Abstract
Neuroimaging research has characterized underlying neural mechanisms of attentional control and cognitive reappraisal, common implicit and explicit forms of emotion regulation, respectively. This research suggests attentional control and reappraisal may engage similar midline and lateral areas in the prefrontal cortex (PFC); however, findings are largely based on separate studies. Therefore, the extent to which mechanisms of implicit versus explicit regulation are independent or overlapping is not clear. In the current study, 49 healthy participants completed well-validated implicit and explicit regulation tasks in the form of attentional control and cognitive reappraisal during functional magnetic resonance imaging. During implicit regulation, participants identified a target letter in a string of letters superimposed on threatening faces. To manipulate attentional control, the letter string either consisted of all targets (‘Threat Low’ perceptual load), or was embedded among non-target letters (‘Threat High’ perceptual load). During cognitive reappraisal, participants were shown aversive images and instructed to use a cognitive approach to down-regulate negative affect (‘Reappraise’) or to naturally experience emotions without altering them (‘Look-Negative’). Order of administration of tasks was counterbalanced across participants. Whole-brain results regarding frontal activity showed ventromedial PFC/rostral anterior cingulate cortex was recruited during Threat Low>Threat High. In contrast, Reappraise>Look-Negative resulted in engagement of the dorsolateral PFC, ventrolateral PFC and dorsomedial PFC. In addition, results showed no relationship between accuracy during attentional control and self-reported negative affect during cognitive reappraisal. Results indicate attentional control in the context of threat distractors and the reappraisal of negative images are supported by discrete, non-overlapping neurocircuitries.
Keywords: emotion regulation, implicit, explicit, attentional control, cognitive reappraisal, fMRI, neuroimaging
Introduction
Negative stimuli are preferentially attended to (LeDoux, 1998; Öhman & Mineka, 2001; Yiend, 2010), therefore, the ability to adaptively manage emotional response to salient negative cues has a positive effect on day-to-day functioning and psychological health (Gross & John, 2003; Gross & Muñoz, 1995; Koole & Rothermund, 2011). As such, understanding the neural underpinnings of effectual emotion regulation is an intensive area of study, due in part to its relevance in psychiatric illnesses that are characterized by disturbances in affect and maladaptive behaviors to cope with negative emotions. Emotions unfold over time and various strategies exist for modulating the emotion-generative process (Gross, 2002; Gross & John, 2003). One way to conceptualize differences in emotion regulation strategies is through a dual-processes framework, which considers regulation in the context of two distinct, yet related, processes defined by implicit and explicit operations (Gyurak, Gross, & Etkin, 2011). Distinction between the two forms stem from variations in the effort used to alter an emotional experience (Mauss, Bunge, & Gross, 2007).
Implicit regulation refers to processes that modify an emotional response without deliberate intent or conscious supervision (Hopp, Troy, & Mauss, 2011; Koole & Rothermund, 2011). An important aspect of implicit regulation is attentional control (e.g., resolution of emotional interference), an antecedent-focused strategy as it inhibits an emotional response to a stimulus before it becomes fully activated (Gross & Thompson, 2007). Due to processing capacity limits (Desimone & Duncan, 1995), sensory-driven, bottom-up stimuli compete with higher-order cognitive goals for neural representation and the stimulus that ‘wins’ the competition guides action and behavior (Pessoa, Kastner, & Ungerleider, 2002). Factors that impact attentional control include the salience of distractors (e.g., negative versus neutral task-irrelevant cues) and task demands as demonstrated with paradigms that vary in perceptual load. For instance, according to load theory, when perceptual load is low (e.g., when cognitive goals are easy to execute), task-relevant resources are available (i.e., ‘leftover’) to process emotional distractors (Lavie, Lin, Zokaei, & Thomas, 2009). Conversely, when a cognitive goal exhausts capacity limits (e.g., when goals are difficult to execute), the processing of salient distractors is attenuated (Lavie et al., 2004, 2009). Put another way, the higher the cognitive effort directed at completing a goal, the lower the emotional interference linked to task-irrelevant stimuli.
At the neural level, effectual implicit regulation is implicated when frontal regions are recruited [e.g., anterior cingulate cortex (ACC), medial prefrontal cortices (MPFC), lateral areas of the prefrontal cortex (PFC)] and activity in central emotion processing regions (e.g., amygdala) are reduced (Etkin, Egner, & Kalisch, 2011a; MacDonald, 2000). In support of load theory, Bishop and colleagues showed ACC, dorsolateral PFC (DLPFC), ventrolateral PFC (VLPFC), and parietal regions were engaged during high perceptual load relative to low perceptual load in the context of briefly-presented threat and neutral face distractors (i.e., 200 milliseconds (ms)), indicating the high load condition was demanding of processing resources (Bishop, Jenkins, & Lawrence, 2007). Differences in cognitive effort were also observed at the behavioral level as accuracy was higher and response times shorter in the low relative to high load condition (Bishop et al., 2007). Moreover, an a priori analysis revealed increased amygdala reactivity to threat face distractors in the low relative to high perceptual load condition (Bishop et al., 2007). Taken together, threat distractors were only processed when the condition allowed for residual task-relevant resources. Here, load was manipulated with letter strings superimposed on faces where the identification of a target letter (X or N) was either embedded in a mixed string of letters (high perceptual load) or a homogeneous string of target letters (low perceptual load). Thus, the letters, a source of neutral information, may have served as a distractor from the emotional face particularly under high load conditions, as evinced by an absence of amygdala reactivity in this condition (Bishop et al., 2007). Recently, using a similar task we observed ACC recruitment during low perceptual load when the cognitive goal was easy to execute and distractors were salient (Bunford, Roberts, Kennedy, & Klumpp, 2017; Wheaton, Fitzgerald, Phan, & Klumpp, 2014). These data are in line with accumulating evidence that effective attentional control engages the ACC (Etkin, Egner, & Kalisch, 2011; MacDonald, 2000; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Kanske & Kotz, 2011).
In contrast to implicit regulation, explicit regulation refers to a deliberate attempt to inhibit or alter an emotional state in a specific direction (Gross & John, 2003; Ochsner & Gross, 2005). A well-studied form of explicit regulation is cognitive reappraisal, also an antecedent-focused emotion regulation approach that involves changing the meaning of an emotion-eliciting stimulus or situation to alter its emotional impact (Gross, 2013; Ochsner, Silvers, & Buhle, 2012). Studies, including meta-analytic investigations (Buhle et al., 2014; Messina, Bianco, Sambin, & Viviani, 2015), have consistently shown that cognitive reappraisal involves widespread cortical recruitment including areas associated with implicit regulation such as the ACC, DLPFC, DMPFC, and VLPFC. Less consistent with implicit regulation is evidence of engagement in the inferior frontal gyrus (IFG), middle and superior temporal gyri, and parietal regions including the angular gyrus (Buhle et al., 2014; Kohn et al., 2014; Messina, Bianco, Sambin, & Viviani, 2015; Ochsner et al., 2012). Involvement of these regions in particular may be due to the fact that cognitive reappraisal involves many higher-order functions (e.g., appraisal, attention, inhibition, working memory) as well as semantic functions to facilitate the generation of alternative representations of emotional stimuli (Messina et al., 2015). Behaviorally, self-reported affective state based on a Likert-type scale is a common index of on-line reappraisal ability. In a seminal study by Ochsner and colleagues (Ochsner, Bunge, Gross, & Gabrieli, 2002) greater reappraisal facility qualified by a larger difference in self-reported affect between reappraising negative images (‘Reappraise’) versus looking at negative images (‘Look Negative’) corresponded with more activation in the ACC and supramarginal gyrus and, in another study, with DMPFC activity (Modinos, Ormel, & Aleman, 2010). In further support of brain-behavioral relationships, we recently demonstrated that greater engagement of frontal regions predicted successful reappraisal using self-reported ratings (Klumpp, Bhaumik, Kinney, & Fitzgerald, 2018).
