microRNA regulators in the kidney among the different treatments. Increased expression of inflammatory markers Il6 and Rorc2, along with Tgfβ, were found in the hypertensive kidney. The glomerular filtration rate (GFR) was improved in mice treated with Ang-II+GYY compared with Ang-II only, indicating improved kidney function. The Erysipelotrichia class of bacteria, linked with high fat diets, was enriched in hypertensive animals but reduced with GYY supplementation. These data point towards a role for miRNA regulation of SCFA receptors in hypertensive kidney and are normalized by H2S supplementation.
Keywords: Gut microbiome, hydrogen sulfide, short chain fatty acid, microRNA
Graphical Abstract
1. Introduction
Hypertension is a complex etiological disease affecting as many as 874 million adults globally with the prevalence increasing annually (Benjamin et al., 2018; Forouzanfar et al., 2017). Hypertension is a noted risk factor for stroke, cardiovascular disease, and chronic kidney disease (CKD), the latter of which can progress to end-stage renal disease (ESRD), with higher incidence rates in individuals who are overweight, diabetic, aged intake high amounts of salt or have altered sleep patterns (Botdorf et al., 2011; Judd and Calhoun, 2015; Townsend and Taler, 2015). One of the primary drivers of increased blood pressure is angiotensin-II (Ang-II), a hormone that is activated, in part, by kidney-produced renin and subsequently travels throughout the body and adversely affecting multiple organ systems through increased inflammation and oxidative damage (Montezano et al., 2014; Pushpakumar et al., 2017; Yim and Yoo, 2008). As hypertension is a multifactorial disease, so are the prescribed methods to combat its affects, including changes to diet, establishing an exercise regimen, renal denervation, as well as pharmaceutical intervention in the form of antihypertensive drug treatments (Appel et al., 2006; Barlow et al., 2006; Chobanian et al., 2003; Oparil and Schmieder, 2015; Pescatello et al., 2004). While these treatment approaches can improve blood pressure, not all individuals are responsive, reinforcing the complexity of hypertension as well as highlighting the need to identify new mechanisms and pathways that can be utilized in regulating blood pressure.
Recent studies have highlighted the importance of gut microbiota on physiological processes, such as metabolism, and have been linked with several chronic diseases, including diabetes, obesity, CKD, cardiovascular disease and hypertension (Aron-Wisnewsky and Clement, 2016; Mell et al., 2015; Tang et al., 2017; Wenzel et al., 2016). Some of the byproducts of gut microbiota are metabolites known as short chain fatty acids (SCFAs), mainly acetic acid, propionic acid, and butyric acid, can diffuse into the bloodstream and affect multiple tissues and processes, including metabolism, energy balance and the immune system (Maslowski et al., 2009; Samuel et al., 2008; Scheithauer et al., 2016; Turnbaugh et al., 2006). Moreover, SCFAs have been implicated in blood pressure regulation through interaction with target receptors. Olfr78 is a SCFA receptor found in the kidney and has been shown to respond to SCFA stimulation by increasing blood pressure through increased renin release and subsequent vasoconstriction (Pluznick et al., 2013). Conversely, Gpr41 SCFA receptor has been shown to have hypotensive effects as Gpr41 knockout mice have increased systolic blood pressure (Natarajan et al., 2016). Evidence from human studies also support a role for the gut microbiome and SCFAs in blood pressure regulation by examining changes in microbiota to interventions such as probiotics (Khalesi et al., 2014). While several associations exist between gut microbiota SCFAs and blood pressure, regulation of these receptors are not clearly defined.
The gaseous molecule, hydrogen sulfide (H2S) is involved in regulating several physiological processes, as well as having a role in blood pressure regulation (Sikora et al., 2014; Tomasova et al., 2016; Weber et al., 2017; Yoo et al., 2015). In the kidney, H2S has been shown to mitigate kidney damage due to aging, hyperhomocysteinemia-induced damage, and hyperglycemic-induced matrix remodeling and deposition (Kundu et al., 2014; Lee et al., 2018; Sen et al., 2012). Recently, H2S production in the colon by gut microbiota has been linked with blood pressure regulation as well as the bioavailability on the whole organism (Shen et al., 2013; Tomasova et al., 2016). Sulfate-reducing bacteria are present in the gut of humans, with one of the main species being Desulfovibrio piger, and is influenced by factors, such as diet (Rey et al., 2013). However, the role and influence of H2S on the gut microbiome genera and on renal function, in particular SCFA receptors, remains to be elucidated.
In this study, we investigated the impact of exogenous administration of the H2S donor GYY 4137 on the gut microbiome composition as well as SCFA target receptors in the hypertensive kidney. MicroRNAs are small, highly conserved non-coding RNAs that regulate gene expression and have been implicated in several kidney diseases and hypertension (Eskildsen et al., 2013; Khella et al., 2013; Marques et al., 2011). In addition, SCFA receptors have been shown to play a role in blood pressure regulation in the kidney; however, the regulation of these receptors has not been evaluated.
2. Materials and Methods
2.1. Animals
WT (C57BL/J) male mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in the animal care facility on a 12 hour light/dark cycle at the University of Louisville and the study was approved by University of Louisville Institutional Animal Care and Use Committee. All mice were fed standard chow (LabDiet 5010, St. Louis, MO), with free access to food and water. Animals aged 10–12 weeks were grouped based on treatment, with an ‘n’ of 5 per group: WT, WT+GYY4137, WT+Ang-II, WT+GYY4137+Ang-II. Administration of Ang-II (Sigma, St. Louis, MO) was at a rate of 1000ng/kg−1/min−1 for 4 weeks via Alzet mini osmotic pumps (Durect Corp., Cupertino, CA) and inserted subcutaneously on back right of mice. GYY4137 (Sigma, St. Louis, MO) treatments were given by intraperitoneally injection (133μM/kg−1/d−1) (Weber et al., 2017).
2.2. Cell culture and transfections and target prediction
Mouse Glomerular Endothelial Cells were maintained in Endothelial Cell Medium + 10% FBS and antibiotics from passage 12–16. Cells were transfected with mimics, or inhibitor, for miR-129-5p, miR-132-3p, and miR-329, as well as controls, obtained from Exiqon (Woburn, MA). Cells were seeded at 30% confluency in growth medium without antibiotics the day before transfections. A concentration of 50nM for mimics, inhibitors, and controls were transfected using Lipofectamine RNAiMAX transfection reagent according to manufacturer’s recommendations. Cells were transfected for a period of 48 hours and subsequently collected in lysis buffer for Western blot analysis. TargetScan (http://www.targetscan.org/) and miRWalk 2.0 (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) were utilized to identify potential targets of microRNAs. Default settings were used interrogating the mouse database and lists were investigated for potential targets of interest.
