Abstract
Study Objectives:
To evaluate both the influence of the volume of the excised base of tongue (BOT) on the surgical outcome after robotic tongue reduction in patients affected by obstructive sleep apnea (OSA) and the role of the lymphatic or muscular predominance within the removed tissue.
Methods:
Fifty-one patients with OSA were included in this study. All patients were treated with a robotic tongue base reduction. Data registered for the analysis were: age, sex, preoperative body mass index, preoperative and postoperative apnea-hypopnea index (AHI), delta AHI (preoperative AHI − postoperative AHI), total volume of the excised BOT, total thickness of excised BOT, isolated lymphatic thickness and soft tissue thickness (including muscular component) of the excised BOT, and lymphatic/soft tissue ratio (lymphatic thickness / soft tissue thickness).
Results:
A statistically significant reduction of AHI values was seen postoperatively, and a success rate of 74.5% was recorded. However, no significant correlations between delta AHI and tongue volume in cubic centimeters, lymphatic/soft tissue ratio, and total thickness were found.
Conclusions:
These findings reinforce the general opinion that OSA is not only influenced by anatomic factors but other phenomena may play a fundamental role in its genesis. A deeper understanding of OSA pathogenesis is needed in order to tailor an individual treatment strategy that could lead to a more effective therapy.
Citation:
Cammaroto G, Meccariello G, Costantini M, Stomeo F, Hoff P, Montevecchi F, Vicini C. Trans-oral robotic tongue reduction for OSA: does lingual anatomy influence the surgical outcome? J Clin Sleep Med. 2018;14(8):1347–1351.
Keywords: base of tongue, obstructive sleep apnea, OSA, trans-oral robotic surgery, TORS
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea is known to be caused by anatomic and physiological factors. The aim of this study is to evaluate the influence of the base of the tongue and its histological components on the effectiveness of trans-oral robotic surgery.
Study Impact: The results highlighted by this study underlines the importance of nonanatomic factors in the genesis of obstructive sleep apnea and express the need for a better understanding of the pathology and a more precise selection of candidates for sleep surgery.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder that affects more than 2% to 4% of the adult population. It is a serious social health problem with significant implications on quality of life as well as long-term health. OSA has consistently been shown to cause a multitude of neurobehavioral issues and is an independent risk factor for cardiopulmonary diseases that significantly increase the risk of death.1,2 Among the most important pathophysiologic causes the following should be cited:
An anatomically compromised or collapsible upper airway (high passive critical closing pressure of the upper airway [Pcrit])3;
Inadequate responsiveness of the upper airway dilator muscles during sleep (minimal increase in electromyography muscular activity to negative pharyngeal pressure)4,5;
Premature awakening to airway narrowing (a low respiratory arousal threshold)6–10;
Oversensitive ventilatory control system (high loop gain).10–12
The gold standard treatment for OSA remains continuous positive airway pressure (CPAP). However, large proportions of patients do not tolerate or show consistent compliance with CPAP and require an alternative treatment.13
In selected patients, trans-oral robotic surgery (TORS) has been shown to be a promising and effective option for the treatment of OSA allowing the resection of the base of the tongue (BOT) and supporting several surgical techniques.14,15
However, the most appropriate selection of patients for TORS is currently the main issue. For instance, the risk of TORS failure rate was reported to be significantly higher in patients with body mass index (BMI) > 30 kg/m2.16
Some studies have also shown correlations between upper airway volumes measured by imaging techniques and polysomnographic parameters.17–22 Thus, these anatomic findings might imply that surgical success could also be related to upper airway volumes.
The aim of this study is to evaluate the influence of the volume of the excised BOT on the surgical outcome after TORS, and the influence of lymphatic or muscular predominance within the removed tissue.
METHODS
The medical charts of consecutive patients who underwent TORS for OSA at the Otolaryngology Head-Neck Surgery and Stomatology Unit of the Hospital Morgagni-Pierantoni in Forlì, Italy were evaluated retrospectively.
The patients were randomly selected from the dataset including patients with OSA treated surgically from June 2010 to April 2015 at the ENT unit of the Hospital Morgagni-Pierantoni, Forlì, Italy.
Patients met inclusion criteria if they were 18 years of age or older, had failed CPAP as a nonsurgical treatment alternative, and had an apnea-hypopnea index (AHI) of 20 events/h or above.
Patients who had undergone prior airway surgery, such as uvulopalatopharyngoplasty or tonsillectomy, were not eligible for the trial. Incomplete or recent cases, with a postoperative polysomnographic evaluation shorter than 1 year, were also excluded.
Preoperative workup included preoperative drug-induced sleep endoscopy. Patients who were found to have significant multilevel collapse at the palatal, lingual, and pharyngeal levels with complete obstruction of the tongue base (lymphatic and nonlymphatic hypertrophy) were included in this study. A total of 51 patients were included in this study.
All patients were treated with a robotic tongue base reduction as previously described with temporary tracheostomy, tonsillectomy, expansion sphincter pharyngoplasty, and septoturbinoplasty.23,24
Data registered for the analysis were: age, sex, preoperative BMI, palatine tonsils grade at clinical examination, pre-operative and postoperative AHI, delta AHI (preoperative AHI − postoperative AHI), total volume of the excised BOT, total thickness of excised BOT, lymphatic and soft tissue thickness (including muscular component) of excised BOT, and lymphatic/soft tissue ratio (lymphatic thickness / soft tissue thickness).
