Abstract
Purpose
The decline in female fecundity with age may be caused by decreased oocyte quality, a factor that may be associated with the altered composition of follicular fluid (FF).
Methods
In an effort to better understand follicular aging and the role of lipids in a given biological system, we present a prospective study that compares lipid profiles of FF from women older than 35 years (aging group, n = 12) to women equal or younger than 35 years old (control group, n = 17). FF lipids were extracted, and mass spectra were generated using a Waters Synapt G1 Q-TOF in MS mode. MS data was evaluated for both multi- and univariate statistics. The lipids identified as potential biomarkers of follicle aging were attributed by the online databases Lipid Maps, followed by pathway network analysis using Cytoscape software.
Results
The in vitro fertilization (IVF) parameters showed significant differences in aging, number of follicles, total number of oocytes and oocytes in MII, and number of injected oocytes. Additionally, FF from the aging group revealed 11 lipids with higher abundance, while FF from the control group included 4 lipids with higher abundance.
Conclusions
We suspect that aging may influence lipid metabolism in a downstream cascade leading, ultimately, to decreased oocyte quality. The discovery of target lipids may assist oocyte selection for IVF in the future. Furthermore, systems biology approach based on post-genomic medicine may help unravel a number of altered mechanisms not previously understood.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1259-5) contains supplementary material, which is available to authorized users.
Keywords: Aging, Oocyte quality, In vitro fertilization, Lipidomics, Mass spectrometry, MALDI-TOF
Introduction
Postponement of motherhood is intrinsically associated with the redefinition of women’s roles in society during recent decades [1]. In addition, advances in reproductive medicine may contribute to the idea that assisted reproductive technologies (ART) compensate for natural fertility decline with aging [2].
With aging, the decline in female fecundity is primarily due to decreases in ovarian follicle numbers or oocyte quality [3]. From the clinical perspective, these factors cannot be changed or controlled [4]. As a result of diminished reproductive capacity, follicular fluid (FF) may present an altered composition, which can be assessed by anti-Mullerian hormone and inhibin levels [5, 6]. However, such biomarkers are not exclusive to reproductive aging. In an effort to better comprehend aging mechanisms in ovaries and to discover potential biomarkers associated with in vitro fertilization (IVF) outcomes for older women, metabolomic analysis of FF may reveal new insights regarding the follicular environment of aging.
Modern metabolomic platforms have been widely used for biomarker discovery in FF, including the identification of lipids and small metabolites that may be associated with reproductive outcomes and oocyte competence [7, 8]. Therefore, the study of follicular fluid metabolites in patients of various ages may uncover metabolic pathways associated with oocyte and embryo quality. Additionally, the identification of new biomarkers offers new opportunities for the development of more sensitive diagnostic tools for the prediction of pregnancy outcomes.
One of these biomarker discovery tools includes matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), which allows untargeted analysis and minimal sample fragmentation for the identification of complex compounds [9]. Furthermore, the association between MALDI-TOF MS and multivariate statistics provide an opportunity to identify biomarkers associated with aging and with reproductive potential during IVF cycles [10]. Here, we present an initial analysis of FF lipid composition in patients older than 35 years of age compared to their younger counterparts, in an effort to identify lipid signatures associated with follicular changes related to aging. Additionally, the application of a systems biology approach introduces a potential altered mechanism not previously described.
Materials and methods
Patients
We conducted a prospective case-control study and enrolled 29 patients undergoing IVF treatment, independent of this study. Participants were divided into two groups, according to age: The control group (n = 17) included women equal and younger than 35 years, and the aging group (n = 12) included women older than 35 years. Inclusion criteria required only normo-ovulatory patients who presented with tubal factors of infertility, such that other factors of infertility would not confound differences attributable to a non-aging clinical condition. Exclusion criteria were defined as patients with a basal follicle stimulating hormone (FSH) concentration ≤ 10 mIU/mL on the third day of the cycle, in order to exclude patients with premature ovarian failure. Other exclusion criteria were patients with polycystic ovary syndrome, diabetes, metabolic syndrome, endometriosis, cancer, and other infertility factors that might compromise ovarian function.
Controlled ovarian stimulation and oocyte collection
Controlled ovarian stimulation was performed for all patients with the use of exogenous recombinant gonadotropins (225 IU/day of Gonal-F, Merck-Serono, Darmstadt, Germany) starting on cycle day 2. When the leading follicle reached 14 mm in diameter, endogenous LH release was suppressed by the GnRH antagonist (Cetrorelix–Cetrotide, Merk-Serono) until the day of hCG administration. As soon as the leading follicle reached 17 mm in diameter, a total dose of 250 μg of hCG was administered. Ultrasound-guided transvaginal oocyte retrieval was performed 36 h after hCG administration. After oocyte isolation, FF from each ovary was collected and pooled and centrifuged at 800×g for 10 min to separate the fluid from follicular cells. The supernatant was transferred to a sterile microtube and stored at −20 °C for further sample analysis.