2.3. RNA isolation
Total RNA was isolated from a portion of the kidney from all animals for each group and was used for downstream assays. Tissue was homogenized in QIAzol reagent using a BenchMark BeadBlaster (Edison, NJ) using 45 second pulses for six times and subsequently extracted and purified using the Qiagen miRNeasy Mini Kit (Valencia, CA) according to manufacturer’s protocol. RNA concentration and quality was assessed using a NanoDrop Instrument (Thermo Scientific, Wilmington, DE)
2.4. DNA isolation and 16S sequencing
Microbial and host genomic DNA was isolated from fecal samples from mice at the end of the treatment period. DNA was extracted utilizing the ThermoFisher PureLink Microbiome DNA Purification Kit (ThermoFisher, Waltham, MA) and sent to the University of Louisville Genomics Core for library preparation and sequencing. Libraries were prepared using Illumina’s 16S library preparation guide and the Nextera Index Kit (FC-121-1012). Sequencing was done using Illumina MiSeq Reagents Kit v3 (MS-102-3003, 600 cycles) on an Illumina MiSeq instrument. Sequencing data was analyzed by the University of Louisville Bioinformatics Core to determine species composition and diversity using Qiime 1.9.
2.5. Polymerase chain reaction
Genomic DNA (gDNA) was extracted from kidney tissue using the Qiagen DNeasy Blood and Tissue Kit (Valencia, CA) according to the manufacturer’s recommendations. The quality and quantity was assessed using a Nanodrop 1000 spectrophotometer. PCR was performed on 100ng of sample using GoTaq Hot Start Master Mix (Promega, Madison, WI) with primers designed through Primer3 software. Products were run out on a 1.0% agarose gel and visualized on a ChemiDoc XRS system (Bio-Rad, Hercules, CA). Primer sequences are listed in Table 1.
Table 1.
Primers used for qPCR.
| Gene | Forward | Reverse |
|---|---|---|
| Olfr78 | ACGGTGCTAAGACCAAACAGAT | AGGGTGATAGGATGGTAAGGGT |
| Gpr41 | GACTCCTCCTAGCTCCTCAGAA | CTGTCTGGAAAACGCTCACAAG |
| Gpr43 | GAAAGGTTTGCTACTGATCCGC | TGTACCCCTTCTGCTTGACTTC |
| Gpr120 | AACAAGACTACCGACTCTTCCG | GGAGGATGGTGATGATGATGGG |
| Rorc1 | AGCAAGGACGGCACCAAG | GATGTCCGGTGGTGTCTCTG |
| Rorc2 | CTTCACCCCACCTCCACTG | CTGAGAACTTGGCTCCCTGTC |
| Tgfβ | GACGAGCTGGTTGAGAGAAGAG | GCAGCGGAAAAGTCTCAAAACT |
| Il6 | CTGCAAGAGACTTCCATCCAGT | CAGGTCTGTTGGGAGTGGTATC |
| Gapdh | GTCAAGGCCGAGAATGGGAA | GGCCTCACCCCATTTGATGT |
| 16S rRNA | GATCATGGCTCAGATTGAACGC | TTTCCCAGACATTACTCACCCG |
2.6. Quantitative PCR
Quantitative PCR was carried out using the Bullseye EvaGreen qPCR MasterMix (MidSci, Valley Park, MO) on RNA samples extracted from tissue. 1 μg of total RNA was transcribed into cDNA using the Bio-Rad iScript Select cDNA Synthesis Kit (Bio-Rad, Hercules, CA) with primers designed against each gene using Primer3 software (Primer3). Subsequent qPCR reactions were setup and run on a Roche LightCycler 96 instrument (Roche, Indianapolis, IN) and run in triplicate using Gapdh as a reference. Table 1 contains primer sequences utilized.
2.7. Western blotting
Whole kidney tissues were homogenized with RIPA buffer (Boston BioProducts, Inc., Ashland, MA) containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and phenylmethylsulfonyl fluoride. 100 μg of protein from each sample were loaded and electrophoresed in sodiumdodecyl sulphate-polyacrylamide gel electrophoresis, and transferred onto PVDF membranes. Membranes were probed with appropriate primary antibody at 4°C overnight. Olfr78 (Abcam, ab140907), Gpr41 (Sigma, SAB4501281), Gpr43 (Santa Cruz, sc293202), Tgfβ (Abcam, ab64715), Rorcγ (Abcam, ab207082), Il6 (Abcam, ab6672), Gpr120, (ThermoFisher, PA5-50973), Gapdh (Santa Cruz, sc32233). Respective Horseradish Peroxidase-conjugated secondary antibody incubation was for 2 h at room temperature. Membranes were developed using chemiluminescence (Pierce ECL Western blotting substrate; Thermo Scientific, Rockford, IL). Gapdh was used as loading control and band intensity was quantified using ImageJ software.
2.8. Immunofluorescence staining
Frozen kidney sections of 5 μm in thickness were fixed in ice cold acetone. Immunofluorescence staining was performed for Olfr78, Gpr41, Gpr43, Gpr120, Il-6, Tgfβ, Rorc with primary antibody incubation for overnight and subsequently incubated with appropriate secondary antibody and counterstained with DAPI. Images were captured by confocal microscope (FluoView1000; Olympus) and analyzed by Image ProPlus, version 7.0 (Media Cybernetics, Inc., Rockville, MD).
2.9. ELISA
Plasma samples collected at the end of the treatment period were measured for the haptoglobin protein using an ELISA kit (Abcam ab157714, Cambridge, MA).
2.10. Glomerular filtration rate measurement
GFR measurements were taken based on previous studies (Schock-Kusch et al., 2013; Schreiber et al., 2012) with slight modifications. Mice were anesthetized with inhaled isoflurane and Nair™ was applied to a small area of the back to remove hair using a dampened gauze pad and sterile water. Next, a detecting device that can measure fluorescent compounds transcutaneously in blood was fixed to the shaved area of the mouse. FITC-sinistrin was dissolved in saline solution and injected into the femoral vein at a dose of 15mg/100 g body weight and monitored for 2 hours after injection. Data was analyzed using MPD Studio software (Mannheim Pharma and Diagnostics, Amtsgericht Mannheim, Germany). GFR was calculated by obtaining the half-life of the FITC-sinistrin curve and using the rate constant value as described (Schreiber et al., 2012).
2.11. Statistical analysis
All results are expressed as ± Standard Deviation. Comparison between the groups was done using one-way ANOVA followed by a least squared difference (LSD) post-hoc test using Prism 5.0 (Graph Software, San Diego, CA). Significance was achieved with a p-value of p < 0.05.