In summary, the effective management of emotions elicited by a salient cue can be attained by means of attentional control modulated by perceptual load or cognitive reappraisal. While neuroimaging studies suggests shared and unique mechanisms underlie these implicit and explicit emotion regulation approaches, information regarding the spatial distribution of these neural circuitries are based on discrete studies completed in parallel, separate samples. Therefore, further study is necessary. Findings have implications in identifying phenotypes among internalizing conditions such as anxiety, depression, or other conditions marked by emotion dysregulation (e.g., excessive negative affect) or individuals at risk of developing internalizing psychopathologies. In particular, in highlighting the relevance of load theory in advancing our understanding of attentional control as it relates to psychological health, we and others have shown that individual differences in the anxiety spectrum (e.g., trait anxiety, social anxiety disorder) and traits that act as a vulnerability factor to anxiety and depression (e.g., behavioral inhibition; Muris, Merckelbach, Schmidt, Gadet, & Bogie, 2001) are frequently observed when perceptual load is low (Bishop et al., 2007; Bunford, Roberts, et al., 2017; Wheaton et al., 2014). It has also been demonstrated that anxious and depressed individuals exhibit aberrant neurofunctional activity during cognitive reappraisal to negative stimuli (Fitzgerald et al., 2017; Goldin, Manber, Hakimi, Canli, & Gross, 2009; Radke et al., 2017; Stephanou, Davey, Kerestes, Whittle, & Harrison, 2017). Furthermore, neural activity (i.e., ACC, amygdala) during reappraisal and to negative distractors under low (relative to high) perceptual load conditions predicts symptom change after cognitive behavioral therapy (CBT) in socially anxious patients (Klumpp, Fitzgerald, et al., 2017).
In addition, findings have implications for the treatment of individuals characterized by emotion dysregulation (e.g., depression, anxiety disorders). For example, CBT is directed at enhancing emotion regulation with an amalgam of approaches (e.g., Socratic questioning, logical empiricism, behavioral ‘experiments’) over the course of multiple sessions (e.g., Arch & Craske, 2009). Evidence reappraisal is used more frequently in anxious patients who participate in CBT (Moscovitch et al., 2012) and reports of increased attentional control following CBT (Lundh & Öst, 2001; Mattia, Heimberg, & Hope, 1993; Pishyar, Harris, & Menzies, 2008) suggest our understanding of treatment mechanisms could be advanced as CBT encompasses strategies analogous to reappraisal but does not directly target attentional control. Indeed, if attentional control and reappraisal engage similar neural pathways, it is expected that attentional bias modification interventions, which are less costly and more readily available than CBT (Price et al., 2017; Yang, Zhang, Ding, & Xiao, 2016), would increase reappraisal capability. Alternatively, if mechanisms are discrete, the rate of response and/or attrition could be improved by tailoring CBT components to a patient's level of regulation facility in terms of implicit and explicit strategies as proficiency in one may not extend to the other. Findings also have inferences for neuromodulation as shared involvement of a targeted region (e.g., DLPFC) would broadly impact regulation capacity regardless of the effort and processes involved. Conversely, the existence of a more distinct distribution of neural function across forms of regulation may necessitate refinement in the selection of neural targets directed at regulation-specific operations.
Therefore, the primary objective of the current study was to examine mechanisms of implicit regulation in accordance with load theory and mechanisms of explicit regulation by way of cognitive reappraisal in the same sample of healthy individuals. We hypothesized that in the context of negative stimuli, both tasks would recruit dorsal midline structures (e.g., DMPFC), lateral frontal regions (e.g., DLPFC, VLPFC), and ACC. We also expected patterns would point to regulation-dependent systems, for example, distinction between regions strongly implicated in reappraisal (e.g., IFG, temporal regions) (Buhle et al., 2014; Kohn et al., 2014; Messina, Bianco, Sambin, & Viviani, 2015; Ochsner et al., 2012) but not attentional control and vice versa (Bishop et al., 2007; Bunford, Roberts, et al., 2017; Etkin, Egner, & Kalisch, 2011b; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Kanske & Kotz, 2011; MacDonald, 2000). Beyond neuroimaging, we explored whether there was a relationship between accuracy related to a cognitive goal (i.e., attentional control) and affective state related to reappraisal to aid in the characterization of distinct or shared mechanisms involving implicit and explicit regulation strategies.
Methods
Participants
Participants consisted of 49 healthy adults between the ages of 18-60 (25.24± 7.98) with 12-22 years of education (15.65±2.41). Sixty-seven percent of the sample was female and racial and ethnic composition consisted of: 45% Caucasian, 29% Asian, 14% African-American, and 12% unknown; 22% percent of the identified as Hispanic. Inclusion criteria were: (1) absence of any major medical or neurological illness or Axis I disorder as confirmed by diagnostic interviewing with the Structured Clinical Interview for Diagnostic Statistical Manual-IV (SCID-IV; (First, Spitzer, Gibbon, & Williams, 2002) conducted at screening by trained master's level clinicians, (2) ability to give informed consent, and (3) free from drugs on the day of testing as confirmed by a urinary drug screen. Exclusion criteria for fMRI included (1) the presence of ferromagnetic objects within the body, (2) being pregnant or actively trying to become pregnant, (3) fear of enclosed spaces (e.g., claustrophobia), and (4) inability to lie still in an enclosed space. All participants completed a consent form approved by the local Institutional Review Board at the University of Illinois at Chicago. Participants were compensated for their time and all procedures complied with the Helsinki Declaration.
fMRI Tasks
All participants completed implicit and explicit emotion regulation tasks during fMRI in the form of the Emotional Faces Interference Task (EFIT) and the Emotion Regulation Task (ERT), respectively. Task order was counterbalanced across all participants to control for order effects.
Emotional Faces Interference Task (EFIT)
EFIT was adapted from Bishop and colleagues (Bishop et al., 2007) for the inclusion of both angry and fearful faces involving both genders as emotional distractors, with the present version previously validated in our laboratory (Bunford et al., 2017ab; Klumpp et al., 2016, 2017; Wheaton et al., 2014). The balance of gender was matched across emotional faces. Participants viewed a string of six letters superimposed on task-irrelevant face distractors (e.g., angry, fearful, neutral) and were asked to identify whether there was an N or X present as quickly and accurately as possible. Participants were not explicitly instructed to ignore faces and responses were collected via button press. For low perceptual load trials, the string was comprised entirely of target letters (NNNNN or XXXXX) and in high perceptual load trials, the string included a single target letter and five non-target consonants (HKMWZ) in randomized order. Thus, low perceptual load was comparatively easier to perform. Distractor faces were drawn from a standardized set of photographs and consisted of fearful, angry, and neutral expressions from 8 different individuals (Ekman & Friesen, 1976). The experiment was completed over two separate runs, each comprising 12 blocks of 5 trials. A mixed block/event-related design was employed whereby perceptual load (low vs. high) varied across blocks and facial expression (fearful, angry, neutral) within blocks on a trial-by-trial basis. Images were presented for 200 ms followed by a fixation cross presented for 1800 ms. Within blocks, trials were separated by a jittered inter-stimulus interval lasting 2–6 seconds (s); trials between blocks were separated by 4–8 s.
