Abstract
Purpose
The purpose of the study was to determine whether the GDF-15 is present in follicular fluid; to evaluate if there is a relation between follicular and serum levels of GDF-15 and fertility status of study subjects; and to test whether granulosa cells, oocytes, or both produce GDF-15.
Methods
This study used follicular fluid (FF, serum, and oocytes obtained under informed consent from women undergoing oocyte retrieval for in vitro fertilization. It also used ovaries from deceased preterm newborns. Collection of FF and blood at the time of oocyte retrieval, ELISA and western blot were performed to determine levels and forms of GDF-15. Concentrations of GDF-15 in FF and serum, its expression in ovarian tissue, and secretion from granulosa cells were analyzed.
Results
GDF-15 concentration in FF ranged from 35 to 572 ng/ml, as determined by ELISA. Western blot analysis revealed the GDF-15 pro-dimer only in FF. Both normal healthy and cancerous granulosa cells secreted GDF-15 into culture media. Primary oocytes displayed cytoplasmic GDF-15 positivity in immunostained newborn ovaries, and its expression was also observed in fully grown human oocytes.
Conclusions
To the best of our knowledge, this is the first documentation of cytokine GDF-15 presence in follicular fluid. Its concentration was not associated with donor/patient fertility status. Our data also show that GDF-15 is expressed and inducible in both normal healthy and cancerous granulosa cells, as well as in oocytes.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1230-5) contains supplementary material, which is available to authorized users.
Keywords: Follicular fluid, Growth/differentiation factor-15, Follicular granulosa cells, IVF
Introduction
Oocytes developing within ovarian follicles are bathed in follicular fluid (FF), immersed in a dynamic milieu enriched with signaling molecules, including hormones, prostanoids, polysaccharides, growth factors, cytokines, chemokines, metabolites, reactive oxygen species (ROS), and antioxidant enzymes [1]. As its primary function, FF supports the development of oocytes and follicular cells and acts to protect them from stress and damage [2, 3]. FF is likely derived from blood passing through thecal capillaries [3] and is thus conditioned by the collective follicular secretome (granulosa, theca cells, and oocytes).
Cytokines in FF are fundamentally important for reproduction. By modulating oocyte development and ovulation, they influence subsequent fertilization, early embryonic development, and the potential for implantation [4]. The transforming growth factor-β (TGF-β) superfamily of pleiotropic cytokines has broad functional ramifications, impacting all stages of folliculogenesis [4].
Growth/differentiation factor-15 (GDF-15, MIC-1, NAG-1, PTGF, or PDF) is a divergent member of the TGF-β family. The highest levels of GDF-15 gene and protein expression have been observed in human placenta and in prostatic epithelial cells [5]. Earlier, we reported on the abundance of GDF-15 protein found in human seminal plasma [6]. Furthermore, GDF-15 expression has been linked to a variety of adverse medical conditions, including cancer, cardiovascular conditions, diabetes, anorexia/cachexia, obesity, and many others (see [7, 8]), serving as an index of all-cause mortality. Recently, orphan receptor GFRAL has been identified as a receptor for GDF-15 that mediates the metabolic effects of GDF-15 [9–11], and GDF-15 Fc fusion proteins showed its potential as a therapeutic agent for the treatment of obesity and related comorbidities [12].
The secretion of biologically active GDF-15 is remarkably complex, and variability in the pool of available GDF-15 forms was suggested to be involved in modulating of tissue microenvironment. The unprocessed translated form of GDF-15 includes the signal sequence, the propeptide, and a mature protein, which contains a cysteine knot typical for the TGF-β family [13]. The generation of the biologically active form requires removal of the hydrophobic signal sequence followed by disulfide-linked dimerization of GDF-15 monomers and a final cleavage by furin-like protease at the canonic RXXR site. This process generates the C-terminal form of GDF-15 with a molecular weight of approximately 20 kDa that subsequently enters secretion pathway [13]. The secreted mature protein is a 25-kDa dimer cleaved from the 62-kDa intracellular precursor [14]. The tissue availability of GDF-15 depends on ECM degradation, histological composition, and architecture or presence of enzymes capable of conversion of pro-GDF-15 to GDF-15, implying a regulation similar to TGF-β [15, 16].
Multiple studies have confirmed that GDF-15 is induced by cellular stress (i.e., tissue damage, inflammation), particularly oxidative stress and a broad spectrum of ROS [17, 18]. Although physiologic amounts of ROS in FF reflect healthy oocyte development [19], oxidative stress has been cited as a possible cause of female infertility (see [20, 21]).
As a general soluble biomarker for stress, GDF-15 is also likely present in FF. However, neither its presence nor an association with fertility status has been previously addressed in either of two published proteomic analyses of FF [2, 22]. To address this issue, here we investigated the concentrations and forms of GDF-15 in FF and serum in patients with infertility and in healthy donors. We also studied the relation of the given levels of GDF-15 with size and a number of follicles, and with BMI of study subjects. Finally, we evaluated production of GDF-15 by somatic follicular cells and oocytes by (i) measuring the concentration of GDF-15 in media harvested from cultured granulosa cells and (ii) by visualizing expression of GDF-15 on ovarian sections. Our data indicate that the GDF-15 cytokine is, in fact, present in FF, also showing expression in granulosa cells and oocytes.
