Abstract
Objective
The goal of this systematic review of the literature was to summarize neurologic outcomes following neonatal and pediatric extracorporeal membrane oxygenation (ECMO).
Data Sources
We conducted electronic searches of PubMed, Scopus, Web of Science, CINAHL, Cochrane, and EMBASE.
Study Selection
Inclusion criteria included publication dates 2000–2016, patient ages 0–18 years, and use of standardized measures to evaluate outcomes after ECMO.
Data Extraction
We identified 3497 unique citations; 60 full-text articles were included in the final review.
Data Synthesis
Studies evaluated patients with congenital diaphragmatic hernia (7), cardiac disease (8), cardiac arrest (13), and mixed populations (32). Follow-up was conducted at hospital discharge in 10 (17%) studies, and at a median of 26 (IQR, 8-61) months after ECMO in 50 (83%) studies. We found 55 outcome measures that assessed: overall health and function (4), global cognitive ability (7), development (4), motor function (5), adaptive function (2), behavior/mood (6), hearing (2), quality of life (2), school achievement (5), speech and language (6), learning and memory (4), and attention and executive function (8). Overall, 10% to as many as 50% of children scored more than 2 SD below the population mean on cognitive testing. Behavior problems were identified in 16% to 46% of children tested, and severe motor impairment was reported in 12% of children. Quality of life of former ECMO patients evaluated at school age or adolescence ranged from similar to healthy peers, to 31%-53% having scores more than 1 SD below the population mean.
Conclusions
This systematic review of the literature suggests that children who have undergone ECMO suffer from a wide range of disabilities. A meta-analysis was not feasible due to heterogeneity in pathologies, outcome measures and age at follow-up, underscoring the importance of developing and employing a core set of outcomes measures in future ECMO studies.
Keywords: Extracorporeal membrane oxygenation, neurodevelopmental outcomes, neurocognitive outcomes, PICU, pediatrics, intensive care unit
INTRODUCTION
The number of pediatric patients who receive extracorporeal membrane oxygenation (ECMO) support each year has been growing, with the Extracorporeal Life Support Organization (ELSO) reporting ECMO use in 3,249 infants and children in 2015 (1, 2). In the early 1990s, the vast majority of ECMO patients were neonates undergoing ECMO for respiratory indications (3). By comparison, in 2016, over half of pediatric ECMO patients were over 28 days of age, and the number of respiratory (1,331), cardiac (1,135) and extracorporeal cardiopulmonary resuscitation (ECPR) (579) cases were more evenly divided (3). Though the characteristics of patients on ECMO have changed during this time, mortality has remained stable (1–3). Among ECMO survivors, neurologic injury is a significant cause of morbidity (4–6). Between 10% and 62% of survivors have been found to have evidence of injury on neuroimaging studies (7–9), but imaging alone is not a good predictor of cognitive and functional abnormalities (7, 10, 11). An estimated 10% to 60% of ECMO survivors have neurologic disability long-term (12–14), including motor deficits (6), behavior problems (11), attention deficits and slow processing speeds (15).
The goals of this systematic review of the literature were to summarize neurologic outcomes following neonatal and pediatric ECMO and to determine the most widely used outcome measures such studies.
MATERIALS AND METHODS
Data Sources and Study Selection
We conducted electronic searches of PubMed, Scopus, Web of Science, CINAHL, Cochrane and EMBASE, using a combination of medical subject headings and text words to capture concepts of extracorporeal life support and neurologic outcomes as detailed in Supplemental Digital Content 1. Eligible studies included those published between January 1, 2000 and November 30, 2016, with enrollment of children from birth to 18 years, during or after ECMO support, who were evaluated for neurofunctional outcomes with a standardized measure at or after hospital discharge. We excluded case reports and case series of fewer than 10 patients, studies for which we could not separate ECMO from other populations, or children from adults, as well as reviews, editorials, and commentaries. Two investigators (K.B. and M.B.) reviewed citations independently, and disagreement regarding eligibility was resolved by consensus.
Data Synthesis
We constructed evidence tables by primary ECMO indication and summarized the data using descriptive statistics.
RESULTS
We identified 3497 unique citations, of which 182 were included for full text review, and 54 met our eligibility criteria. We also identified 6 citations from hand searching review references. Ultimately, 60 studies were included in the study (Figure 1).
Figure 1.
