Abstract
Background
Quantitative computed tomography (QCT) biomarkers of airway morphology hold potential for understanding and monitoring regional airway remodeling in asthma.
Objective
We sought to determine if the change of airway lumen area between TLC and FRC lung volumes measured from CT imaging data was correlated with severe outcomes in asthma patients.
Methods
We studied 152 asthma patients (90 female and 62 male) and 33 normal subjects (12 female and 21 male) with QCT. Post-processing of airways at generations 1–5 (1 = trachea) was performed for wall area percent (WA%), wall thickness percent (WT%), lumen area at baseline TLC (LATLC) and FRC (LAFRC) and low attenuation area at FRC. A new metric (reflecting remodeling and/or distal air trapping), Delta Lumen, was determined from the percent normalized difference in lumen area defined as (LATLC − LAFRC) / LATLC × 100.
Results
Post-processing of 4501 airway segments was performed (3681 segments in the 152 subjects with asthma and 820 segments in the 33 normal subjects, range 17–28 segments per subject). Delta Lumen was negatively correlated with WT% and low attenuation area (p<0.01) in asthma subjects. Delta Lumen was significantly lower for airway generations 3–5 (segmental airways) in subjects undergoing hospitalization due to exacerbation and in refractory asthma requiring treatment with systemic corticosteroids. WT% and low attenuation area were positively, and Delta Lumen was negatively, associated with systemic corticosteroid treatment (p<0.05) suggesting reduced Delta Lumen is a potential outcomes biomarker in severe asthma.
Conclusion
Reduced Delta Lumen of the central airways measured on QCT is a promising exploratory biomarker of unstable refractory asthma that warrants further study.
Keywords: Quantitative CT, Asthma, Airway Remodeling, Severe Asthma, Air Trapping
INTRODUCTION
Quantitative computed tomographic (QCT) imaging is emerging as a useful tool in the study of asthma that can provide structural and functional information not readily available through traditional measures.1,2 QCT measures of airway wall remodeling and air trapping correlate well with lung function, asthma severity, and histology.3–5 Specifically, the airway parameters of wall thickness percentage (WT%), wall area percentage (WA%) and air trapping (i.e. percent low attenuation area < −850 HU at functional residual capacity, hereafter “low attenuation area” (LAA)) derived from single coached breath-hold QCT of the lungs are increased in asthma subjects compared to the normal control subjects.3,6,7 Additionally, these measures are typically greater in those patients with more severe asthma.8,9
Despite prior QCT imaging studies focusing on airway remodeling due to increased WT% and WA% in asthma, there are only a few reports addressing potential mechanical changes in the airway lumen area as a function of lung volume, a measure thought to be related to static airway compliance.10 Other works define airway lumen area change with lung volume variously using terms such as collapsibility, distensibility, and elasticity and have shown positive correlation with lung function.11–14 However, there are multiple factors that may contribute to lumen area change between total lung capacity (TLC) and functional reserve capacity (FRC) including airway remodeling and distal air trapping serving to reduce the FRC/TLC lung volume ratio; because of this issue, we here choose a descriptive term, hereafter “Delta Lumen.” Delta Lumen is specifically defined here as, “the percent change in airway lumen area between functional reserve capacity FRC and total lung capacity TLC as measured from the mid portion of the airway perpendicular to the centerline.” Additionally, prior results were derived from a single cross section of a specific bronchus.12,14 Newer post processing algorithms are capable of measuring Delta Lumen at multiple segments of the tracheobronchial tree from the trachea to the 5th generation bronchi.
The purpose of this study was to quantify Delta Lumen measures, from the trachea to the 5th generation of the central airways, and correlate these measurements with clinical and other QCT based imaging biomarkers of airway disease in asthmatics. We hypothesized that decreased Delta Lumen at different airway generations would be associated with poorer asthma outcome, especially for severe asthmatics where there is known to be significant airway remodeling and increased air trapping.5
MATERIALS AND METHODS
Study Population
Subjects were enrolled at 7 sites that comprise the Severe Asthma Research Program (SARP) network15 during SARP I and SARP II between June, 2003 and May, 2011. A total of 88/123 subjects acquired in SARP I overlap with prior published work.4 Another 97 subjects are new and derive the SARP II study. Both inspiratory (supine total lung capacity (TLC)) and expiratory (supine functional residual capacity (FRC)) lung volumes were required for the Delta Lumen measurement. This was a HIPAA compliant and IRB approved prospective study. All subjects signed an informed consent. One hundred fifty-two asthma patients (90 female and 62 male) and 33 normal subjects (12 female and 21 male) were included in the study, and their demographics are summarized in Table 1. Outcome metrics analyzed for comparison to image measures included asthma exacerbations, emergency department visits, intubation and hospitalizations.
Table 1.