The oocytes were incubated for 3 h before intracytoplasmic sperm injection procedure (ICSI). Fertilization was assessed 18 h after ICSI, by observing the presence of two polar bodies and two pronuclei. Embryo cleavage was observed 48 h after sperm injection. Seventy-two hours after sperm injection, the embryo quality was assessed in order to select embryos of best morphology for transfer. Evaluation of embryo morphology was performed on days 2 and 3 of culture, which considered the number of blastomeres, the regularity, and the fragmentation rate. On day 3, high-quality embryos were classified as presenting 7–9 cells, less than 15% of fragmentation, presence of symmetric blastomeres, and the absence of multinucleation. Beta subunit of human chorionic gonadotropin (βhCG) was measured 15 days after embryo transfer, considering a minimum of 25 mIU/mL of βhCG level as pregnancy positive for all patients.
Sample preparation
The FF samples were submitted to lipid extraction according to the Bligh and Dyer protocol with minor modifications [11, 12]. Briefly, 50 μL of distilled water was placed in a microtube with 50 μL of FF. Next, 125 μL of chloroform (CHCl3) and 250 μL of methanol (MeOH) were added. The mixture was vortexed for 1 min, followed by the addition of 100 μL of distilled water and 125 μL of chloroform. The final mixture was centrifuged at 300×g for 1 min. The apolar phase containing the lipids was recovered and transferred to a clean microtube, which was left open overnight for solvent evaporation.
Lipidomic analysis by mass spectrometry
The FF lipids in the microtube were reconstituted by adding 10 μL of CHCl3. For the MALDI-TOF MS analysis, 2 μL of the recovered lipids from each sample was deposited on the MALDI target plate and covered with 1 μL of 2,5-dihydroxybenzoicacid (DHB 0.5 M), dissolved in 90% methanol. Mass spectra were acquired in the positive ion mode using a Q-ToF Premier (Synapt HDMS) mass spectrometer (Waters, Manchester, UK) equipped with a 200-Hz solid-state laser in the m/z 700–1200, in the reflectron mode. Operating conditions included laser energy of 250 a.u., sample plate 20 V, and Trap and Transfer collision energies of 6 and 4 V, respectively (Q-TOF-MS mode).
Statistical analysis
Clinical data was normalized by Z-Score and analyzed by Students’ t-test. Pregnancy rates of controls and aging groups were compared by chi-square test, excluding patients whose embryo transfer was canceled in the respective IVF cycle. Both analyses were conducted by PASW 20.0 software package (SPSS Chicago, IL, United states), with significance set by p ≤ 0.05.
For MS data, the mass spectrum from each sample was processed by the MarkerLynx Software 4.1 (Waters, Manchester, UK) and exported to Microsoft® Excel (version 2016) for normalization by total ion count (TIC). For multivariate statistical analysis, data was normalized by PARETO prior to partial least square–discriminant analysis (PLS-DA), which was performed by MetaboAnalyst 3.0 software (http://www.metaboanalyst.ca). The PLS-DA regression model was applied to the dataset in order to generate sample classification. In addition, a set of biomarkers was defined by the variables of importance for the projection of the model (VIPs Scores), followed by a box plot analysis of individual biomarkers, so that the biomarker variance within groups could be observed.
Lipid attribution was performed using the Lipid Maps database (http://www.lipidmaps.org/), considering the main subclass, with a maximum mass tolerance of 0.1 Da and a maximum mass error of 50 ppm. For the attribution, only molecules containing hydrogen (M + H+), sodium (M + Na+), and potassium (M + K+) as adducts were considered.
Results
The clinical data demonstrated that the aging and control groups differed significantly by age. The aging group showed a significantly lower number of punctured follicles (p = 0.02), oocytes (p = 4.10E−04), oocytes in metaphasis II (MII—p = 7.92E−04), and injected oocytes (p = 6.46E−04). No differences were observed for hormonal or embryo parameters. Considering implantation rates, no differences were observed between groups (Table 1).
Table 1.
