Abstract
Osteosarcoma is a malignant bone tumor that occurs mainly in children and adolescents. Because Wnt signaling has been implicated in the pathogenesis of osteosarcoma, we have investigated the circulating and local levels of the Wnt antagonist protein, Secreted Frizzled Related Protein (sFRP)3, in osteosarcoma patients. Enzyme linked immunosorbent assay (ELISA) analysis of 67 osteosarcoma and age-matched non-diseased control sera showed that sFPR3 protein levels were significantly lower in osteosarcoma than in normal. Analysis of tumor and adjacent normal tissues (9 pairs) from osteosarcoma patients showed a decrease in sFRP3 expression in 5 out of 9 tumor samples compared to normal tissues. Furthermore, immunohistochemical analysis of tissue microarray revealed a significant decrease in sFRP3 levels in tumor compared to normal bone. RNA sequencing analysis in osteosarcoma cells shows suppression of sFRP3 and concomitant expression of multiple Wnt family members mediating canonical or non-canonical Wnt signaling. Taken together, our findings show that the systemic and local levels of sFRP3 protein are downregulated in osteosarcoma and sFRP3 levels could be explored further in the diagnosis and the care of osteosarcoma patients.
Keywords: Frzb, sFRP3, Wnt, Osteosarcoma
1. Introduction
Osteosarcoma is a primary bone malignancy that affects predominantly children and young adults. The standard treatment for osteosarcoma involves a combination of surgery and chemotherapy (Arndt et al., 2012). Despite this treatment, one-third of the patients diagnosed with osteosarcoma will develop metastatic diseases (O’Reilly et al., 1996). Therefore, there is a critical need to define prognostic biomarkers with predictive potential for osteosarcoma progression.
The canonical and non-canonical Wnt signaling pathways play key roles in cell differentiation, survival, stem cell self-renewal and the homeostasis of many tissues (Logan and Nusse, 2004; Clevers, 2006; Monroe et al., 2012). The canonical pathway is mediated by β-catenin and activation of T-cell factor (TCF)/lymphoid enhancer fator-1(LEF) transcription pathways, while the non-canonical pathways involve planar-cell polarity (PCP-like pathway) and the Wnt/Ca2+ pathway. The Wnt pathway is a key regulator of bone formation and bone remodeling (Monroe et al., 2012). Activation of Wnt signaling has been implicated in many malignant diseases. A deregulated canonical Wnt pathway has been demonstrated in osteosarcoma and several other cancers (Morin et al., 1997; Fujie et al., 2001; Woo et al., 2001; Polakis, 2007; MacDonald et al., 2009). In addition, some reports show that non-canonical Wnt pathway is involved in certain malignancies (Jessen, 2009). Suppression of the Wnt pathway as a potential treatment approach has been explored in many tumors and Wnt antagonists have been studied for their anti-tumor effects (Suzuki et al., 2004; He et al., 2005; Guo et al., 2008; Chen et al., 2010; Saraswati et al., 2012). The Wnt antagonist sFRP3, also called Frizzled-related protein (FRZB), acts as a tumor suppressor in osteosarcoma and other cancers (Zi et al., 2005; Mandal et al., 2007; Guo et al., 2008). The sFRPs are secreted by osteocytes and inhibit the Wnt pathway as antagonistic decoy receptors. Because they share a high degree of similarity with the seven-transmembrane domain-spanning frizzled receptors, they are capable of sequestering agonistic Wnt glycoproteins thus preventing activation of Wnt signaling.
Our group and others have investigated examined pharmacological strategies (Benedikt et al., 2010; Maran et al., 2013; Yang et al., 2013; Bravo et al., 2017; Gustafson et al., 2017; Mamo et al., 2017) diagnostic markers (Pereira et al., 2009; Riester et al., 2017)and molecular mechanisms (van der Deen et al., 2012; van der Deen et al., 2013; Vega et al., 2017) linked to osteosarcoma formation and metastasis. These studies addressed the interplay between Wnt-signaling, transcriptional control and microRNA mediated regulation of gene expression in osteosarcoma. However, there are no reliable serum tumor markers for early diagnosis and prediction of metastasis. In this study, we assessed the potential of sFRP3/FRZB protein as a potential prognostic marker for osteosarcoma by investigating the levels of sFRP3 protein in normal and osteosarcoma tissue specimens.
2. Materials and Methods
Osteosarcoma sample collection
The serum samples were obtained through a study protocol approved by the institutional review board (IRB). To quantify the sFRP3 levels in human serum, we have analyzed samples from 134 patients (67 osteosarcoma patients + 67 sex and age-matched, non-diseased controls) and clinical data from the medical records was correlated with experimental results. Baseline demographic and characteristics are shown in Table 1. Samples from thirty-nine male and twenty-eight females (Age range 8–75 years; Mean: 30 years; Median: 23 years) patients were analyzed, and six patients had low grade tumors, while sixty-one patients had high grade tumors (Table 1). Also, 45 patients had metastatic diseases and 22 had only local disease.
Table 1.
Details on tissue samples
| Patient Information | Number (%) |
|---|---|
| Number of patients | |
| Normal | 67 |
| Osteosarcoma | 67 |
| Age | |
| Mean | 30 |
| Median | 21 |
| Range | 8–75 |
| Gender | |
| Female | 28 (42) |
| Male | 39 (58) |
| Grade | |
| Low grade | 6 (9) |
| High Grade | 61 (91) |
| Metastatic status | |
| Metastatic | 45 (67) |
| Non-metastatic | 22 (33) |
Osteosarcoma tissues and adjacent normal tissues were obtained by surgical resection through Mayo Clinic Institute Review Board (IRB)-approved protocol. Prior to use, the histological diagnosis of the tissues was confirmed by certified musculoskeletal pathologists at Mayo Clinic.