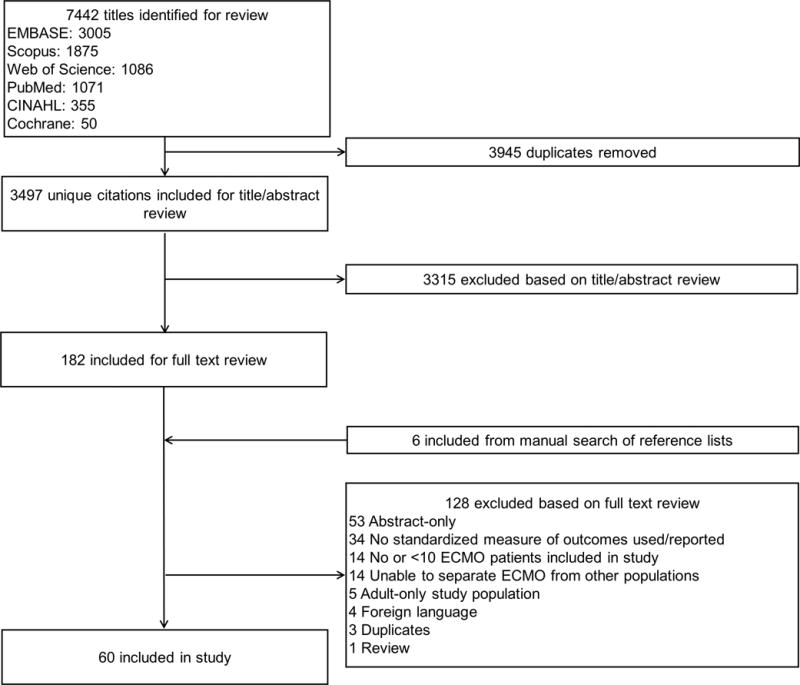
Systematic Review Flowsheet
Thirty-four of the 60 studies (57%) were from North America, 17 (28%) were from Europe, 6 (10%) were from Asia, and 3 (5%) were from Australia. Forty-five (75%) were single center and 15 (25%) were multicenter studies. Several articles presented data from the same cohort followed over time, and were included as separate studies. Thirty studies (50%) were published between 2000 and 2010 (5, 6, 13, 16, 18–42), and 30 studies (50%) were published between 2011 and 2016 (7, 11, 15, 17, 43–68).
The median sample size was 80 (interquartile range [IQR], 36-154), and the median number of children evaluated at follow-up was 36 (IQR, 18-76). Neurologic evaluations were conducted only at hospital discharge in 10 (17%) studies and at a median of 26 (IQR, 8-61) months after ECMO in 50 (83%) studies. Twenty-nine studies (48%) enrolled newborns exclusively and were all long-term outcome studies (5–7, 15, 18–21, 23–30, 32, 34–36, 43, 44, 54–56, 60). Supplemental Digital Content 2, Supplemental Table 1 presents the types and frequencies of the 55 distinct measures used to evaluate neurodevelopmental outcomes in ECMO survivors, organized by domains evaluated (e.g., overall health and function, global cognitive ability, adaptive behavior, etc). The most commonly used outcome measures were the Pediatric Cerebral Performance Category (PCPC; 30% of studies), the Wechsler Preschool and Primary Scale of Intelligence (WPPSI; 18%) and the Bayley Scales of Infant Development (BSID; 17%).
ECMO Populations
Study type, years of study, country, patient age, numbers of patients enrolled, of survivors to discharge, and of patients seen in follow-up, age at follow-up, time to follow-up, outcome measures used, and main study results, are presented in Supplemental Digital Content 3, Supplemental Table 2, organized by the main diagnostic categories as presented below.
Congenital Diaphragmatic Hernia
There were 7 studies that evaluated CDH patients (7, 15, 19–21, 43, 44). Unfavorable neurodevelopmental outcomes defined as Mental Development Index (MDI) <70 using BSID-II (general population mean 100, SD 15), were found in 17% (3/18) and 100% (4/4) of children evaluated at a mean of 2 years, and median of 4.9 years, respectively (21, 43). Rasheed et al (20) showed that 3 of 15 survivors evaluated at a median of 7 years after neonatal ECMO had at least one IQ measure more than 2 SD below the mean, using the Wechsler Intelligence Scale for Children (WISC-III). The mean Developmental Quotient (DQ) using the Brunet-Lezine test in 18 toddlers evaluated at 2 years was 96 (range, 77-113), and the IQ using the WISC or Revised Amsterdam Intelligence Test (RAKIT) tests in 16 children evaluated at 8 years was 91.7 (SD 19.5) (population mean, 100, SD, 15) (15, 19).
The quality of life in children who underwent ECMO for CDH as newborns was evaluated in 2 studies (15, 44). In a 1999-2003 Dutch cohort of 35 patients with CDH who required ECMO as newborns, 12 had lower quality of life in multiple domains at the age of 8 years, compared to children who had CDH managed without ECMO, using PedsQL (mean difference in total functioning score, −13.43, p=0.024) (15). In 10 children evaluated at a mean age of 5.5 years who underwent neonatal ECMO for CDH between 2002-2014, the mean score for physical functioning was 91 and for psychosocial functioning was 82, which was not significantly different from a non-ECMO CDH control group (44).
Cardiac Disease
In the 8 studies that evaluated patients who required ECMO support for acquired or congenital heart disease, patients were diverse in age and disease presentations (i.e., cyanotic and non-cyanotic congenital heart disease or acquired heart disease from myocarditis or cardiomyopathy) (22, 45–48, 50, 51). Three studies used the PCPC and Pediatric Overall Performance Category (POPC) to determine outcomes either at discharge or within 3 years of discharge and showed that 81%-91% were favorable, based on PCPC/POPC <3 or <4 or no change from baseline (46, 47, 50). More detailed evaluations that included neuropsychological testing revealed that, at 1 to 5 years after discharge, 25% to 50% of those seen in follow-up tested in the severely disabled range, defined as more than 2 SD below the mean for the population on the given test (i.e., WPPSI, WISC, BSID) (16, 22, 51). Studies that evaluated quality of life showed that 34% to 53% of cardiac ECMO survivors reported significantly diminished quality of life compared to age-matched, healthy peers (45, 48). One of two studies showed that quality of life was also significantly diminished compared to that of peers with cardiac disease but no history of ECMO (48).