IVF characteristics of patients from aging and control groups
| Control group Average; SD | Aging group Average; SD | P | |
|---|---|---|---|
| Age (years) | 26.24; 2.88 | 37.67; 1.31 | 1.67E−11 |
| BMI (Kg/m2) | 24.73; 2.80 | 24.60; 2.3 | 0.92 |
| LH (mIU/mL) | 4.56; 1.49 | 3.99; 1.36 | 0.39 |
| FSH (mIU/mL) | 5.86; 1.72 | 5.43; 2.25 | 0.64 |
| Endometrial Thickness (mm) | 10.10; 1.21 | 9.92; 1.83 | 0.80 |
| Punctured follicles (n) | 25.47; 8.64 | 14.50; 10.92 | 0.02 |
| Oocyte retrieval rate | 0.54; 0.18 | 0.46; 0.20 | 0.11 |
| Oocytes (n) | 14.41; 6.20 | 5.75; 3.17 | 4.10E−04 |
| Oocytes MII (n) | 11.35; 4.65 | 4.92; 3.04 | 7.92E−04 |
| Oocytes MI (n) | 0.88; 0.83 | 0.33; 0.62 | 0.16 |
| Injected (n) | 12.24; 5.07 | 5.25; 2.98 | 6.46E−04 |
| Fertilized (n) | 5.71; 3.14 | 3.83; 3.21 | 0.13 |
| Fertilization rate | 0.63; 0.28 | 0.63; 0.36 | 0.98 |
| Cleavage rate | 0.93; 0.10 | 0.82; 0.39 | 0.24 |
| Embryos D2 (n) | 5.18; 2.72 | 3.75; 3.19 | 0.21 |
| Good-quality embryos D2 (n) | 3.82; 2.10 | 2.25; 2.38 | 0.07 |
| Poor-quality embryos D2 (n) | 1.35; 1.46 | 1.08; 1.68 | 0.65 |
| Embryos D3 (n) | 2.44; 1.17 | 2.33; 1.33 | 0.86 |
| Good-quality embryos D3 (n) | 2.53; 1.50 | 2.75; 2.31 | 0.90 |
| Poor-quality embryos D3 (n) | 1.35; 1.81 | 1.00; 1.53 | 0.82 |
| βhCG (% of positive) | 29.0 | 8.0 | 0.17 |
Embryos number and quality were evaluated in the third day of culture. Fertilization rate was determined considering the number of fertilized per number of injected. Cleavage rate considered number of embryos in the second day of culture per number of fertilized embryos
mIU/mL milli-international units per milliliter, (mm) millimeter, (n) number, MII Metaphasis II, MI metaphasis I, βhCG β-subunit of human chorionic gonadotropin, D2 second day of culture, D3 third day of culture
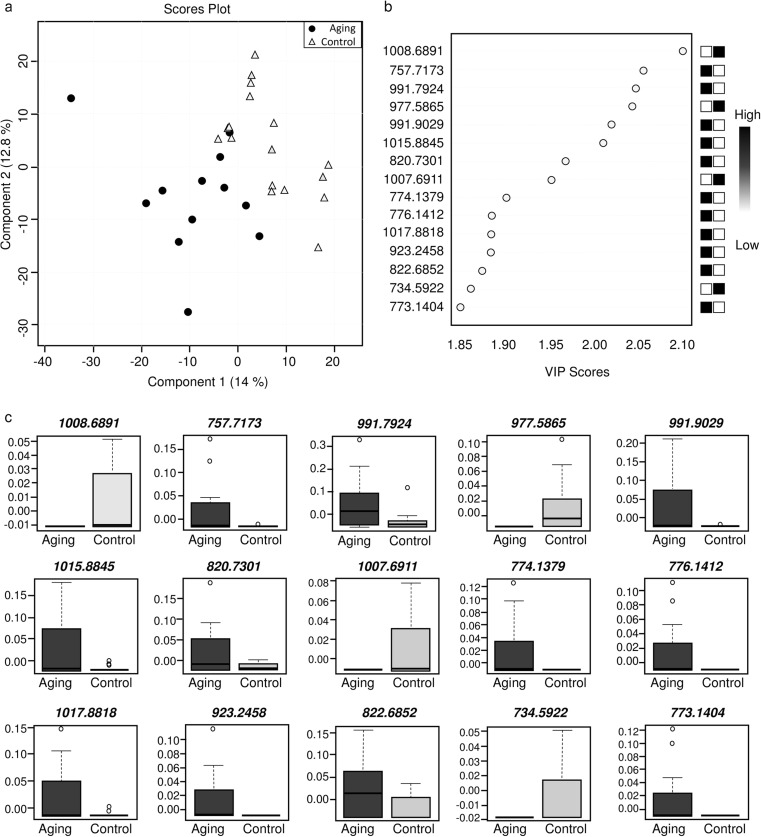
The lipidomic analysis was initially evaluated according to the mass spectra signals, which showed minor differences in the signal intensities that can be observed in the representative mass spectrum from each group (Fig. 1). Multivariate analysis by PLS-DA demonstrated a separation between groups according to the Scores Plot chart (Fig. 2a). From this analysis, the VIP Scores chart showed that 11 out of 15 biomarkers were of higher abundance in FF from the aging group, while 4 biomarkers were of higher abundance in FF from the control group (Fig. 2b). The variance of each biomarker within groups is shown in Fig. 2c.
Fig. 1.