Demographics and pulmonary function test results and indices of study subjects
| Index | Normal (n=33) |
Asthma | ||
|---|---|---|---|---|
| Non-severe (n=63) |
Severe (n=89) |
All Asthma (n=152) |
||
| Age(years) | 31.6 (9.2) | 33.3 (11.2) | 38.4 (13.2) | 36.4 (12.5) |
| Gender, F:M | 12:21 | 39:24 | 51:38 | 90:62 |
| Smoking history (pack years) | 0.27 (0.48) | 1.31 (1.48) | 1.99 (2.09) | 1.73 (1.87) |
| Asthma duration (years) | - | 19.5 (11.4) | 23.8(13.1) | 22.1 (12.6) |
| Body mass index (kg/m2) | 27.4 (8.8) | 28.4 (6.9) | 30.2 (7.2) | 29.4 (7.1) |
| Baseline FEV1 (% predicted) | 99.4 (9.2) | 86.2 (17.3) | 63.1 (22.4) | 72.5 (23.1) |
| Baseline FVC (%predicted) | 99.7 (9.7) | 97.5 (13.6) | 80.3 (18.5) | 87.4 (18.7) |
| Baseline FEV1/FVC (% predicted) | 99.6 (6.4) | 88.0 (11.8) | 74.7 (19.4) | 80.2 (17.9) |
| Post- bronchodilator FEV1 (% predicted) | - | 92.4 (17.8) | 70.9 (21.3) | 80.1 (22.4) |
Data expressed as mean (SD). FEV1-forced expiratory volume in 1 second; FVC = forced vital capacity.
The QCT acquisition methodology is covered in the online supplement.
Image Analysis
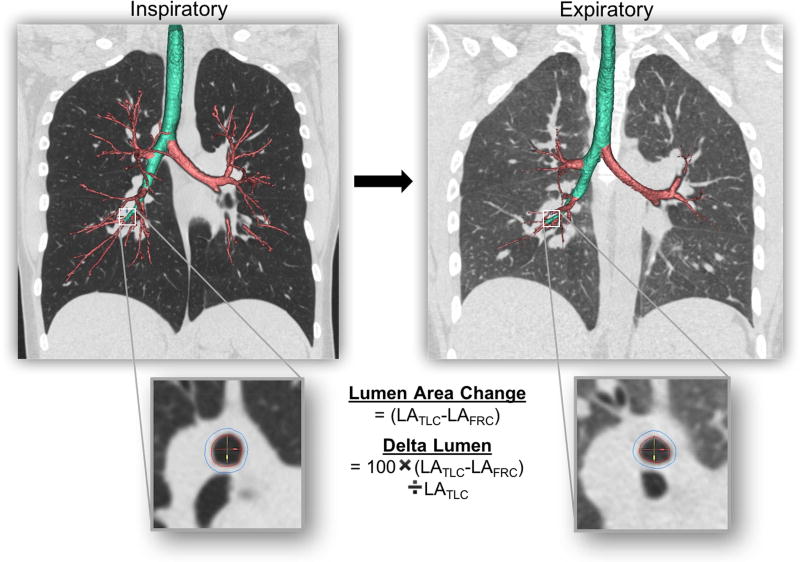
All anonymized CT scan data were downloaded to the VIDA software platform (PW version 2.0, Coralville, IA) where airway lumen area (LA), wall area percent (WA%), and wall thickness percent (WT%) were measured per airway generations (from trachea = 1, 2 (main bronchi), 3 (lobar), 4 (segmental) and 5 (first subsegmental bronchi)) for each subject using differences in segmented average outer and inner area and diameter (Figure 1). The airway lumen area was derived by subtracting the average lumen area over the middle third of each segment at TLC (LATLC) and FRC (LAFRC) lung volumes to mitigate the impact of local variations. Delta Lumen was normalized as a percent of LATLC, mathematically [(LATLC − LAFRC) / LATLC × 100], for each respective airway segment. Airway segment measures were then grouped by airway generation given the competing needs for sufficient statistical power, normalization of expected anatomic variations in airway size16, and the desire to capture regional variations in disease. Whole lung air trapping was measured as the percent low attenuation area < −850 HU at the FRC lung volume.
Figure 1.
Airway lumen area (LA) were measured per airway generation for each subject using differences in inner area (red lines) averaged over the middle third of each segment at TLC (LATLC) and FRC (LAFRC) lung volumes using commercial software (VIDA Diagnostics, PW version 2.0, Coralville, IA). Delta Lumen was normalized as a percent of LATLC, [(LATLC − LAFRC) / LATLC × 100], for each respective airway segment. Airway segment measures were then grouped by airway generation for analysis.
Statistical analysis
SPSS (version 12.0; Chicago, IL, USA), SAS (version 9.4; Cary, NC, USA), and R (version 3.2.0; Vienna, Austria) were used for analysis. Airway generations 1 through 5 were represented by up to 1, 2, 3, 9, and 11 measurements, respectively, in each subject. Average CT measures for each generation were calculated by averaging across all available segments in a generation for each subject. To compare airway measures by clinical characteristics (asthma severity, emergency room (ER), hospitalization, or systemic corticosteroids (CS)) at each airway generation, mixed effect linear models were fit with fixed effect covariates for clinical characteristic, airway generation, characteristic-by-generation interaction, gender, age, BMI, and study (SARP 1 or 2), and a random effect covariate for subject to account for correlation of multiple measurements in each subject. The results are reported as least squares means with 95% confidence intervals. Correlations among LAA, WT%, and Delta Lumen in each airway generation were evaluated by Spearman rank test. A P-value less than 0.05 was considered to be statistically significant.
RESULTS
Analysis of airway trees segmented from CT scans included 4501 total airway segments with a range of 17–28 segments represented per subject. The number of segments represented did not differ by asthma severity or between normal and asthma subjects.