Cardiac Arrest
Ten of the 13 studies that evaluated outcomes of infants and children who suffered cardiac arrest prior to ECMO enrolled only patients who underwent ECPR (37–39, 42, 49, 61–67, 69), and 3 studies included patients who achieved return of spontaneous circulation prior to cannulation for ECMO. Twelve of the 13 studies used PCPC and POPC. Favorable outcomes were defined as a PCPC/POPC < 3 or < 4, and were found in 14% to 89% of survivors to follow-up, where time to follow-up ranged from hospital discharge to 2.5 years (37–39, 61–67, 69). In a study that evaluated 17 survivors at a median of 52 months post-ECPR, over 23% of survivors were 2 or more SD below the population mean for full scale IQ measured by WPPSI-III (49). None of these 13 studies evaluated HRQOL in survivors.
Mixed ECMO Populations
Thirty-two studies included mixed patient populations. The outcome measures used and individual study results are presented below and in Supplemental Digital Content 3, Supplemental Table 2.
Overall Cognition/Development/Adaptive Skills
Most studies concluded that global ability of former ECMO patients was not severely affected, with mean scores for the ECMO patients falling within 1 SD of the population mean (5, 6, 11, 15, 17, 19, 24, 26, 27, 29, 34–36, 55, 58). Four studies demonstrated mean scores for cognitive ability <80 (population mean, 100, SD, 15) (13, 16, 49, 51).
Five studies combined results for cognitive skills tested by WPPSI, WISC or BSID as appropriate for age (13, 15, 16, 20, 36). Among 672 cumulative individuals with IQ or MDI scores reported, the mean of the mean IQ or MDI scores was 87 (SD 10.3), at a median of 60 (IQR, 9-84) months after ECMO. An additional five Dutch studies used the RAKIT to evaluate cognitive outcomes of ECMO patients. Reporting of results was not uniform. In 3 of these studies, the mean IQ was 99.7 (SD 17.7) (n=125), 100.5 (SD 19.7) (n=79), and 99.7 (SD 18.1) (n=131), at mean follow-up times of 96, 62, and 62 months, respectively (6, 11, 34). The median IQ was 104 in a study of 99 children seen at 60 months following neonatal ECMO (58). Lastly, in a study of 102 children evaluated at 60 months following neonatal ECMO, 10% had IQ scores more than 2 SD below the mean (56).
Behavior
Estimates of the prevalence of any behavioral problems were significantly higher than in the general population and ranged from 10% to 50% of former ECMO patients (5, 34). “Clinically significant behavior problems” were reported in 16% to 46% of former ECMO patients. Of note, “clinically significant behavior problems” definitions were different in these studies, with cutoff scores of 60, 70 and 74 on the Achenbach Child Behavior Checklist (CBCL) (11, 24, 27, 34, 56).
Quality of Life
Eight studies evaluated quality of life for a total of 315 former ECMO patients using the PedsQL (13, 15, 17, 44, 48, 56, 59, 60). The PedsQL questionnaire evaluates functional categories including physical, social, emotional and school (if age appropriate). Between 31% and 53% of former ECMO patients had mean scores more than 1 SD below the population mean (13, 48, 56, 59, 60). PedsQL in these studies was conducted between 5 and 8 years of age, with 31 patients evaluated at 17 years of age. The single study which evaluated former ECMO patients at 17 years of age reported that quality of life for this cohort did not differ from healthy peers (17).
Motor Skills
Reported rates of motor disability ranged between 16% and 33%, and from mild (e.g., delayed in reaching motor milestones, weakness without impaired activity) to severe (e.g., quadriparesis, wheelchair dependence) (5, 6, 13, 16, 27, 30, 34, 56, 60). Almost all cohort studies reported several severely impaired survivors who were unable to engage in testing. Of a cumulative total of 532 who had undergone ECMO as newborns and were evaluated by the Motor Assessment Battery for Children (MABC) in 6 studies, 64 (12%) scored below the 5th percentile, indicating severe motor impairment at mean follow-up ages that ranged between 5 to 8 years (5, 6, 15, 34, 56, 60).
School Performance
In informal evaluations, more ECMO survivors than their healthy peers had additional help in school or were enrolled in special courses (11, 31, 42). Formal evaluations of school performance and school readiness were conducted in 5 studies (5, 24, 26, 29, 36). Three studies evaluated former neonatal ECMO patients at 5 to 8 years age using the Wide Range Achievement Test (WRAT) (24, 26, 29). The mean reading score and mean arithmetic score in these three studies (cumulative n=173) fell within 1 SD below the mean (24, 26, 29). Patients who had seizures while on ECMO had significantly lower mean scores on the WRAT and on the Bracken Basic Concept Scale of School Readiness than survivors in the same cohort who did not have seizures (29). One study evaluated 56 former neonatal ECMO patients at 7 years of age; these children were found to have deficits in reading comprehension on the Wechsler Objective Reading Dimensions (WORD) (5). Lastly, one study evaluated 11 patients 7 years after neonatal ECMO using the Kaufman Test of Educational Achievement. Mean, reading and arithmetic scores were within 1 SD below the population mean and were not significantly different from a group who received inhaled nitric oxide and mechanical ventilation as neonates but not ECMO (36).