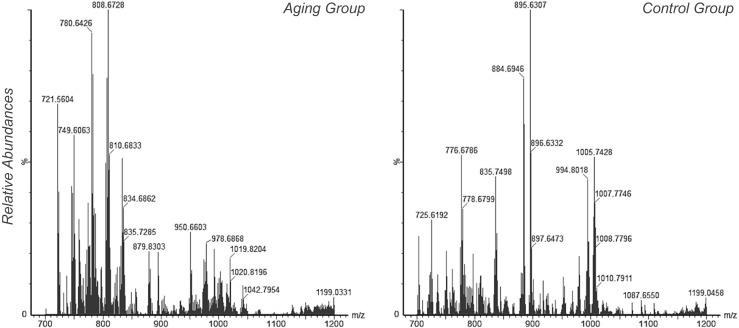
Representative mass spectra of follicular fluid analysis by MALDI-TOF-MS for each group included in the present study. The x axis indicates m/z, while y axis indicates relative abundance of ions. Mass spectrum selection for each group considered the spectrum of a sample that was visually similar to the spectrum of other samples
Fig. 2.
Multivariate statistical analysis of the lipid profile of follicular fluid. a Partial least square-discriminant analysis. b VIP Scores indicating potential ions as biomarkers. c Box plot evaluation of individual distribution and variance of ions suggested as biomarkers by the PLS-DA
The biomarker attribution demonstrated that FF from the aging group had increased abundance of phosphatidic acid (PA), phosphatidylinositol (PI), monogalactosyldiacylglycerol (MGDG), phosphatidylglycerol (PG), sphingomyelin (SM), diacylglycerol (DG), and triacylglycerol (TG—Table 2). The FF from controls had higher abundance of phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol phosphate (PIP), digalactosyldiacylglycerol (DGDG), PG, PI, PA, MGDG, and phosphatidylserine (PS—Table 3). Although some attributions are common between groups, the molecules present different m/z. In addition, some of the potential biomarkers were not attributed to a metabolite class in both groups, whereas others could have been attributed to more than one class, as can be observed in Tables 2 and 3. Detailed information regarding attribution is present in Supplementary Table 1.
Table 2.
Attribution of lipids of high abundance in FF from the aging group. The attribution considered the m/z, presence of adducts, tolerance of 0.1 of exact mass, and a maximum error of 50 ppm
| m/z | Attribution | Formula | Adduct | Error |
|---|---|---|---|---|
| 757.7173 | Not attributed | – | – | – |
| 773.1404 | Not attributed | – | – | – |
| 774.1379 | Not attributed | – | – | – |
| 776.1412 | Not attributed | – | – | – |
| 820.7301 | Not attributed | – | – | – |
| 822.6852 | Not attributed | – | – | – |
| 923.2458 | Not attributed | – | – | – |
| 991.7924 | Phosphatidic acid | C59H108O9P | M + H+ | 19.96 |
| C57H109O9PNa | M + Na+ | 22.48 | ||
| C57H109O8PK | M + K+ | 43.56 | ||
| Phosphatidylinositol | C55H108O12P | M + H+ | 35.39 | |
| Monogalactosyldiacylglycerol | C57H108O10K | M + K+ | 35.19 | |
| Phosphatidylglycerol | C56H112O11P | M + H+ | 1.31 | |
| Sphingomyelin | C57H113N2O6PK | M + K+ | 4.44 | |
| Diacylglycerol | C65H108O5Na | M + Na+ | 16.64 | |
| Triacylglycerol | C67H107O5 | M + H+ | 19.06 | |
| C65H108O5Na | M + Na+ | 16.64 | ||
| 991.9029 | Triacylglycerol | C65H115O6 | M + H+ | 34.38 |
| C63H116O6Na | M + Na+ | 36.80 | ||
| 1015.8845 | Phosphatidylglycerol | C60H120O9P | M + H+ | 17.72 |
| triacylglycerol | C67H115O6 | M + H+ | 15.45 | |
| C65H116O6Na | M + Na+ | 17.82 | ||
| C65H116O5K | M + K+ | 38.39 | ||
| Diacylglycerol | C65H116O5K | M + K+ | 38.39 | |
| 1017.8818 | Phosphatidylglycerol | C60H122O9P | M + H+ | 0.29 |
| Diacylglycerol | C65H118O5K | M + K+ | 20.34 | |
| Triacylglycerol | C67H117O6 | M + H+ | 2.65 | |
| C65H118O6Na | M + Na+ | 0.20 | ||
| C65H118O5K | M + K+ | 20.34 |
The maximum error allowed for attribution was 50 ppm
M + H+ protonated molecule, M + Na+ molecule with sodium as adduct, M + K+ molecule with potassium as adduct
Table 3.