Enzyme Linked Immunosorbent Assay (ELISA)
ELISA analysis was performed to estimate sFRP3 levels as described in the manufacturer’s protocol (Aviscera Bioscience, Santa Clara, CA).
Immunohistochemical staining of osteosarcoma arrays
Tissue microarrays representing malignant and normal bone were purchased from US Biomax, Inc (Rockville, MD) and analyzed by immunostaining, using anti-sFRP3 (1: 25 dilution), anti-axin2 (1: 50 dilution)and non-immune immunoglobulin (IgG) (1:200 dilution) (Santa Cruz Biotechnology, Dallas, TX). The anti-sFRP3- and anti-axin2-stained tissue arrays were normalized using IgG staining and the quantitation of signals was carried out using BIOQUANT OSTEO Image analysis system (Bioquant Image Analysis Corporation, Nashville, TN). The average densities for sFRP3 and Axin staining were calculated in normal and osteosarcoma tissues. The average density was determined by calculating the intensities of all significant pixels in the object and dividing that value by the number of pixels as described in the manufacturer’s protocol (Bioquant Image Analysis Corporation).
Protein isolation and western blot hybridization
Cytoplasmic extracts were prepared by homogenizing the tissues in lysis buffer as described (Wimbauer et al., 2012). The protein concentration was determined by Bradford protein assay, and cytoplasmic extracts containing protein (60 μg) were analyzed by western blot hybridization as previously shown (Benedikt et al., 2010; Wimbauer et al., 2012) using anti-sFRP3 (1:2000 dilution) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:5000 dilution) antibodies (Santa Cruz Biotechnology, Dallas, TX). The expression levels of proteins on the western blots were quantified using densitometer and Imagelab software (BioRad, Hercules, CA).
Cell Culture, RNA isolation and sequencing
Osteosarcoma cells (MG63, SAOS and U2OS) were cultured in DMEM/F12 media as described (Bravo et al., 2017). RNA isolation was carried out with the RNeasy mini kit (Qiagen, Germantown, MD) and subsequent RNA sequencing was performed using an established pipeline at the Mayo Clinic RNA sequencing Core facility as described previously (Dudakovic et al., 2014; Paradise et al., 2018)
Statistical Analysis
All values were expressed as means + standard error. Samples were analyzed using a Wilcoxon signed rank test for matched pairs to test the difference of the means, and the likelihood ratio was used to test difference in probabilities of sFRP3 differential regulation. P≤0.05 was considered statistically significant. For tumor patients, a nonparametric Mann-Whitney test was used to analyze intragroup variations.
3. Results
ELISA Analysis of sFRP3 levels in osteosarcoma patients
Serum sFRP3 protein levels were measured by ELISA in osteosarcoma patients (N=67) and age-matched controls (N=67). Our results show that patients with osteosarcoma have significantly decreased levels of sFRP3 compared to normal without the disease (P ≤ 0.005) (Table.2). Quantitation of sFRP3 concentration revealed a mean concentration of 1715 ± 194 pg/ml and 2290 ± 165 pg/ml, in osteosarcoma and control sera, respectively (Table 2). The control group levels ranged from 731 to 6339 pg/mL with a median value of 1922 pg/ml and the values for osteosarcoma patients ranged from 13 to 6216 pg/mL with a median value of 1237 pg/ml (Table 2). Our analysis shows that sFRP3 protein was down regulated in 67.2% (45 patients), upregulated in 26.8% (18 patients) and remained unchanged in 6% (4 patients) of osteosarcoma patients (Fig.2).
Table 2.
Mean serum sFRP3 levels for normal and osteosarcoma samples.
| Variables of SFRP3 levels | Osteosarcoma patients (pg/ml) | Control patients (pg/ml) | P value |
|---|---|---|---|
| Mean ± SE | 1715 ± 194 | 2290 ± 165 | 0.005* |
| Median | 1237 | 1922 | |
| Range | 13–6216 | 730–6339 |
significant in tumor compared to age matched control
Fig.2.
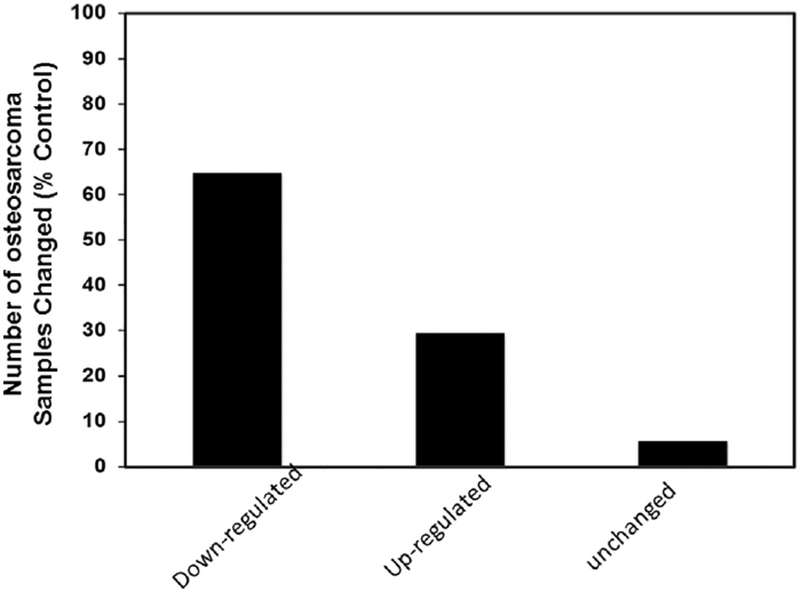
Percent change in sFRP3 levels in osteosarcoma patients. Serum sFRP3 levels determined by ELISA were compared to age-matched normal samples.