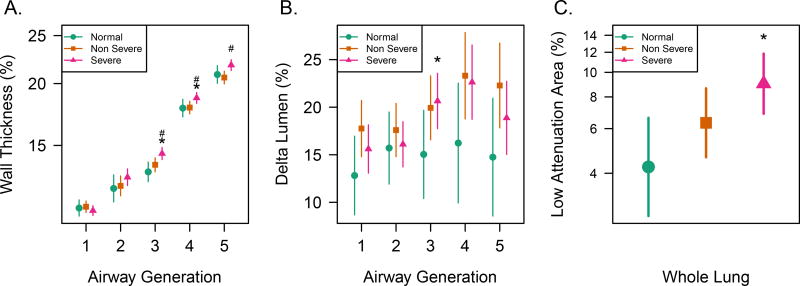
The Delta Lumen, WT%, and LAA are compared for different asthma severities by airway generation in Figure 2. As was reported in prior studies4,5, WT% was found to be increased for the subsegmental airways (20.6% vs. 21.8% at generation 5 for non-severe vs. severe asthma, p = 0.004) and LAA was globally increased in severe asthma compared to normal groups (9.0% vs. 4.2%; p = 0.004) but not compared to non-severe asthma (9.0% vs. 6.3%; p = 0.09). Note that WA% showed nearly identical trends to WT%. Absolute measures of WT% and WA% are summarized in Supplementary Table e-2. The Delta Lumen also increased with airway generation in asthma compared to the normal group at generations 3 and 4 (p<0.05), while Delta Lumen trended higher in both severe and non-severe asthma compared to the normal group (22.6% severe and 23.3% non-severe vs 16.2% normal at generation 4, respectively; p<0.09). It should be noted that absolute measures of lumen area at TLC and FRC (Supplementary Table e-3) were smaller in asthma vs. normal groups, especially at FRC, but their change upon lung inflation was overall greater, for both Delta Lumen and the absolute difference in airway lumen area (i.e. LATLC− LAFRC) by airway generation (Supplementary Figure e-1).
Figure 2.
Asthma severity groups and CT biomarkers where * indicates significance for severe asthma vs. normal groups and # indicates significance for severe vs. non-severe asthma groups at p<0.05. A) Mean and 95% confidence interval (CI) for wall thickness as a percent of airway diameter measured at inspiratory lung volume (Wall Thickness Percent (WT%)) is greater in severe asthma vs. other groups for generations 3 and 4, generations 3 and 4 mark the transition to subsegmental airways. B) Mean change and 95% confidence interval (CI) for airway lumen area between expiratory and inspiratory lung volumes as a percentage (Delta Lumen) is significantly greater in asthma vs. normal groups for generations 3 and 4, which are predominantly lobar and segmental airways, respectively. C) Mean and 95% confidence interval (CI) for percent of the lung with low attenuation area (LAA) <−850 HU for expiratory lung volume is significantly greater in severe asthma vs. normal groups.
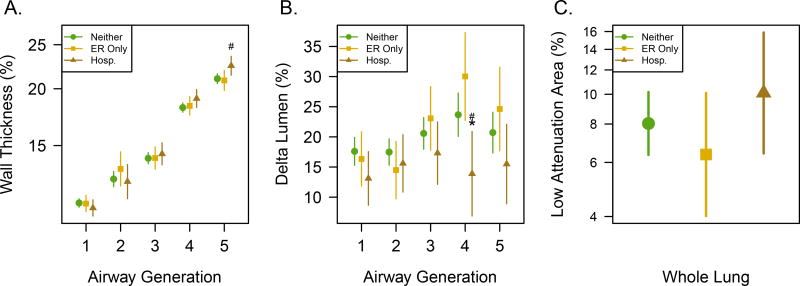
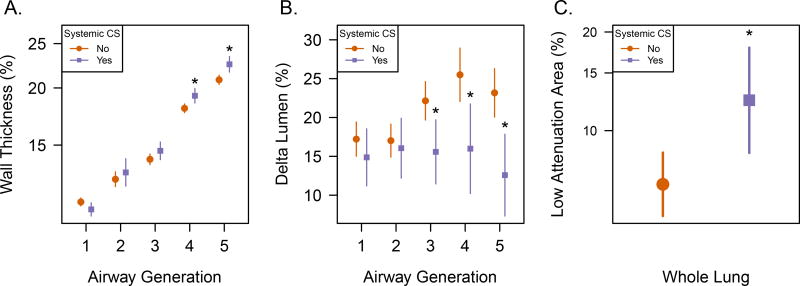
Delta Lumen differed markedly for subpopulations of asthmatics. Delta Lumen was typically smaller in asthma subjects exhibiting severe outcomes of asthma exacerbation requiring hospitalization within 12 months of imaging (Figure 3) and refractory asthma requiring systemic corticosteroid therapy (Figure 4). These subpopulations with more severe outcomes are summarized in Table 2. Subjects undergoing exacerbations leading to hospitalization were associated with decreased Delta Lumen for the segmental airways, generation 4 (14% hospitalization vs 30% ER visit without hospitalization, p <0.0001), in conjunction with increased WT% in the subsegmental airways, generation 5 (p = 0.03), and trended towards decreased Delta Lumen in the subsegmental airways (p = 0.08) with no significant differences in LAA (p = 0.16). Subjects undergoing systemic CS treatment showed a more pronounced association with decreased Delta Lumen at multiple airway generations that had the largest difference at subsegmental generation 5 (12.6% vs. 23.2%; p <0.0001), and included significantly increased WT% (p=0.005) also at generation 5, and increased whole lung LAA (p = 0.008) compared to non-systemic CS, implying segmental and subsegmental airway remodeling and overall increased air trapping. Importantly, a higher exposure to systemic CS (systemic CS dose × number of months taken)was found to be associated with reduced Delta Lumen at generation 5 (Supplementary Table e-4), further supporting the association of reduced Delta Lumen with less well-controlled asthma.