Hearing and Vision
Hearing loss was identified in 5% to 28% of ECMO survivors during follow-up testing (5, 6, 16, 18–20, 22, 30, 32, 54–56, 58). Severe visual impairment, defined as blindness or abnormal vision after correction, was reported in 0% to 5% of ECMO survivors who underwent vision evaluations (5, 6, 16, 18, 22, 31, 35, 55).
Speech and Language
Ten studies reported that mean expressive and receptive language scores or composite verbal scores fell between 90 and 100, and within 1 SD of the population mean (5, 20, 24, 26, 27, 35, 36, 43, 55, 58). In two studies from a Canadian cohort, one reporting solely on ECPR survivors (49), 4-5 year old ECMO survivors had a mean verbal IQ on the WPPSI-III more than 1 SD below the mean for age (51). Parish et al reported that patients with a history of seizures scored below expected for age on their verbal index with a mean of 85.4 (SD 17) at preschool age and 84.8 (SD 19.3) at school age (29). ECMO survivors who did not have seizures had a mean score of 96.9 (SD 12) at preschool age and 93.7 (SD 12) at school age (29).
Higher Level Cognitive Skills
Seven studies reported results of executive function, attention and memory testing (5, 11, 15, 17, 26, 29, 36). Two studies that employed the Bourdon-Vos test reported that up to 70% of 8 year old ECMO survivors who underwent testing (n=10, n=123) had slow to very slow working memory processing speed (11, 15). Using the Rey Complex Figure Test (RCFT) and the Rey Auditory Verbal Learning Test (RAVLT), Madderom et al found that deficits in memory remained substantial after controlling for the mean IQ of 28 children who underwent neonatal ECMO and were evaluated at 17 years of age (17). In contrast, Goodman et al found normal mean scores for 66 neonatal ECMO survivors with mean age of 8 years at follow-up, using the Wide Range Assessment of Memory and Learning (WRAML) (26).
General Outcomes
In total, 754 survivors of neonatal and pediatric ECMO were evaluated by PCPC at hospital discharge (742 individuals from 16 studies) and/or at follow up appointments (76 individuals from 4 studies). Results were available for all but one of these studies (n=69) (46). Cumulatively, at hospital discharge, 321/673 (48%) had PCPC <3 or unchanged from baseline, and 471/673 (70%) had PCPC<4 (33, 37–39, 41, 50, 52, 53, 57, 62, 63, 65–67, 69). At post-discharge follow–up, 44/76 (58%) had PCPC <3 or unchanged from baseline (33, 38, 41, 61).
DISCUSSION
This systematic review of the literature on outcomes for critically ill infants and children supported with ECMO showed a wide range of deficits described in survivors, as well as variability in the types of outcome measures used and in the timing of follow-up evaluations, even among children with similar pathologies and ages.
Studies that concluded at time of discharge included more patients but less detailed neuropsychological evaluations, relying on the PCPC and POPC to evaluate outcomes. These scales are both 6-point scales that provide information on overall neurologic functioning and health, respectively, but do not allow for an understanding of more subtle cognitive and developmental ability. Their advantage is that they can be easily assessed, often with review of medical records, and they correlate with more thorough measures such as VABS (70). However, especially at young ages, emerging neurologic deficits may first manifest long after brain injury (71).
Follow-up studies in this review showed variable outcomes for ECMO survivors, but the data point to a risk for ongoing neurologic deficits and cognitive delays no matter the initial reason for ECMO cannulation. Owing to study heterogeneity and the large number of outcomes measures used, we were unable to conduct a meta-analysis. Several themes emerged from our review of the literature, however. The majority of studies reported IQ in the normal range in ECMO survivors, although 10% to as many as 50% of children, in selected cohorts, had scores that were more than 2 SD below the population mean. Behavior problems were identified in 16% to 46% of children tested, and severe motor impairment was reported in 12% of children. Quality of life of former ECMO patients evaluated at school age or adolescence ranged from similar to healthy peers, to 31%-53% having scores more than 1 SD below the population mean. Generally, functional outcomes reported for CDH, cardiac disease, and cardiac arrest populations appeared worse when compared to mixed ECMO populations.
To gain a full understanding of ECMO patient outcomes, it will be necessary for study teams and research networks to collaborate and develop outcome measure guidelines, which could be modeled on existing similar initiatives (e.g., for pediatric traumatic brain injury) (72). Outcome measures would need to be easy to implement and validated in several languages. Timing of follow-up could be harmonized with existing recommendations for populations at high risk for critical illness requiring ECMO (e.g., CHD) (73), with serial evaluations at toddler, preschool and school age. Based on the results of this systematic review, a combination of measures to evaluate cognition and development (e.g., BSID, WPPSI, WISC), behavior (e.g., CBCL) and motor (e.g., MABC) skills, school achievement (e.g., WRAML, WRAT) and hearing would constitute a sensible starting point for a core set of ECMO outcomes measures.
Our study has several limitations. The number of participants in each study included in the systematic literature review was low, making it difficult to draw robust conclusions from any one study. The studies included in the review used a variety of outcome measures conducted at varied time points, and thus could not be combined in a meta-analysis; many measures were used in only a single study. Studies that used the same tests reported results differently and with various levels of detail (e.g., as mean [SD] vs percent patients with scores more than −1 SD or −2 SD below the mean, etc), which made comparison and aggregation of results difficult.