sFRP3 regulation by age, gender, stage and grade in osteosarcoma
When stratified by age, two groups were considered based on the median value (23 years). The groups were: (1) adult ≥23; and (2) young adult/pediatric population <23 (Table. 3). Results showed that osteosarcoma patients had down-regulation in both age groups, but only a statistically significant difference (P≤0.023) was observed in the adult populations when compared to age-matched normal population (Table 3). Stratification of data based on gender points out significant decreases in female osteosarcoma patients but not in male patients (P<0.008), compared to age-matched normal population (Table 3). Patient disease characteristics gathered from our Mayo Clinic serum sample database were compared with differences in sFRP3 levels, and further divided into subcategories based on stage (metastasis vs non-metastasis) and tumor grade (low vs high grade). The results showed a down-regulation of sFRP3 levels in patients with metastatic disease (P≤0.0083) (Table 3), whereas in non-metastatic patients, no significant difference was observed (Table 3). Further a significant difference was noticed (P<0.01) in patients with high grade tumors but not in low grade osteosarcoma when compared to corresponding age-matched control specimens (P:0.006) (Table 3). Thus, this shows that in the osteosarcoma patients sFRP3 levels were significantly lower than in normal group. This difference was statistically significant in several subgroups including in patients older than 23 years, females, metastatic conditions, and high grade osteosarcomas.
Table 3.
Mean serum sFRP3 levels for normal and osteosarcoma patients in different subgroups
| Variables of SFRP3 levels | Osteosarcoma patients Mean±SE (pg/ml) | Control patients mean±SE (pg/ml) | P value (Wilcoxon test) |
|---|---|---|---|
| Age | |||
| ≤23 age (N: 35/67) | 1646±292 | 1981±156 | >0.05 |
| >23 age (N: 32/67) | 1792±255 | 2629±291 | 0.007* |
| Gender | |||
| Female (N: 28/67) | 1790±295 | 2556±281 | 0.008* |
| Male (N: 39/67) | 1662±260 | 2099±196 | >0.05 |
| Stage | |||
| Metastatic (45/67) | 1825±248 | 2525±214 | 0.008* |
| Non-metastatic (22/67) | 1492±303 | 1810±219 | >0.05 |
| Grade | |||
| Low & moderate grade (6/67) | 1937±730 | 2452±790 | >0.05 |
| High grade (61/67) | 1694±202 | 2274±166 | 0.003* |
Significant in tumor compared to age matched control
Analysis of sFRP3 expression by immunohistochemistry
Tissue microarrays containing osteosarcoma and normal tissues were analyzed for sFRP3 expression. Immunostaining, followed by quantitation of signals, showed decreased staining in tumor compared to normal tissues (Fig. 3A and 3B). In order to further verify our findings and determine whether downstream Wnt signal is regulated, we have analyzed the expression of axin2 using the tissue microarrays. Our results show that axin2 expression is increased in osteosarcoma tissues compared to the normal bone tissues (Fig.4A and 4B).
Fig.3.
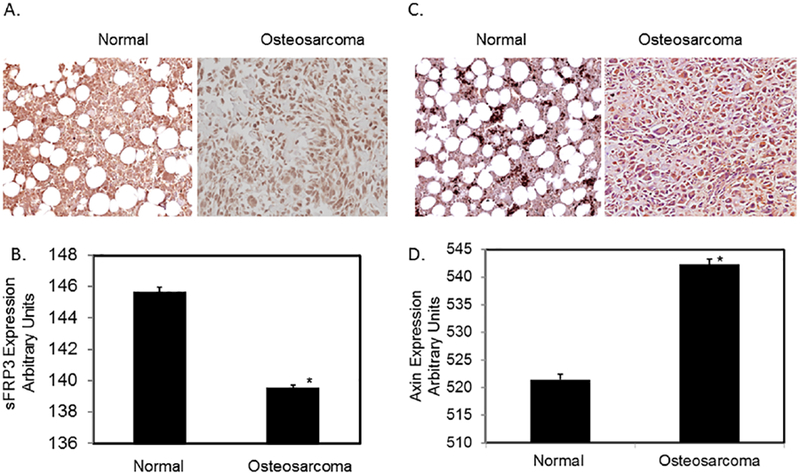
Tissue microarray analysis of osteosarcoma and normal bone samples.
Tissue arrays were analyzed by immunostaining with anti-sFRP3 and anti-axin2 antibodies and quantitated using digital image software analysis as described in Methods. A & C) Representative images of Normal bone and osteosarcoma samples. B & D) Quantitation of immunostaining signals through Bioquant Image analysis. *P< 0.05 vs normal.
Fig.4.