Material and methods
Female patients
The design of this prospective study was approved by the Faculty of Medicine’s Ethics Committee at Masaryk University. Written informed consent was granted by all donor participants. A total of 26 paired blood and FF samples were obtained from women undergoing assisted reproduction procedures at the Reprofit International Clinic of Reproductive Medicine, Brno, Czech Republic. Causes of infertility were recorded as follows: tubal, 8% (2/26); hormonal, 23% (6/26); male origin, 19% (5/26); male/female factors, 35% (9/26); and other etiologies (endometriosis, genetic, idiopathic, immune), 15% (4/26). A total of 31 samples were provided by healthy female donors (enrolled to the egg donation program). The study size was considered as an initial set of analyses (Supplementary Fig. 1). All samples were collected and handled in agreement with EU Act No. 296/2008 Coll. on Safeguarding the Quality and Safety of Human Tissues and Cells Intended for Use in Man and on Amendments to Related Acts (Act on Human Tissues and Cells) and Decree No. 422/2008 Coll. on Detailed Requirements for the Safeguarding of the Quality and Safety of Human Tissues and Cells Intended for Use in Man.
Follicular fluid aspiration, blood processing, and oocyte isolation
As standard procedure, all test subjects received the follitropin α agonist Gonal-f (175 ± 12.5 units/day; EMD Serono Inc., Rockland, MA, USA), followed by the gonadorelin (LHRH) analog Decapeptyl (2 × 0.1 mg) for induction of follicular maturation. Blood samples were collected on the same day of oocyte retrieval and processed within 30 min. FF was aspirated by puncture of dominant single follicles. Only FF samples without bloody contamination were used for further analyses. Both FF and blood samples were centrifuged (1000 g, 15 min), collecting supernatants for storage (− 80 °C) in aliquots until analysis. Oocytes were isolated from cumulus-oocyte complexes by manual denudation in Sydney IVF Fertilization medium (Cook Medical, Bloomington, IN, USA). Oocytes were developmental status assessed under a microscope (SMZ 1500; Nikon, Tokyo, Japan), reserving only those unsuitable for in vitro fertilization (GV or MI stage) or matured but unsuccessfully fertilized in Sydney IVF Cleavage medium (Cook) for study purposes.
Cell culture and harvesting of conditioned media
Five unrelated lines of human granulosa cells (COV434, GC8, GC15, GC671, and GC672) were used and cultured as described by Bruckova and colleagues [23, 24]. COV434 cell line represented cancerous cells originating in a granulosa cell tumor of ovary [25]. Instead, GC8, GC15, GC671, and GC672 lines represented normal healthy granulosa cells, since they were established by in vitro expansion of granulosa cells recovered from FF during oocyte retrieval [23, 24]. The cells were grown (24 h) at a subconfluent density in complete media (without serum) and conditioned media was harvested for storage (− 80 °C) in aliquots. Cells were counted using a CASY TT Cell Counter analyzer (OLS OMNI Life Science, Bremen, Germany). To obtain conditioned media after p53 activation, GC8 and COV434 cells were treated by nutlin-3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and cytosine-β-D-arabinofuranoside (cytarabine, AraC), respectively, before harvesting. Prostatic cancer LNCaP cells (DSMZ) were cultivated as earlier described [26].
Determination of quantity of GDF-15 in follicular fluid, serum, and cell culture media
Quantification of GDF-15 in FF, serum, and conditioned cell culture media was achieved using a commercially available ELISA kit (#DY957; R&D Systems, Minneapolis, MN, USA) according to manufacturer’s instructions.
Quantification of GDF-15 mRNA in oocytes by real-time RT-PCR
Harvested oocytes (four and seven oocytes, respectively, per sample) were frozen on dry ice. Lysis, cDNA synthesis, preamplification, and PCR analysis were all performed according to manufacturer’s recommendations (Single Cell-to-CT™ Kit; Thermo Fisher Scientific Inc., Waltham, MA, USA). TaqMan Gene Expression Assays (Thermo Fisher Scientific) for GDF15 (Hs00171132_m1, ref. seq. NM_004864.2) and GAPDH (Hs03929097_g1, ref. seq. NM_001256799.1, NM_001289745.1, NM_001289746.1, NM_002046.4) were used for preamplification and PCR reactions. cDNA synthesis and preamplification were conducted using a Thermocycler PTC-200 (MJ Research Inc., Waltham, MA, USA), using a LightCycler 480 Instrument (Roche Diagnostics, Basel, Switzerland) for PCR reactions. As a positive control, 1,4,7, and 10 cells, respectively, of LNCaP cell line were sorted by BD FACSAria II 4L Sorp (BD Biosciences, Franklin Lakes, NJ, USA; 100-mm nozzle, dead cells differentiated via propidium iodide staining) directly into lysis buffer and processed similarly to oocyte samples (Single Cell-to-CT™ Kit). LNCaP cells were also used to generate calibration curves, from which the efficiency of both GAPDH and GDF15 TaqMan Gene Expression Assays were counted (GDF-15, 2.073; GAPDH, 2.066). Cp calculations relied on proprietary software (LightCycler 480 v1.5.0 [absolute quantification 2nd derivative maximum analysis]).