Figure 3.
Outcomes of severe exacerbations in terms of emergency room visits (ER Only) and those leading to hospitalizations (Hosp.) or neither in the last 12 months compared to CT biomarkers where * indicates significance for Hosp vs. neither groups and # indicates significance for Hosp vs. ER Only groups at p<0.05. A) Mean and 95% CI for WT% is greater for hospitalization at generations 5 (sub-segmental airways) compared to those leading to emergency room (ER) visits only and subjects reporting neither outcome. B) Mean and 95% CI for Delta Lumen is significantly reduced in exacerbations leading to hospitalization at generation 4 compared to both ER Only visits and subjects reporting neither outcome. C) Mean and 95% CI for LAA in percent of expiratory lung volume does not differ for these outcomes.
Figure 4.
Refractory asthma as indicated by systemic corticosteroid treatment (CS) compared to subjects on other non-systemic therapies is starkly different for all CT biomarkers where * indicates significance at the p<0.05 level. A) Mean and 95% CI show significantly greater WT% for systemic CS treatment at generations 4 and 5, the subsegmental airways. B) Mean and 95% CI for Delta Lumen is reduced in subjects treated with systemic corticosteroids (CS) at generations 3–5. C) Mean and 95% CI for LAA in percent of expiratory lung volume is greater in subjects treated with systemic corticosteroids (CS).
Table 2.
Fractions of severe vs. non severe asthma groups that fall into different treatment category outcomes that were compared to CT biomarkers.
| Outcome | Non Severe | Severe |
|---|---|---|
| Neither | 90% (56/62) | 52% (45/87) |
| ER Only | 10% (6/62) | 22% (19/87) |
| Hosp | 0% (0/62) | 26% (23/87) |
| Systemic CS | 0% (0/63) | 45% (40/89) |
Emergency room (ER), hospitalization (Hosp), and subjects undergoing neither in the previous 12 months add to 100%. Systemic corticosteroid (CS) treatment is a distinct group exclusive to severe asthmatics compared to subjects on other non-systemic therapies as defined in our study cohort.
Interestingly, Delta Lumen correlates only weakly with whole lung spirometry measures of airflow obstruction (Supplementary Table e-5). Delta Lumen is moderately and inversely correlated WT% for the segmental and subsegmental airways, most prominently in severe asthma (r = −0.224 to −0.349, P<0.05; Table 3). Delta Lumen is also moderately and inversely correlated to LAA in both non-severe and severe asthma, most prominently for the trachea through lobar airway segments (r = −0.246 to −0.630, P<0.05; Table 4). Sputum WBC measurements were available in 87 of 185 total subjects in our study. We found no correlations between Delta Lumen and sputum inflammatory markers in our study population (Supplementary Table e-6).
Table 3.
Correlation of airway delta lumen area with WT% by airway generation in normal andasthma subjects
| G | Normal (n=33) | Non-severe (n=63) | Severe (n=89) | Total asthma (n=152) | ||||
|---|---|---|---|---|---|---|---|---|
| rs | P | rs | P | rs | P | rs | P | |
| 1 | 0.03 | 0.87 | −0.18 | 0.15 | −0.07 | 0.51 | −0.11 | 0.19 |
| 2 | −0.20 | 0.27 | −0.21 | 0.10 | −0.22* | 0.04 | −0.24* | <0.01 |
| 3 | −0.07 | 0.70 | −0.03 | 0.83 | −0.03 | 0.81 | −0.00 | 0.99 |
| 4 | −0.19 | 0.28 | 0.14 | 0.29 | −0.22* | 0.04 | −0.05 | 0.56 |
| 5 | −0.31 | 0.08 | −0.07 | 0.56 | −0.51* | <0.01 | −0.35* | <0.01 |
indicates Spearman correlation p<0.05;
G = generation of the airway; rs = Spearman rank correlation coefficient; P = p-value; N = number of airway segments
Table 4.
Correlation of airway delta lumen area with low attenuation area (LAA) by airway generation in normal and asthma subjects
| G | Normal (n=33) | non-severe (n=63) | Severe (n=89) | total asthma (n=152) | ||||
|---|---|---|---|---|---|---|---|---|
| rs | P | rs | P | rs | P | rs | P | |
| 1 | −0.13 | 0.48 | −0.63* | <0.01 | −0.34* | <0.01 | −0.45* | <0.01 |
| 2 | −0.22 | 0.23 | −0.37* | <0.01 | −0.32* | <0.01 | −0.34* | <0.01 |
| 3 | −0.26 | 0.16 | −0.44* | <0.01 | −0.25* | 0.02 | −0.30* | <0.01 |
| 4 | −0.04 | 0.84 | −0.16 | 0.20 | −0.23* | 0.04 | −0.20* | 0.01 |
| 5 | −0.05 | 0.78 | −0.22 | 0.08 | −0.24* | 0.02 | −0.25* | <0.01 |
Indicates Spearman correlation P<0.05;
G = generation of the airway; rs = Spearman rank correlation coefficient; P = p-value; N = number of airway segments.