3. Results
3.1. Gut microbiota alterations
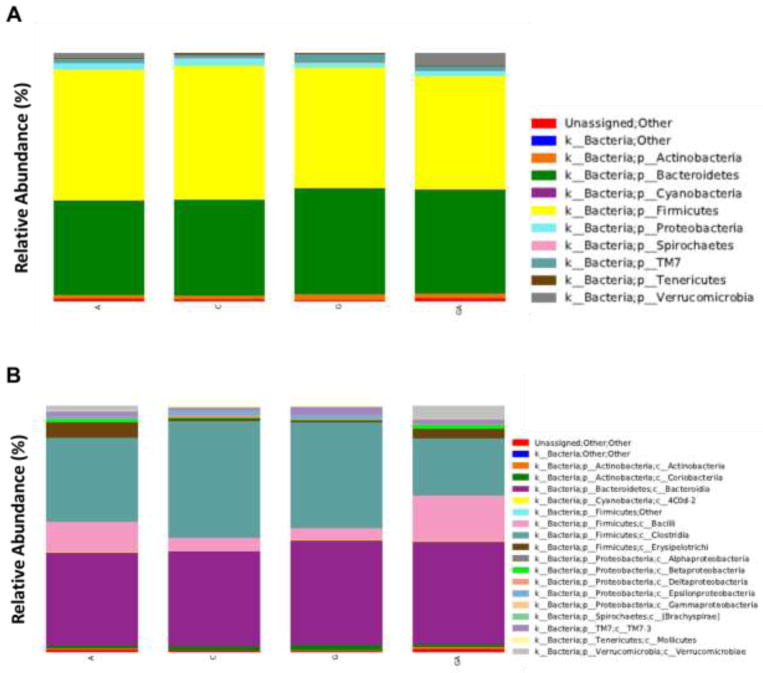
We employed 16S rRNA sequencing to samples extracted from stool from each of the four groups at the end of the treatment period to gain a perspective on genra abundance in each treatment group in each phyla. We did not observe a substantial shift in the relative abundance of Firmicutes or Bacteroidetes in the control (52.7%) and Ang-II (53.7%) treatment groups (Fig. 1A). However, the abundance of Firmicutes was decreased in GYY only (48.5%) and Ang-II+GYY (45.7%) groups with an accompanying increase in Bacteroidetes adundance (Fig. 1A). Examining more in depth, we did observe a substancial higher abundance in Erysipelotrichi of the Firmicutes phylum compared to the other three groups (Fig. 1B).
Fig. 1.
16S rRNA profiling of gut microbiome in mice treated with Ang-II and GYY. (A) Relative abundance of total bacteria at the genus level in each treatment group. (B) Relative abundance of total bacteria at the class level for each treatment group. Abundance is expressed as a percentage. C – Control, A – Ang-II, G – GYY, AG – Ang-II+GYY.
3.2. Short chain fatty acid receptors are altered in hypertension kidney
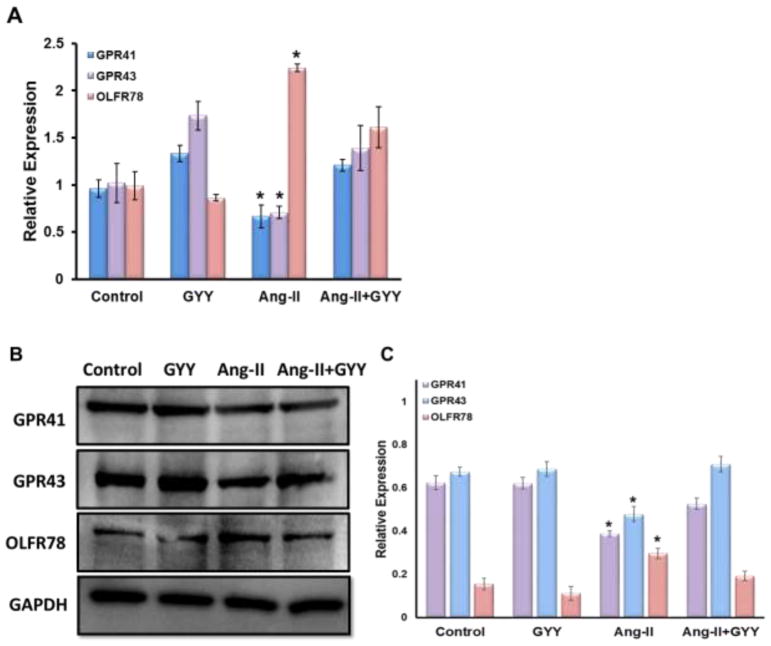
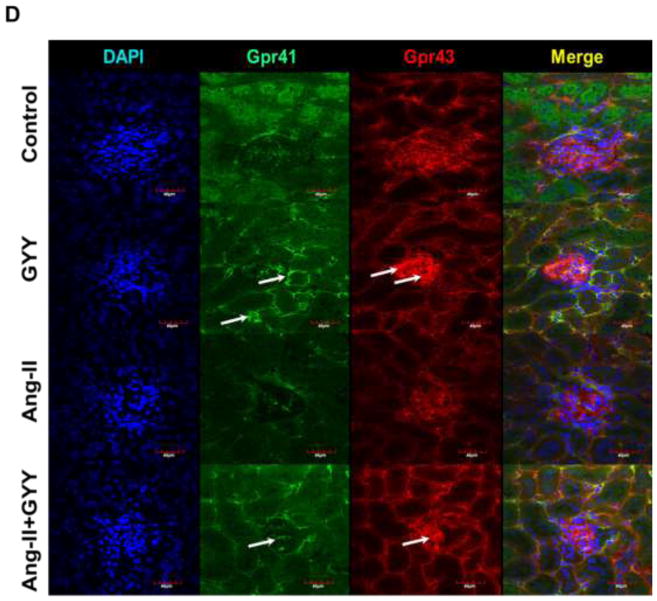
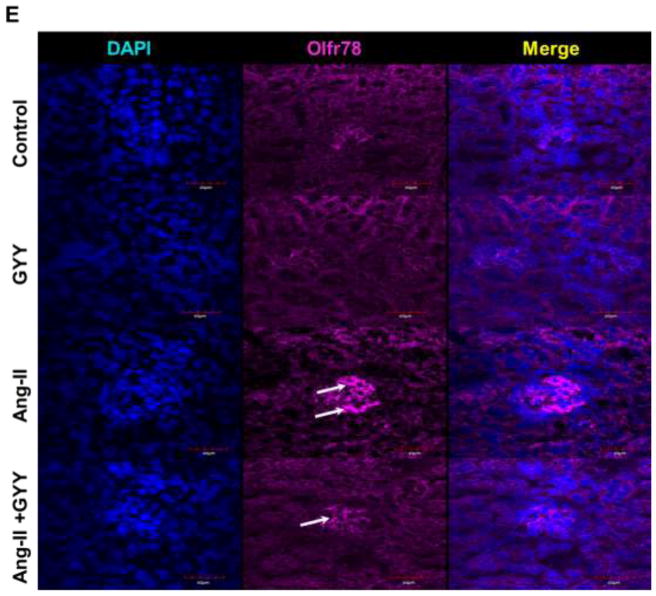
To determine whether Ang-II induced hypertension affected the expression of SCFA receptors in the kidney, we performed qPCR analysis on three different SCFA receptors that have been shown to have a role in hypertension and immunity. Olfr78 expression, which was previously shown to promote vasoconstriction, was found to be upregulated in the hypertension kidney (Fig. 2A). Gpr41 and Gpr43 expression, on the other hand, were shown to be downregulated in the hypertensive kidney (Fig. 2A). GYY supplementation reduced the levels of Olfr78 as well as induced Gpr41 and Gpr 43 expression, bringing them closer to control levels (Fig. 2A). These results were supported by Western blot analysis with Ang-II treated animals having higher expression of Olfr78 and lower levels of Gpr41 and Gpr43 (Fig. 2B–C). Protein levels of all three receptors were normalized in mice supplemented with GYY. We observed that Gpr41 was localized to tubules and vessels in the kidney while Gpr43 was more concentrated in the glomerulus (Fig. 2D). We found Olfr78 to be expressed in the glomerulus as well, with minimal expression in all but hypertensive kidney (Fig. 2E).