SDS-PAGE and western blot analysis of GDF-15
All samples (FF, serum, somatic/granulosa cells, and oocytes) were harvested and frozen on dry ice. Dithiothreitol-reduced and non-reduced lysates in radio-immunoprecipitation assay buffer were prepared and analyzed by western blotting as described previously [27]. LNCaP cells (2.5 μg of total protein) and seminal plasma were used as positive controls. To detect GDF-15, membranes were incubated for overnight at 4 °C with primary antibody against GDF-15 (07-217, 1:250 dilution; EMD Millipore [Merck], Billerica, MA, USA,), washed once in TBS-Tween, and then incubated for 1 h at room temperature with HRP-conjugated secondary antibody (NA934V, 1:6000 dilution; GE Healthcare Bioscience, Chicago, IL, USA). Final visualization was achieved using Immobilon Western Chemiluminiscence HRP Substrate (WBKLS0500; EMD Millipore) and X-ray films (Agfa Healthcare, Mortsel, Belgium).
Immunohistochemical detection of GDF-15 and HIF-1α in ovaries
This aspect of the study, which used ovaries obtained from two deceased preterm newborns, was approved by the Ethics Committee of the University Hospital and Medical Faculty of Palacky University in Olomouc (Reference No. 205/14). The cases were retrieved from archive and informed consent was not available. Therefore, the Ethics Committee was asked for permission with the immunohistochemical staining of these two samples. Newborn 1 died 65 days after cesarean birth (29 weeks of gestation) due to respiratory failure. Newborn 2 died the second day after cesarean birth (24 weeks of gestation) due to respiratory failure and prematurity. Both neonates were developed appropriately for gestational age. The autopsies were performed 28 and 47 h after exitus, respectively. Four-micrometer sections were cut from formalin-fixed paraffin-embedded tissues for standard immunohistochemical (IHC) staining. Rabbit polyclonal anti-GDF15 (HPA011191; Sigma-Aldrich [Merck], St Louis MO, USA) and rabbit monoclonal anti-HIF1α (clone EP1215Y; Abcam, Cambridge, MA, USA) antibodies were used after antigen retrieval by microwave treatment. The EnVision+Dual Link System (Dako, Glostrup, Denmark) and diaminobenzidine (Liquid DAB+Substrate Chromogen System, Dako) were applied for visualization. Validation of the anti-GDF15 antibody was performed on formalin-fixed paraffin-embedded cell lines with known expression of GDF15 (Supplementary Fig. 2).
Statistical analysis
Baseline patient characteristics were summarized using descriptive statistics: mean, median, standard deviation, minimum, maximum, and 25 and 75% percentiles (Fig. 1a). The t test was used for analysis of baseline patient characteristics (Supplementary Table 1), non-parametric Kruskal-Wallis test was applied to assess differences in continuous variables, using the Pearson correlation coefficient to measure relations between two variables.
Fig. 1.
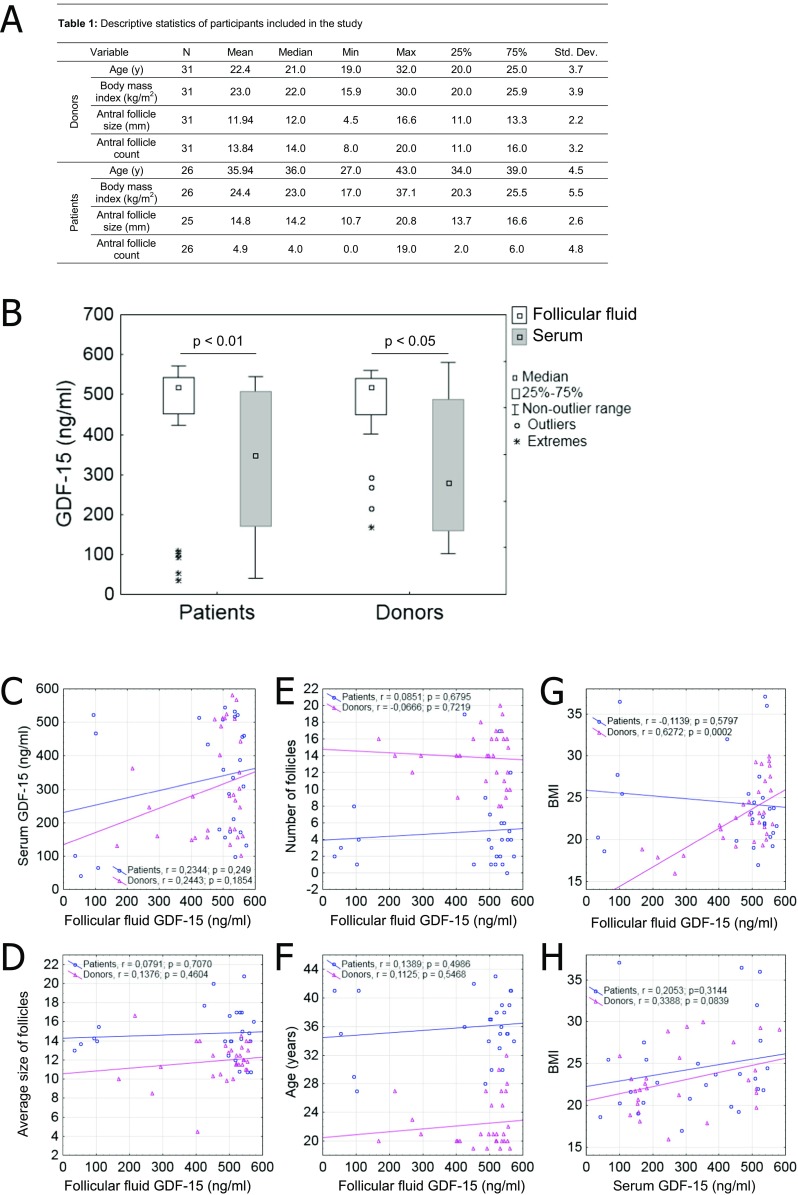
a Donor and patient characteristics; b GDF-15 concentrations (ng/mL) in follicular fluid (FF), comparing/correlating healthy donors and patients; c relation between concentrations of GDF-15 in FF and serum; d–g relation between GDF-15 concentration in FF and average follicular size, number of follicles, subject age, and BMI; and (H) BMI vs. concentrations of GDF-15 in serum (Pearson’s correlations calculated)
Results
We first determined the concentrations of GDF-15 by ELISA in FF and serum of patients and healthy donors. The concentrations varied from 35 to 571 ng/mL, with GDF-15 being always significantly higher in FF then in corresponding serum (Fig. 1b). Still, no significant association between FF and serum concentrations emerged in correlation analysis (Fig. 1c), nor was there an association between GDF-15 concentration and number or size of follicles obtained or donor/patient age (Fig. 1d–f). The only positive correlation to surface was that between BMIs of healthy donors and concentration of GDF-15 in FF (r = 0.63; p = 0.0002) (Fig. 1g). A similar trend also applied to healthy donors in terms of BMI and concentrations of GDF-15 in serum (Fig. 1h, r = 0.34; p = 0.08).