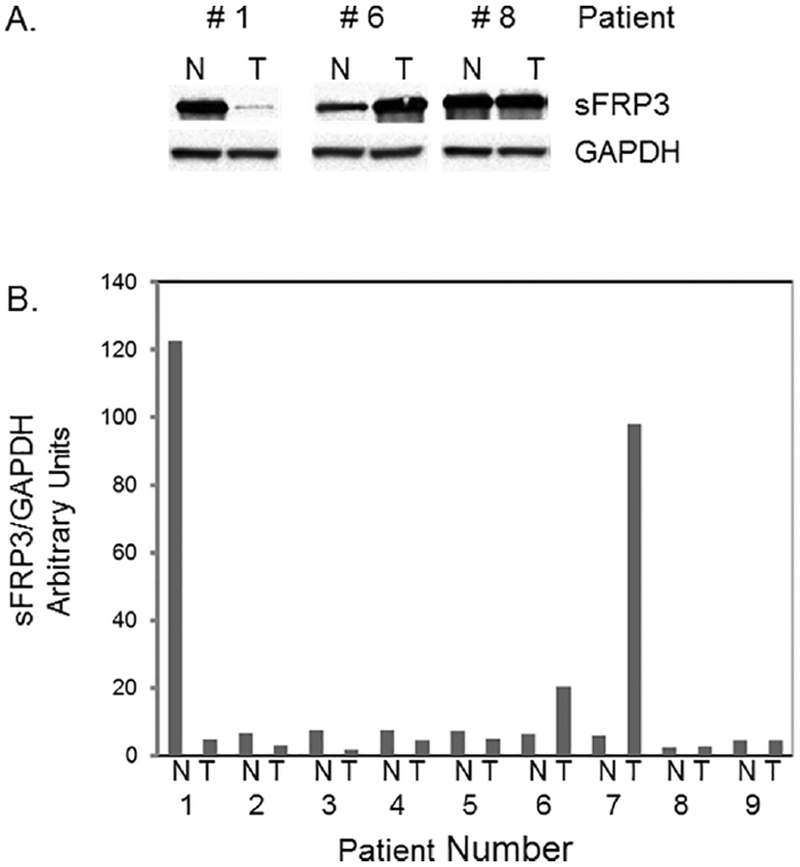
sFRP3 levels of tissue samples analyzed by western blot. Cytoplasmic extracts prepared from tumor and adjacent normal tissues from patients (9 sets) were analyzed using anti-sFRP3 and anti-GAPDH antibodies and quantitated by densitometry as described in Methods. T,tumor; N, normal. A) Representative blots; B) Quantitation of densitometry signals
Measurement of sFRP3 protein levels by western blot analysis
To further verify above findings, cytoplasmic extracts from osteosarcoma and adjacent normal tissues from patients were analyzed by western blot analysis. Figures 5A and 5B show representative blots from tissues and quantitation of signals from 9 sets of tissues, respectively. The results showed that the osteosarcoma tissue specimens had decreased sFRP3 levels compared to the control samples in 5 out of 9 sets. The sFRP3 protein levels were upregulated in 2 specimens and remained unchanged in 2 specimens (Fig. 5B).
Fig.5.
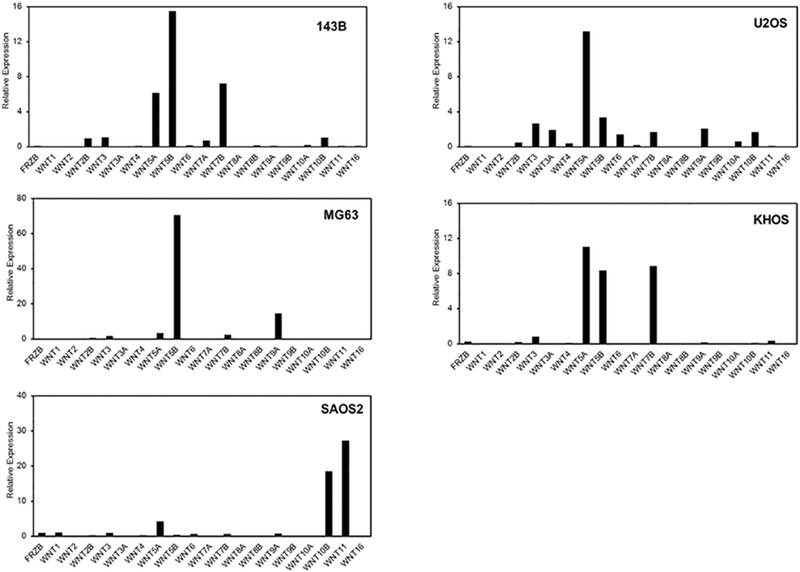
sFRP3 and Wnt mRNA levels in osteosarcoma cells. Total RNA isolated from 143B, U2OS, MG63, KHOS and SAOS2 cells were analyzed by RNA sequencing as described in Methods.
Analysis of sFRP3 and Wnt mRNA levels in osteosarcoma cell lines
To further investigate the impact of sFRP3 downregulation, we examined the gene expression profiles and patterns of Wnt family genes using RNA sequencing in osteosarcoma cells. Our analysis reveal that sFRP3 expression is very low or at undetectable levels in 5 different cell osteosarcoma cell lines (143B, U2OS, MG63, KHOS and SAOS2). In contrast, a number of Wnt family members (e.g., Wnt2B, Wnt3, Wnt4, Wnt5 A, Wnt5b, Wnt6, Wnt7A, Wnt7B, Wnt9A, Wnt10A, Wnt10B and Wnt11) are robustly expressed to different degrees depending on the cell type. Importantly, the results show that Wnt5A and Wnt5B are most consistently expressed in all osteosarcoma cell types examined.
4. Discussion
We show that the sFRP3 proteins levels are significantly decreased in osteosarcoma patients. Using various techniques (ELISA, immunohistochemistry and western blot analysis), we have demonstrated that both systemic and local levels of sFRP3 are decreased in osteosarcoma patients compared to normal. Thus, this study corroborates our previous results on mRNA levels in osteosarcoma (Mandal et al., 2007) indicating that monitoring sFRP3 expression levels could be a valuable approach in the care of osteosarcoma patients.