Attribution of lipids of high abundance in FF from the control group. The attribution considered the m/z, presence of adducts, tolerance of 0.1 of exact mass, and a maximum error of 50 ppm
| m/z | Attribution | Formula | Adduct | Error |
|---|---|---|---|---|
| 734.5922 | Phosphatidylcholine | C40H81NO8P | M + H+ | 30.90 |
| Phosphatidylethanolamine | C41H85NO7P | M + H+ | 18.51 | |
| 977.5865 | Phosphatidylinositol phosphate | C49H87O15P2 | M + H+ | 35.80 |
| C47H95O16P2 | M + H+ | 23.02 | ||
| C47H88O15P2Na | M + Na+ | 38.26 | ||
| Phosphatidylinositol | C53H86O14P | M + H+ | 11.76 | |
| C51H87O14PNa | M + Na+ | 14.32 | ||
| C51H87O13PK | M + K+ | 35.70 | ||
| C49H95O14PK | M + K+ | 23.12 | ||
| Digalactosyldiacylglycerol | C51H86O15K | M + K+ | 27.21 | |
| Phosphatidylglycerol | C56H91O10PNa | M + Na+ | 38.56 | |
| 1007.6911 | Digalactosyldiacylglycerol | C56H95O15 | M + H+ | 24.31 |
| Phosphatidylglycerol | C60H96O10P | M + H+ | 17.37 | |
| C58H104O11P | M + H+ | 39.69 | ||
| C58H97O10PNa | M + Na+ | 19.85 | ||
| C56H105O11PNa | M + Na+ | 37.31 | ||
| C56H105O10PK | M + K+ | 16.47 | ||
| Phosphatidic acid | C61H100O9P | M + H+ | 18.76 | |
| C59H101O9PNa | M + Na+ | 16.27 | ||
| C59H101O8PK | M + K+ | 4.47 | ||
| Phosphatidylinositol | C57H100O12P | M + H+ | 3.57 | |
| C55H101O12PNa | M + Na+ | 1.19 | ||
| C55H101O12PNa | M + Na+ | 1.19 | ||
| Monogalactosyldiacylglycerol | C59H100O10K | M + K+ | 3.77 | |
| 1008.6891 | Phosphatidylethanolamine | C59H104NO8PNa | M + Na+ | 49.67 |
| C57H96NO9PK | M + K+ | 43.22 | ||
| Phosphatidylcholine | C60H99NO9P | M + H+ | 15.96 | |
| C58H100NO9PNa | M + Na+ | 13.58 | ||
| C58H100NO8PK | M + K+ | 7.14 | ||
| C56H108NO9PK | M + K+ | 49.87 | ||
| Phosphatidylserine | C56H108NO9PK | M + K+ | 49.87 | |
| C54H100NO11PK | M + K+ | 22.31 |
The maximum error allowed for attribution was 50 ppm
M + H+ protonated molecule, M + Na+ molecule with sodium as adduct, M + K+ molecule with potassium as adduct
From the lipids attribution, an interaction network was built to visualize potential enhanced pathways in each group. The aging group showed enhanced metabolism of glycosphingolipid, phosphatidylinositol phosphate, and glycerophospholipid (Fig. 3). The control group demonstrated enhanced metabolism of linoleate, arachidonic acid, glycerophospholipid, and phosphatidylinositol phosphate (Fig. 4).
Fig. 3.
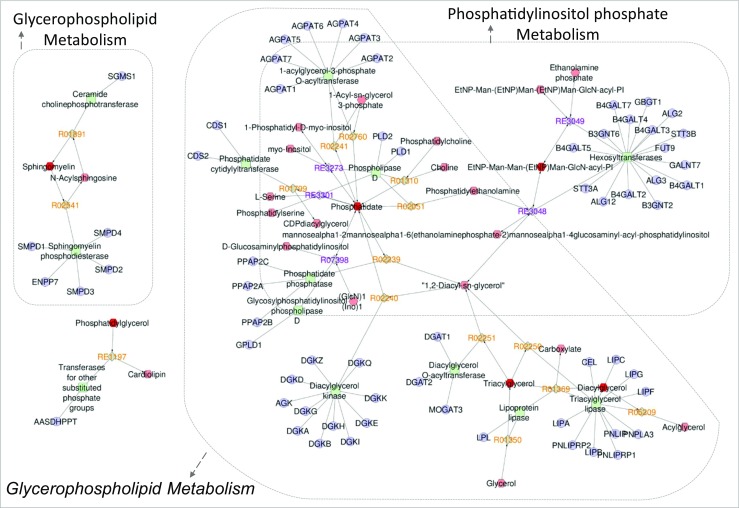
Network interaction analysis considering the lipid attributions of the aging group. The Metscape application indicated three enhanced pathways: glycosphingolipid metabolism; phosphatidylinositol phosphate metabolism, and glycerophospholipid metabolism. The metabolites of increased abundance in the aging group are represented by hexagons. Different relations were observed with enzymes (squares), genes (circles), and associated reactions (diamonds)
Fig. 4.