Emotion Regulation Task (ERT)
In addition to EFIT, participants completed ERT validated in other labs (Ochsner, Bunge, Gross, & Gabrieli, 2002) as well as our own (Fitzgerald et al., 2016, 2017; Klumpp et al., 2016; MacNamara et al., 2016; Phan et al., 2005; Rabinak et al., 2014). During ERT, participants used cognitive reappraisal to down-regulate negative affect state in response to negative images. Participants were shown 64 negative and 32 neutral images from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert BN, 2008) across three conditions (Reappraise, Look-Negative, Look-Neutral). Participants were instructed to: 1) use a cognitive strategy to reduce negative affect to aversive images (‘Reappraise’ condition); 2) attend to the emotional state elicited by aversive images (‘Look-Negative’ condition); or 3) view neutral images (‘Look-Neutral’ condition). Prior to scanning and as per protocol (Fitzgerald et al., 2016, 2017; Klumpp et al., 2016; MacNamara et al., 2016; Phan et al., 2005; Rabinak et al., 2014), participants were instructed to employ the strategy of cognitive reappraisal to “reduce [their] negative affect by making the image appear less emotional”. Consistent with prior studies (Fitzgerald et al., 2016, 2017; Gorka et al., 2016; Klumpp, Roberts, et al., 2017; MacNamara et al., 2016; K. N. Ochsner et al., 2002; Rabinak et al., 2014) participants were provided examples to promote understanding of cognitive approaches to reduce a negative emotional response to an aversive image. For example, transforming the scenario depicted by an image into positive terms (e.g., women crying outside of a church could be alternatively interpreted as expressing tears of joy from a wedding ceremony rather than of sorrow from a funeral) and rationalizing or objectifying the content of the pictures (e.g., a woman with facial bruises could be translated as an actor wearing makeup rather than a victim of domestic abuse; Phan et al., 2005, p. 211). Participants completed eight practice trials using images not shown in the actual task to confirm understanding of task instructions.
The ERT comprised two separate runs, each lasting a total of 5 min. Each run consisted of two 20 s blocks of each condition (four images presented for 5 s each without inter-stimulus interval). Blocks were interspersed by 20 s blocks of a fixation cross. Prior to each block, an instruction screen (“Reappraise” or “Look”) was presented for 5 s. To assess behavioral responses, participants viewed a screen that asked them to answer the question “How negative do you feel?” for the collection of on-line subjective negative affect ratings immediately following each block. Participants indicated their response on a 5-item Likert scale (1 = not at all; 5 = extremely) via a 5-button response.
Behavioral Data Analysis
To verify participants followed EFIT and ERT task instructions, behavioral data were submitted to Analysis of Variances (ANOVAs) for each paradigm. Significant ANOVA findings were followed-up with simple effects analysis. Two-tailed Pearson's correlations were used to explore potential relationships between accuracy during EFIT Threat Low > Threat High trials and self-reported affective state during ERT Reappraise > Look-Negative trials.
fMRI Acquisition, Preprocessing, and Analysis
FMRI scanning was performed on a 3 Tesla Discovery System (General Electric Healthcare; Waukesha, WI) using a standard radiofrequency coil. Whole-brain functional images (i.e., blood oxygen level-dependent [BOLD]) were collected using the following parameters: TR = 2 s, TE = 25 ms, flip angle = 90°, field of view = 22 × 22 cm2, acquisition matrix 64 × 64; 44 axial, 3-mm-thick slices with no gap. The first 4 volumes from each run were discarded to allow magnetization to reach equilibrium.
Conventional preprocessing steps were completed in Statistical Parametric Mapping (SPM8) software package (Wellcome Trust Centre for Neuroimaging, London www.fil.ion.ucl.ac.uk/spm). Images were temporally corrected to account for slice time acquisition differences and spatially realigned to the mean image to correct for head movement while motion realignment parameters were entered as regressors of no-interest to control for minimal head movement during scanning. Functional images from all participants included in analysis met criteria for quality with minimal motion correction (movements were < 3 mm and < 3 degrees rotation in any one direction). Images were then normalized to a Montreal Neurological Institute (MNI) template using the echo-planar imaging template, resampled to 2 × 2 × 2 voxels and smoothed with an 8 mm isotropic Gaussian kernel.
For each task, a general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128 s high-pass filter. For EFIT, blocks of low and high perceptual load were modeled separately based on task-irrelevant face type (angry, fearful, and neutral) resulting in six regressors (angry low, angry high, fearful low, fearful high, neutral low, neutral high), the effects of which were estimated for each voxel for each participant and taken to the second level for random effects analysis. Since negative stimuli are more salient than neutral stimuli, fearful and angry emotional distractors were combined (i.e., ‘threat’ distractors) to maximize power. Our primary contrast of interest was Threat Low > Threat High perceptual load in order to isolate neural activation during attentional control when distractors were more salient (e.g., high emotional interference due to low perceptual load). The analyses was repeated using Neutral Low > Neutral High to confirm that any significant effects were driven by threat distractors. For ERT, blocks of Reappraise, Look-Negative, and Look-Neutral were modeled separately in relation to implicit baseline (i.e., fixation cross), the effects of which were estimated for each voxel for each participant and taken to the second level for random effects analysis. Our primary objective was to examine neural engagement significantly related to cognitive reappraisal in relation to emotional reactivity; therefore, the primary contrast of interest was Reappraise > Look-Negative.
One-sample t-tests were performed in order to characterize the activation driving the primary linear contrasts of interest, as opposed to interaction effects. Following recent guidelines in response to concerns about false positives resulting from lenient significance thresholds (Eklund, Nichols, & Knutsson, 2016; Woo, Krishnan, & Wager, 2014), all clusters were considered significant only when they surpassed family-wise error (FWE) correction adjusted for multiple comparisons across the entire brain at p<0.05 and comprising at least 20 contiguous voxels. The Automatic Anatomical Labeling (AAL) system (Tzourio-Mazoyer et al., 2002) was used to aid in the interpretation of regions within large clusters of activity.
To examine potential overlap in neural engagement between EFIT and ERT, binary masks based on significant activation detected in one form of regulation were created and subjected to a conjunction analysis by looking for overlap using the logical AND operator in the SPM MarsBar toolbox (Brett, Anton, Valabregue, & Poline, 2002), in keeping with the approach used by prior guidelines (Nichols, Brett, Andersson, Wager, & Poline, 2005). Additionally, overlap was examined with the common region function in the xjView toolbox (http://www.alivelearn.net/xjview), which displays the product of values from the contrasts of interest. Overlap was assessed using the minimum statistic for significance generated by SPM using a whole-brain FWE-correction.
In subsequent exploratory analyses, we controlled for individual differences in behavior by adding the difference score between Threat Low and Threat High accuracy (for EFIT) and the difference score between self-report ratings during Reappraise and Look-Negative (for ERT) as covariates of no-interest in the respective one-sample t-tests (i.e., Threat Low > Threat High; Reappraise > Look Negative). In addition, we also explored whether neural activation could be explained by variability in accuracy in EFIT and self-reported affective state in ERT. These analyses were completed to test the possibility that individual differences in behavior and reappraisal ability may account for variability in the distribution of neural activation. All planned and exploratory analyses controlled for age and gender.
Results
Behavioral Results
EFIT
With regard to accuracy, a 2 (Perceptual Load: Low, High) × 2 (Distractor Type: Threat, Neutral) repeated measures ANOVA demonstrated a main effect of load (F(1, 48)=310.96, p<0.001), a main effect of distractor type (F(1, 48)=5.46), p=0.02) but no load-by-distractor type interaction (p=0.31). Follow-up, paired-tests showed participants were more accurate during low load (90±15%) compared to high load conditions (62±13%) [t(48)=17.63, p<0.001] and when neutral distractors were present (77±14%) compared to threatening distractors (75±13%) [t(48)=2.34, p=0.02]).