Establishing a valid diagnostic marker in osteosarcoma can serve many purposes: a) help improve the prognosis for osteosarcoma by early detection; and b) provide molecular targets for developing novel therapies. The markers reported represent an extensive mixture of compounds including carbohydrates, glycoproteins, polyamines, proteins and immunoglobulins. Preclinical and clinical studies have revealed that a few serum proteins are associated with osteosarcoma. Various in vitro, in vivo and patient tissue investigations have identified that the expression of MMP-2, MMP-9 (Foukas et al., 2002), uPA (Clark et al., 2008), CXCR4(Laverdiere et al., 2005), Survivin (Osaka et al., 2006), Ezrin (Park et al., 2006) and RUNX2 (Pereira et al., 2009; van der Deen et al., 2013)are upregulated, and the expression of P53 (Park et al., 2001; Pereira et al., 2009)and Rb (Wadayama et al., 1994; Pereira et al., 2009)are down regulated in osteosarcoma. Our findings show that sFRP3 is down regulated in 67% of the cases studied indicating that sFRP3 may be useful in both diagnosis and monitoring of osteosarcoma.
Stratification of serum data showed a significant decrease in sFRP3 levels in adult patients over 23 years. Also, the current study, revealed a significant decrease in sFRP3 protein expression in females. Earlier reports show that the incidence of osteosarcoma occurs in males more frequently than in females (Gatta et al., 2002; Jessen, 2009). These investigations point out that osteosarcoma could occur in females due to the earlier onset of growth spurt. However, it remains to be determined whether earlier growth spurt directly contributes to down regulation of sFRP3 expression and activation of Wnt signaling that lead to the development of osteosarcoma.
Furthermore, our results show a statistically significant decrease of sFRP3 protein levels in high grade osteosarcomas compared to low and moderate grade osteosarcomas. It is histologically challenging to diagnose low grade osteosarcomas and often will be mistaken for fibrous dysplasia or myositis ossificans. The diagnosis can be facilitated with the use of MDM2 and cyclin-dependent kinase 4 (CDK4) genes which have been identified as candidate markers for low grade osteosarcomas (Zi et al., 2005). Also, co-expression has been specifically associated with low grade tumors that progressed to high-grade osteosarcomas (Yoshida et al., 2012). It is unclear whether similar correlation exists between sFRP3 down regulation and progression to high grade osteosarcomas in low grade tumors. In addition, our data suggest that sFRP3 down regulation is significant in metastatic diseases compared to local diseases. Upregulation of the genes uPA, CXCR4 and Ezrin during osteosarcoma invasion and metastasis have been demonstrated before. Verifying our finding in an animal model would help establish the regulation of sFRP3 in metastasis, could lead to a better understanding of the role of sFRP3 in tumor induction and progression.
In this report, the results from serum studies have been corroborated by immunohistochemistry and quantitation of tissue microarray analysis from 11 osteosarcoma patients. Significant downregulation of sFRP3 protein was observed in osteosarcoma patients compared to normal without the disease. Also, these results have been further confirmed by higher Axin2 levels in osteosarcoma compared to normal indicating increased Wnt signaling in osteosarcoma. In addition, analysis of osteosarcoma tissues and adjacent normal tissues from patients showed a downregulation of sFRP3 in 5 out of 9 osteosarcoma patients (1.5 to 24 fold). This observation supports the serum results, but warrants further studies in a larger cohort of osteosarcoma patients. These results in tissues and serum samples which show downregulation of sFRP3 in majority of patients and upregulation in certain cases support the possibility of a context-dependent dual role for sFRP3 protein in osteosarcoma as observed in certain malignancies (Mii and Taira, 2011; Surana et al., 2014). We have previously demonstrated that sFRP3 transcription is suppressed in osteosarcoma patients (Mandal et al., 2007). Current results further extend these observations and show that sFRP3 protein level is decreased in osteosarcoma patients. Our recent report shows that sFRP3 expression is increased in osteosarcoma cells treated with anti-tumor drugs, and reveals that sFRP3 contributes to tumor suppression (Bravo et al., 2017).
Down regulation of sFRP3 protein levels and its potential function as a tumor suppressor has been studied in cancers other than osteosarcoma. It exhibits anti-tumor activity and reverses epithelial to mesenchymal transition in prostate cancer cells (Zi et al., 2005). Methylation and loss of sFRP3 enhances cell migration and invasion in melanoma (Ekstrom et al., 2011). Other evidences point out epigenetic silencing and tumor suppressor roles for sFRP3 in medulloblastoma (Kongkham et al., 2010), as well as in colorectal cancer (Qi et al., 2006). The level of sFRP3 protein was found to be high in normal kidney, low in primary renal cancer tissues and high in metastatic renal cancer tissues. The changes in sFRP3 expression levels suggest that this protein may function as a tumor suppressor and oncogene during renal cancer progression.