CONCLUSION
This systematic review of the literature suggests that children who have undergone ECMO suffer from a wide range of disabilities. A meta-analysis was not feasible due to heterogeneity in pathologies, outcome measures and age at follow-up, underscoring the importance of developing and employing a core set of outcomes measures in future ECMO studies.
Supplementary Material
Supplemental Digital Content 1. Systematic Review Search Strategy
Supplemental Digital Content 2, Supplemental Table 1. Neurofunctional Outcome Measures Used in Studies of Children After ECMO
Supplemental Digital Content 3, Supplemental Table 2. Study, Population, and Follow-up Characteristics and Results
Acknowledgments
The authors would like to acknowledge the contribution of Katie Lobner, MLIS, who assisted with the literature search.
Financial Support: Support for this work included funding from: the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number K23NS076674 (MMB)
Copyright form disclosure: This is the authors’ own work. Dr. Bembea’s institution received funding from the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke K23NS076674; she received support for article research from the NIH; and she disclosed off-label product use of ECMO (not FDA-approved for longer than 6 hours).
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Barbaro RP, Paden ML, Guner YS, et al. Asaio Journal. Publish Ahead of Print; 2017. Pediatric extracorporeal life support organization registry international report 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63:60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 3.Extracorporeal Life Support Organization. Extracorporeal life support organization registry international summary. 2017 [Google Scholar]
- 4.Cengiz P, Seidel K, Rycus PT, et al. Central nervous system complications during pediatric extracorporeal life support: Incidence and risk factors*. Crit Care Med. 2005;33:2817–2824. doi: 10.1097/01.ccm.0000189940.70617.c3. [DOI] [PubMed] [Google Scholar]
- 5.McNally H, Bennett CC, Elbourne D, et al. United kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: Follow-up to age 7 years. Pediatrics. 2006;117:e854. doi: 10.1542/peds.2005-1167. [DOI] [PubMed] [Google Scholar]
- 6.Nijhuis-van der Sanden MW, van der Cammen-van Zijp MH, Janssen AJ, et al. Motor performance in five-year-old extracorporeal membrane oxygenation survivors: A population-based study. Crit Care. 2009;13:R47. doi: 10.1186/cc7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rollins MD, Yoder BA, Moore KR, et al. Utility of neuroradiographic imaging in predicting outcomes after neonatal extracorporeal membrane oxygenation. J Pediatr Surg. 2012;47:76–80. doi: 10.1016/j.jpedsurg.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Wien MA, Whitehead MT, Bulas D, et al. Patterns of brain injury in newborns treated with extracorporeal membrane oxygenation. AJNR Am J Neuroradiol. 2017;38:820–826. doi: 10.3174/ajnr.A5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Heijst Arno FJ, de Mol AC, Ijsselstijn H. ECMO in neonates: Neuroimaging findings and outcome. Semin Perinatol. 2014;38:104–113. doi: 10.1053/j.semperi.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Lazar EL, Abramson SJ, Weinstein S, et al. Neuroimaging of brain injury in neonates treated with extracorporeal membrane oxygenation: Lessons learned from serial examinations. J Pediatr Surg. 1994;29:1. doi: 10.1016/0022-3468(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 11.Madderom MJ, Reuser JJ, Utens EM, et al. Neurodevelopmental, educational and behavioral outcome at 8 years after neonatal ECMO: A nationwide multicenter study. Intensive Care Med. 2013;39:1584–1593. doi: 10.1007/s00134-013-2973-1. [DOI] [PubMed] [Google Scholar]
- 12.Waitzer E, Riley SP, Perreault T, et al. Neurologic outcome at school entry for newborns treated with extracorporeal membrane oxygenation for noncardiac indications. J Child Neurol. 2009;24:801–806. doi: 10.1177/0883073808330765. [DOI] [PubMed] [Google Scholar]
- 13.Wagner K, Risnes I, Berntsen T, et al. Clinical and psychosocial follow-up study of children treated with extracorporeal membrane oxygenation. Ann Thorac Surg. 2007;84:1349–1355. doi: 10.1016/j.athoracsur.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Glass P, Bulas DI, Wagner AE, et al. Severity of brain injury following neonatal extracorporeal membrane oxygenation and outcome at age 5 years. Dev Med Child Neurol. 1997;39:441–448. doi: 10.1111/j.1469-8749.1997.tb07463.x. [DOI] [PubMed] [Google Scholar]
- 15.Madderom MJ, Toussaint L, van der Cammen-van Zijp MH, et al. Congenital diaphragmatic hernia with(out) ECMO: Impaired development at 8 years. Arch Dis Child Fetal Neonatal Ed. 2013;98:316. doi: 10.1136/archdischild-2012-303020. [DOI] [PubMed] [Google Scholar]