A 2 (Load: Low, High) × 2 (Distractor Type: Threat, Neutral) repeated measures ANOVA on reaction time revealed a main effect of load [F(1, 48)=191.97, p<0.001] but no main effect for distractor type (p=0.20); there was no load-by-distractor type interaction (p=0.45). Follow-up paired t-tests revealed participants were faster during low load trials (7,412±247ms) compared to high load trials (1,058±347ms) [t(48)=13.86, p<0.001]. Results for behavioral data for both tasks across all conditions are listed in Table 1.
Table 1. Mean (M) and standard deviation (SD) for Emotional Faces Interference Task and Emotion Regulation Task.
| Accuracy | Reaction Time (ms) | ||
|---|---|---|---|
|
| |||
| Condition | M (SD) | M (SD) | |
| Emotional Faces Interference Task | |||
| Threat Low | 0.90 (0.15) | 749.64 (255.62) | |
| Threat High | 0.60 (0.13) | 1,058.97 (353.81) | |
| Neutral Low | 0.91 (0.16) | 733.56 (241.82) | |
| Neutral High | 0.63 (0.15) | 1,056.41(347.49) | |
| Subjective Affective State | |||
| Condition | M (SD) | ||
| Emotion Regulation Task | |||
| Look-Neutral | 1.20 (0.32) | ||
| Look-Negative | 2.53 (0.82) | ||
| Reappraise | 2.18 (0.78) | ||
ERT
Behavioral data for ERT was available for all participants except in the case of one participant who had missing ratings during the Look-Neutral condition. As this participant's ratings were present for the primary contrast of interest, Reappraise (> Look-Negative), the participant was retained in all analyses. A repeated measures ANOVA for Reappraise, Look-Negative, Look-Neutral revealed a main effect of condition [F(2, 94)=70.71, p<0.001]. In follow-up paired t-tests, participants rated feeling more negative following Look-Negative conditions (2.53±0.82) compared to Look-Neutral (1.20±0.32) conditions [t(47) = 10.24, p<0.001]. As expected, participants showed a reduction in negative affect following Reappraise (2.18±0.78) in comparison to Look-Negative (t(48) = 3.33, p<0.01).
Correlation Between EFIT and ERT Behavioral Rata
Pearson's correlation showed no relationship between accuracy during EFIT Threat Low (> Threat High) and self-reported affective state during Reappraise (> Look-Negative) (p>0.48).
fMRI Results: Independent Task Engagement
EFIT
Whole-brain findings for Threat Low > Threat High revealed engagement of the left VMPFC encompassing rostral ACC (rACC; peak MNI coordinates: -2, 52, -2; Z = 5.45; volume = 5,752 mm3; p<0.05 FWE corrected), left posterior cingulate cortex (PCC; peak MNI coordinates: -8, -46, 32; Z = 5.24; volume = 1,248 mm3; p<0.05 FWE corrected), and left inferior parietal lobe (IPL; peak MNI coordinates: -44, -72, 38; Z = 4.93, volume = 1,008 mm3; p<0.05 FWE corrected). No significant clusters were observed for Neutral Low > Neutral High (p>0.05 FWE corrected).
Results did not change when adding the difference score reflecting changes in accuracy between Threat Low and Threat High conditions as a covariate of no-interest. Similarly, we did not find a significant association between neural activation and accuracy.
ERT
Whole-brain findings for Reappraise > Look-Negative revealed recruitment of the left DLPFC (peak MNI coordinates: -42, 12, 60; Z = 5.89; volume = 2,464 mm3; p<0.05 FWE corrected), right DLPFC (peak MNI coordinates: 18, 40, 56; Z = 5.23; volume = 1,408 mm3; p<0.05 FWE corrected), left VLPFC (peak MNI coordinates: -50, 22, 12 & -46, 36, -10; Z = 4.70 & 4.59; volume = 672 mm3 & 480 mm3; p<0.05 FWE corrected) right VLPFC (peak MNI coordinates: 40, 34, -16; Z = 4.95; volume = 800 mm3; p<0.05 FWE corrected), and a cluster spanning the left supplementary motor cortex and DMPFC (peak MNI coordinates: -6, 16, 64, Z = 5.56, volume = 4,136 mm3, p<0.05 FWE corrected). Beyond frontal engagement, we observed recruitment in temporal regions, parahippocampal gyrus, and cerebellum.
Results did not change when adding the difference in self-reported negative affect between Reappraise and Look-Negative conditions as a covariate and no significant association between neural activation and differences in reported negative affect were found. Figure 1 depicts the spatial location of ERT and EFIT results; see Table 2 for all results.
Figure 1.
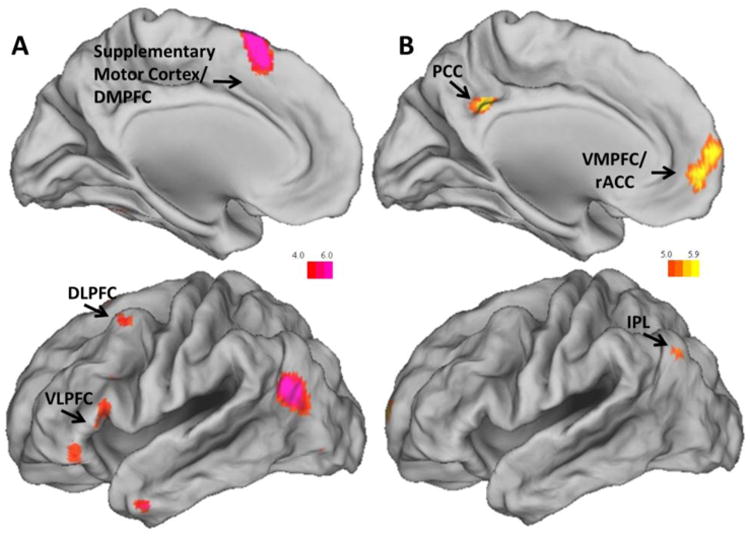
(A) Significant activation during ERT Reappraise > Look-Negative, reflecting involvement of the left DLPFC (peak MNI coordinate: -42, 12, 60), left VLPFC (peak MNI coordinates: -50, 22, 12 & -46, 36, -10), and supplemental motor cortex/DMPFC (peak MNI coordinate: -6, 16, 64). (B) Significant activation during EFIT Threat Low > Threat High, reflecting involvement of the VMPFC/rACC (peak MNI coordinate: -2, 52, -2), PCC (peak MNI coordinate: -8, -46, 32), and IPL (peak MNI coordinate: -44, -72, 38). DLPFC = dorsolateral prefrontal cortex; VLPFC = ventrolateral prefrontal cortex; DMPFC = dorsomedial prefrontal cortex; VMPFC = ventromedial prefrontal cortex; rACC = rostral anterior cingulate cortex; PCC = posterior cingulate cortex; IPL = inferior parietal lobe.
Table 2.