RNA-seq analysis of osteosarcoma cells reveals that sFRP3 is not expressed at appreciable levels, while there are a number of mRNAs encoding Wnt proteins that are robustly expressed in at least five different osteosarcoma cell lines. The expression of these Wnt proteins provides a paracrine microenvironment for osteosarcoma cells that could be blocked by sFRP3. The mRNA expression profile for all Wnt members indicates that both canonical and non-canonical Wnt signaling pathways may be supported in each of the different osteosarcoma cell types we analyzed. We find that Wnt5A, which activates non-canonical Wnt signaling, is expressed in all cell types examined. The latter is notable because the non-canonical Wnt pathway, controls the planar cell polarity pathway in proliferating osteoblasts in relation to mechanical stimulation (Galea et al., 2013; Galea et al., 2015)The RNA-seq data suggest that sFRP3 may antagonize Wnt signaling through both canonical and non-canonical pathways to control osteosarcoma progression.
Our investigation has limitations and needs to be verified using a larger cohort of serum samples, as well as with specimens covering the primary tumor and adjacent tissues. However, our findings offer valuable indications that local and systemic levels of sFRP3 are downregulated in osteosarcoma and could be useful in monitoring the progression in osteosarcoma. From a mechanistic perspective, the absence of sFRP3/FRZB expression in osteosarcoma cells and elevated expression of both canonical and non-canonical Wnt members suggests that serum levels of sFRP3/FRZB may not only be diagnostic of disease, but also further point towards the importance of Wnt paracrine regulatory events that control proliferation during the disease progression in osteosarcoma.
5. Conclusion
Our results show that systemic and local levels of sFRP3 are decreased in osteosarcoma patients and estimation of circulating sFRP3 protein levels could be explored further in the diagnosis and treatment of osteosarcoma.
Fig.1.
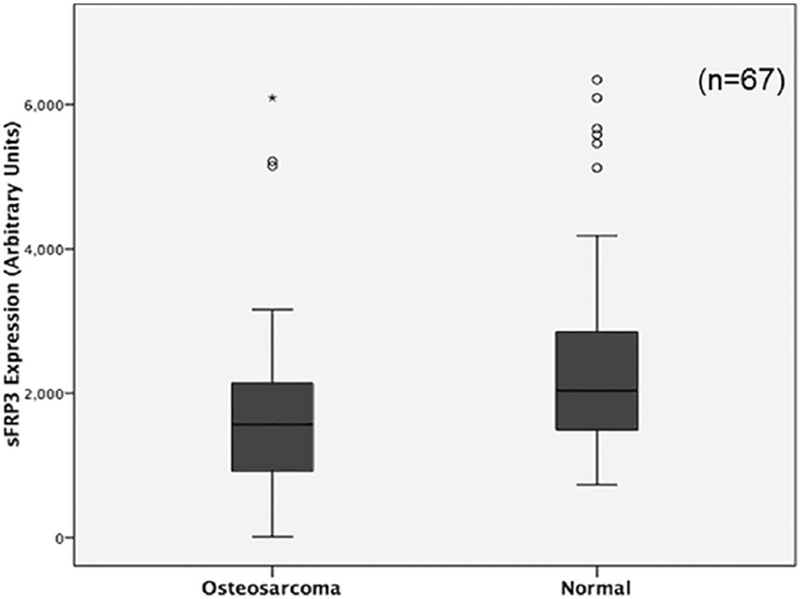
Analysis of serum sFRP3 levels. Mean sFRP3 levels in the serum of normal and osteosarcoma patients were determined by ELISA. *P≤0.05 vs normal
Acknowledgment
This work was supported by funding from the Mayo Clinic and National Institutes of Health R34 grant DE025593 (AM and MJY) and R01 grant AR049069 (AJvW). DB was supported by Center for Clinical and Translational Science Master program. AS was supported by The Scientific and Technological Research Council of Turkey.
Abbreviations List
- sFRP3
Secreted Frizzled Related Protein 3
- ELISA
Enzyme linked immunosorbent assay
- IgG
immunoglobulin
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- CDK4
cyclin-dependent kinase 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arndt CA, Rose PS, Folpe AL and Laack NN Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc 87 (2012), pp. 475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedikt MB, Mahlum EW, Shogren KL, Subramaniam M, Spelsberg TC, Yaszemski MJ and Maran A 2-methoxyestradiol-mediated anti-tumor effect increases osteoprotegerin expression in osteosarcoma cells. J Cell Biochem 109 (2010), pp. 950–6. [DOI] [PubMed] [Google Scholar]
- Bravo D, Shogren KL, Zuo D, Wagner ER, Sarkar G, Yaszemski MJ and Maran A 2- Methoxyestradiol-Mediated Induction of Frzb Contributes to Cell Death and Autophagy in MG63 Osteosarcoma Cells. J Cell Biochem 118 (2017), pp. 1497–1504. [DOI] [PubMed] [Google Scholar]
- Chen W, Chen M and Barak LS Development of small molecules targeting the Wnt pathway for the treatment of colon cancer: a high-throughput screening approach. Am J Physiol Gastrointest Liver Physiol 299 (2010), pp. G293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JC, Dass CR and Choong PF A review of clinical and molecular prognostic factors in osteosarcoma. Journal of cancer research and clinical oncology 134 (2008), pp. 281–97. [DOI] [PubMed] [Google Scholar]
- Clevers H Wnt/beta-catenin signaling in development and disease. Cell 127 (2006), pp. 469–80. [DOI] [PubMed] [Google Scholar]
- Dudakovic A, Camilleri E, Riester SM, Lewallen EA, Kvasha S, Chen X, Radel DJ, Anderson JM, Nair AA, Evans JM, Krych AJ, Smith J, Deyle DR, Stein JL, Stein GS, Im HJ, Cool SM, Westendorf JJ, Kakar S, Dietz AB and van Wijnen AJ High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem 115 (2014), pp. 1816–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom EJ, Sherwood V and Andersson T Methylation and loss of Secreted Frizzled- Related Protein 3 enhances melanoma cell migration and invasion. PloS one 6 (2011), p. e18674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foukas AF, Deshmukh NS, Grimer RJ, Mangham DC, Mangos EG and Taylor S Stage-IIB osteosarcomas around the knee. A study of MMP-9 in surviving tumour cells. J Bone Joint Surg Br 84 (2002), pp. 706–11. [DOI] [PubMed] [Google Scholar]