Fig. 2.
Short chain fatty acid receptors are altered in the hypertensive kidney. (A) qPCR analysis of Olfr78, Gpr41, and Gpr43 expression in kidney of Ang-II and GYY treated mice. (B) Western blot analysis and (C) bar graph of protein levels of SCFA receptors. Band intensities were quantified using ImageJ. Bar graphs are the mean intensities ± SD. n=5/group. *p < 0.05 Control vs. Ang-II; (D–E) Immunostaining for Gpr41 and Gpr 43 showed increased expression in glomeruli and proximal tubules (white arrows). (F) Immunostaining for Olfr78 showed increased expression and localization in the glomerulus. Magnification, ×60. Scale bars, 40 μm.
3.3. Ang-II promotes inflammation in the hypertensive kidney and is suppressed by GYY supplementation
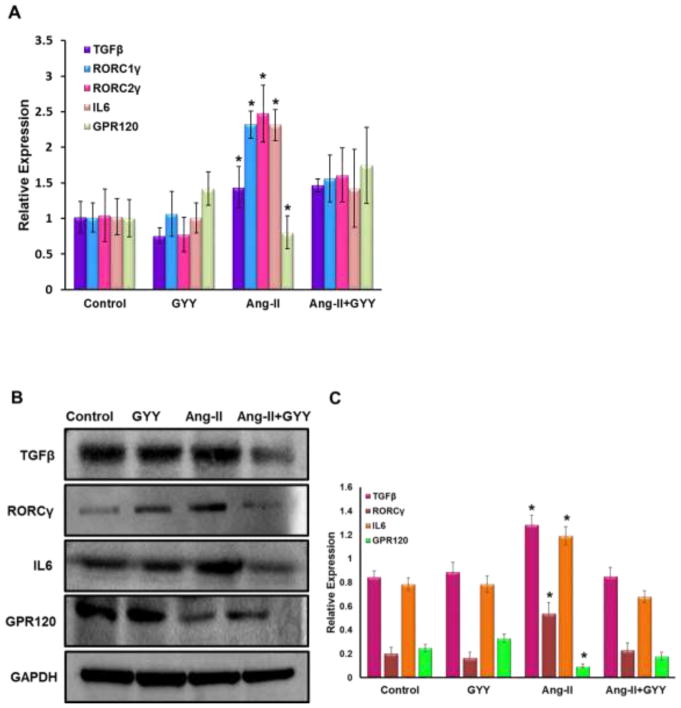
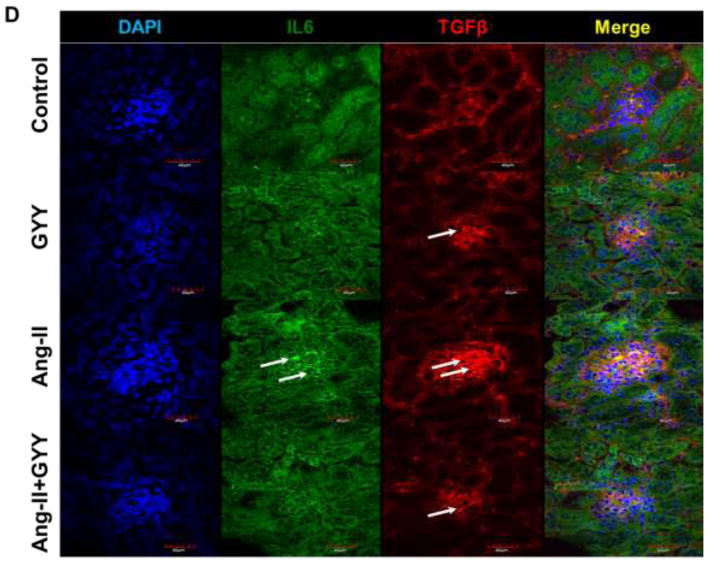
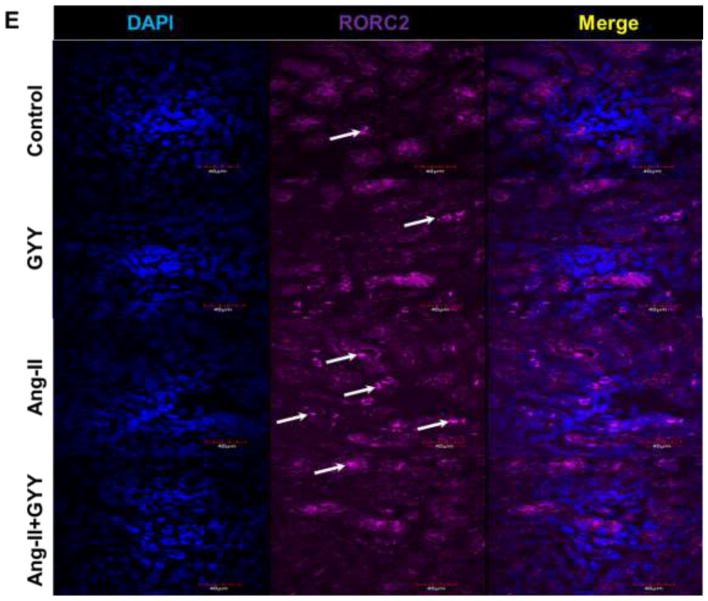
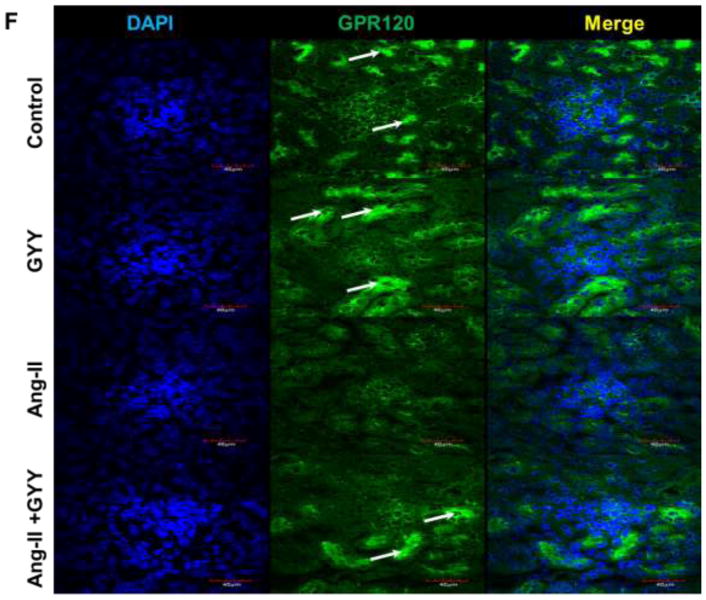
Previous work has shown Ang-II promotes inflammation-induced kidney injury, in part, through Tgfβ pathway (Pushpakumar et al., 2017). In addition, Retinoic acid-related orphan receptor gamma t (Rorγt) is a transcription factor important in T-cell development as well as promoting Il-17a productions, a cytokine implicated in hypertension (Madhur et al., 2010; Rutz et al., 2016). Gene expression analysis revealed inflammation-promoting targets Tgfβ, Il-6, and both isoforms of Rorγ (Rorc1γ/Rorc2γ) were upregulated in the hypertensive kidney (Fig. 3A). Gpr120 expression, which is a long chain fatty acid receptor with reported anti-inflammatory effects (Oh et al., 2010), was found to be downregulated in the hypertensive kidney (Fig. 3A). GYY supplementation reduced levels of inflammatory markers as well as increased Gpr120 expression (Fig. 3A). These results were also observed at the protein level (Fig. 3B–C). Tgfβ and Il6 expression were found to be localized in the glomerulus whereas Rorc2γ was increased in the vessels and tubules (Fig. 3D–E). Gpr120 expression followed a similar pattern to that of Rorc2γ and was elevated in GYY treated kidneys (Fig. 3F).