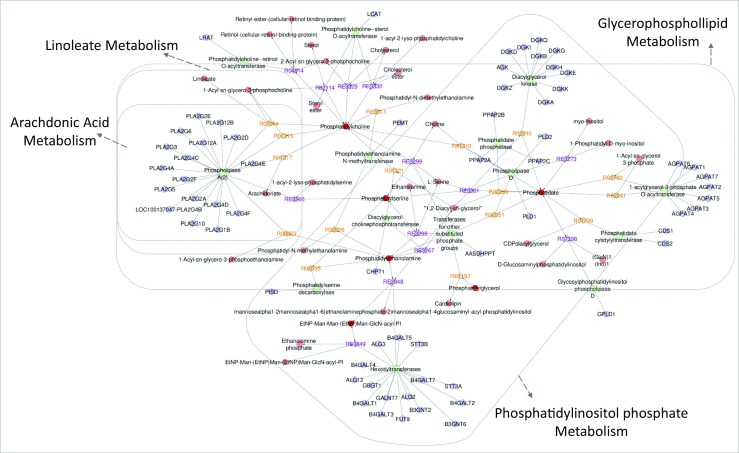
Network interaction analysis considering the lipid attributions of the control group. The Metscape application highlighted four main pathways: linoleate metabolism, arachidonic acid metabolism, glycerophospholipid metabolism, and phosphatidylinositol phosphate metabolism. The metabolites of increased abundance in the control group are represented by red hexagons. Different relations were also observed with enzymes (squares), genes (circles), and associated reactions (diamonds)
Discussion
The research and development for assisted reproductive treatments (ART) has steadily expanded over the years, which may be associated with the increasing average age of patients [13]. In addition, the age-associated decline in female fertility is often attributed to ovarian and oocyte aging. Until now, non-invasive oocyte assessment has only shown alterations in the metabolism of cumulus-oocyte complex and blood markers for ovarian aging are mostly related to measurement of AMH for premature ovarian aging [14, 15]. Thus, the search for potential biomarkers and their related metabolic pathways in FF from patients of different ages may improve our understanding of ovarian and oocyte aging.
Pregnancy outcomes after IVF treatments are influenced by age, and a significant decrease is common after 40 years of age, suggesting that IVF treatment is still not achieving the desired success rate [16]. In accordance with these findings, we observed a decrease in the pregnancy rates for patients in the aging group compared to the control group, although the difference was not significant. Diverging from literature, the average age of patients included in our aging group was lower, which could explain why our clinical results were not significant. Moreover, clinical pregnancy and live birth rates were not considered in the present study, as the main goal was to identify FF alteration that could be associated with oocyte aging and consequent embryo alterations.
With aging, the oocyte quality represents the main factor associated with infertility and potentially with the lower mitochondrial levels detected in the oocytes of these women [17]. As expected, we observed that aging may be associated with the production of follicles, evidenced by a significantly lower number of overall oocytes and oocytes in MII. Furthermore, FF from the aging group showed a higher abundance of SM, DG, and TG. Of these lipids, SM is intrinsically associated with the metabolism of glycosphingolipids (Fig. 3). Interestingly, some of the potential lipid biomarkers of increased abundance in both groups showed similar attribution. This finding suggests that different structural molecules belonging to the same class can be found in both groups, although physiological implications remain unknown.
The increased abundance of SM has been previously found in FF of patients with polycystic ovary syndrome (PCOS) compared to patients without PCOS, indicating that this lipid may be associated with poor oocyte quality [18]. The hydrolysis of membrane SM generates ceramide, and the sphingolipid breakdown is an important event during apoptosis. Nonetheless, the precise mechanism is still unclear [19, 20]. Interesting, the SM cycle occurs such that the same enzyme (sphingomyelinase, SMase) hydrolyzes and resynthesizes SM [21]. Thus, both consumption and synthesis of SM could indicate apoptosis, which could be a consequence of poor oocyte quality with aging.
The increased abundance of glycerolipids in the FF from the aging group patients may also be associated with oocyte impairment. TG has been associated with a decrease in follicular maturation and oocyte competence [22], which could result in a lower number of oocytes in MII, as observed in our study. More recently, the presence of higher levels of TG in FF from obese patients undergoing IVF was associated with altered follicular environment and oocyte gene expression [23]. Similar to poor ovarian responders undergoing IVF, the patients of the aging group had increased abundance of DG in FF [24], suggesting that DG may stimulate the apoptosis of granulosa cells, causing low oocyte production [25].
In addition to better oocyte quality, patients from the control group showed significant metabolism of linoleate (LA) and arachidonic acid (AA), considering that both pathways were associated with high abundance of PC in FF. As shown in the Fig. 4, the arachidonic acid and linoleate pathways may occur via the activity of phospholipase A2 (PLA2) [26]. High activity of PLA2 was associated with degenerated or non-fertilized oocytes, suggesting that an increase in the activity of PLA2 may be related to oocyte failure to form pronuclei [27]. Therefore, we believe that an increased abundance of PC indicates an association with oocyte competence in the control group.