DISCUSSION
In this work we explored a potential new QCT biomarker based on the percentage change in each generation’s airway lumen area derived from their respective change in measurements between inspiratory and expiratory lung volumes (Delta Lumen). Automated post-processing of CT scans enabled bronchial lumen extraction up to the 5th generation bronchi.17 Similar to wall thickness measures, Delta Lumen was found to vary with bronchial generation. Reduced subsegmental (bronchial) Delta Lumen was found to be significantly associated with asthma exacerbations leading to hospitalization and asthma that was refractory to treatment. These subpopulations were exclusively severe asthma subjects representing 26% and 45% of our total severe asthma study population, respectively (Table 2). The decrease in Delta Lumen found in subpopulations with severe outcomes generally coincided with other CT biomarkers of airway remodeling (e.g. WT% and LAA). We also found that Delta Lumen negatively correlated with QCT biomarkers of remodeling (i.e. WA% and WT%) and obstructive physiology (LAA). The main value of the current work is as a feasibility study to guide future prospective studies to further establish Delta Lumen as a reliable biomarker of severe outcomes in asthma. Airway lumen area offers potential advantages as a biomarker because it can be more accurately and precisely measured than wall thickness or wall area due to the current spatial resolution limitations of CT imaging.18
Taken together, the increase in wall thickness, air trapping and decrease in Delta Lumen is highly suggestive of airway remodeling that is associated with severe clinical outcomes. The source of the reduced Delta Lumen in a subset of subjects with severe, refractory asthma may result from increased wall thickening in concert with regional air trapping; measures that are all detectable on QCT. The coincidence of all three markers may provide a functional measure of more persistent airway remodeling vs. purely airway inflammation. Measuring Delta Lumen in more persistent vs. reversible airway segments may be useful for predicting which subjects are at-risk for severe exacerbations enabling follow-up with more intensive or alternative therapy approaches for individual patients. Observations of WT%, Delta Lumen, and LAA could eventually guide future treatments by targeting airway fibrosis to improve outcomes in severe asthma19 Improving asthma patient outcomes through the use of personalized clinical and imaging biomarkers that are shown to cluster with phenotype severity has been one of the primary goals of the SARP Project.15
Delta Lumen is distinguished from other QCT biomarkers by its potential relationship to the biomechanical properties of the central airways from the bronchial to the subsegmental generations. Delta Lumen in the current work is a composite of airway segment lengthening, remodeling and coupling to the lung parenchyma. Structural remodeling leading to compliance changes in the asthma group may encompass both features of airways stiffening, abnormal segment lengthening, and smooth muscle changes leading to regional heterogeneity in muscle tone despite bronchodilation20–24 We suspect that Delta Lumen is probably most sensitive to subepithelial fibrosis, a well-known cause of increased airway stiffness, as opposed to other sources of remodeling such as smooth muscle hyperplasia because inhaled bronchodilator was given prior to CT scanning. Regional bronchial inflammation and changes in airway parenchymal coupling in the presence of air trapping likely also play a role in our Delta Lumen measures arguing for evaluation by individual segments, rather than by generation in future studies. Moreover, the order of lung inflation volumes acquired with CT scanning and presence of smooth muscle tone are potential confounders due to the known hysteresis25 of lung inflation and dependence of airway compliance on smooth muscle tone in asthma.26 However, we believe these sources of variation are relatively well-controlled for in this study given that all asthma subjects were scanned after bronchodilation and with TLC lung volume followed by FRC lung volume. These results suggest that discrete changes in airway lumen after bronchoconstriction or bronchodilation, even for just two lung volumes, may add additional information related to the biomechanical and functional changes indicative of severe outcomes in asthma due to increased airway stiffness either related to remodeling27,28 or volume changes secondary to air trapping.29
Airway remodeling encompassing increased airway smooth muscle, subepithelial fibrosis, submucosal gland hyperplasia, increased airway vascularization, and inflammation makeup the phenomenon of airway wall thickening on QCT and are considered markers of severe asthma.30 However, among these, subepithelial fibrosis has been observed in patients with moderate to severe asthma while being absent in patients with mild persistent asthma and normal controls.22 The results of our study in severe asthmatics requiring systemic corticosteroids for asthma control supports the hypothesis that the remodeling process thickens the airways in severe refractory asthma possibly making them stiffer and less compliant leading to persistent airflow obstruction during expiration.23–25 Our results also confirm that WA% and WT% is positively associated with disease severity and negatively to lung function. Brackel et al. have also shown that asthmatic airways may be less collapsible or distensible and concluded that airway remodeling may result in stiffer dynamic elastic properties of the central airway walls in asthmatics.31 Subsequently, the use of WA% and WT% for airway assessment in asthma has been extensively investigated by others.6,11,32,33 A number of studies have shown that the degree of airway thickening was related to the duration, severity of disease, and the level of the airflow limitation.3,4,34,35
LAA, a measure of air trapping, and Delta Lumen were negatively correlated to each other in our study. A similar relationship was also observed by Yamashiro et al. for the trachea in which they found that the expiration/inspiration ratio of tracheal volume showed negative correlations with FEV1/FVC and RV/TLC. They hypothesized that greater changes in tracheal lumen area are associated with less severe airflow limitation and lower amounts of air trapping.14 Since overall lumen area is smaller in asthma (Supplementary Table e-3), these results can be potentially explained by the higher closing volume for end–expiratory scans in more severe asthmatics.36 A prior study that divided asthmatics by LAA greater vs. less than a median threshold found associations between higher LAA and severe outcomes, including intubation history and hospitalizations.5 In our study, associations of Delta Lumen and LAA were analyzed using mixed effect linear models. Delta Lumen was negatively associated with asthma exacerbations leading to hospitalizations and the use of systemic corticosteriods, while LAA was positively associated only with steroid use.