- 16.Lequier L, Joffe AR, Robertson CM, et al. Two-year survival, mental, and motor outcomes after cardiac extracorporeal life support at less than five years of age. J Thorac Cardiovasc Surg. 2008;136:983.e3. doi: 10.1016/j.jtcvs.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Madderom MJ, Schiller RM, Gischler SJ, et al. Growing up after critical illness: Verbal, visual-spatial, and working memory problems in neonatal extracorporeal membrane oxygenation survivors. Crit Care Med. 2016;44:1182–1190. doi: 10.1097/CCM.0000000000001626. [DOI] [PubMed] [Google Scholar]
- 18.Jaillard S, Pierrat V, Truffert P, et al. Two years’ follow-up of newborn infants after extracorporeal membrane oxygenation (ECMO) Eur J Cardiothorac Surg. 2000;18:328–333. doi: 10.1016/s1010-7940(00)00514-5. [DOI] [PubMed] [Google Scholar]
- 19.Jaillard SM, Pierrat V, Dubois A, et al. Outcome at 2 years of infants with congenital diaphragmatic hernia: A population-based study. Ann Thorac Surg. 2003;75:250–256. doi: 10.1016/s0003-4975(02)04278-9. [DOI] [PubMed] [Google Scholar]
- 20.Rasheed A, Tindall S, Cueny DL, et al. Neurodevelopmental outcome after congenital diaphragmatic hernia: Extracorporeal membrane oxygenation before and after surgery. J Pediatr Surg. 2001;36:539–544. doi: 10.1053/jpsu.2001.22278. [DOI] [PubMed] [Google Scholar]
- 21.Buesing KA, Kilian AK, Schaible T, et al. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia: Follow-up MRI evaluating carotid artery reocclusion and neurologic outcome. AJR Am J Roentgenol. 2007;188:1636–1642. doi: 10.2214/AJR.06.1319. [DOI] [PubMed] [Google Scholar]
- 22.Hamrick SEG, Gremmels DB, Keet CA, et al. Neurodevelopmental outcome of infants supported with extracorporeal membrane oxygenation after cardiac surgery. Pediatrics. 2003;111:671. doi: 10.1542/peds.111.6.e671. [DOI] [PubMed] [Google Scholar]
- 23.Nield TA, Langenbacher D, Poulsen MK, et al. Neurodevelopmental outcome at 3.5 years of age in children treated with extracorporeal life support: Relationship to primary diagnosis. J Pediatr. 2000;136:338–344. doi: 10.1067/mpd.2000.103359. [DOI] [PubMed] [Google Scholar]
- 24.Rais-Bahrami K, Wagner AE, Coffman C, et al. Neurodevelopmental outcome in ECMO vs near-miss ECMO patients at 5 years of age. Clin Pediatr (Phila) 2000;39:145–152. doi: 10.1177/000992280003900302. [DOI] [PubMed] [Google Scholar]
- 25.Bennett CC, Johnson A, Field DJ, et al. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: Follow-up to age 4 years. Lancet. 2001;357:1094–1096. doi: 10.1016/S0140-6736(00)04310-5. [DOI] [PubMed] [Google Scholar]
- 26.Goodman M, Gringlas M, Baumgart S, et al. Neonatal electroencephalogram does not predict cognitive and academic achievement scores at early school age in survivors of neonatal extracorporeal membrane oxygenation. J Child Neurol. 2001;16:745–750. doi: 10.1177/088307380101601007. [DOI] [PubMed] [Google Scholar]
- 27.Langenbacher D, Nield T, Poulsen MK. Neurodevelopmental outcome of ECMO survivors at five years of age: The potential for academic and motor difficulties. The Journal of Special Education. 2001;35:156–160. [Google Scholar]
- 28.Cheung P, Etches PC, Weardon M, et al. Use of plasma lactate to predict early mortality and adverse outcome after neonatal extracorporeal membrane oxygenation: A prospective cohort in early childhood. Crit Care Med. 2002;30:2135–2139. doi: 10.1097/00003246-200209000-00030. [DOI] [PubMed] [Google Scholar]
- 29.Parish AP, Bunyapen C, Cohen MJ, et al. Seizures as a predictor of long-term neurodevelopmental outcome in survivors of neonatal extracorporeal membrane oxygenation (ECMO) J Child Neurol. 2004;19:930–934. doi: 10.1177/08830738040190120401. [DOI] [PubMed] [Google Scholar]
- 30.Lamers LJ, Rowland DG, Seguin JH, et al. The effect of common origin of the carotid arteries in neurologic outcome after neonatal ECMO. J Pediatr Surg. 2004;39:532–536. doi: 10.1016/j.jpedsurg.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Amigoni A, Pettenazzo A, Biban P, et al. Neurologic outcome in children after extracorporeal membrane oxygenation: Prognostic value of diagnostic tests. Pediatr Neurol. 2005;32:173–179. doi: 10.1016/j.pediatrneurol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Fligor BJ, Neault MW, Mullen CH, et al. Factors associated with sensorineural hearing loss among survivors of extracorporeal membrane oxygenation therapy. Pediatrics. 2005;115:1519–1528. doi: 10.1542/peds.2004-0247. [DOI] [PubMed] [Google Scholar]
- 33.Litsenburg RV, Mos ND, Edgell D, et al. Resource use and health outcomes of paediatric extracorporeal membrane oxygenation. Archives of Disease in Childhood – Fetal and Neonatal Edition. 2005;90:F177. doi: 10.1136/adc.2003.047779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanekamp MN, Mazer P, van der Cammen-van Zijp Monique HM, et al. Follow-up of newborns treated with extracorporeal membrane oxygenation: A nationwide evaluation at 5 years of age. Crit Care. 2006;10:R127. doi: 10.1186/cc5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khambekar K, Nichani S, Luyt DK, et al. Developmental outcome in newborn infants treated for acute respiratory failure with extracorporeal membrane oxygenation: Present experience. Arch Dis Child Fetal Neonatal Ed. 2006;91:21. doi: 10.1136/adc.2004.066290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg AA, Lee NR, Vaver KN, et al. School-age outcomes of newborns treated for persistent pulmonary hypertension. J Perinatol. 2010;30:127–134. doi: 10.1038/jp.2009.139. [DOI] [PubMed] [Google Scholar]