Whole-brain voxel-wise t-test results.
| Volume | Z-Score | Peak MNI Coordinates | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Contrast | Region | Laterality | (mm3) | x | y | z | |
| Emotional Faces Interference Task | |||||||
| Threat Low > Threat High | Ventromedial prefrontal cortex/Rostral anterior cingulate cortex | L | 5,752 | 5.45 | -2 | 52 | -2 |
| Posterior cingulate cortex | L | 1,248 | 5.24 | -8 | -46 | 32 | |
| Inferior parietal lobe | L | 1,008 | 4.93 | -44 | -72 | 38 | |
| Emotion Regulation Task | |||||||
| Reappraise > Look-Negative | Dorsolateral prefrontal cortex | L | 2,464 | 5.89 | -42 | 12 | 60 |
| Supplemental motor cortex/Dorsomedial prefrontal cortex | L | 4,136 | 5.56 | -6 | 16 | 64 | |
| Middle temporal gyrus | L | 3,560 | 5.40 | -48 | -66 | 20 | |
| Cerebellum posterior and anterior lobes | L | 1,568 | 5.26 | -42 | -54 | -28 | |
| Dorsolateral prefrontal cortex | R | 1,408 | 5.23 | 18 | 40 | 56 | |
| Superior temporal gyrus | R | 1,520 | 5.12 | 50 | -60 | 18 | |
| Temporal pole | R | 320 | 5.03 | 44 | 18 | -34 | |
| Inferior temporal gyrus | R | 384 | 5.01 | 40 | 0 | -46 | |
| Ventrolateral prefrontal cortex | R | 800 | 4.95 | 40 | 34 | -16 | |
| Parahippocampal gyrus | R | 304 | 4.87 | 16 | -2 | -20 | |
| Middle temporal gyrus | L | 200 | 4.83 | -52 | 2 | -34 | |
| Ventrolateral prefrontal cortex | L | 672 | 4.70 | -50 | 22 | 12 | |
| Ventrolateral prefrontal cortex | L | 480 | 4.59 | -46 | 36 | -10 | |
Note. L = left; R = right. Significant results correcting for multiple comparisons across the entire brain at family-wise error (FWE) at p<0.05 with minimum cluster size > 20 voxels.
fMRI Results: Common Task Engagement
When assessing overlap between the masks derived from Threat Low > Threat High and Reappraise > Look-Negative, no significant clusters were detected. In addition, no overlapping clusters were identified using the xjView common region approach.
Discussion
The present study evaluated independent and common neural functioning at the whole-brain level with validated paradigms of attentional control, a form of implicit emotion regulation, and cognitive reappraisal, a form of explicit regulation. During attentional control, we observed engagement of VMPFC/rACC, PCC, and IPL when threat interference was high (perceptual load was low) compared to when threat interference was low (perceptual load was high) (i.e., Threat Low > Threat High). No significant clusters were detected for neutral face distractors suggesting regions implicated in attentional control were recruited in the presence of threatening distractors. During cognitive reappraisal of negative images compared to naturally experiencing emotions evoked by negative images (i.e., Reappraise > Look-Negative), we observed recruitment of DLPFC, VLPFC, DMPFC, temporal regions, parahippocampal gyrus, and cerebellar activations. There was no evidence of an overlap in neural engagement between these emotion regulation approaches when correcting for multiple comparisons across the entire brain. Behavioral data confirmed participants followed instructions as exemplified by higher accuracy and faster response times in the low relative to high perceptual load condition and reduced negative affective state during reappraise compared to viewing negative images. Similar to neurofunctional findings, behavioral data pointed to task-dependent effects as accuracy related to attentional control did not correlate with affective state during cognitive reappraisal.
Neural Signature of Implicit Regulation
Data provide partial support for hypotheses insofar as distinct, task-dependent mechanisms are concerned. Consistent with previous studies (Bishop et al., 2007; Bunford, Kinney, Michael, & Klumpp, 2017; Wheaton et al., 2014), attentional control recruited the VMPFC extending to rACC. The VMPFC possesses heavy reciprocal connections with key emotion processing regions (e.g., the amygdala; (Sergerie, Chochol, & Armony, 2008; Zald, 2003) as well as with the lateral cortex involved in goal-directed behavior (Vogt, Finch, & Olson, 1992). Similarly, the rACC is strongly connected with the amygdala as well as with dorsal ACC and involved in conflict control, response selection, and error detection (Bush, Luu, & Posner, 2000). Therefore, ventral regions are perceived to serve as relay-stations for “bottom-up” information from limbic and sub-cortical structures signaling emotion detection, and lateral PFC and dorsal ACC regions signaling response selection and control (Banks, Eddy, Angstadt, Nathan, & Phan, 2007). In the context of negative stimuli, engagement of ventral midline regions may resolve threat interference through the integration of these two sources of information (Etkin et al., 2011b, 2006; Kanske & Kotz, 2011).
In addition to the involvement of hypothesized frontal regions, we also found evidence of the IPL and PCC recruitment during attentional control. The IPL plays a role in maintaining attention as well as responding to new, salient information specific to visual stimuli (Singh-Curry & Husain, 2009). Its involvement in implicit regulation in the present study likely suggests engagement in general attention processes and/or response to face distractors. In contrast, the involvement of the PCC in the context of implicit regulation is difficult to discern given that the function of the region in general is not entirely clear (Pearson, Heilbronner, Barack, Hayden, & Platt, 2011). Nevertheless, involvement of the PCC in the context of emotion processing is in line with prior reports that the PCC is activated in response to emotional content (Maddock, Garrett, & Buonocore, 2003), and specifically in response to negative stimuli in comparison to neutral stimuli (Maddock et al., 2003). The co-activation of the PCC and rACC in the present study is particularly interesting in light of prior work demonstrating that the PCC is connected with the rACC (Cole, Pathak, & Schneider, 2010). Both regions are also a part of the default mode network (DMN), a network of brain regions that are engaged during internally-directed focus (Raichle et al., 2001). The PCC is also a hub that interacts with attentional focus and arousal (Leech & Sharp, 2014). Accordingly, its activation may be tied to identifying target letters in the context of distractors or the state of arousal that results from engaging in a challenging cognitive task (i.e., attempting to respond both accurately and rapidly to stimuli presented for 200 ms). More research is needed to delineate the role of this region in this paradigm. Interestingly, we did not observe amygdala reactivity in the low relative to high load threat condition, which may be due to our whole-brain approach in determining significant activation.
Neural Signature of Explicit Regulation
With regard to explicit regulation, we observed engagement of the DLPFC, VLPFC, and DMPFC during cognitive reappraisal, which replicates prior reports (Buhle et al., 2014; Kanske, Heissler, Schonfelder, Bongers, & Wessa, 2011; Messina et al., 2015; Ochsner et al., 2012). The DLPFC is involved in executive functioning broadly-defined, and is implicated in working memory (Arnsten & Jin, 2014), decision making (Lee, Seo, & Jung, 2012), attentional control (Brosnan & Wiegand, 2017), and response selection (Yamagishi et al., 2016). Given these functions, DLPFC's role may be described as the active generation of strategies in order to execute goal-directed behavior (Corbetta, Patel, & Shulman, 2008; Corbetta & Shulman, 2002). In contrast, the VLPFC is involved in semantic processing (Marumo et al., 2014), categorization of objects (Corbetta et al., 2008; Corbetta & Shulman, 2002), and memory for semantic information (Nozari & Thompson-Schill, 2016). During emotion regulation, the VLPFC is also implicated in the generation of inner speech (Geva et al., 2011; Jones & Fernyhough, 2007; Morin & Hamper, 2012), which is believed to aid in the categorization of emotions (Kohn et al., 2014). The joint recruitment of DLPFC and VLPFC during cognitive reappraisal may represent the involvement of joint processes of emotional appraisal (sub-served by VLPFC) and reappraisal through the selection of an alternative emotional meaning (sub-served by DLPFC) to a negative image. With regard to DMPFC activation during cognitive reappraisal (Buhle et al., 2014; Diekhof, Geier, Falkai, & Gruber, 2011; Kalisch, 2009), this region is thought to monitor changing emotional states, a feature of reappraisal (Ochsner & Gross, 2005; Ochsner et al., 2012).
