Abstract
Density functional theory calculations of magnetically induced current densities have revealed high diatropic ring currents in unsubstituted isocorrole consistent with homoaromatic character. An examination of the Kohn-Sham molecular orbitals showed clear evidence of homoconjugative interactions in four occupied π-type molecular orbitals as well as in the LUMO. Remarkably, substituents at the saturated meso position were found to exert a dramatic influence on the overall current density pattern. Thus, whereas bis(trimethylsilyl)-substitution strongly enhanced the peripheral diatropic current (consistent with enhanced homoaromaticity), difluoro-substitution engendered a strong, net paratropic current (consistent with antihomoaromaticity). In this respect, isocorroles stand in sharp contrast to benzenoid aromatics, for which substituents typically exert a small influence on the current density distribution.
Introduction
Isocorroles are fascinating macrocyclic ligands with a sterically constrained N4 cavity characteristic of corroles and with the 2– charge of porphyrins (Fig. 1)1–5. With significant absorption in 700–1000 nm range, they are of considerable interest as near-IR dyes6. They also exhibit a Soret-like band in the 400–500 nm range, with an intensity comparable to those of porphyrins and corroles. These characteristics are exemplified in Fig. 2, which depicts the UV-vis spectra of selected 5/10-methoxy-5,10,15-triphenylisocorrole derivatives, H2[iso-5/10-MeO-TPC] and Ni[iso-5/10-MeO-TPC]. In addition, the 1H NMR spectra of many free-base isocorroles (including H2[iso-5/10-MeO-TPC]) exhibit moderately upfield-shifted β-pyrrole resonances and dramatically downfield-shifted NH resonances (relative to analogous corroles) (Fig. 3). These spectroscopic features are suggestive of either homoaromaticity or antihomoaromaticity, which are associated with the presence of a ring current in organic molecules in which an sp3 atom interrupts the conjugation7–9. Two density functional theory-based approaches have been employed here to examine the potential homoaromaticity of select isocorrole derivatives (Fig. 4), magnetically induced current density analysis and time-dependent density functional theory (TDDFT) calculations.
Figure 1.
Isocorroles (with atom numbering of the carbon skeleton) as hybrid ligands with characteristics of both porphyrins and corroles.
Figure 2.

UV-vis spectra of representative isocorrole derivatives.
Figure 3.
1H NMR spectra of representative isocorrole derivatives.
Figure 4.
Corrole and isocorrole derivatives examined in this study.
Results and Discussion
Current density analyses
Figure 5 depicts B3LYP/def2-TZVP current densities for unsubstituted gold corrole (Au[Cor])10 and free-base (H2[10-isoCor]) and nickel 10-isocorrole (Ni[10-isoCor]). Because the current density in all fully conjugated porphyrin-type molecules bifurcates at the pyrrole α-carbons, we will use the term ‘peripheral current’ to refer to the current along either the C9-C10 or the C1-C19 bond. The general features of the current density pathways for the molecules examined here are similar to those of other porphyrinoids; diatropic currents circulate along the outer rim of the molecules, while paratropic ones flow around the inner C11N4 framework11,12. Figure 5 shows that Au[Cor] sustains a strong diatropic peripheral current of ~26 nA·T−1 comparable to that of porphyrins. The current density passing between nitrogens and the central Au atom is almost negligible, reminiscent of current density pathways in porphyrins11. By comparison, the peripheral ring current in the unsubstituted metalloisocorrole Ni[10-isoCor] is ~9.8 nA·T−1 for the C9-C10 bond, which is about a third of that calculated for Au[Cor]. The reduced peripheral ring current in Ni[10-isoCor] is nevertheless far from insignificant and is just under that calculated for benzene (~11 nA·T−1). Qualitatively similar peripheral currents were also observed for the corresponding free-base isocorrole H2[10-isoCor] (Fig. 5). These data strongly suggest that Ni[10-isoCor] and H2[10-isoCor] are homoaromatic. Indeed, an examination of the π-type molecular orbitals of isocorrole derivatives provides conclusive proof of homoconjugation (hyperconjugative interactions); as discussed later in the paper, a total of 4 occupied MOs and the LUMO were found to exhibit with significant amplitudes at the saturated meso position.
Figure 5.