Fig. 3.
Ang-II induced inflammatory response in kidney. (A) Inflammatory markers were assessed by qPCR, including Tgfβ, Il6, Rorc1γ, Rorc2γ, and reported anti-inflammatory fatty acid receptor Gpr120. (B) Western blot analysis and (C) bar graph of protein levels inflammatory markers and long chain fatty acid receptor Gpr120. Bar graphs are the mean intensities ± SD. n=5/group. *p < 0.05 Control vs. Ang-II; (D) Immunostaining for Tgfβ and Il6 showed increased expression and localization in glomeruli (white arrows). (E) Immunostaining for Rorcγ showed increased expression and localization in the tubules and vessels around the glomerulus while (F) Gpr120 was less in hypertensive kidney (white arrows). GYY increased expression of GPR 120. Magnification, ×60. Scale bars, 40 μm.
3.4. miRNA regulation of short chain fatty acid receptors
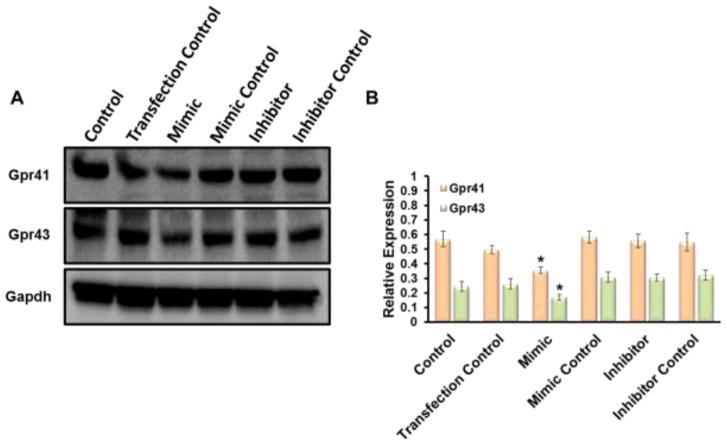
In an effort to determine if miRNAs had a regulatory role for short chain fatty acid receptors in the kidney, we transfected mouse glomerular endothelial cells with miRNA mimics and inhibitors for miR-132 and miR-329. These miRNAs were previously found to have been altered in the hypertensive kidney (Weber et al., 2017) and in silico analysis of these miRNAs revealed potential targeting of SCFA receptors by these miRNAs, with miR-132 targeting Gpr43 and miR-329 and Gpr41. Mimics of all three miRNAs transfected into cells showed a moderate decrease in target protein expression (Fig. 4A–B). Inhibitors and controls transfected into cells revealed no significant changes in target protein expression (Fig. 4B).
Fig. 4.
miRNAs target SCFA receptors in vitro. Mouse Glomerular Endothelial Cells were transfected with mimics, inhibitors, and controls, against miR-132 and miR-329. In silico analysis using target software predicted miR-132 would target Gpr43 and miR-329 binding to Gpr41. (A) One hundred μg of protein was resolved on SDS-PAGE gels and probed with appropriate antibodies overnight. Band intensities were quantified using ImageJ. (B) Bar graphs are the mean intensities ± SD. n=4/group. *p < 0.05.
3.5. Kidney function is improved with GYY supplementation
Glomerular filtration rate (GFR) remains an important measurement in determining kidney function. To determine if kidney function is reduced in Ang-II treated mice, we injected fluorescently-labeled sinistrin (FITC-sinistrin) into the femoral vein of anesthetized mice and attached a detector to transcuteaneously measure the efficiency of FITC-sinistrin being filtered by the kidney. We found the GFR to be significantly reduced in mice treated with Ang-II, but was normalized when supplemented with GYY (Fig. 5). GYY treatment alone induced GFR, albeit not significantly (Fig. 5).
Fig. 5.
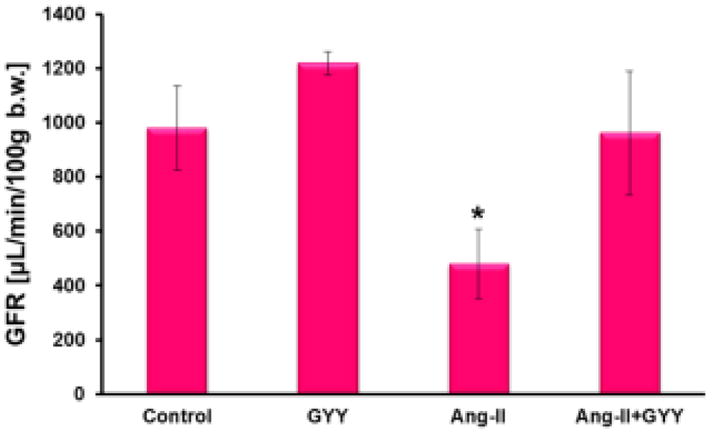
Glomeruluar filtration rate is improved in mice supplemented with GYY. Live, conscious mice were anesthetized with isoflurane and injected with FITC-sinistrin (15 mg/100 g) into the femoral artery. Detector was fixed to area removed of hair and mice were put back into cages while the detector took measurements for 2 hours. GFR was calculated after obtaining half-life of excreted FITC-sinistrin. Bar graph, n=5/group. *p < 0.05
3.6. Intestinal integrity
Zonulin is an intestinal structural protein known to have a role in maintaining intestinal integrity by maintaining tight junction proteins as well as a noted promoter of inflammation (Kim et al., 2018; Li et al., 2016; Lukaszyk et al., 2018). Haptoglobin is a preproprotein which can be processed into zonulin and a potential marker for barrier integrity (Ficek et al., 2017). Using an ELISA, we found circulating plasma levels of haptoglobin were increased in hypertensive mice compared to controls (Fig. 6A). Mice supplemented with GYY in either group had reduced levels of haptoglobin compared to Ang-II (Fig. 6A). As this protein is a marker for intestinal barrier integrity, we tested whether contents from the gut were leaking into the bloodstream and being carried to other organ systems, i.e. the kidney. Primers targeting bacterial specific rRNA were used in a PCR reaction to determine if bacteria had leaked from the gut to the kidney. We found higher expression in Ang-II kidney samples compared to other groups, indicating bacteria may be leaking from the gut into the blood.