Other lipid classes were observed in FF from controls, including PE, PS, PIP, and DGDG. These lipids together are involved in the PIP metabolism (Fig. 4). Although it is known that FSH regulates oocyte growth and follicle development, the precise mechanism is still not clear [28]. Recently, FSH was demonstrated to activate the phosphatidylinositol phosphate cascade in mouse oocytes, confirming the hypothesis that FSH improves oocyte developmental competence by regulating translation of its mRNA, which could improve oocyte quality [29]. In the present study, we observed a slight decrease in FSH levels in the aging group when compared with controls, although it was not significant. In this regard, the aging group has a higher standard deviation value, which indicates more variability of FSH levels in this group, although the difference observed is not enough to suggest some metabolic alteration at this moment. According to the literature, we believe that enhanced PIP metabolism in the control group may suggest a synergic action involving FSH with the presence of PIP and PI in FF from these patients, which could contribute to the higher number of overall oocytes and oocytes in MII for the control group.
In the present study, embryo quality was found to be similar for both groups, indicating that IVF may facilitate the proper generation of embryos for women over 35 years of age without any other related fertility issues. However, this initial assessment of follicular fluid suggests that aging affects the follicular environment by decreasing follicle production even during ART, with a resulting lower number of competent oocytes. The alterations in the follicular environment were associated with alterations in the lipid profiles of different groups and their respective pathway analysis. In spite of the low sample size, a limiting factor that mainly affected the evaluation of clinical data, lipidomics followed by post-genomic pathway analysis has contributed an improved understanding of a potential underlying mechanism of ovarian aging and poor oocyte quality. In summary, lipids seem to play a crucial role in ovary aging and further analysis and validation of potential lipid biomarkers may facilitate improved IVF in the future.
Electronic supplementary material
(PDF 534 kb)
Compliance with ethical standards
The authors comply with Springer’s Ethical Policies. The study received approval by the Ethics in Research Committee of São Paulo Federal University. Informed consent was obtained from all individual participants included in the study. All experiments were performed in accordance with relevant guidelines and regulations.
References
- 1.Luk J, Greenfeld DA, Seli E. Third party reproduction and the aging couple. Maturitas. 2010;66:389–396. doi: 10.1016/j.maturitas.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril. 2015;103:1136–1143. doi: 10.1016/j.fertnstert.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Wang T, Gao YY, Chen L, Nie ZW, Cheng W, Liu X, Schatten H, Zhang X, Miao YL. Melatonin prevents postovulatory oocyte aging and promotes subsequent embryonic development in the pig. Aging. 2017;9:1552–1564. doi: 10.18632/aging.101252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt T, Sobotka JG, Bentzen A, Nyboe A. On behalf of the ESHRE reproduction and society task force. Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update. 2012;1:29–43. doi: 10.1093/humupd/dmr040. [DOI] [PubMed] [Google Scholar]
- 5.Kedem A, Yung Y, Yerushalmi GM, Haas J, Maman E, Hanochi M, Hemi R, Orvieto R, Dor J, Hourvitz A. Anti Müllerian hormone (AMH) level and expression in mural and cumulus cells in relation to age. J Ovarian Res. 2014;11:113. doi: 10.1186/s13048-014-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein NA, Houmard BS, Hansen KR, Woodruff TK, Sluss PM, Bremner WJ, Soules MR. Age-related analysis of inhibin A, inhibin B, and activin a relative to the intercycle monotropic follicle-stimulating hormone rise in normal ovulatory women. J Clin Endocrinol Metab. 2004;89:2977–2981. doi: 10.1210/jc.2003-031515. [DOI] [PubMed] [Google Scholar]
- 7.Bracewell-Milnes T, Saso S, Abdalla H, Nikolau D, Norman-Taylor J, Johnson M, Holmes E, Thum MY. Metabolomics as a tool to identify biomarkers to predict and improve outcomes in reproductive medicine: a systematic review. Hum Reprod Update. 2017;23:723–736. doi: 10.1093/humupd/dmx023. [DOI] [PubMed] [Google Scholar]
- 8.O'Gorman A, Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction. 2013;146:389–395. doi: 10.1530/REP-13-0184. [DOI] [PubMed] [Google Scholar]
- 9.Schiller J, Arnhold J, Benard S, Müller M, Reichl S, Arnold K. Lipid analysis by matrix-assisted laser desorption and ionization mass spectrometry: a methodological approach. Anal Biochem. 1999;267:46–56. doi: 10.1006/abio.1998.3001. [DOI] [PubMed] [Google Scholar]