Current density pathways (a, c, and e) and plots (b, d, and f) for Au[Cor], Ni[10-isoCor], and H2[10-isoCor]. The plots refer to a displacement of 1 bohr above the molecular plane, where the π ring current is most intense. Colors ranging from blue (corresponding to 0.001 au) to red (0.0 au) represent stronger to weaker current densities.
Remarkably, substituents at the saturated meso position C10 by fluoro and trimethylsilyl groups were found to result in striking changes in the calculated current densities (Fig. 6). Thus, fluoro substituents effectively quench the diatropic ring current; indeed, the difluorinated compound Ni[10-F2-isoCor] sustains a net paratropic peripheral current and is legitimately viewed as antihomoaromatic. The paratropic current in this compound flows largely around the 15-membered inner C11N4 ring, paralleling similar behavior observed for other antiaromatic porphyrinoids13. Trimethylsilyl groups on the other hand behave oppositely; the hypothetical bis(trimethylsilyl) compound Ni[10-(Me3Si)2-isoCor] sustains a greatly enhanced diatropic peripheral current and may be regarded as strongly homoaromatic. This diverse range of behavior is relatively simply attributed to the hyperconjugative effects of C-F σ* orbitals and of C-Si σ orbitals, as discussed by von Schleyer and coworkers14,15. Nevertheless, given that substituent effects on ring currents in aromatic systems are typically quite small16–20, the present dramatic variations as a function of substituents at the saturated meso carbon are unusual indeed.
Figure 6.

Integrated current densities (a, c, and e) and current density plots (b, d, and f) for Ni[10-F-isoCor], Ni[10-isoCor], and Ni[10-(Me3Si)2-isoCor]. The plots refer to a displacement of 1 bohr above the molecular plane. Colors ranging from blue (corresponding to 0.001 au) to red (0.0 au) represent stronger to weaker current densities. Negative values in entry (c) indicate net paratropic currents.
TDDFT calculations
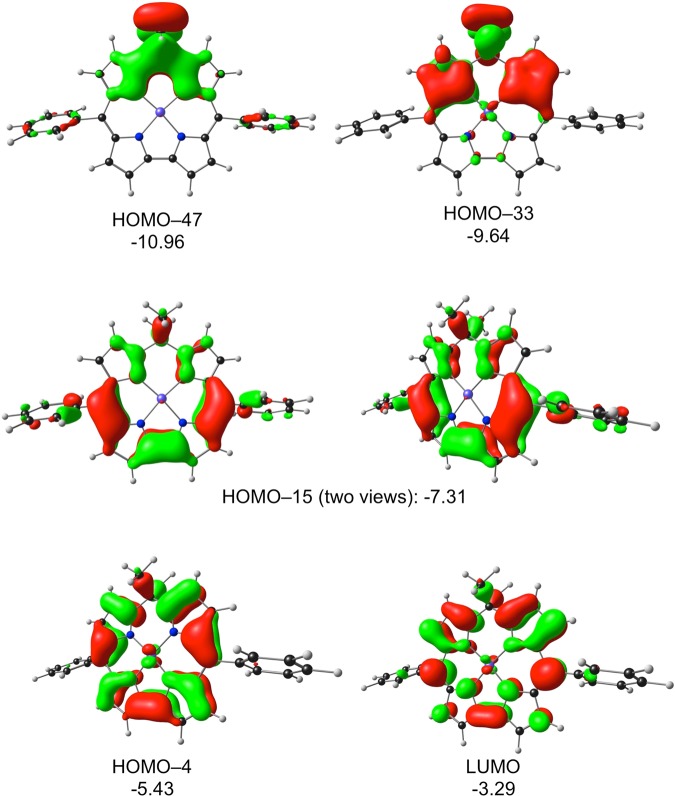
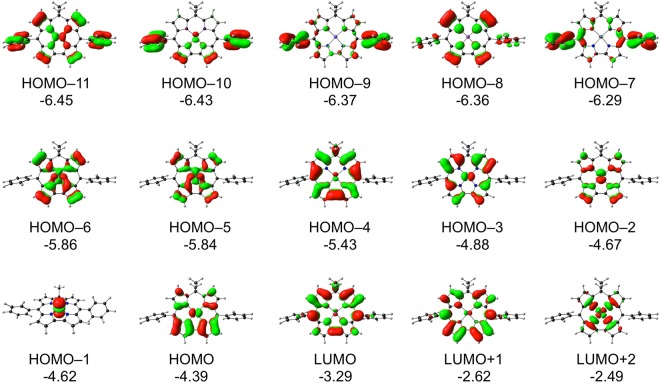
Molecular orbital and TDDFT21,22 analyses were carried out on a number of isocorrole derivatives with all-electron OLYP/STO-TZP calculations. The various systems chosen yielded very similar qualitative insights; the discussion below is based on our results for nickel 10,10-dimethyl-5,15-diphenylisocorrole, Ni[iso-Ph2MeCor]. The ground-state calculations readily identified four π-type occupied MOs and the LUMO as having significant hyperconjugative