- 37.Morris MC, Wernovsky G, Nadkarni VM. Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2004;5:440–446. doi: 10.1097/01.pcc.0000137356.58150.2e. [DOI] [PubMed] [Google Scholar]
- 38.Prodhan P, Fiser RT, Dyamenahalli U, et al. Outcomes after extracorporeal cardiopulmonary resuscitation (ECPR) following refractory pediatric cardiac arrest in the intensive care unit. Resuscitation. 2009;80:1124–1129. doi: 10.1016/j.resuscitation.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kane DA, Thiagarajan RR, Wypij D, et al. Rapid-response extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in children with cardiac disease. Circulation. 2010;122:241. doi: 10.1161/CIRCULATIONAHA.109.928390. [DOI] [PubMed] [Google Scholar]
- 40.Maclaren G, Butt W, Best D, et al. Extracorporeal membrane oxygenation for refractory septic shock in children: One institution’s experience. Pediatr Crit Care Med. 2007;8:447–451. doi: 10.1097/01.PCC.0000282155.25974.8F. [DOI] [PubMed] [Google Scholar]
- 41.Jan S, Lin S, Fu Y, et al. Extracorporeal life support for treatment of children with enterovirus 71 infection-related cardiopulmonary failure. Intensive Care Med. 2010;36:520–527. doi: 10.1007/s00134-009-1739-2. [DOI] [PubMed] [Google Scholar]
- 42.Huang S, Wu E, Chen Y, et al. Extracorporeal membrane oxygenation rescue for cardiopulmonary resuscitation in pediatric patients. Crit Care Med. 2008;36:1607–1613. doi: 10.1097/CCM.0b013e318170b82b. [DOI] [PubMed] [Google Scholar]
- 43.Benjamin JR, Gustafson KE, Smith PB, et al. Perinatal factors associated with poor neurocognitive outcome in early school age congenital diaphragmatic hernia survivors. J Pediatr Surg. 2013;48:730–737. doi: 10.1016/j.jpedsurg.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheikh F, Akinkuotu A, Clark SJ, et al. Assessment of quality of life outcomes using the pediatric quality of life inventory survey in prenatally diagnosed congenital diaphragmatic hernia patients. J Pediatr Surg. 2016;51:545–548. doi: 10.1016/j.jpedsurg.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Costello JM, O’Brien M, Wypij D, et al. Quality of life of pediatric cardiac patients who previously required extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2012;13:428–434. doi: 10.1097/PCC.0b013e318238ba21. [DOI] [PubMed] [Google Scholar]
- 46.Chrysostomou C, Maul T, Callahan PM, et al. Neurodevelopmental outcomes after pediatric cardiac ECMO support. Front Pediatr. 2013;1:47. doi: 10.3389/fped.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chrysostomou C, Morell VO, Kuch BA, et al. Short- and intermediate-term survival after extracorporeal membrane oxygenation in children with cardiac disease. J Thorac Cardiovasc Surg. 2013;146:317–325. doi: 10.1016/j.jtcvs.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Garcia Guerra G, Robertson CMT, Alton GY, et al. Health-related quality of life in pediatric cardiac extracorporeal life support survivors. Pediatr Crit Care Med. 2014;15:720–727. doi: 10.1097/PCC.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 49.Garcia Guerra G, Zorzela L, Robertson CMT, et al. Survival and neurocognitive outcomes in pediatric extracorporeal-cardiopulmonary resuscitation. Resuscitation. 2015;96:208–213. doi: 10.1016/j.resuscitation.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 50.Nosaka N, Muguruma T, Fujiwara T, et al. Effects of the elective introduction of extracorporeal membrane oxygenation on outcomes in pediatric myocarditis cases. Acute Medicine & Surgery. 2015;2:92–97. doi: 10.1002/ams2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryerson LM, Guerra GG, Joffe AR, et al. Survival and neurocognitive outcomes after cardiac extracorporeal life support in children less than 5 years of age: A ten-year cohort. Circ Heart Fail. 2015;8:312–321. doi: 10.1161/CIRCHEARTFAILURE.114.001503. [DOI] [PubMed] [Google Scholar]
- 52.Bembea MM, Savage W, Strouse JJ, et al. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:572–579. doi: 10.1097/PCC.0b013e3181fe3ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bembea MM, Rizkalla N, Freedy J, et al. Plasma biomarkers of brain injury as diagnostic tools and outcome predictors after extracorporeal membrane oxygenation. Crit Care Med. 2015;43:2202–2211. doi: 10.1097/CCM.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 54.Murray M, Nield T, Larson-Tuttle C, et al. Sensorineural hearing loss at 9-13 years of age in children with a history of neonatal extracorporeal membrane oxygenation. Arch Dis Child Fetal Neonatal Ed. 2011;96:128. doi: 10.1136/adc.2010.186395. [DOI] [PubMed] [Google Scholar]
- 55.Field D, Juszczak E, Linsell L, et al. Neonatal ECMO study of temperature (NEST): A randomized controlled trial. Pediatrics. 2013;132:1247. doi: 10.1542/peds.2013-1754. [DOI] [PubMed] [Google Scholar]