Proposed mechanism of dynamic airway response in asthmatics
With respect to asthma overall, our data show a qualitative increase in Delta Lumen for segmental and subsegmental bronchi compared to normal subjects that is difficult to explain. One possibility is that normalization by the LATLC value is artificially amplifying the percent change in Delta Lumen because the LATLC is smaller overall in asthma. From supplementary Table e-3, asthma subjects have lower LAFRC for segments 3–5 compared to normal, but not, in general, for LATLC, especially for non-severe asthma. Moreover, for the absolute difference measured between LATLC and LAFRC (in mm2) at different airway generations, the qualitative and quantitative relationships for asthma subgroups and outcomes are still observed without normalization (supplementary e-Figure 1). One interpretation is that the central airways in asthma are more compliant overall than normal airways after bronchodilation, with a sub-population of severe asthma demonstrating relative stiffening that is associated with severe outcomes. For example, reduced delta lumen area is associated with increased wall thickness (Table 4) despite the regional heterogeneity introduced by averaging across segments within an airway generation. But multiple factors can influence Delta Lumen, including air trapping which has been demonstrated to be increased in severe asthma.5 The complexity introduced by regional air trapping and impracticality of measuring transbronchial pressure in conjunction with QCT in the clinical setting complicates the mechanistic interpretation of the Delta Lumen measure. The sample size for normal subjects (N = 33) is substantially smaller and predominantly male compared to the asthma subgroups in our study and therefore may not represent the full range of variability in the general normal population. Future work developing this exploratory biomarker will necessarily depend on creation of a model that includes more normative data and accounts for disease heterogeneity using statistical modeling that considers individual airway segments rather than grouping by generation.
Limitations
Several design limitations of the current study are acknowledged. First, the sample size for bronchi at each generation was not equivalent in this analysis across subjects. This was due to smaller, more distal airway pruning at the 5th generation for FRC “expiratory” CT lung volumes simply due to inadequate spatial resolution (i.e. small airway and lumen size), mucous plugging and/or cardiac motion. However, it should be noted that the number of airway segments represented did not differ across groups, or by asthma severity. Second, other CT parameters in this study, such as WA% and WT%, showed relatively lower SD than Delta Lumen measures even at the 5th airway generation. Grouping airway segments by airway generation likely contributed to this variability and future studies could seek to analyze by airway segment rather than grouping by generation. Third, an unknown fraction of the variability in airway measures is almost certainly due to the multi-center design of SARP that necessitated breath-hold coaching by the local research coordinator without spirometric control due to the situation in which most sites performed the studies on clinical scanners, not research scanners with more time flexibility. Additionally, the sites used different CT scanner platforms and there was evolution in CT scanner architecture and software over the time interval that CT scans were included. Finally, inflammatory state was not specifically controlled, although a major criterion for severe asthma was based on corticosteroid dose necessary to control symptoms.15 No withdrawal or dose tapering of steroid use was required prior to imaging. From a conceptual perspective, there will clearly be an impact of inflammation on multiple factors affecting airway mechanics. We hypothesize that Delta Lumen could complement WT% as a means to monitor response to interventions to control inflammation as well as subepithelial fibrosis. Recent advances in CT detector technology and image reconstruction18,37,38 have made low dose lung imaging feasible, thus supporting safer acquisition of multiple CT lung volumes pre- and post-intervention. From a translational perspective, distinguishing likely fibrosis from inflammation by imaging pre- and post-treatment would potentially be valuable for monitoring individual patient response.
Despite the limitations of the present work, the evidence presented supports Delta Lumen as a promising exploratory biomarker that may capture mechanical changes due to airway remodeling and parenchymal coupling in severe outcomes of asthma. Further study will use Delta Lumen to complement wall thickness and air trapping measures in longitudinal studies of severe asthma, especially to test the hypothesis that airway remodeling in severe asthma subpopulations can be used as a prospective marker for likelihood of severe exacerbations and other adverse outcomes.
Conclusion
We studied the change in airway lumen area from TLC to FRC (Delta Lumen) by airway generation on QCT as an exploratory biomarker of severe outcomes in asthmatic subjects. We showed that Delta Lumen has morphological correlates to wall thickness measures that are known to reflect airway remodeling on CT and was significantly decreased in asthma subpopulations that reflect severe clinical outcomes. Future work will further study Delta Lumen and other QCT biomarkers longitudinally as a prospective biomarker for predicting severe asthma exacerbations and for monitoring response to therapy.
Supplementary Material
KEY MESSAGES.
Airway remodeling is a known feature of severe asthma as is increased air trapping; in this work a new QCT biomarker is developed that measures the percent change in airway lumen area of the central airways between two lung volumes (Delta Lumen) as a biomarker associated with severe asthma, reflective of both airway remodeling and air trapping.