Beyond these regions, we observed reappraisal-related activation in various structures spanning temporal and cerebellar regions, which is consistent with evidence that reappraisal relies on a widely distributed neural network. These regions in particular are consistent with meta-analytic work showcasing their involvement in reappraisal (Buhle et al., 2014; Kanske et al., 2011; Messina et al., 2015; K. N. Ochsner et al., 2012). The temporal cortex likely contributes to reappraisal through semantic and perceptual associations (Aggelopoulos & Rolls, 2005; Levy, Bayley, & Squire, 2004) as both processes are involved in cognitive reappraisal (Ochsner & Gross, 2005; Ochsner et al., 2012). With regard to reappraisal-related cerebellar activity, we hesitate to interpret its involvement, as the function of the cerebellum during cognitive reappraisal is not clear. Nevertheless, there is increasing recognition that the cerebellum plays a role in emotion processing (Snider & Maiti, 1976) and emotion regulation (e.g., ‘cerebellar cognitive-affective syndrome’; Parvizi, Anderson, Martin, Damasio, & Damasio, 2001; Schmahmann & Sherman, 1998). Therefore, its known function as a regulator of motor behavior (Bastian, Mugnaini, & Thach, 1999) may extend to functions involved in reappraisal (Sacchetti, Scelfo, & Strata, 2009).
Relationship between Implicit and Explicit Regulation
Counter to expected results, there was no overlap in neural activity in regions involved in implicit and explicit emotion regulation. While surprising, evidence that neural involvement across the two tasks was spatially discrete is in line with some prior work that assessed overlap in brain regions involved in distraction (i.e., working memory task during the viewing of negative stimuli) and cognitive reappraisal (Dörfel et al., 2014). In this prior work, although similar brain regions were recruited in both regulation strategies (e.g., insula), a conjunction analysis failed to find spatially-similar activation using a whole-brain FWE-corrected threshold. In contrast, another study found similar VLPFC, DLPFC, and DMPFC engagement between implicit regulation via affect-labeling and cognitive reappraisal (Burklund, Torre, Lieberman, Taylor, & Craske, 2017), whereas a meta-analysis found overlap in the insula, pre-supplementary motor area (SMA), and VLPFC in assessing distraction by various means and affect-labeling as implicit strategies alongside reappraisal, expressive suppression, and mindfulness (Morawetz, Bode, Derntl, & Heekeren, 2017). Based on this work, results are mixed in terms of whether there is neural overlap between implicit and explicit regulation strategies. Studies that do find overlap highlight the involvement of several regions spanning the prefrontal cortex and insula in both strategies.
Inconsistencies among studies may be driven by the way in which implicit regulation is differentially operationalized, although the employment of cognitive reappraisal is relatively uniform. Implicit regulation is an umbrella term that includes attentional control, distraction, affect labeling, reinforcer reevaluation, and extinction (Braunstein, Gross, & Ochsner, 2017). In the present study, attentional control was manipulated by perceptual load, a form of implicit regulation that may not be comparable to other implicit regulation strategies (e.g., affect-labeling, distraction by means of working memory). Also, differences in regulation approaches include the extent to which participants were made aware of emotional content. For example, in previous studies participants were instructed that they would be viewing negative images and engaging in implicit regulation via distraction or affect-labeling (Burklund et al., 2017; Dörfel et al., 2014). In contrast, participants in our study were not told that face distractors conveyed emotional meaning. Appraising emotional content prior to regulation may alter the engagement of specific brain regions. Specifically, the insula, MPFC, and VLPFC are involved in emotional appraisal, in addition to emotion regulation. The insula is involved in interoceptive awareness of emotional states (Craig, 2009; Xu, Xu, & Yang, 2016), while the MPFC is involved in evaluating one's own emotional experience and is activated to a greater extent during self-focused emotional monitoring (Ochsner et al., 2004). In addition, the VLPFC plays a role in semantic processing and action inhibition of emotional states, the former of which involves the appraisal of emotion (Kohn et al., 2014). Therefore, these regions are involved both in appraising and regulating emotions. Their shared involvement in distraction (e.g., when rehearsing a 9-digit number during the viewing of negative stimuli; Dörfel et al., 2014), affect-labeling and cognitive reappraisal in previous work may be due to the fact that these tasks made emotional content explicit. However, more work is needed on this topic. In particular, one of these prior studies found overlap between distraction/affect-labeling and reappraisal in the DLPFC, a region involved more in regulation than emotional processing (Golkar et al., 2012). Therefore, future studies are needed to precisely parse-out the contribution of appraisal-related neural activation.
In addition, these prior studies tested implicit and explicit regulation strategies within the same task design, requiring participants to switch between strategies within a shorter timeframe (e.g., on the order of seconds). In contrast, two separate tasks of attentional control and cognitive reappraisal were utilized in the current study. As a result, this approach gave participants a period of rest between the collection of fMRI signal specific to implicit and explicit strategies and did not require participants to switch between strategies. Temporal lag and dissociation of tasks may have reduced carryover effects. Relatedly, there may be individual differences with regard to whether participants are able to effectively switch strategies within short timeframes (e.g., on the order of seconds) which may contribute to the sustained use of one strategy over another, even when instructed to switch. In particular, prior behavioral studies have found individual differences in the ability to switch between distraction and reappraisal (e.g., dynamic emotion regulation) (Birk & Bonanno, 2016). As a result, controlling for the ability to switch strategies is an important consideration for future studies mapping distinct versus shared neural networks of regulation.
To note, distinct neurocircuitries of implicit and explicit regulation is supported by prior work. That is, prior reports qualify that these two regulation strategies are dissimilar in terms of effort, time course, and efficiency (Gyurak et al., 2011). In terms of the separation of neural substrates, prior work emphasizes the role of the VMPFC/rACC in regulation of affective experiences, in contrast to dorsal regions (e.g., DMPFC) playing a stronger role in the evaluation of stimuli (Etkin et al., 2011b). That we found VMPFC/rACC involvement during implicit regulation, but DMPFC involvement in explicit regulation fits with this framework, as the cognitive reappraisal process inherently involves appraisal of emotional state for reinterpretation purposes. In contrast, implicit regulation in the form of attentional control over emotional distractors does not likely involve appraisal, but rather, quick management of competing stimuli that demand conflict resolution.
Study Limitations
Findings from the present study should be considered in light of important limitations. First, implicit emotion regulation was studied with an emotional interference task that varied in perceptual load and cognitive reappraisal was used to examine explicit emotion regulation. Also, participants were given examples of cognitive approaches that provided alternative paths to cognitive change to aid in clarifying what was meant by ‘reappraisal’. These factors may reduce generalizability. Second, while these tasks were validated across different laboratories, a limitation of this approach is the use of different task stimuli and experimental design. In other words, faces were used for implicit regulation in a mixed/block event-related design (i.e., EFIT) and images were used for explicit regulation in a block design (i.e., ERT). Furthermore, the duration of these stimuli differed due to differences in the function of the task. For example, the brief presentation of string-plus-face stimuli in EFIT (200 ms) is in line with automaticity, a component of implicit regulation (Gross & Thompson, 2007); moreover, the short stimulus duration reduces the potential of shifting of attention from one location to another. Yet, the duration of stimuli in EFIT would likely be too brief for reappraisal, an effortful, cognitive strategy involving various executive functions. Therefore, a failure to detect significant overlap in the spatial distribution of brain activation during these forms of emotion regulation may be the consequence of differences between paradigms. Another factor that may have reduced our ability to identify shared mechanisms is the use of a stringent whole-brain threshold, which differs in approach from prior work (Burklund et al., 2017). What constitutes significant neural activity in fMRI research is on-going (Eklund et al., 2016; Woo et al., 2014) and in lieu of a gold standard criterion, we followed current guidelines (Woo et al., 2014) to protect against Type I error, which may be overly conservative (Lieberman & Cunningham, 2009). Lastly, results are based on healthy participants, therefore, findings may not generalize to a clinical population.