interactions, i.e., relatively large amplitudes at the saturated meso position (Fig. 7). The TDDFT results (Table 1 and Figs 8 and 9) led to several additional insights. First, the energy spacing of the Kohn-Sham MO eigenvalues clearly does not correspond to Gouterman’s four-orbital model23. That said, the HOMO-4, HOMO-3, LUMO, and LUMO + 1 do resemble the four frontier orbitals of a porphyrin or corrole in terms of qualitative shape24,25. Of these, the HOMO-4 and LUMO exhibit significant hyperconjugative interactions, i.e., relatively large amplitudes at the saturated meso position. The most intense calculated transitions all involve substantial HOMO-1/HOMO → LUMO/LUMO + 1 character as well as smaller amounts of HOMO-4 character. The lowest-energy transition exhibits a Q-like transition energy of ~2.0 eV and has predominantly HOMO-3 → LUMO character. Furthermore, multiple transitions with a similar intensity then cluster in the typical Soret region (~3.0 eV), whose cumulative effect is a deceptively porphyrin-like overall spectrum. Finally, since the LUMO has large amplitudes at the meso positions and the majority of the low-energy transitions have significant LUMO character, it stands to reason that the UV-vis-NIR spectra should exhibit a strong dependence on meso substituents, as is indeed observed1–5.
Figure 7.
OLYP/STO-TZP π-type MOs of Ni[IsoPh2MeCor], which involve homoconjugative interactions at the C10 meso position, along with their orbital energies (eV).
Table 1.
TDDFT (OLYP/STO-TZP) results for the main “Q” and “Soret” transitions of Ni[Iso10Me2–5,15Ph2C].
| E (eV) | Symmetry | λ (nm) | f | From | To | % contribution |
|---|---|---|---|---|---|---|
| 1.988 | B | 624 | 1.46 × 10–1 | HOMO-3 | LUMO | 84.0 |
| HOMO-2 | LUMO + 1 | 6.4 | ||||
| HOMO | LUMO | 3.9 | ||||
| HOMO | LUMO + 2 | 2.7 | ||||
| HOMO-3 | LUMO + 2 | 0.6 | ||||
| 3.033 | A | 409 | 8.87 × 10–2 | HOMO | LUMO + 4 | 54.9 |
| HOMO-4 | LUMO | 10.0 | ||||
| HOMO-3 | LUMO + 1 | 8.9 | ||||
| HOMO-8 | LUMO | 6.3 | ||||
| HOMO-9 | LUMO | 5.4 | ||||
| 3.081 | A | 402 | 9.63 × 10–2 | HOMO | LUMO + 4 | 40.1 |
| HOMO-9 | LUMO | 27.8 | ||||
| HOMO-4 | LUMO | 8.4 | ||||
| HOMO-3 | LUMO + 1 | 6.2 | ||||
| 3.166 | B | 392 | 6.84 × 10–2 | HOMO-4 | LUMO + 1 | 37.7 |
| HOMO-7 | LUMO | 24.2 | ||||
| HOMO-11 | LUMO | 16.0 | ||||
| HOMO-14 | LUMO | 9.9 | ||||
| 3.169 | A | 391 | 8.16 × 10–2 | HOMO-9 | LUMO | 35.1 |
| HOMO-10 | LUMO | 19.4 | ||||
| HOMO | LUMO + 6 | 9.4 | ||||
| HOMO-4 | LUMO | 7.1 | ||||
| HOMO-8 | LUMO | 5.6 |
Figure 8.