To also address a possible link between GDF-15 with fertility, we compared GDF-15 concentrations in healthy donors (n = 31) against those of patients with proven infertility (n = 26). With this set of individuals, no significant correlation between GDF-15 present in FF or serum of donors or patients and fertility status was evident (Fig. 1b).
GDF-15 is synthesized as an inactive precursor and dimerized in the endoplasmic reticulum, then transported to the Golgi apparatus for proteolytic cleavage and eventual secretion as an active dimer [28]. However, some cell types are capable of secreting large pools of unprocessed GDF-15, which thereafter inhabit the extracellular matrix [16]. To determine the nature of GDF-15 in FF and serum, we electrophoresed two paired samples of both liquids under reducing (Fig. 2a) and non-reducing (Fig. 2b) conditions and then western blotted them for GDF-15. Seminal plasma, known to contain both processed and unprocessed forms was used as an etalon. As obviated in Fig 2a, b, both FF and serum contained only the pro-GDF-15 monomer/dimer and no mature GDF-15 monomer/dimer, proving that these body liquids completely lack mature forms of GDF-15.
Fig. 2.

Analysis of GDF-15 in follicular fluid, serum, and granulosa cell lines: Follicular fluid (FF) and serum (S) of two subjects electrophoresed under a reduced or b non-reduced conditions (seminal plasma [SP] serving as positive control); conditioned media harvested from c control and e, f nutlin-3- or AraC-treated granulosa cell lines, quantification by ELISA; d expression of GDF-15 in granulosa cell lines as determined by western blotting under reduced condition (β-actin serves as loading control). *~ p < 0.05
The presence of GDF-15 in FF shown here brought up the question of whether or not this molecule is produced by granulosa cells. We addressed this issue by measuring by ELISA the concentration of GDF-15 in media conditioned by cultured granulosa cells (five independent lines, the details are in M + M section). As shown in Fig. 2c, COV434, GC671, and GC 672 but not GC8 and GC15 produced GDF-15 to the level for GDF-15 to be detectable by ELISA in conditioned media. This variance was well matched by the quantities of GDF-15 contained in granulosa cells as determined by western blot (Fig. 2d). Cell of low-producer lines GC8 and GC15 contained only negligible (hard to visualize) amounts of GDF-15, while cells of high-producer lines contained easily detectable amounts. It is also of note that the cancerous granulosa cells (COV434) expressed the mature monomer form, whereas normal healthy granulosa cells (GC671, GC672) expressed the pro-monomer form only.
The production of GDF-15 is known to be induced by external stimuli causing unfavorable conditions to cells. To assess this possibility, we exposed granulosa cells of both low- and high-producer lines (represented by GC8 and COV434) to two types of insults. One was the treatment by nutlin-3, an inhibitor of MDM2 and p53 activator [29], the other one was the treatment by cytarabine (AraC), an antimetabolite antineoplastic agent [30]. Both nutlin-3 and AraC induced GDF-15 in COV434 cells, increasing its concentration in conditioned media (Fig. 2e, f). In contrast, in GC8 cells a significant induction of GDF-15 was observed only after nutlin-3 treatment. We conclude that GDF-15 is produced by normal healthy and cancerous granulosa cells, with its expression being inducible by DNA damage or p53 activation.
Finally, we have employed immunostaining of ovarian sections, and qRT-PCR and western blot on isolated oocytes, to determine whether or not oocytes themselves might express GDF-15 to also enrich FF. Initially, the quality of immunostaining in post-oophorectomy tissue samples proved unsatisfactory (data not shown), so the cadaveric ovarian tissue of two deceased preterm newborns was utilized instead. Cytoplasmic GDF-15 positivity was confirmed by immunostaining in normal primary oocytes (Fig. 3a). We also assessed ovarian HIF1-α expression to exclude the potential of a hypoxic basis for GDF-15 expression in this material. Delayed (autopsy) procurement entailed higher HIF-1α expression in follicular and stromal cells but not in oocytes (Fig. 3b and Supplementary Fig. 3). Expression of GDF-15 in oocytes was then investigated using both qRT-PCR and western blot. For these analyses, we could only use oocytes that were rejected from insemination (GV and MI stage) and/or failed to be fertilized (MII stage). All these oocytes are here categorized uniformly as fully grown. Figure 4a, b shows the expression of mRNA in two independent samples of fully grown oocytes containing 4 and 7 oocytes, respectively. The calculated Cp values were comparable with such values obtained from single-cell qRT-PCR analysis of LNCaP cells serving as positive controls. For western blot quantification of GDF-15, we have used mixed sample of 17 fully grown oocytes. As shown in Fig. 4c, fully grown oocytes contained low but still detectable amount of pro-monomeric GDF-15. Mature form, however, was not seen in this experiment. Although this set of analyses was accomplished only once because of the hard-to-obtain nature of the biological material, we may conclude that human oocytes synthesize GDF-15 at least when they reach their fully grown state.