Conclusions
Despite these limitations, results from this work provide support for distinct neural circuits characterized by VMPFC in the context of implicit emotion regulation and DLPFC, VLPFC, and DMPFC in the context of explicit emotion regulation. The uniqueness of these neural circuits is also supported by behavioral data as no relationship between accuracy related to attentional control and self-reported affective state during reappraisal was detected. The implications of this work are relevant for the treatment of psychiatric illnesses that possess difficulty in down-regulating negative affect by means of implicit and explicit regulation strategies.
Supplementary Material
Highlights.
Healthy participants completed implicit and explicit regulation tasks during fMRI
Results indicated tasks recruited non-overlapping, discrete neurocircuitries
Findings are relevant for psychiatric illnesses with difficulty in down-regulation
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health grants K23MH093679 (to HK), R01MH101497 (to KLP) and 1S10RR028898 and Center for Clinical and Translational Science (CCTS) UL1RR029879.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggelopoulos NC, Rolls ET. Scene perception: inferior temporal cortex neurons encode the positions of different objects in the scene. European Journal of Neuroscience. 2005;22(11):2903–2916. doi: 10.1111/j.1460-9568.2005.04487.x. [DOI] [PubMed] [Google Scholar]
- Arch JJ, Craske MG. First-line Treatment: A Critical Appraisal of Cognitive Behavioral Therapy Developments and Alternatives. Psychiatric Clinics of North America. 2009;32(3):525–547. doi: 10.1016/j.psc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Jin LE. Progress in Molecular Biology and Translational Science. Vol. 122. Elsevier; 2014. Molecular Influences on Working Memory Circuits in Dorsolateral Prefrontal Cortex; pp. 211–231. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian A, Mugnaini E, Thach W. Fundamental Neuroscience. San Diegno, CA: Academic Press; 1999. Cerebellum; pp. 973–992. [Google Scholar]
- Birk JL, Bonanno GA. When to throw the switch: The adaptiveness of modifying emotion regulation strategies based on affective and physiological feedback. Emotion. 2016;16(5):657–670. doi: 10.1037/emo0000157. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Jenkins R, Lawrence AD. Neural Processing of Fearful Faces: Effects of Anxiety are Gated by Perceptual Capacity Limitations. Cerebral Cortex. 2007;17(7):1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Braunstein LM, Gross JJ, Ochsner KN. Explicit and implicit emotion regulation: a multi-level framework. Social Cognitive and Affective Neuroscience. 2017;12(10):1545–1557. doi: 10.1093/scan/nsx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of Interest Analysis Using an SPM Toolbox [Abstract] NeuroImage. 2002;16(2) [Google Scholar]
- Brosnan MB, Wiegand I. The Dorsolateral Prefrontal Cortex, a Dynamic Cortical Area to Enhance Top-Down Attentional Control. The Journal of Neuroscience. 2017;37(13):3445–3446. doi: 10.1523/JNEUROSCI.0136-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Ochsner KN. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunford N, Kinney KL, Michael J, Klumpp H. Threat distractor and perceptual load modulate test-retest reliability of anterior cingulate cortex response. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2017;77:120–127. doi: 10.1016/j.pnpbp.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunford N, Roberts J, Kennedy AE, Klumpp H. Neurofunctional correlates of behavioral inhibition system sensitivity during attentional control are modulated by perceptual load. Biological Psychology. 2017;127:10–17. doi: 10.1016/j.biopsycho.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund LJ, Torre JB, Lieberman MD, Taylor SE, Craske MG. Neural responses to social threat and predictors of cognitive behavioral therapy and acceptance and commitment therapy in social anxiety disorder. Psychiatry Research: Neuroimaging. 2017;261:52–64. doi: 10.1016/j.pscychresns.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain's most globally connected regions. NeuroImage. 2010;49(4):3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nature Reviews Neuroscience. 2002;3(3):215–229. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18(1):193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows. NeuroImage. 2011;58(1):275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Dörfel D, Lamke JP, Hummel F, Wagner U, Erk S, Walter H. Common and differential neural networks of emotion regulation by Detachment, Reinterpretation, Distraction, and Expressive Suppression: A comparative fMRI investigation. NeuroImage. 2014;101:298–309. doi: 10.1016/j.neuroimage.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols T, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 2016;113(28):7900–7095. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Measuring facial movement. Environmental Psychology and Nonverbal Behavior. 1976;1(1):56–75. [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011a;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011b;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: 2002. [Google Scholar]
- Fitzgerald JM, MacNamara A, Kennedy AE, Rabinak CA, Rauch SAM, Liberzon I, Phan KL. Individual differences in cognitive reappraisal use and emotion regulatory brain function in combat-exposed veterans with and without PTSD. Depression and Anxiety. 2016 doi: 10.1002/da.22551. [DOI] [PMC free article] [PubMed]
- Fitzgerald JM, Phan KL, Kennedy AE, Shankman SA, Langenecker SA, Klumpp H. Prefrontal and amygdala engagement during emotional reactivity and regulation in generalized anxiety disorder. Journal of Affective Disorders. 2017;218:398–406. doi: 10.1016/j.jad.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva S, Jones PS, Crinion JT, Price CJ, Baron JC, Warburton EA. The neural correlates of inner speech defined by voxel-based lesion-symptom mapping. Brain. 2011;134(10):3071–3082. doi: 10.1093/brain/awr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural Bases of Social Anxiety Disorder: Emotional Reactivity and Cognitive Regulation During Social and Physical Threat. Archives of General Psychiatry. 2009;66(2):170. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, et al. Öhman A. Distinct Contributions of the Dorsolateral Prefrontal and Orbitofrontal Cortex during Emotion Regulation. PLoS ONE. 2012;7(11):e48107. doi: 10.1371/journal.pone.0048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Luan Phan K, Lyons M, Mori S, Angstadt M, Rabinak CA. Cannabinoid Modulation of Frontolimbic Activation and Connectivity During Volitional Regulation of Negative Affect. Neuropsychopharmacology. 2016;41:1888–1896. doi: 10.1038/npp.2015.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/S0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: taking stock and moving forward. Emotion. 2013;13(3):359–365. doi: 10.1037/a0032135. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Muñoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2(2):151–164. [Google Scholar]
- Gross JJ, Thompson RA. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. Emotion Regulation: Conceptual Foundations; pp. 3–24. [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: A dual-process framework. Cognition & Emotion. 2011;25(3):400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp H, Troy AS, Mauss IB. The unconscious pursuit of emotion regulation: Implications for psychological health. Cognition & Emotion. 2011;25(3):532–545. doi: 10.1080/02699931.2010.532606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Fernyhough C. Neural correlates of inner speech and auditory verbal hallucinations: A critical review and theoretical integration. Clinical Psychology Review. 2007;27(2):140–154. doi: 10.1016/j.cpr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: Time matters. Neuroscience & Biobehavioral Reviews. 2009;33(8):1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to Regulate Emotion? Neural Networks for Reappraisal and Distraction. Cerebral Cortex. 2011;21(6):1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Emotion Speeds up Conflict Resolution: A New Role for the Ventral Anterior Cingulate Cortex? Cerebral Cortex. 2011;21(4):911–919. doi: 10.1093/cercor/bhq157. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Bhaumik R, Kinney KL, Fitzgerald JM. Principal component analysis and neural predictors of emotion regulation. Behavioural Brain Research. 2018;338:128–133. doi: 10.1016/j.bbr.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald JM, Kinney KL, Kennedy AE, Shankman SA, Langenecker SA, Phan KL. Predicting cognitive behavioral therapy response in social anxiety disorder with anterior cingulate cortex and amygdala during emotion regulation. NeuroImage: Clinical. 2017;15:25–34. doi: 10.1016/j.nicl.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Roberts J, Kapella MC, Kennedy AE, Kumar A, Phan KL. Subjective and objective sleep quality modulate emotion regulatory brain function in anxiety and depression: KLUMPP et al. Depression and Anxiety. 2017 doi: 10.1002/da.22622. [DOI] [PMC free article] [PubMed]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation — An ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole SL, Rothermund K. “I feel better but I don't know why”: The psychology of implicit emotion regulation. Cognition & Emotion. 2011;25(3):389–399. doi: 10.1080/02699931.2010.550505. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual Technical Report A-8. Gainesville, FL: 2008. [Google Scholar]