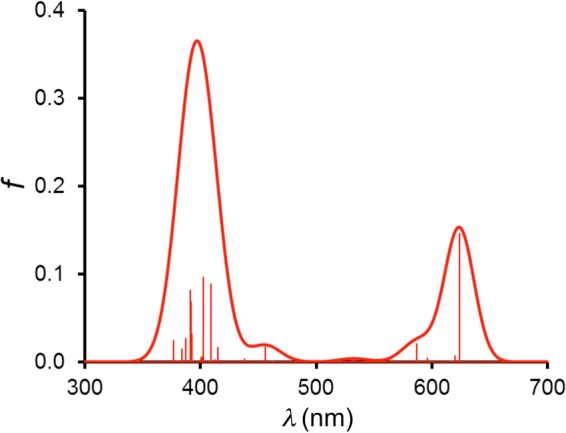
TDDFT oscillator strengths (f) plotted against wavelength (λ, nm) and an artificially broadened spectrum with Gaussians with FWHM = 30 nm.
Figure 9.
Selected OLYP/STO-TZP MOs relevant to Table 1, along with their orbital energies (eV).
Conclusion
A first detailed DFT investigation has clearly implicated homoconjugation as a critical determinant of the observed spectroscopic features of isocorroles. Thus, the calculations indicated unsubstituted free-base 10-isocorrole and its nickel complex as clearly homoaromatic. That said, substituents at the saturated meso carbon were found to dramatically affect the homoconjugation. Thus, while fluoro substituents were found to quench the diatropic peripheral current, leading in some cases to net antihomoaromatic character, trimethylsilyl substituents were found to greatly enhance homoaromatic character. The calculations further revealed homoconjugative/hyperconjugative interactions in four π-type occupied MOs as well as in LUMO. The strong Soret-like feature of isocorroles was found to arise from the clustering of several near-degenerate transitions with individual Q-like intensities. Finally, the large amplitude of the LUMO at the meso positions provides a simple rationale for the observed large variations in the UV-vis-NIR spectral profiles of isocorroles as a function of meso substituents.
Methods
All structures were fully optimized at B3LYP26–28/def2-TZVP29 computational level by Gaussian 09 rev. D130. (All optimized Cartesian coordinates are listed in the Supplementary information.) Eigenvalues of the Hessian matrix of energy were checked to ensure that all structures correspond to local minima. To obtain current density plots and intensities GIAO NMR computations were performed at the same level of theory by Gaussian 09 rev. D1 and the wave function of the NMR computations were further analyzed by AIMAll (version 16.05.18) suite of programs31. The current density were obtained within the context of quantum theory of atoms in molecules as developed by Keith and Bader32–36. TDDFT calculations were performed with ADF201737,38 on OLYP27,39/STO-TZP optimized geometries.
Free-base H2[iso-5/10-MeO-TPC] was synthesized according to the method described by the Kadish and Paolesse groups2. Although both isomeric free bases were isolated in reasonable yields, only the 10-methoxy compound (surprisingly) proved readily amenable to nickel insertion.
Synthesis of H2[iso-5/10-MeO-TPC]
To a solution of 5,10,15-triphenylcorrole (46.7 mg) in a mixture of dichloromethane (20 mL) and methanol (10 mL) was added DDQ (20.4 mg, 1 eq) and the resulting solution was stirred for 10 min. The solvents were removed under vacuum and the solids were washed down through a plug of silica with dichloromethane. The two isomers were then separated with preparative thin-layer chromatography on silica plates employing 2:1 dichloromethane/hexane as solvent. Yields: 32 mg of the 5-isomer (64.8 %) and 5.5 mg (11.1%) of the 10-isomer.
Spectroscopic data for H2[iso-5-MeO-TPC]
1H NMR (400 MHz, CDCl3, δ): 16.19 (s, 1H, NH), 15.85 (s, 1H, NH), 7.72 – 7.67 (m, 2H, 5-o-Ph), 7.53 – 7.48 (m, 2H, 15-o-Ph), 7.48 – 7.37 (m, 9H, 10-o-Ph and Ph), 7.25 – 7.22 (m, 2H, Ph), 6.93 (d, J = 4.6 Hz, 1H, β-H), 6.84 (d, J = 4.5 Hz, 1H, β-H), 6.56 (dd, J = 3.6, 2.6 Hz, 1H, β-H), 6.53 (d, J = 4.6 Hz, 1H, β-H), 6.50 (d, J = 4.6 Hz, 1H, β-H), 6.27 (dd, J = 4.3, 2.0 Hz, 1H, β-H), 6.11 (dd, J = 4.3, 2.6 Hz, 1H, β-H), 6.03 (dd, J = 3.6, 2.5 Hz, 1H, β-H), 3.43 (s, 3H, 5-MeO). UV-Vis (CH2Cl2) λmax [nm; ϵ × 10-4 (M-1cm-1)]: 337 (2.42), 401 (3.93), 678 (0.60), 739 (0.56). MS (MALDI-TOF): m/z calcd for C38H28N4O 556.2263 [M+]; found 556.2272.