- Fujie H, Moriya K, Shintani Y, Tsutsumi T, Takayama T, Makuuchi M, Kimura S and Koike K Frequent beta-catenin aberration in human hepatocellular carcinoma. Hepatol Res 20 (2001), pp. 39–51. [DOI] [PubMed] [Google Scholar]
- Galea GL, Meakin LB, Savery D, Taipaleenmaki H, Delisser P, Stein GS, Copp AJ, van Wijnen AJ, Lanyon LE and Price JS Planar cell polarity aligns osteoblast division in response to substrate strain. J Bone Miner Res 30 (2015), pp. 423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H, Stein GS, van Wijnen AJ, Lanyon LE and Price JS Estrogen receptor alpha mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor beta. J Biol Chem 288 (2013), pp. 9035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta G, Capocaccia R, Coleman MP, Ries LA and Berrino F Childhood cancer survival in Europe and the United States. Cancer 95 (2002), pp. 1767–72. [DOI] [PubMed] [Google Scholar]
- Guo Y, Xie J, Rubin E, Tang YX, Lin F, Zi X and Hoang BH Frzb, a secreted Wnt antagonist, decreases growth and invasiveness of fibrosarcoma cells associated with inhibition of Met signaling. Cancer Res 68 (2008), pp. 3350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson CT, Mamo T, Shogren KL, Maran A and Yaszemski MJ FH535 Suppresses Osteosarcoma Growth In Vitro and Inhibits Wnt Signaling through Tankyrases. Front Pharmacol 8 (2017), p. 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F and Jablons DM Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene 24 (2005), pp. 3054–8. [DOI] [PubMed] [Google Scholar]
- Jessen JR Noncanonical Wnt signaling in tumor progression and metastasis. Zebrafish 6 (2009), pp. 21–8. [DOI] [PubMed] [Google Scholar]
- Kongkham PN, Northcott PA, Croul SE, Smith CA, Taylor MD and Rutka JT The SFRP family of WNT inhibitors function as novel tumor suppressor genes epigenetically silenced in medulloblastoma. Oncogene 29 (2010), pp. 3017–3024. [DOI] [PubMed] [Google Scholar]
- Laverdiere C, Hoang BH, Yang R, Sowers R, Qin J, Meyers PA, Huvos AG, Healey JH and Gorlick R Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res 11 (2005), pp. 2561–7. [DOI] [PubMed] [Google Scholar]
- Logan CY and Nusse R The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20 (2004), pp. 781–810. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K and He X Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17 (2009), pp. 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo T, Mladek AC, Shogren KL, Gustafson C, Gupta SK, Riester SM, Maran A, Galindo M, van Wijnen AJ, Sarkaria JN and Yaszemski MJ Inhibiting DNA-PKCS radiosensitizes human osteosarcoma cells. Biochem Biophys Res Commun 486 (2017), pp. 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal D, Srivastava A, Mahlum E, Desai D, Maran A, Yaszemski M, Jalal SM, Gitelis S, Bertoni F, Damron T, Irwin R, O’Connor M, Schwartz H, Bolander ME and Sarkar G Severe suppression of Frzb/sFRP3 transcription in osteogenic sarcoma. Gene 386 (2007), pp. 131–8. [DOI] [PubMed] [Google Scholar]
- Maran A, Dadsetan M, Buenz CM, Shogren KL, Lu L and Yaszemski MJ Hydrogel- PLGA delivery system prolongs 2-methoxyestradiol-mediated anti-tumor effects in osteosarcoma cells. J Biomed Mater Res A 101 (2013), pp. 2491–9. [DOI] [PubMed] [Google Scholar]
- Mii Y and Taira M Secreted Wnt “inhibitors” are not just inhibitors: regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev Growth Differ 53 (2011), pp. 911–23. [DOI] [PubMed] [Google Scholar]
- Monroe DG, McGee-Lawrence ME, Oursler MJ and Westendorf JJ Update on Wnt signaling in bone cell biology and bone disease. Gene 492 (2012), pp. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B and Kinzler KW Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275 (1997), pp. 1787–90. [DOI] [PubMed] [Google Scholar]
- O’Reilly R, Cheung NK, Bowman L, Castle V, Hoffer F, Kapoor N, Kletzel M, Lindsley K, Shamberger R and Tubergen D NCCN pediatric neuroblastoma practice guidelines. The National Comprehensive Cancer Network. Oncology (Williston Park) 10 (1996), pp. 1813–1822. [PubMed] [Google Scholar]
- Osaka E, Suzuki T, Osaka S, Yoshida Y, Sugita H, Asami S, Tabata K, Hemmi A, Sugitani M, Nemoto N and Ryu J Survivin as a prognostic factor for osteosarcoma patients. Acta Histochem Cytochem 39 (2006), pp. 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradise CR, Galeano-Garces C, Galeano-Garces D, Dudakovic A, Milbrandt TA, Saris DBF, Krych AJ, Karperien M, Ferguson GB, Evseenko D, Riester SM, van Wijnen AJ and Noelle Larson A Molecular characterization of physis tissue by RNA sequencing. Gene (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Jung WW, Bacchini P, Bertoni F, Kim YW and Park YK Ezrin in osteosarcoma: comparison between conventional high-grade and central low-grade osteosarcoma. Pathol Res Pract 202 (2006), pp. 509–15. [DOI] [PubMed] [Google Scholar]