Delta Lumen at the level of the segmental airways is associated with clinical outcomes of severe asthma, including exacerbations leading to hospitalization and systemic corticosteroid (CS) therapy as an indicator of severe disease that has not been controlled by inhaled CS.
Delta Lumen on QCT is an accessible and measureable feature of the central airways in asthma with potential for identifying patients with less stable asthma.
Acknowledgments
Funding Sources:
NIH-NHLBI R01 HL069116
NIH-NHLBI U10 HL109168
NIH U01 HL114494
NIH UL1TR000427
The authors would like to acknowledge Drs. Serpil Erzurum and Jason Lempel for supporting CT acquisition at the Lerner Research Institute and the Department of Radiology, Cleveland Clinic, Cleveland OH; the Data Coordinating Center (DCC) of the Severe Asthma Research Program, especially Dave Mauger and Brenda Philips; the CT technologists who participated in this work, and the research volunteers who participated in the CT substudy.
Acronyms/Abbreviations
- ER
Emergency room
- FEV1
Forced expiratory volume in 1 s
- FRC
Functional residual capacity
- FVC
Forced vital capacity
- CT
Multi-detector computed tomography
- QCT
Quantitative computed tomography
- CS
Corticosteroid
- Delta Lumen
Percent difference in lumen area (LATLC − LAFRC) / LATLC × 100
- LATLC
Lumen area at baseline TLC
- LAFRC
Lumen area at baseline FRC
- LAA
Low attenuation area at FRC (air trapping)
- PFT
Pulmonary function test
- RV
Residual volume
- SARP
Severe Asthma Research Program
- TLC
Total lung capacity
- WA%
Wall area percent
- WT%
Wall thickness percent
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castro M, Fain SB, Hoffman EA, et al. Lung imaging in asthmatic patients: the picture is clearer. J Allergy Clin Immunol. 2011;128(3):467–478. doi: 10.1016/j.jaci.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeBoer EM, Spielberg DR, Brody AS. Clinical potential for imaging in patients with asthma and other lung disorders. J Allergy Clin Immunol. 2017;139(1):21–28. doi: 10.1016/j.jaci.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Niimi A, Matsumoto H, Amitani R, et al. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1518–1523. doi: 10.1164/ajrccm.162.4.9909044. [DOI] [PubMed] [Google Scholar]
- 4.Aysola RS, Hoffman EA, Gierada D, et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest. 2008;134(6):1183–1191. doi: 10.1378/chest.07-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busacker A, Newell JD, Jr, Keefe T, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009;135(1):48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little SA, Sproule MW, Cowan MD, et al. High resolution computed tomographic assessment of airway wall thickness in chronic asthma: reproducibility and relationship with lung function and severity. Thorax. 2002;57(3):247–253. doi: 10.1136/thorax.57.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YM, Park JS, Hwang JH, et al. High-resolution CT findings in patients with near-fatal asthma: comparison of patients with mild-to-severe asthma and normal control subjects and changes in airway abnormalities following steroid treatment. Chest. 2004;126(6):1840–1848. doi: 10.1378/chest.126.6.1840. [DOI] [PubMed] [Google Scholar]
- 8.Paganin F, Seneterre E, Chanez P, et al. Computed tomography of the lungs in asthma: influence of disease severity and etiology. Am J Respir Crit Care Med. 1996;153(1):110–114. doi: 10.1164/ajrccm.153.1.8542102. [DOI] [PubMed] [Google Scholar]
- 9.Bumbacea D, Campbell D, Nguyen L, et al. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur Respir J. 2004;24(1):122–128. doi: 10.1183/09031936.04.00077803. [DOI] [PubMed] [Google Scholar]
- 10.Papandrinopoulou D, Tzouda V, Tsoukalas G. Lung compliance and chronic obstructive pulmonary disease. Pulm Med. 2012;2012:542769. doi: 10.1155/2012/542769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med. 2001;163(4):994–1001. doi: 10.1164/ajrccm.163.4.2007119. [DOI] [PubMed] [Google Scholar]
- 12.Chae EJ, Kim TB, Cho YS, et al. Airway Measurement for Airway Remodeling Defined by Post-Bronchodilator FEV1/FVC in Asthma: Investigation Using Inspiration-Expiration Computed Tomography. Allergy Asthma Immunol Res. 2011;3(2):111–117. doi: 10.4168/aair.2011.3.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson JP, McLaughlin RA, Noffsinger WJ, et al. Elastic properties of the central airways in obstructive lung diseases measured using anatomical optical coherence tomography. Am J Respir Crit Care Med. 2011;183(5):612–619. doi: 10.1164/rccm.201002-0178OC. [DOI] [PubMed] [Google Scholar]