Spectroscopic data for H2[iso-10-MeO-TPC]
1H NMR (400 MHz, CDCl3, δ): 15.58 (s, 2H, NH), 7.69 (d, J = 7.0 Hz, 2H, 10-o-Ph), 7.59 – 7.55 (m, 4H, 5,15-o-Ph), 7.48 – 7.42 (m, 6H, 5,15-m-Ph and 5,15-p-Ph), 7.25 – 7.16 (m, 3H, 10-m-Ph and 10-p-Ph), 6.69 – 6.67 (m, 4H, β-H), 6.61 (d, J = 4.3 Hz, 2H, β-H), 6.40 (d, J = 4.3 Hz, 2H, β-H), 3.49 (s, 3H, 10-MeO). UV-Vis (CH2Cl2) λmax [nm; ϵ × 10-4 (M-1cm-1)]: 351 (2.24), 430 (4.09), 668 (0.49), 721 (0.53). MS (MALDI-TOF): m/z calcd for C38H28N4O 556.2263 [M+]; found: 556.2272.
Synthesis of Ni[iso-5/10-MeO-TPC]
Free-base isocorrole (12.8 mg, mixture of isomers) and Ni(OAc)2 ∙ 4H2O (48.9 mg, 6 eq) were dissolved in dry DMF (5 ml) and refluxed for 1 h. The solvent was removed under vacuum and the solids were washed down with dichloromethane through a silica gel plug. The resulting product, upon preparative thin-layer chromatography on a silica plate with 2:1 dichloromethane/hexane as eluent, yielded a brown band composed of Ni[5,10,15-triphenyl-10-methoxyisocorrole]. Yield 1.2 mg (8.5%).
Spectroscopic data for Ni[iso-10-MeO-TPC]
1H NMR (400 MHz, CDCl3, δ): 7.86 (d, J = 7.6 Hz, 2H, Ph), 7.45 – 7.35 (m, 13H, Ph), 6.41 (d, J = 4.5 Hz, 2H, β-H), 6.27 – 6.23 (m, 4H, β-H), 6.15 (d, J = 4.5 Hz, 2H, β-H), 3.39 (s, 3H, 10-MeO). UV-Vis (CH2Cl2) λmax [nm; ϵ × 10-4 (M-1cm-1)]: 356 (1.23), 430 (2.51), 533 (0.45), 818 (0.18), 909 (0.39); MS (MALDI-TOF): m/z calcd for C38H26N4ONi: 612.1460 [M+]; found 612.1638.
Electronic supplementary material
Acknowledgements
Financial support from the Research Council of Norway (grant no. 262229 to AG) and the National Research Foundation of South Africa (grant no. 113327 to JC) is gratefully acknowledged. C.F.-N. acknowledges (1) “Projects of Large Research, Development, and Innovations Infrastructures” for access to the computational resources provided by the CESNET LM2015042 and the CERIT Scientific Cloud LM2015085 and (2) project CEITEC 2020 LQ1601 with financial support from the Ministry of Education, Youth, and Sports of the Czech Republic under the National Sustainability Programme II.
Author Contributions
C.F.N. performed the current density calculations and J.C. carried out the TDDFT and MO analyses. S.L. carried out all syntheses and spectroscopic analyses. A.G. planned and coordinated the project. All authors contributed to the writing of the paper.
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cina Foroutan-Nejad, Email: cina.foroutannejad@ceitc.muni.cz.
Jeanet Conradie, Email: conradj@ufs.ac.za.
Abhik Ghosh, Email: abhik.ghosh@uit.no.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29819-3.