Fig. 3.
a Cytoplasmic expression of GDF-15 in primary oocytes of preterm newborn ovaries as determined by immunohistochemistry. b Expression of HIF-1α in somatic ovarian cells as determined by immunohistochemistry. c Negative control, processed without primary antibody. Gestational age of the preterm newborn 1 was 29 weeks and lived for 65 days. Preterm newborn 2 (24 weeks of gestation) died the second day after cesarean. Magnification × 400 for all pictures, scale bars represent 20 μm
Fig. 4.

Quantitative RT-PCR and western blot analysis of GDF-15 expression in fully grown human oocytes: a amplification curves re-constructed using data exported from LightCycler 480 to SigmaPlot software, presented as an average of all amplified curves obtained for particular sample (from all replicates) and gene (LNCaP cells sorted in set numbers serving as positive and calibration controls); b calculated Cp values (by LightCycler 480 software [absolute quantification 2nd derivative maximum analysis]). $ ~ 5 replicates; § ~ 2 replicates; and c expression of GDF-15 in oocytes (OO, 17 oocytes) analyzed by western blot under reduced condition (LNCaP cells and seminal plasma [SP] samples serving as positive controls)
Discussion
To our knowledge, this is the first report explicitly documenting that a distinctive TGF-β superfamily member, cytokine GDF-15, is present in human FF. Moreover, it also shows that GDF-15 is expressed and inducible in both normal healthy and cancerous granulosa cells, as well as in oocytes.
To date, the presence of GDF-15 has gone undocumented in previously published complex proteomic studies analyzing human FF composition [2, 22, 31–33]. Other members of the TGF-β superfamily, inhibin A and anti-Mullerian hormone, have been measured in the FF of patients undergoing IVF, ranging from 15 to 75 ng/mL [34] and 1.1–3.5 ng/mL [35], respectively. However, our findings show that GDF-15 is an even more abundant cytokine, with concentrations between 35 and 571 ng/mL. Such levels are consonant to those recorded by Fried et al. [36], who measured the TGF-β family cytokine inhibin B in FF. Unlike inhibin B, however, we found that concentrations of GDF-15 in FF were significantly higher than those in serum. Moreover, GDF-15 concentrations in FF and serum showed no significant correlation. Given these results, one may presume that follicular GDF-15 levels are at least partially independent of the blood.
Curiously, our study did not reveal any obvious correlation between GDF-15 and fertility status. We did find a positive correlation between BMI of healthy donors and GDF-15 levels in FF, which was not in agreement with other published reports of inverse correlations between serum levels of GDF-15 and BMI in both humans and mice [37, 38]. Moreover, our results also showed that suggested links between GDF-15 as a possible marker of oxidative stress induced by elevated BMI [39] and obesity associated with reduced fecundity [40] is too simplified. The limited number of donors/patients in our study and the apparently independent nature of follicular GDF-15 regulation may account for such discrepancies, not to mention that prior to FF collection; the donors were subjected to hormonal stimulation.
GDF-15 is synthesized as an inactive precursor that is dimerized in the endoplasmic reticulum, then transported to the Golgi apparatus for proteolytic cleavage by furin-like proteases, and eventual secretion as an active dimer [28]. However, certain cells secrete large pools of unprocessed GDF-15 that subsequently inhabit the extracellular matrix, perhaps undergoing processing via extracellular MMP-26 [16, 41]. Preprocessed and/or mature GDF-15 forms have been suggested to be differentially deposited in extracellular matrix [16]. Clinical relevance has been suggested for both mature and pro-dimer forms of GDF-15 [16, 42]. Western blot analysis under reducing and non-reducing conditions confirmed that the GDF-15 pro-dimer only is present in FF. It is also notable that the activated form of GDF-15 was secreted only by cancerous (COV434) cells, possibly explaining the worse prognosis of furin-overexpressing ovarian cancers [43]. Recently, orphan receptor GFRAL has been identified as a receptor for GDF-15 [9–11]. Immunohistochemical analysis of GFRAL expression in clinical samples presented at the Human Protein Atlas portal (www.proteinatlas.org) [44] shows negative staining in normal ovary, but high and medium expression in ovarian cancer tissue. Therefore, further study of GFRAL-GDF-15 signaling is necessary for understanding of this signaling pathway in normal physiology and pathophysiology.