Fig. 6.
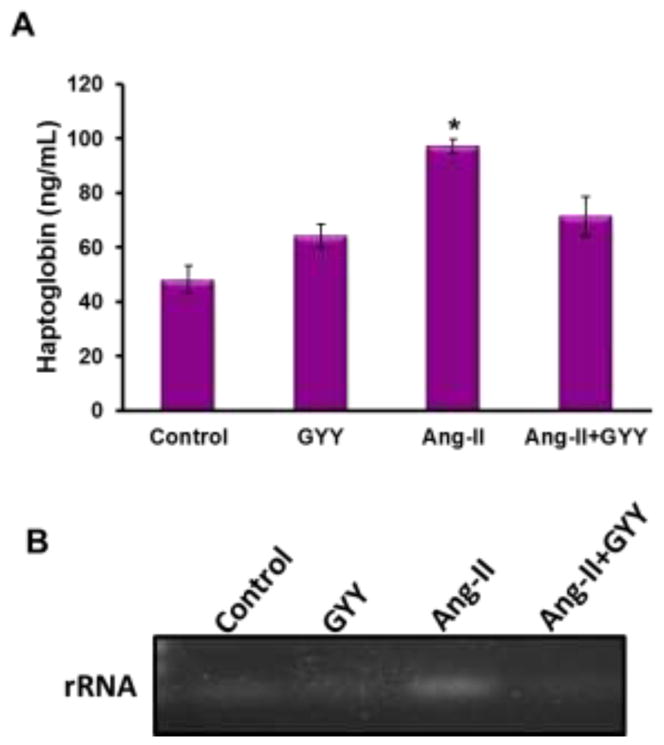
Intestinal barrier integrity is compromised in hypertensive mice. (A) Plasma samples were diluted 1/10,000 and added to ELISA sandwich with antibody to haptoglobin. The plate was washed and then exposed to substrate to reveal expression. Absorbance was detected at 480 nm wavelength. Bar graph, n=5/group. *p < 0.05 (B) gDNA extracted from kidney was purified and set up for a PCR reaction, using 100ng sample and primers specific to bacteria. Samples at the end of the PCR run were loaded onto a 1% agarose gel and imaged.
4. Discussion
The role(s) of gut microbiota in regulating physiological processes and linking changes in microbiome composition to varies diseases and disorders have been received a great deal of attention and investigation in the past several years. Understanding how, why and, importantly, what affects gut microbiota may lend insight into therapeutic intervention of multiple diseases. One of the factors that influences gut microbiome composition is diet (David et al., 2014). This change in composition will yield different amounts of normal byproducts and metabolites that are produced in the gut. One major category of metabolites produced in the gut are SCFAs, which are derived from dietary fibers digested mainly in the colon (den Besten et al., 2013). SCFAs can enter into the bloodstream and tissues through diffusion or through monocarboxylate transporters (Coady et al., 2004). Three of the main studied SCFAs are butyrate, acetic and propionic acids. Once these SCFAs enter the bloodstream, they will bind to their respective receptors and elicit a physiological response. Some of these receptors have been shown to play a role in blood pressure regulation, including Olfr78 and Gpr41 (Natarajan et al., 2016; Pluznick et al., 2013). Both of these receptors reside in the kidney and when stimulated, have different effects on the tissue, with Olfr78 shown to have hypertensive effects and Gpr41 hypotensive. However, little is known about the regulation of the SCFA receptors in the kidney. In this study, we found Olfr78 increases in expression in the hypertensive kidney, which likely enhances vasoconstriction, leading to kidney injury and a sustained hypertensive state. Moreover, Gpr41, which is reported to have hypotensive effects, was decreased in the kidney of mice treated with Ang-II, further amplifying the effects of Ang-II on the kidney.
With respect to how these SCFA receptors might be regulated in the kidney, we identified several miRNAs miR-132, and miR-329, which have been previously found to be altered in the hypertensive kidney (Weber et al., 2017) that target and potentially bind to SCFA receptors reported in this study. In silico analysis predicted miR-329 would target Gpr41 and miR-132 would bind to Gpr43. We found through transfection experiments for miR-132 and miR-329 that overexpression via mimics led to a decrease in protein expression for each miRNA-gene target. It is interesting to note that our previous study found miR-329 and miR-132 to be upregulated in the hypertensive kidney whereas miR-129 was downregulated. miR-129 was predicted to target Olfr78, however, the expression of this receptor was low in this cell line and we were unable to get a signal to observe any change in expression. In addition, GYY supplementation normalized the levels of these miRNAs, further supporting regulatory roles of miRNAs in the kidney and specifically of SCFA receptors.
We, as well as others, have investigated the role inflammation has in hypertension and the damage it causes to the kidney. Tgfβ has been shown to be responsive to Ang-II treatment and has been linked with renal fibrosis in hypertension (Border and Noble, 1998). A recent study showed that Tgfβ and Il6 were both upregulated in the hypertensive kidney through Toll-like receptor 4 (TLR4) immune response pathway (Pushpakumar et al., 2017). Here, we recapitulate the upregulation of both these molecules in the kidney of Ang-II treated wild-type mice. Moreover, supplementation with GYY reduces expression of both these inflammatory mediators. In addition, we also explored a role for Rorc2 (Rorcγt), which is involved in the maturation and regulation of Th17 cells (Castro et al., 2017; Rutz et al., 2016). This process appears to occur in CD4+ lymphocytes and promotes Il-17 production, a prominent cytokine in hypertension (Madhur et al., 2010; Zhang et al., 2008). Here, we assessed both isoforms of Rorc, which were found to be upregulated the kidney of Ang-II treated animals. We followed up with protein and immunofluorescence assays for Rorc2, as this isoform is believed to be responsible for Il-17 regulation, and found this protein localized to the tubules and vessels of the kidney. While Rorc2 regulates Il-17 in lymphocytes, the role of this transcription factor in kidney is still unknown. GYY supplementation normalized the expression of Rorc2, supporting the role of hydrogen sulfide as an anti-inflammatory agent. As we were interested in SCFA receptors, we also investigated the long chain fatty acid receptor Gpr120. This receptor binds omega-3 fatty acids and may act as an anti-inflammatory receptor by suppressing macrophage-mediated inflammation (Oh et al., 2010). Here, we investigated a role of Gpr120 in the kidney and found that this receptor is downregulated in hypertension but induced with GYY supplementation. While the mechanism of action is not known, our results suggest this receptor has a role in the kidney during hypertension, possibly as an anti-inflammatory mediator.