- Park YB, Kim HS, Oh JH and Lee SH The co-expression of p53 protein and P-glycoprotein is correlated to a poor prognosis in osteosarcoma. Int Orthop 24 (2001), pp. 307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira BP, Zhou Y, Gupta A, Leong DT, Aung KZ, Ling L, Pho RW, Galindo M, Salto-Tellez M, Stein GS, Cool SM, van Wijnen AJ and Nathan SS Runx2, p53, and pRB status as diagnostic parameters for deregulation of osteoblast growth and differentiation in a new pre-chemotherapeutic osteosarcoma cell line (OS1). J Cell Physiol 221 (2009), pp. 778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P The many ways of Wnt in cancer. Curr Opin Genet Dev 17 (2007), pp. 45–51. [DOI] [PubMed] [Google Scholar]
- Qi J, Zhu YQ, Luo J and Tao WH Hypermethylation and expression regulation of secreted frizzled-related protein genes in colorectal tumor. World J Gastroenterol 12 (2006), pp. 7113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riester SM, Torres-Mora J, Dudakovic A, Camilleri ET, Wang W, Xu F, Thaler RR, Evans JM, Zwartbol R, Briaire-de Bruijn IH, Maran A, Folpe AL, Inwards CY, Rose PS, Shives TC, Yaszemski MJ, Sim FH, Deyle DR, Larson AN, Galindo MA, Cleven AGH, Oliveira AM, Cleton-Jansen AM, Bovee J and van Wijnen AJ Hypoxia-related microRNA-210 is a diagnostic marker for discriminating osteoblastoma and osteosarcoma. J Orthop Res 35 (2017), pp. 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswati S, Deskins DL, Holt GE and Young PP Pyrvinium, a potent small molecule Wnt inhibitor, increases engraftment and inhibits lineage commitment of mesenchymal stem cells (MSCs). Wound Repair Regen 20 (2012), pp. 185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana R, Sikka S, Cai W, Shin EM, Warrier SR, Tan HJ, Arfuso F, Fox SA, Dharmarajan AM and Kumar AP Secreted frizzled related proteins: Implications in cancers. Biochim Biophys Acta 1845 (2014), pp. 53–65. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG and Baylin SB Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 36 (2004), pp. 417–22. [DOI] [PubMed] [Google Scholar]
- van der Deen M, Akech J, Lapointe D, Gupta S, Young DW, Montecino MA, Galindo M, Lian JB, Stein JL, Stein GS and van Wijnen AJ Genomic promoter occupancy of runt-related transcription factor RUNX2 in Osteosarcoma cells identifies genes involved in cell adhesion and motility. J Biol Chem 287 (2012), pp. 4503–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Deen M, Taipaleenmaki H, Zhang Y, Teplyuk NM, Gupta A, Cinghu S, Shogren K, Maran A, Yaszemski MJ, Ling L, Cool SM, Leong DT, Dierkes C, Zustin J, Salto-Tellez M, Ito Y, Bae SC, Zielenska M, Squire JA, Lian JB, Stein JL, Zambetti GP, Jones SN, Galindo M, Hesse E, Stein GS and van Wijnen AJ MicroRNA-34c inversely couples the biological functions of the runt-related transcription factor RUNX2 and the tumor suppressor p53 in osteosarcoma. J Biol Chem 288 (2013), pp. 21307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega OA, Lucero CMJ, Araya HF, Jerez S, Tapia JC, Antonelli M, Salazar-Onfray F, Las Heras F, Thaler R, Riester SM, Stein GS, van Wijnen AJ and Galindo MA Wnt/beta-Catenin Signaling Activates Expression of the Bone-Related Transcription Factor RUNX2 in Select Human Osteosarcoma Cell Types. J Cell Biochem 118 (2017), pp. 3662–3674. [DOI] [PubMed] [Google Scholar]
- Wadayama B, Toguchida J, Shimizu T, Ishizaki K, Sasaki MS, Kotoura Y and Yamamuro T Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res 54 (1994), pp. 3042–8. [PubMed] [Google Scholar]
- Wimbauer F, Yang C, Shogren KL, Zhang M, Goyal R, Riester SM, Yaszemski MJ and Maran A Regulation of interferon pathway in 2-methoxyestradiol-treated osteosarcoma cells. BMC Cancer 12 (2012), p. 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo DK, Kim HS, Lee HS, Kang YH, Yang HK and Kim WH Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int J Cancer 95 (2001), pp. 108–13. [DOI] [PubMed] [Google Scholar]
- Yang C, Shogren KL, Goyal R, Bravo D, Yaszemski MJ and Maran A RNA-dependent protein kinase is essential for 2-methoxyestradiol-induced autophagy in osteosarcoma cells. PloS one 8 (2013), p. e59406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Ushiku T, Motoi T, Beppu Y, Fukayama M, Tsuda H and Shibata T MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol 36 (2012), pp. 423–31. [DOI] [PubMed] [Google Scholar]
- Zi X, Guo Y, Simoneau AR, Hope C, Xie J, Holcombe RF and Hoang BH Expression of Frzb/secreted Frizzled-related protein 3, a secreted Wnt antagonist, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasiveness. Cancer Res 65 (2005), pp. 9762–70. [DOI] [PubMed] [Google Scholar]