For our purposes, multiple methods were engaged to determine the expression of GDF-15 in human oocytes, which was not associated with HIF-1 expression. We also demonstrated that GDF-15 is secreted by both normal healthy and cancerous granulosa cells, its expression inducible through DNA damage or activation of p53 signaling. This collective evidence suggests that GDF-15 levels in FF reflect its secretion by granulosa cells and by oocytes as well. The signaling pathways pertaining to GDF-15 are still poorly understood, owing to as yet unidentified specific receptors. GDF-15 has been shown to transduce signals through stimulation of TGF-β receptors type I and II and intracellular Smad signal transduction protein complexes [45] as well as through Akt/ERK [46] or EGFR [47]. Greater insight into GDF-15 signaling is needed to delineate its presumptive context-dependent role in folliculogenesis and oocyte development. Although our efforts have shown that GDF-15 is present in FF, the levels measured did not correlate with donor fertility status. Patients presented in the study demonstrated a variety of causes of infertility. These variations may have influenced analysis of the association between GDF-15 level and fertility status. Still, production of GDF-15 by granulosa cells and oocytes was clearly demonstrated. Further studies are needed to clarify the role of GDF-15 in folliculogenesis and oocyte development.
Electronic supplementary material
Supplementary Fig. 1 Flow-chart diagram. Supplementary Fig. 2 Validation of the anti-GDF15 antibody was performed on formalin-fixed paraffin-embedded cell lines with known expression of GDF15. High (LNCaP cell line) and low expression (BPH1 cell line) of GDF15 was detected both by immunohistochemistry and western blotting. Magnification 200X, scale bars represent 50 μm. Supplementary Fig. 3 Immunohistochemical staining of HIF-1α in the ovary from the preterm newborn 2. The staining pattern of some follicles is different from the preterm newborn 1, probably due to longer time to autopsy (the autopsies were performed 28 and 47 h after exitus of newborns 1 and 2, respectively). Importantly, the staining pattern of both HIF-1α and GDF15 in the cytoplasm of oocytes is similar in both cases (please compare to the Fig. 3). Magnification 400X, scale bar represents 20 μm. Supplementary Table 1. Results of T-test of donor vs. patient characteristics. (PDF 652 kb)
Acknowledgements
The authors would like to thank Iva Lišková, Martina Urbánková, and Kateřina Svobodová for their superb technical assistance; Petra Ovesná for helping with statistical analyses, Monika Smějová, and Veronika Toporcerová for helping with protein quantifications and for western blot analyses respectively.
Author’s roles
KS carried out ELISA procedures and conceptualized the study, contributing to its design, coordination, and manuscript draft. Tsu and JR performed western blot testing; ZK and ES conducted qRT-PCR analysis, and IHC analysis was undertaken by JB and DK. TSo established the primary granulosa cell lines. AM and ZH completed sample preparation. AH participated in study design and with ES helped to draft the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the following grants: 15-11707S from Czech Science Foundation, by Ministry of Health of the Czech Republic, grant no. 15-33999A, all rights reserved (KS); project PROGRES Q40/06 (Charles University [TSo]); project mobility no. 7AMB16AT022 from the Ministry of Education, Youth and Sports; project HistoPARK (CZ.1.07/2.3.00/20.0185, [KS, AH]), CELLBIOL (CZ.1.07/2.3.00/30.0030 [TS]); project no. LQ1605 from the National Program of Sustainability II (MEYS CR; KS, AH); project no. LO1304 from the National Program of Sustainability I (MEYS CR; JB, DK); and the European Union—project ICRC-ERA-HumanBridge (no. 316345; KS, AH).
Compliance with ethical standards
The authors declare that they have no conflict of interest.
Footnotes
Topic: follicular fluid, IVF
Contributor Information
Karel Souček, Phone: +420 541 517 166, Email: ksoucek@ibp.cz.
Aleš Hampl, Phone: +420 549 49 1362, Email: ahampl@med.muni.cz.
References
- 1.Revelli A, Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7(1):40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambekar AS, Nirujogi RS, Srikanth SM, Chavan S, Kelkar DS, Hinduja I, Zaveri K, Prasad TSK, Harsha HC, Pandey A, Mukherjee S. Proteomic analysis of human follicular fluid: a new perspective towards understanding folliculogenesis. J Proteome. 2013;87:68–77. doi: 10.1016/j.jprot.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82(6):1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 4.Field SL, Dasgupta T, Cummings M, Orsi NM. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol Reprod Dev. 2014;81(4):284–314. doi: 10.1002/mrd.22285. [DOI] [PubMed] [Google Scholar]
- 5.Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-β superfamily expression and actions in the endometrium and placenta. Reproduction. 2006;132(2):217–232. doi: 10.1530/rep.1.01076. [DOI] [PubMed] [Google Scholar]
- 6.Soucek K, Slabakova E, Ovesna P, Malenovska A, Kozubik A, Hampl A. Growth/differentiation factor-15 is an abundant cytokine in human seminal plasma. Hum Reprod. 2010;25(12):2962–2971. doi: 10.1093/humrep/deq264. [DOI] [PubMed] [Google Scholar]