Alteration of the gut microbiome has been implicated in a number of diseases, including hypertension (Marques et al., 2018; Pevsner-Fischer et al., 2017; Richards et al., 2017). Two of the major phyla composing the gut microbiome are Bacteroidetes and Firmicutes, with a shift in the ratio of these bacteria associated with hypertension. (Yang et al., 2015). While we did not observe this increase in F/B ratio in hypertensive mice, we did find GYY supplementation substantially reduced the overall abundance of Firmicutes while increasing Bacteroidetes. Moreover, we found the highest abundance of Erysipelotrichi, which is a class of bacteria in the Firmicutes phylum and has been linked with high fat diet and obesity (Greiner and Backhed, 2011). The context in which this enrichment has in hypertension remains to be studied and it is worth speculating that the smaller classes and bacteria may influence the microbiome in critical ways that are not necessarily reflected at the phyla level. It is also known that hypertension can cause intestinal barrier damage and result in leakage of the gut contents into the bloodstream. Zonulin is one marker that has been associated with loss of barrier integrity along with its precursor haptoglobin (Ficek et al., 2017; Kim et al., 2018). We observed a significant increase in haptoglobin protein in plasma of mice treated with Ang-II compared to other groups, suggesting the gut barrier was damaged as zonulin is structural tight junction protein. Supporting this result was the presence of bacteria in the kidney as detected by PCR in hypertensive mice whereas GYY supplemented animals did not appear to have a bacterial presence in the kidney. The presence of localized bacteria in the kidney may account for part of the inflammatory response markers observed as well.
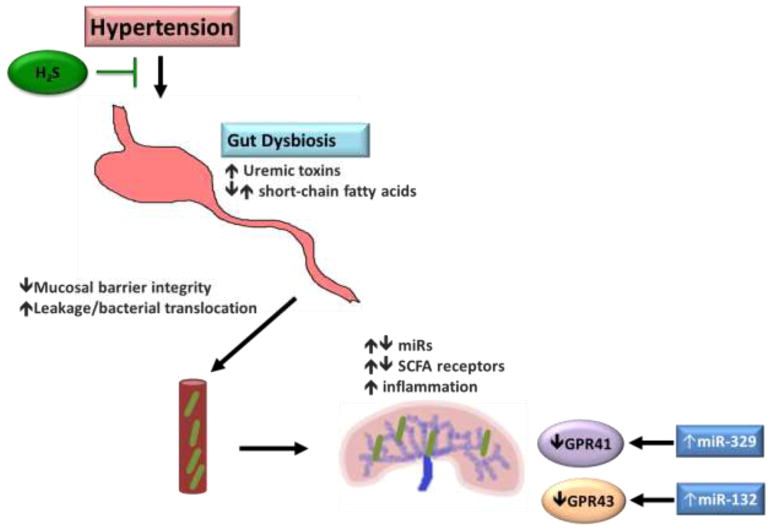
In summary, we have provided evidence for miRNA regulation of SCFA receptors in the kidney causing an enhanced hypertensive response along with bacterial leakage from the gut from a compromised intestinal barrier lead to a localized inflammatory response and further causing kidney damage. Small changes in the gut microbiome may also affect the intestinal barrier and promote leakage, thereby amplifying the overall hypertensive affect. Figure 7 is a proposed model of these events whereby hypertension can cause gut dysbiosis, damage the intestinal barrier, allow bacteria to leak out and attach to other organs, such as the kidney, and cause inflammation-induced damage. Further, SCFA can stimulate vasoconstriction through increased Olfr78 expression, causing further damage and perpetuating the cycle, which can be alleviated through hydrogen sulfide supplementation.
Fig. 7.
Schematic or proposed model of hypertension-induced gut dysbiosis, leakage of contents into bloodstream and miRNA regulation of SCFA receptors in the kidney.
Supplementary Material
Acknowledgments
The authors wish to thank Sabine Waigel, Eric Rouchka and Xiaohong Li for their work on the 16S sequencing and bioinformatics analysis.
Funding
This study was supported in part by U.S. National Institutes of Health (NIH) Grants DK104653 (U.S.) and P20GM103436 (N.C.), and American Heart Association Scientist Development Grant 15SDG25840013 (S.P.).
Abbreviations
- Ang-II
angiotensin-II
- CKD
chronic kidney disease
- H2S
hydrogen sulfide
- SCFA
short chain fatty acid
- GYY
GYY4137 Dichloromethane complex
- WT
wild type
- GFR
glomerular filtration rate
- Olfr78
olfactory receptor 78
- Rorc
RAR-related orphan receptor gamma
- Gpr41
free fatty acid receptor 3
- Gpr43
free fatty acid receptor 2
- Gpr120
free fatty acid receptor 4
- Il-6
interleukin 6
- Tgfβ
transforming growth factor beta
- qPCR
quantitative polymerase chain reaction
- RNA
ribonucleic acid
- DNA
deoxyribonucleic acid
- FITC
fluorescein isothiocyanate
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM American Heart A. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- Aron-Wisnewsky J, Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12:169–181. doi: 10.1038/nrneph.2015.191. [DOI] [PubMed] [Google Scholar]
- Barlow CE, LaMonte MJ, Fitzgerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. 2006;163:142–150. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–188. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- Botdorf J, Chaudhary K, Whaley-Connell A. Hypertension in Cardiovascular and Kidney Disease. Cardiorenal Med. 2011;1:183–192. doi: 10.1159/000329927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro G, Liu X, Ngo K, De Leon-Tabaldo A, Zhao S, Luna-Roman R, Yu J, Cao T, Kuhn R, Wilkinson P, Herman K, Nelen MI, Blevitt J, Xue X, Fourie A, Fung-Leung WP. RORgammat and RORalpha signature genes in human Th17 cells. PLoS One. 2017;12:e0181868. doi: 10.1371/journal.pone.0181868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention DE, Treatment of High Blood Pressure. National Heart L, Blood I, National High Blood Pressure Education Program Coordinating C. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Coady MJ, Chang MH, Charron FM, Plata C, Wallendorff B, Sah JF, Markowitz SD, Romero MF, Lapointe JY. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol. 2004;557:719–731. doi: 10.1113/jphysiol.2004.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PB, Jensen CH, Hansen ML, Marcussen N, Rasmussen LM, Bie P, Andersen DC, Sheikh SP. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci. 2013;14:11190–11207. doi: 10.3390/ijms140611190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficek J, Wyskida K, Ficek R, Wajda J, Klein D, Witkowicz J, Rotkegel S, Spiechowicz-Zaton U, Kocemba-Dyczek J, Ciepal J, Wiecek A, Olszanecka-Glinianowicz M, Chudek J. Relationship between plasma levels of zonulin, bacterial lipopolysaccharides, D-lactate and markers of inflammation in haemodialysis patients. Int Urol Nephrol. 2017;49:717–725. doi: 10.1007/s11255-016-1495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, Ali R, Alvis-Guzman N, Azzopardi P, Banerjee A, Barnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catala-Lopez F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang YH, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas-Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki ME, Murray CJ. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- Greiner T, Backhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab. 2011;22:117–123. doi: 10.1016/j.tem.