- 10.Montani DA, Cordeiro FB, Regiani T, Victorino AB, Pilau EJ, Gozzo FC, Ferreira CR, Fraietta R, Lo Turco EG. The follicular microenvironment as a predictor of pregnancy: MALDI-TOF MS lipid profile in cumulus cells. J Assist Reprod Genet. 2012;29:1289–1297. doi: 10.1007/s10815-012-9859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 12.Cordeiro FB, Cataldi TR, Perkel KJ, do Vale Teixeira da Costa L, Rochetti RC, Stevanato J, Eberlin MN, Zylbersztejn DS, Cedenho AP, Turco EG. Lipidomics analysis of follicular fluid by ESI-MS reveals potential biomarkers for ovarian endometriosis. J Assist Reprod Genet. 2015;32:1817–1825. doi: 10.1007/s10815-015-0592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi T, Igarashi H, Amita M, Hara S, Kurachi H. Cellular and molecular mechanisms of various types of oocyte aging. Reprod Med Biol. 2011;10:239–249. doi: 10.1007/s12522-011-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103:303–316. doi: 10.1016/j.fertnstert.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Lin PY, Huang FJ, Kung FT, Chiang HJ, Lin YJ, Lin YC, Lan KC. Evaluation of serum anti-Mullerian hormone as a biomarker of early ovarian aging in young women undergoing IVF/ICSI cycle. Int J Clin Exp Pathol. 2014;7(9):6245–6253. [PMC free article] [PubMed] [Google Scholar]
- 16.Ng EH, Ho PC. Ageing and ART: a waste of time and money? Best Pract Res Clin Obstet Gynaecol. 2007;21:5–20. doi: 10.1016/j.bpobgyn.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Keilty D, Zhang ZF, Chian RC. Mitochondria in oocyte aging: current understanding. Facts Views Vis Obgyn. 2017;9:29–38. [PMC free article] [PubMed] [Google Scholar]
- 18.Cordeiro FB, Cataldi TR, do Vale Teixeira da Costa L, de Lima CB, Stevanato J, Zylbersztejn DS, Ferreira CR, Eberlin MN, Cedenho AP, Turco EG. Follicular fluid lipid fingerprinting from women with PCOS and hyper response during IVF treatment. J Assist Reprod Genet. 2015;32:45–54. doi: 10.1007/s10815-014-0375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrieu-Abadie N, Levade T. Sphingomyelin hydrolysis during apoptosis. Biochim Biophys Acta. 2002;1585:126–134. doi: 10.1016/S1388-1981(02)00332-3. [DOI] [PubMed] [Google Scholar]
- 20.Lucki NC, Sewer MB. The interplay between bioactive sphingolipids and steroid hormones. Steroids. 2010;75:390–399. doi: 10.1016/j.steroids.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;4:3125–3128. [PubMed] [Google Scholar]
- 22.Yang X, Wu LL, Chura LR, Liang X, Lane M, Norman RJ, Robker RL. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil Steril. 2012;97:1438–1443. doi: 10.1016/j.fertnstert.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Ruebel ML, Cotter M, Sims CR, Moutos DM, Badger TM, Cleves MA, Shankar K, Andres A. Obesity modulates inflammation and lipid metabolism oocyte gene expression: a single-cell transcriptome perspective. J Clin Endocrinol Metab. 2017;102:2029–2038. doi: 10.1210/jc.2016-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cataldi T, Cordeiro FB, Costa Ldo V, Pilau EJ, Ferreira CR, Gozzo FC, Eberlin MN, Bertolla RP, Cedenho AP, Turco EG. Lipid profiling of follicular fluid from women undergoing IVF: young poor ovarian responders versus normal responders. Hum Fertil. 2013;16:269–277. doi: 10.3109/14647273.2013.852255. [DOI] [PubMed] [Google Scholar]
- 25.Onalan G, Selam B, Baran Y, Cincik M, Onalan R, Gündüz U, Ural AU, Pabuccu R. Serum and follicular fluid levels of soluble Fas, soluble Fas ligand and apoptosis of luteinized granulosa cells in PCOS patients undergoing IVF. Hum Reprod. 2005;20:2391–2395. doi: 10.1093/humrep/dei068. [DOI] [PubMed] [Google Scholar]
- 26.Wonnacott KE, Kwong WY, Hughes J, Salter AM, Lea RG, Garnsworthy PC, Sinclair KD. Dietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction. 2010;139:57–69. doi: 10.1530/REP-09-0219. [DOI] [PubMed] [Google Scholar]
- 27.Ciepiela P, Bączkowski T, Drozd A, Kazienko A, Stachowska E, Kurzawa R. Arachidonic and linoleic acid derivatives impact oocyte ICSI fertilization—a prospective analysis of follicular fluid and a matched oocyte in a ‘one follicle—one retrieved oocyte—one resulting embryo’ investigational setting. PLoS One. 2015;10(3):e0119087. doi: 10.1371/journal.pone.0119087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol (Paris) 2010;71:132–143. doi: 10.1016/j.ando.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Franciosi F, Manandhar S, Conti M. FSH regulates mRNA translation in mouse oocytes and promotes developmental competence. Endocrinology. 2016;157:872–882. doi: 10.1210/en.2015-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 534 kb)