- 14.Yamashiro T, San Jose Estepar R, Matsuoka S, et al. Intrathoracic tracheal volume and collapsibility on inspiratory and end-expiratory ct scans correlations with lung volume and pulmonary function in 85 smokers. Acad Radiol. 2011;18(3):299–305. doi: 10.1016/j.acra.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarjour NN, Erzurum SC, Bleecker ER, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185(4):356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S, Hoffman EA, Wenzel SE, et al. Quantitative assessment of multiscale structural and functional alterations in asthmatic populations. J Appl Physiol (1985) 2015;118(10):1286–1298. doi: 10.1152/japplphysiol.01094.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.San Jose Estepar R, Reilly JJ, Silverman EK, Washko GR. Three-dimensional airway measurements and algorithms. Proc Am Thorac Soc. 2008;5(9):905–909. doi: 10.1513/pats.200809-104QC. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez A, Ranallo FN, Judy PF, Fain SB. The effects of iterative reconstruction and kernel selection on quantitative computed tomography measures of lung density. Med Phys. 2017;44(6):2267–2280. doi: 10.1002/mp.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy CL, Nguyen HA, Mohamud R, et al. The activin A antagonist follistatin inhibits asthmatic airway remodelling. Thorax. 2013;68(1):9–18. doi: 10.1136/thoraxjnl-2011-201128. [DOI] [PubMed] [Google Scholar]
- 20.Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Pare PD. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol (1985) 1993;74(6):2771–2781. doi: 10.1152/jappl.1993.74.6.2771. [DOI] [PubMed] [Google Scholar]
- 21.Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology. 2006;11(4):388–406. doi: 10.1111/j.1440-1843.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 22.Saetta M, Turato G. Airway pathology in asthma. Eur Respir J Suppl. 2001;34:18s–23s. doi: 10.1183/09031936.01.00229501. [DOI] [PubMed] [Google Scholar]
- 23.Woodruff PG, Dolganov GM, Ferrando RE, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169(9):1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 24.James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J. 2007;30(1):134–155. doi: 10.1183/09031936.00146905. [DOI] [PubMed] [Google Scholar]
- 25.Burns CB, Taylor WR, Ingram RH., Jr Effects of deep inhalation in asthma: relative airway and parenchymal hysteresis. J Appl Physiol (1985) 1985;59(5):1590–1596. doi: 10.1152/jappl.1985.59.5.1590. [DOI] [PubMed] [Google Scholar]
- 26.Wang YT, Thompson LM, Ingenito EP, Ingram RH., Jr Effects of increasing doses of beta-agonists on airway and parenchymal hysteresis. J Appl Physiol (1985) 1990;68(1):363–368. doi: 10.1152/jappl.1990.68.1.363. [DOI] [PubMed] [Google Scholar]
- 27.Okazawa M, Muller N, McNamara AE, Child S, Verburgt L, Pare PD. Human airway narrowing measured using high resolution computed tomography. Am J Respir Crit Care Med. 1996;154(5):1557–1562. doi: 10.1164/ajrccm.154.5.8912780. [DOI] [PubMed] [Google Scholar]
- 28.Okazawa M, Pare PD, Lambert RK. Compliance of peripheral airways deduced from morphometry. J Appl Physiol (1985) 2000;89(6):2373–2381. doi: 10.1152/jappl.2000.89.6.2373. [DOI] [PubMed] [Google Scholar]
- 29.Choi S, Hoffman EA, Wenzel SE, et al. Registration-based assessment of regional lung function via volumetric CT images of normal subjects vs. severe asthmatics. J Appl Physiol (1985) 2013;115(5):730–742. doi: 10.1152/japplphysiol.00113.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shifren A, Witt C, Christie C, Castro M. Mechanisms of remodeling in asthmatic airways. J Allergy (Cairo) 2012;2012:316049. doi: 10.1155/2012/316049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brackel HJ, Pedersen OF, Mulder PG, Overbeek SE, Kerrebijn KF, Bogaard JM. Central airways behave more stiffly during forced expiration in patients with asthma. Am J Respir Crit Care Med. 2000;162(3 Pt 1):896–904. doi: 10.1164/ajrccm.162.3.9905034. [DOI] [PubMed] [Google Scholar]
- 32.Gono H, Fujimoto K, Kawakami S, Kubo K. Evaluation of airway wall thickness and air trapping by HRCT in asymptomatic asthma. Eur Respir J. 2003;22(6):965–971. doi: 10.1183/09031936.03.00085302. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Siddiqui S, Haldar P, et al. Quantitative analysis of high-resolution computed tomography scans in severe asthma subphenotypes. Thorax. 2010;65(9):775–781. doi: 10.1136/thx.2010.136374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Awadh N, Muller NL, Park CS, Abboud RT, FitzGerald JM. Airway wall thickness in patients with near fatal asthma and control groups: assessment with high resolution computed tomographic scanning. Thorax. 1998;53(4):248–253. doi: 10.1136/thx.53.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasahara K, Shiba K, Ozawa T, Okuda K, Adachi M. Correlation between the bronchial subepithelial layer and whole airway wall thickness in patients with asthma. Thorax. 2002;57(3):242–246. doi: 10.1136/thorax.57.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorkness RL, Bleecker ER, Busse WW, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol (1985) 2008;104(2):394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez A, Ranallo FN, Judy PF, Gierada DS, Fain SB. CT reconstruction techniques for improved accuracy of lung CT airway measurement. Med Phys. 2014;41(11):111911. doi: 10.1118/1.4898098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sieren JP, Hoffman EA, Fuld MK, Chan KS, Guo J, Newell JD., Jr Sinogram Affirmed Iterative Reconstruction (SAFIRE) versus weighted filtered back projection (WFBP) effects on quantitative measure in the COPDGene 2 test object. Med Phys. 2014;41(9):091910. doi: 10.1118/1.4893498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.