References
- 1.Hohlneicher G, et al. Spiroconjugation in Spirodicorrolato‐Dinickel(II) Chem. Eur. J. 2003;9:5636–5642. doi: 10.1002/chem.200305094. [DOI] [PubMed] [Google Scholar]
- 2.Pomarico G, et al. Synthesis and Characterization of Free-Base, Copper, and Nickel Isocorroles. Inorg. Chem. 2010;49:5766–5774. doi: 10.1021/ic100730j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa R, III Geier,GR, Ziegler CJ. Structure and spectroscopic characterization of free base and metal complexes of 5,5-dimethyl-10,15-bis(pentafluorophenyl)isocorrole. Dalton Trans. 2011;40:4384–4386. doi: 10.1039/c1dt10112a. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann, M. et. al. Template Synthesis of Alkyl‐Substituted Metal Isocorroles. Eur. J. Inorg Chem. 3076–3085 (2016).
- 5.Thomas KE, Beavers CM, Gagnon KJ, Ghosh A. β‐Octabromo‐ and β‐Octakis(trifluoromethyl)isocorroles: New Sterically Constrained Macrocyclic Ligands. ChemistryOpen. 2017;6:402–409. doi: 10.1002/open.201700035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omori H, Hiroto S, Shinokubo H. 10‐Silacorroles Exhibiting Near‐Infrared Absorption and Emission. Chem. Eur. J. 2017;23:7866–7870. doi: 10.1002/chem.201701474. [DOI] [PubMed] [Google Scholar]
- 7.Winstein S. Homo-Aromatic Structures. J. Am. Chem. Soc. 1959;81:6524–6525. doi: 10.1021/ja01533a052. [DOI] [Google Scholar]
- 8.Warner P, Harris DL, Bradley CH, Winstein S. Further evidence on the nature of the monohomotropylium ion. Tetrahedron Lett. 1970;11:4013–4016. doi: 10.1016/S0040-4039(01)98653-8. [DOI] [Google Scholar]
- 9.Williams RV. Homoaromaticity. Chem. Rev. 2001;101:1185–1204. doi: 10.1021/cr9903149. [DOI] [PubMed] [Google Scholar]
- 10.Au[Cor] has been chosen as a paradigmatic, innocent metallocorrole: Thomas, K. E., Alemayehu, A. B., Conradie, J., Beavers, C. & Ghosh, A. Synthesis and Molecular Structure of Gold Triarylcorroles. Inorg. Chem. 50, 12844–12851 (2011). [DOI] [PubMed]
- 11.Fliegl H, Sundholm D. Aromatic Pathways of Porphins, Chlorins, and Bacteriochlorins. J. Org. Chem. 2012;77:3408–3414. doi: 10.1021/jo300182b. [DOI] [PubMed] [Google Scholar]
- 12.Franzke YJ, Sundholm D, Weigend F. Phys. Chem. Chem. Phys. 2017;19:12794–12803. doi: 10.1039/C7CP00624A. [DOI] [PubMed] [Google Scholar]
- 13.Fliegl H, Pichierri F, Sundholm D. Calculations of current densities and aromatic pathways in cyclic porphyrin and isoporphyrin arrays. J. Phys. Chem. A. 2015;119:2344–2350. doi: 10.1021/jp5067549. [DOI] [PubMed] [Google Scholar]
- 14.Nyulászi L, Schleyer PvR. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1999;121:6872–6875. doi: 10.1021/ja983113f. [DOI] [PubMed] [Google Scholar]
- 15.Fernández I, Wu JI. & Schleyer, P. v. R. Substituent Effects on “Hyperconjugative” Aromaticity and Antiaromaticity in Planar Cyclopolyenes. Org. Lett. 2013;15:2990–2993. doi: 10.1021/ol401154r. [DOI] [PubMed] [Google Scholar]
- 16.Krygowski TM, et al. Relation between the Substituent Effect and Aromaticity. J. Org. Chem. 2004;69:6634–6640. doi: 10.1021/jo0492113. [DOI] [PubMed] [Google Scholar]
- 17.Krygowski TM, Dobrowolski MA, Zborowski K, Cyrański MK. Relation between the substituent effect and aromaticity. Part II. The case of meta- and para-homodisubstituted benzene derivatives. J. Phys. Org. Chem. 2006;19:889–895. doi: 10.1002/poc.1039. [DOI] [Google Scholar]
- 18.Krygowski TM, Palusiak M, Płonka A, Zachara-Horeglad JE. Relationship between substituent effect and aromaticity – Part III: naphthalene as a transmitting moiety for substituent effect. J. Phys. Org. Chem. 2007;20:297–306. doi: 10.1002/poc.1127. [DOI] [Google Scholar]