- 7.Corre J, Hébraud B, Bourin P. Concise review: growth differentiation factor 15 in pathology: a clinical role? Stem Cells Transl Med. 2013;2(12):946–952. doi: 10.5966/sctm.2013-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai VW, Lin S, Brown DA, Salis A, Breit SN. Anorexia–cachexia and obesity treatment may be two sides of the same coin: role of the TGF-b superfamily cytokine MIC-1/GDF15. Int J Obes. 2016;40(2):193–197. doi: 10.1038/ijo.2015.242. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjaer SB, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017;23(10):1158–1166. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- 10.Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. 2017;23(10):1215–1219. doi: 10.1038/nm.4393. [DOI] [PubMed] [Google Scholar]
- 11.Hsu JY, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, Kutach A, Joo W, Gao Z, Fu D, To C, Mondal K, Li B, Kekatpure A, Wang M, Laird T, Horner G, Chan J, McEntee M, Lopez M, Lakshminarasimhan D, White A, Wang SP, Yao J, Yie J, Matern H, Solloway M, Haldankar R, Parsons T, Tang J, Shen WD, Alice Chen Y, Tian H, Allan BB. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550(7675):255–259. doi: 10.1038/nature24042. [DOI] [PubMed] [Google Scholar]
- 12.Xiong Y, Walker K, Min X, Hale C, Tran T, Komorowski R, Yang J, Davda J, Nuanmanee N, Kemp D, Wang X, Liu H, Miller S, Lee KJ, Wang Z, Véniant MM. Long-acting MIC-1/GDF15 molecules to treat obesity: evidence from mice to monkeys. Sci Transl Med. 2017;9(412):eaan8732. doi: 10.1126/scitranslmed.aan8732. [DOI] [PubMed] [Google Scholar]
- 13.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94(21):11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairlie WD, Moore AG, Bauskin AR, Russell PK, Zhang HP, Breit SN. MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J Leukoc Biol. 1999;65(1):2–5. doi: 10.1002/jlb.65.1.2. [DOI] [PubMed] [Google Scholar]
- 15.Abd El-Aziz SH, Endo Y, Miyamaori H, Takino T, Sato H. Cleavage of growth differentiation factor 15 (GDF15) by membrane type 1-matrix metalloproteinase abrogates GDF15-mediated suppression of tumor cell growth. Cancer Sci. 2007;98(9):1330–1335. doi: 10.1111/j.1349-7006.2007.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauskin AR, Brown DA, Junankar S, Rasiah KK, Eggleton S, Hunter M, Liu T, Smith D, Kuffner T, Pankhurst GJ, Johnen H, Russell PJ, Barret W, Stricker PD, Grygiel JJ, Kench JG, Henshall SM, Sutherland RL, Breit SN. The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res. 2005;65(6):2330–2336. doi: 10.1158/0008-5472.CAN-04-3827. [DOI] [PubMed] [Google Scholar]
- 17.Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, Metz J, Kinscherf R. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318(2):325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 18.Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation. Circulation. 2014;130(21):1847–1858. doi: 10.1161/CIRCULATIONAHA.114.011204. [DOI] [PubMed] [Google Scholar]
- 19.Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, Sharma RK. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45(5):314–320. [PubMed] [Google Scholar]
- 20.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10(1):49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Ghulmiyyah J, Sharma R, Halabi J, Agarwal A. Power of proteomics in linking oxidative stress and female infertility. Biomed Res Int. 2014;2014:26. doi: 10.1155/2014/916212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamah AM, Hassis ME, Albertolle ME, Williams KE. Proteomic analysis of human follicular fluid from fertile women. Clin Proteomics. 2015;12(1):5. doi: 10.1186/s12014-015-9077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruckova L, Soukup T, Visek B, Moos J, Moosova M, Pavelkova J, Rezabek K, Kucerova L, Micuda S, Brcakova E, Mokry J. Proliferative potential and phenotypic analysis of long-term cultivated human granulosa cells initiated by addition of follicular fluid. J Assist Reprod Genet. 2011;28(10):939–950. doi: 10.1007/s10815-011-9617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruckova L, Soukup T, Moos J, Moosova M, Pavelkova J, Rezabek K, et al. The cultivation of human granulosa cells. Acta Med Austriaca. 2008;51(3):165–172. doi: 10.14712/18059694.2017.19. [DOI] [PubMed] [Google Scholar]
- 25.van den Berg-Bakker CA, Hagemeijer A, Franken-Postma EM, Smit VT, Kuppen PJ, van Ravenswaay Claasen HH, et al. Establishment and characterization of 7 ovarian carcinoma cell lines and one granulosa tumor cell line: growth features and cytogenetics. Int J Cancer. 1993;53(4):613–620. doi: 10.1002/ijc.2910530415. [DOI] [PubMed] [Google Scholar]
- 26.Pernicová Z, Slabáková E, Kharaishvili G, Bouchal J, Král M, Kunická Z, Machala M, Kozubík A, Součcek K. Androgen depletion induces senescence in prostate cancer cells through down-regulation of Skp2. Neoplasia. 2011;13(6):526–536. doi: 10.1593/neo.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slabakova E, Pernicova Z, Slavickova E, Starsichova A, Kozubik A, Soucek K. TGF-beta 1-induced EMT of non-transformed prostate hyperplasia cells is characterized by early induction of SNAI2/Slug. Prostate. 2011;71(12):1332–1343. doi: 10.1002/pros.21350. [DOI] [PubMed] [Google Scholar]
- 28.Bauskin AR, Zhang HP, Fairlie WD, He XY, Russell PK, Moore AG, Brown DA, Stanley KK, Breit SN. The propeptide of macrophage inhibitory cytokine (MIC-1), a TGF-beta superfamily member, acts as a quality control determinant for correctly folded MIC-1. EMBO J. 2000;19(10):2212–2220. doi: 10.1093/emboj/19.10.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 30.Database PC. Cytarabine. National Center for Biotechnology Information. 2017. https://pubchem.ncbi.nlm.nih.gov/compound/6253 Accessed Apr 19 2017.