- 56.Madderom MJ, Gischler SJ, Duivenvoorden H, et al. Neonatal extracorporeal membrane oxygenation: Impaired health at 5 years of age. Pediatr Crit Care Med. 2013;14:183–193. doi: 10.1097/PCC.0b013e3182601453. [DOI] [PubMed] [Google Scholar]
- 57.Turek JW, Andersen ND, Lawson DS, et al. Outcomes before and after implementation of a pediatric rapid-response extracorporeal membrane oxygenation program. Ann Thorac Surg. 2013;95:2147. doi: 10.1016/j.athoracsur.2013.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Hondel D, Madderom MJ, Goedegebure A, et al. Sensorineural hearing loss and language development following neonatal extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2013;14:62–69. doi: 10.1097/PCC.0b013e31825b54ae. [DOI] [PubMed] [Google Scholar]
- 59.Chandler HK, Teppa B, Johnson KA, et al. Determining comorbidities and quality of life among pediatric survivors of extracorporeal life support. Journal of Critical Care. 2015;30:1085–1089. doi: 10.1016/j.jcrc.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 60.Toussaint LCC, van der Cammen-van Zijp Monique HM, Janssen AJ, et al. Perceived motor competence differs from actual performance in 8-year-old neonatal ECMO survivors. Pediatrics. 2016;137:e20152724. doi: 10.1542/peds.2015-2724. [DOI] [PubMed] [Google Scholar]
- 61.Sivarajan VB, Best D, Brizard CP, et al. Duration of resuscitation prior to rescue extracorporeal membrane oxygenation impacts outcome in children with heart disease. Intensive Care Med. 2011;37:853–860. doi: 10.1007/s00134-011-2168-6. [DOI] [PubMed] [Google Scholar]
- 62.Huang S, Wu E, Wang C, et al. Eleven years of experience with extracorporeal cardiopulmonary resuscitation for paediatric patients with in-hospital cardiac arrest. Resuscitation. 2012;83:710–714. doi: 10.1016/j.resuscitation.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 63.Tsukahara K, Toida C, Muguruma T. Current experience and limitations of extracorporeal cardiopulmonary resuscitation for cardiac arrest in children: A single-center retrospective study. J Intensive Care. 2014;2:68. doi: 10.1186/s40560-014-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Philip J, Burgman C, Bavare A, et al. Nature of the underlying heart disease affects survival in pediatric patients undergoing extracorporeal cardiopulmonary resuscitation. J Thorac Cardiovasc Surg. 2014;148:2367–2372. doi: 10.1016/j.jtcvs.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 65.Mattke AC, Stocker CF, Schibler A, et al. A newly established extracorporeal life support assisted cardiopulmonary resuscitation (ECPR) program can achieve intact neurological outcome in 60% of children. Intensive Care Med. 2015;41:2227–2228. doi: 10.1007/s00134-015-4036-2. [DOI] [PubMed] [Google Scholar]
- 66.Lasa JJ, Rogers RS, Localio R, et al. Extracorporeal cardiopulmonary resuscitation (E-CPR) during pediatric in-hospital cardiopulmonary arrest is associated with improved survival to discharge: A report from the american heart association’s get with the guidelines-resuscitation (GWTG-R) registry. Circulation. 2016;133:165–176. doi: 10.1161/CIRCULATIONAHA.115.016082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ostendorf AP, Hartman ME, Friess SH. Early electroencephalographic findings correlate with neurologic outcome in children following cardiac arrest. Pediatr Crit Care Med. 2016;17:667–676. doi: 10.1097/PCC.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee H, Chi C, Jan S, et al. Extracorporeal life support for critical enterovirus 71 rhombencephalomyelitis: Long-term neurologic follow-up. Pediatric Neurology. 2012;46:225–230. doi: 10.1016/j.pediatrneurol.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 69.Raymond TT, Cunnyngham CB, Thompson MT, et al. Outcomes among neonates, infants, and children after extracorporeal cardiopulmonary resuscitation for refractory inhospital pediatric cardiac arrest: A report from the national registry of cardiopulmonary resuscitation. Pediatr Crit Care Med. 2010;11:362–371. doi: 10.1097/PCC.0b013e3181c0141b. [DOI] [PubMed] [Google Scholar]
- 70.Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 71.Westmacott R, MacGregor D, Askalan R, et al. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke. 2009;40:2012–2019. doi: 10.1161/STROKEAHA.108.533976. [DOI] [PubMed] [Google Scholar]
- 72.Hicks R, Giacino J, Harrison-Felix C, et al. Progress in developing common data elements for traumatic brain injury research: Version two–the end of the beginning. J Neurotrauma. 2013;30:1852–1861. doi: 10.1089/neu.2013.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the american heart association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Systematic Review Search Strategy
Supplemental Digital Content 2, Supplemental Table 1. Neurofunctional Outcome Measures Used in Studies of Children After ECMO
Supplemental Digital Content 3, Supplemental Table 2. Study, Population, and Follow-up Characteristics and Results