- 19.Curutchet C, Poater J, Solà M, Elguero J. Analysis of the Effects of N-Substituents on Some Aspects of the Aromaticity of Imidazoles and Pyrazoles. J. Phys. Chem. A. 2011;115:8571–8577. doi: 10.1021/jp204263p. [DOI] [PubMed] [Google Scholar]
- 20.Radula-Janik K, Kopka K, Kupka T, Ejsmont K. Substituent Effect Of Nitro Group On Aromaticity Of Carbazole Rings. Chem. Heterocycl. Compd. 2014;50:1244–1251. doi: 10.1007/s10593-014-1586-0. [DOI] [Google Scholar]
- 21.Alemayehu AB, Conradie J, Ghosh A. A First TDDFT Study of Metallocorrole Electronic Spectra: Copper meso‐Triarylcorroles Exhibit Hyper Spectra. Eur. J. Inorg. Chem. 2011;12:1857–1864. doi: 10.1002/ejic.201001026. [DOI] [Google Scholar]
- 22.Rhoda HM, Crandall LA, Geier GR, III, Ziegler CJ, Nemykin VN. Combined MCD/DFT/TDDFT Study of the Electronic Structure of Axially Pyridine Coordinated Metallocorroles. Inorg. Chem. 2015;54:4652–4662. doi: 10.1021/ic502946t. [DOI] [PubMed] [Google Scholar]
- 23.Gouterman M, Wagniére GH, Snyder LC. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 1963;11:108–115. doi: 10.1016/0022-2852(63)90011-0. [DOI] [Google Scholar]
- 24.Ghosh A, Wondimagegn T, Parusel ABJ. Electronic Structure of Gallium, Copper, and Nickel Complexes of Corrole. High-Valent Transition Metal Centers versus Noninnocent Ligands. J. Am. Chem. Soc. 2000;122:5100–5104. doi: 10.1021/ja9943243. [DOI] [Google Scholar]
- 25.Ghosh A. Electronic Structure of Corrole Derivatives: Insights from Molecular Structures, Spectroscopy, Electrochemistry, and Quantum Chemical Calculations. Chem. Rev. 2017;117:3798–3881. doi: 10.1021/acs.chemrev.6b00590. [DOI] [PubMed] [Google Scholar]
- 26.Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A. 1988;38:3098–3100. doi: 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- 27.Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- 28.Miehlich B, Savin A, Stoll H, Preuss H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989;157:200–206. doi: 10.1016/0009-2614(89)87234-3. [DOI] [Google Scholar]
- 29.Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005;7:3297–3305. doi: 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- 30.Frisch, M. J. et al. Gaussian 09, Gaussian, Inc., Wallingford CT, 2013.
- 31.Keith, T. A. AIMAll, Gristmill Software: Overland Park KS, USA, 2017.
- 32.Keith TA, Bader RFW. Calculation of magnetic response properties using atoms in molecule. sChem. Phys. Lett. 1992;194:1–8. doi: 10.1016/0009-2614(92)85733-Q. [DOI] [Google Scholar]
- 33.Keith TA, Bader RFW. Calculation of magnetic response properties using a continuous set of gauge transformations. Chem. Phys. Lett. 1993;210:223–231. doi: 10.1016/0009-2614(93)89127-4. [DOI] [Google Scholar]
- 34.Keith TA, Bader RFW. Topological analysis of magnetically induced molecular current distributions. J. Chem. Phys. 1993;99:3669–3682. doi: 10.1063/1.466165. [DOI] [Google Scholar]
- 35.Keith TA. Calculation of magnetizabilities using GIAO current density distributions. Chem. Phys. 1996;213:123–132. doi: 10.1016/S0301-0104(96)00272-8. [DOI] [Google Scholar]
- 36.Keith TA, Bader RFW. Properties of atoms in molecules: nuclear magnetic shielding. Can. J. Chem. 1996;74:185–200. doi: 10.1139/v96-022. [DOI] [Google Scholar]
- 37.te Velde G, et al. Chemistry with ADF. J. Comput. Chem. 2001;22:931–967. doi: 10.1002/jcc.1056. [DOI] [Google Scholar]
- 38.Guerra CF, Snijders JG, te Velde G, Baerends EJ. Towards an order-N DFT method. Theor. Chem. Acc. 1998;99:391–403. [Google Scholar]
- 39.Handy NC, Cohen AJ. Left-right correlation energy. Mol. Phys. 2001;99:403–412. doi: 10.1080/00268970010018431. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.