- 31.Ambekar AS, Kelkar DS, Pinto SM, Sharma R, Hinduja I, Zaveri K, Pandey A, Prasad TSK, Gowda H, Mukherjee S. Proteomics of follicular fluid from women with polycystic ovary syndrome suggests molecular defects in follicular development. J Clin Endocrinol Metab. 2015;100(2):744–753. doi: 10.1210/jc.2014-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fahiminiya S, Labas V, Roche S, Dacheux J-L, Gérard N. Proteomic analysis of mare follicular fluid during late follicle development. Proteome Sci. 2011;9(1):54. doi: 10.1186/1477-5956-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh JW, Kim SK, Cho K-C, Kim M-S, Suh CS, Lee JR, Kim KP. Proteomic analysis of human follicular fluid in poor ovarian responders during in vitro fertilization. Proteomics. 2017;17(6):1600333-n/a. doi: 10.1002/pmic.201600333. [DOI] [PubMed] [Google Scholar]
- 34.Moos J, Filova V, Pavelkova J, Moosova M, Peknicova J, Rezabek K. Follicular fluid and serum levels of Inhibin A and pregnancy-associated plasma protein A in patients undergoing IVF. Fertil Steril. 2009;91(5):1739–1744. doi: 10.1016/j.fertnstert.2008.01.102. [DOI] [PubMed] [Google Scholar]
- 35.Lee M-S, Tzeng S-L, Yang S-F, Lin Y-P, Cheng E-H, Huang C-C, Yang YS, Lee TH. Correlation of serum anti-Mullerian hormone to follicular follicle stimulating hormone and implantation potential of the ensuing embryos. Clin Chim Acta. 2017;471:327–333. doi: 10.1016/j.cca.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Fried G, Remaeus K, Harlin J, Krog E, Csemiczky G, Aanesen A, Tally M. Inhibin B predicts oocyte number and the ratio IGF-I/IGFBP-1 may indicate oocyte quality during ovarian hyperstimulation for in vitro fertilization. J Assist Reprod Genet. 2003;20(5):167–176. doi: 10.1023/A:1023656225053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai VW-W, Macia L, Johnen H, Kuffner T, Manadhar R, Jørgensen SB, Lee-Ng KKM, Zhang HP, Wu L, Marquis CP, Jiang L, Husaini Y, Lin S, Herzog H, Brown DA, Sainsbury A, Breit SN. TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS One. 2013;8(2):e55174. doi: 10.1371/journal.pone.0055174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai VW-W, Macia L, Feinle-Bisset C, Manandhar R, Astrup A, Raben A, Lorenzen JK, Schmidt PT, Wiklund F, Pedersen NL, Campbell L, Kriketos A, Xu A, Pengcheng Z, Jia W, Curmi PMG, Angstmann CN, Lee-Ng KKM, Zhang HP, Marquis CP, Husaini Y, Beglinger C, Lin S, Herzog H, Brown DA, Sainsbury A, Breit SN. Serum levels of human MIC-1/GDF15 vary in a diurnal pattern, do not display a profile suggestive of a satiety factor and are related to BMI. PLoS One. 2015;10(7):e0133362. doi: 10.1371/journal.pone.0133362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156(3):274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 40.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414–420. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, Wang Y, Cao B, Wu Y, Ji L, Li Y-x, et al. Maturation of growth differentiation factor 15 in human placental trophoblast cells depends on the interaction with matrix metalloproteinase-26. J Clin Endocrinol Metab. 2014;99(11):E2277–E2E87. doi: 10.1210/jc.2014-1598. [DOI] [PubMed] [Google Scholar]
- 42.Brown DA, Lindmark F, Stattin P, Balter K, Adami HO, Zheng SL, et al. Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clin Cancer Res. 2009;15(21):6658–6664. doi: 10.1158/1078-0432.CCR-08-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page RE, Klein-Szanto AJ, Litwin S, Nicolas E, Al-Jumaily R, Alexander P, et al. Increased expression of the pro-protein convertase furin predicts decreased survival in ovarian cancer. Cell Oncol. 2007;29(4):289–299. doi: 10.1155/2007/930321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circul Res. 2006;98(3):342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 46.Kim K-K, Lee JJ, Yang Y, You K-H, Lee J-H. Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells. Carcinogenesis. 2008;29(4):704–712. doi: 10.1093/carcin/bgn031. [DOI] [PubMed] [Google Scholar]
- 47.Carrillo-García C, Prochnow S, Simeonova IK, Strelau J, Hölzl-Wenig G, Mandl C, et al. Growth/differentiation factor 15 promotes EGFR signalling, and regulates proliferation and migration in the hippocampus of neonatal and young adult mice. Development. 2014;141(4):773–783. doi: 10.1242/dev.096131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Flow-chart diagram. Supplementary Fig. 2 Validation of the anti-GDF15 antibody was performed on formalin-fixed paraffin-embedded cell lines with known expression of GDF15. High (LNCaP cell line) and low expression (BPH1 cell line) of GDF15 was detected both by immunohistochemistry and western blotting. Magnification 200X, scale bars represent 50 μm. Supplementary Fig. 3 Immunohistochemical staining of HIF-1α in the ovary from the preterm newborn 2. The staining pattern of some follicles is different from the preterm newborn 1, probably due to longer time to autopsy (the autopsies were performed 28 and 47 h after exitus of newborns 1 and 2, respectively). Importantly, the staining pattern of both HIF-1α and GDF15 in the cytoplasm of oocytes is similar in both cases (please compare to the Fig. 3). Magnification 400X, scale bar represents 20 μm. Supplementary Table 1. Results of T-test of donor vs. patient characteristics. (PDF 652 kb)