- Lavie N, Lin Z, Zokaei N, Thoma V. The role of perceptual load in object recognition. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:1346–1358. doi: 10.1037/a0016454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. Simon & Schuster; 1998. [Google Scholar]
- Lee D, Seo H, Jung MW. Neural Basis of Reinforcement Learning and Decision Making. Annual Review of Neuroscience. 2012;35(1):287–308. doi: 10.1146/annurev-neuro-062111-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DA, Bayley PJ, Squire LR. The anatomy of semantic knowledge: medial vs. lateral temporal lobe. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6710–6715. doi: 10.1073/pnas.0401679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh LG, Öst LG. Attentional Bias, Self-consciousness and Perfectionism in Social Phobia Before and After Cognitive-Behaviour Therapy. Scandinavian Journal of Behaviour Therapy. 2001;30(1):4–16. doi: 10.1080/028457101300140428. [DOI] [Google Scholar]
- MacDonald AW. Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Rabinak CA, Kennedy AE, Fitzgerald DA, Liberzon I, Stein MB, Phan KL. Emotion Regulatory Brain Function and SSRI Treatment in PTSD: Neural Correlates and Predictors of Change. Neuropsychopharmacology. 2016;41(2):611–618. doi: 10.1038/npp.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumo K, Takizawa R, Kinou M, Kawasaki S, Kawakubo Y, Fukuda M, Kasai K. Functional abnormalities in the left ventrolateral prefrontal cortex during a semantic fluency task, and their association with thought disorder in patients with schizophrenia. NeuroImage. 2014;85:518–526. doi: 10.1016/j.neuroimage.2013.04.050. [DOI] [PubMed] [Google Scholar]
- Mattia JI, Heimberg RG, Hope DA. The revised stroop color-naming task in social phobics. Behav Res Ther. 1993;31(3):305–313. doi: 10.1016/0005-7967(93)90029-t. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Bunge SA, Gross JJ. Automatic Emotion Regulation. Social and Personality Psychology Compass. 2007;1(1):146–167. doi: 10.1111/j.1751-9004.2007.00005.x. [DOI] [Google Scholar]
- Messina I, Bianco S, Sambin M, Viviani R. Executive and semantic processes in reappraisal of negative stimuli: insights from a meta-analysis of neuroimaging studies. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Social Cognitive and Affective Neuroscience. 2010;5(4):369–377. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Derntl B, Heekeren HR. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews. 2017;72:111–128. doi: 10.1016/j.neubiorev.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Morin A, Hamper B. Self-reflection and the inner voice: activation of the left inferior frontal gyrus during perceptual and conceptual self-referential thinking. The Open Neuroimaging Journal. 2012;6:1. doi: 10.2174/1874440001206010078. Retrieved from http://benthamopen.com/FULLTEXT/TONIJ-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch DA, Gavric DL, Senn JM, Santesso DL, Miskovic V, Schmidt LA, et al. Antony MM. Changes in judgment biases and use of emotion regulation strategies during cognitive-behavioral therapy for social anxiety disorder: Distinguishing treatment responders from nonresponders. Cognitive Therapy and Research. 2012;36(4):261–271. doi: 10.1007/s10608-011-9371-1. [DOI] [Google Scholar]
- Muris P, Merckelbach H, Schmidt H, Gadet B, Bogie N. Anxiety and depression as correlates of self-reported behavioural inhibition in normal adolescents. Behaviour Research and Therapy. 2001;39(9):1051–1061. doi: 10.1016/s0005-7967(00)00081-4. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nozari N, Thompson-Schill SL. Neurobiology of Language. Elsevier; 2016. Left Ventrolateral Prefrontal Cortex in Processing of Words and Sentences; pp. 569–584. [DOI] [Google Scholar]
- Ochsner K, Gross J. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking Feelings: An fMRI Study of the Cognitive Regulation of Emotion. Journal of Cognitive Neuroscience. 2002;15(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion: Functional imaging studies of emotion regulation. Annals of the New York Academy of Sciences. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108(3):483–522. doi: 10.1037//0033-295X.108.3.483. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Anderson SW, Martin CO, Damasio H, Damasio AR. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124:1708–1719. doi: 10.1093/brain/124.9.1708. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends in Cognitive Sciences. 2011;15(4):143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res. 2002;15(1):31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Pishyar R, Harris LM, Menzies RG. Responsiveness of measures of attentional bias to clinical change in social phobia. Cognition & Emotion. 2008;22(7):1209–1227. doi: 10.1080/02699930701686008. [DOI] [Google Scholar]
- Price RB, Kuckertz JM, Amir N, Bar-Haim Y, Carlbring P, Wallace ML. Less is more: Patient-level meta-analysis reveals paradoxical dose-response effects of a computer-based social anxiety intervention targeting attentional bias. Depression and Anxiety. 2017;34(12):1106–1115. doi: 10.1002/da.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, MacNamara A, Kennedy AE, Angstadt M, Stein MB, Liberzon I, Phan KL. Focal And Aberrant Prefrontal Engagement During Emotion Regulation In Veterans With Posttraumatic Stress Disorder: Research Article: Prefrontal Regulation of Affect in PTSD. Depression and Anxiety. 2014;31(10):851–861. doi: 10.1002/da.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S, Hoffstaedter F, Löffler L, Kogler L, Schneider F, Blechert J, Derntl B. Imaging the up's and down's of emotion regulation in lifetime depression. Brain Imaging and Behavior. 2017 doi: 10.1007/s11682-017-9682-2. [DOI] [PubMed]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Scelfo B, Strata P. Cerebellum and emotional behavior. Neuroscience. 2009;162(3):756–762. doi: 10.1016/j.neuroscience.2009.01.064. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47(6):1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. Journal of Neuroscience Research. 1976;2(2):133–146. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- Stephanou K, Davey CG, Kerestes R, Whittle S, Harrison BJ. Hard to look on the bright side: neural correlates of impaired emotion regulation in depressed youth. Social Cognitive and Affective Neuroscience. 2017;12(7):1138–1148. doi: 10.1093/scan/nsx039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2(6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wheaton MG, Fitzgerald DA, Phan KL, Klumpp H. Perceptual load modulates anterior cingulate cortex response to threat distractors in generalized social anxiety disorder. Biological Psychology. 2014;101:13–17. doi: 10.1016/j.biopsycho.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Xu G, Yang Y. Neural Systems Underlying Emotional and Non-emotional Interference Processing: An ALE Meta-Analysis of Functional Neuroimaging Studies. Frontiers in Behavioral Neuroscience. 2016;10 doi: 10.3389/fnbeh.2016.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T, Takagishi H, Fermin AdeSR, Kanai R, Li Y, Matsumoto Y. Cortical thickness of the dorsolateral prefrontal cortex predicts strategic choices in economic games. Proceedings of the National Academy of Sciences. 2016;113(20):5582–5587. doi: 10.1073/pnas.1523940113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Zhang JX, Ding Z, Xiao L. Attention bias modification treatment for adolescents with major depression: a randomized controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2016;55(3):208–218. doi: 10.1016/j.jaac.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Yiend J. The effects of emotion on attention: A review of attentional processing of emotional information. Cognition & Emotion. 2010;24(1):3–47. doi: 10.1080/02699930903205698. [DOI] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