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Judd E, Calhoun DA. Management of hypertension in CKD: beyond the guidelines. Adv Chronic Kidney Dis. 2015;22:116–122. doi: 10.1053/j.ackd.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- Khella HW, Bakhet M, Lichner Z, Romaschin AD, Jewett MA, Yousef GM. MicroRNAs in kidney disease: an emerging understanding. Am J Kidney Dis. 2013;61:798–808. doi: 10.1053/j.ajkd.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond) 2018;132:701–718. doi: 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Pushpakumar S, Khundmiri SJ, Sen U. Hydrogen sulfide mitigates hyperglycemic remodeling via liver kinase B1-adenosine monophosphate-activated protein kinase signaling. Biochim Biophys Acta. 2014;1843:2816–2826. doi: 10.1016/j.bbamcr.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Feliers D, Barnes JL, Oh S, Choudhury GG, Diaz V, Galvan V, Strong R, Nelson J, Salmon A, Kevil CG, Kasinath BS. Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience. 2018 doi: 10.1007/s11357-018-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Gao M, Zhang W, Chen C, Zhou F, Hu Z, Zeng C. Zonulin Regulates Intestinal Permeability and Facilitates Enteric Bacteria Permeation in Coronary Artery Disease. Sci Rep. 2016;6:29142. doi: 10.1038/srep29142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszyk E, Lukaszyk M, Koc-Zorawska E, Bodzenta-Lukaszyk A, Malyszko J. Zonulin, inflammation and iron status in patients with early stages of chronic kidney disease. Int Urol Nephrol. 2018;50:121–125. doi: 10.1007/s11255-017-1741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ, Morris BJ. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension. 2011;58:1093–1098. doi: 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol. 2018;15:20–32. doi: 10.1038/nrcardio.2017.120. [DOI] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM. Angiotensin II and vascular injury. Curr Hypertens Rep. 2014;16:431. doi: 10.1007/s11906-014-0431-2. [DOI] [PubMed] [Google Scholar]
- Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics. 2016;48:826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparil S, Schmieder RE. New approaches in the treatment of hypertension. Circ Res. 2015;116:1074–1095. doi: 10.1161/CIRCRESAHA.116.303603. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA American College of Sports M. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- Pevsner-Fischer M, Blacher E, Tatirovsky E, Ben-Dov IZ, Elinav E. The gut microbiome and hypertension. Curr Opin Nephrol Hypertens. 2017;26:1–8. doi: 10.1097/MNH.0000000000000293. [DOI] [PubMed] [Google Scholar]
- Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushpakumar S, Ren L, Kundu S, Gamon A, Tyagi SC, Sen U. Toll-like Receptor 4 Deficiency Reduces Oxidative Stress and Macrophage Mediated Inflammation in Hypertensive Kidney. Sci Rep. 2017;7:6349. doi: 10.1038/s41598-017-06484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci U S A. 2013;110:13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EM, Pepine CJ, Raizada MK, Kim S. The Gut, Its Microbiome, and Hypertension. Curr Hypertens Rep. 2017;19:36. doi: 10.1007/s11906-017-0734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz S, Eidenschenk C, Kiefer JR, Ouyang W. Post-translational regulation of RORgammat-A therapeutic target for the modulation of interleukin-17-mediated responses in autoimmune diseases. Cytokine Growth Factor Rev. 2016;30:1–17. doi: 10.1016/j.cytogfr.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheithauer TP, Dallinga-Thie GM, de Vos WM, Nieuwdorp M, van Raalte DH. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol Metab. 2016;5:759–770. doi: 10.1016/j.molmet.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock-Kusch D, Geraci S, Ermeling E, Shulhevich Y, Sticht C, Hesser J, Stsepankou D, Neudecker S, Pill J, Schmitt R, Melk A. Reliability of transcutaneous measurement of renal function in various strains of conscious mice. PLoS One. 2013;8:e71519. doi: 10.1371/journal.pone.0071519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S, Koenig S, Heinrich R, Hoecklin F, Pill J, Friedemann J, Schweda F, Gretz N, Schock-Kusch D. Transcutaneous measurement of renal function in conscious mice. Am J Physiol Renal Physiol. 2012;303:F783–788. doi: 10.1152/ajprenal.00279.2012. [DOI] [PubMed] [Google Scholar]
- Sen U, Sathnur PB, Kundu S, Givvimani S, Coley DM, Mishra PK, Qipshidze N, Tyagi N, Metreveli N, Tyagi SC. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. Am J Physiol Cell Physiol. 2012;303:C41–51. doi: 10.1152/ajpcell.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Carlstrom M, Borniquel S, Jadert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med. 2013;60:195–200. doi: 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora M, Drapala A, Ufnal M. Exogenous hydrogen sulfide causes different hemodynamic effects in normotensive and hypertensive rats via neurogenic mechanisms. Pharmacol Rep. 2014;66:751–758. doi: 10.1016/j.pharep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasova L, Dobrowolski L, Jurkowska H, Wrobel M, Huc T, Ondrias K, Ostaszewski R, Ufnal M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide. 2016;60:50–58. doi: 10.1016/j.niox.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Townsend RR, Taler SJ. Management of hypertension in chronic kidney disease. Nat Rev Nephrol. 2015;11:555–563. doi: 10.1038/nrneph.2015.114. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Weber GJ, Pushpakumar SB, Sen U. Hydrogen sulfide alleviates hypertensive kidney dysfunction through an epigenetic mechanism. Am J Physiol Heart Circ Physiol. 2017;312:H874–H885. doi: 10.1152/ajpheart.00637.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel U, Turner JE, Krebs C, Kurts C, Harrison DG, Ehmke H. Immune Mechanisms in Arterial Hypertension. J Am Soc Nephrol. 2016;27:677–686. doi: 10.1681/ASN.2015050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim HE, Yoo KH. Renin-Angiotensin system - considerations for hypertension and kidney. Electrolyte Blood Press. 2008;6:42–50. doi: 10.5049/EBP.2008.6.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo D, Jupiter RC, Pankey EA, Reddy VG, Edward JA, Swan KW, Peak TC, Mostany R, Kadowitz PJ. Analysis of cardiovascular responses to the H2S donors Na2S and NaHS in the rat. Am J Physiol Heart Circ Physiol. 2015;309:H605–614. doi: 10.1152/ajpheart.00171.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180:6988–6996. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.