Postoperative polysomnography was performed 1 year after surgery in all patients. All the sleep studies were carried out with an unattended type 3 home sleep study (Polymesam 8-channel) and reviewed and scored by the same expert in sleep medicine according to the American Academy of Sleep Medicine Guidelines.25
Apnea was defined as the cessation of nasal airflow of more than 90% for a period of ≥ 10 seconds in the presence of respiratory efforts. A hypopnea was scored whenever there was a > 30% reduced oronasal airflow for at least 10 seconds, accompanied by ≥ 4% oxygen desaturation from preevent baseline.
Measurement of the volume of tissue removed was performed by means of a graduated syringe filled partially with water into which the excised BOT was placed, thus causing the level of the water to rise. Volume displacement was measured in cubic centimeters (cm3).
Excised BOT specimens were fixed in 10% neutral buffered formalin for a minimum of 24 hours, followed by paraffin embedding, cut at maximum thickness and stained with hematoxylin and eosin for microscopic examination. The most representative slide showing the point of the more hyperplastic and thicker lymphoid component was selected for each case.
The measurements were made in millimeters taking into account that the surface of the BOT mucosa is rather irregular, drawing lines perpendicular to the mucosal surface. On the same section, the measurement of the component of the soft tissues was carried out from the limit of lymphoid tissue until the deep margin (Figure 1).
Figure 1. Excised BOT specimens stained with hematoxylin and eosin.
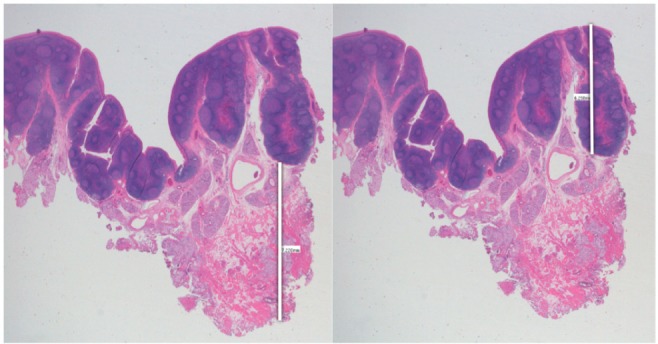
The measurements of lymphatic and soft tissue portions are highlighted (white lines).
The definition of surgical response and success was a reduction from the preoperative AHI of at least 50% (response) and a value less than 20 events/h (success).
The association of delta AHI with tongue volume, tongue thickness, and lymphatic/soft tissue ratio was analyzed using univariate linear regression test.
Adjusted odds ratios (95% confidence intervals) were calculated for independent variables. Pearson correlation coefficients were also tested.
The pretreatment and posttreatment AHI values were compared by t test. A two-sample t test was also used to compare the excised BOT volume of two groups: patients with successful outcomes (postoperative AHI < 20 events/h) (group A) and patients with postoperative AHI > 20 events/h (group B).
Probability values lower than .05 were considered statistically significant. Statistical analyses were performed with SPSS version 2.0 (IBM Corporation, Armonk, New York, United States).
This study was approved by the Institutional Review Board of the Hospital Morgagni-Pierantoni in Forlì, Italy.
RESULTS
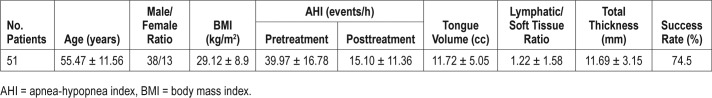
Table 1 shows data about age, M/F ratio, BMI, pretreatment and posttreatment AHI values, resected tongue volume (cm3), lymphatic/soft tissue ratio and total thickness (mm) of the resected tongue volume, and, success rate (%) of treated patients. Palatine tonsils grade at clinical examination was less than 3 in all patients.
Table 1.
Age, male/female ratio, BMI, pretreatment and posttreatment AHI values, resected tongue volume, lymphatic/soft tissue ratio and, total thickness of the resected tongue volume, and success rate of treated patients.
An average of 11.72 cm3 of resected tongue volume was recorded in our series.
A statistically significant reduction of AHI values was seen postoperatively (from 39.97 ± 16.78 to 15.10 ± 11.36 events/h), and a success rate of 74.5% was also recorded (Table 2).
Table 2.
Pretreatment and posttreatment AHI values compared by t test.

However, no significant correlations between delta AHI and tongue volume (cm3), delta AHI and lymphatic/soft tissue ratio, delta AHI and total thickness were found (Figures 2–4).
Figure 2. Univariate linear regression test correlating delta AHI to BOT volume.
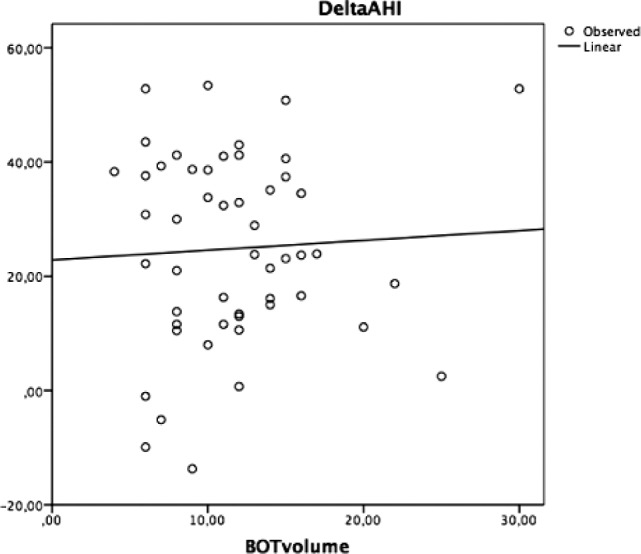
AHI = apnea-hypopnea index, BOT = base of tongue.
Figure 3. Univariate linear regression test correlating delta AHI to lymphatic/soft tissue ratio.
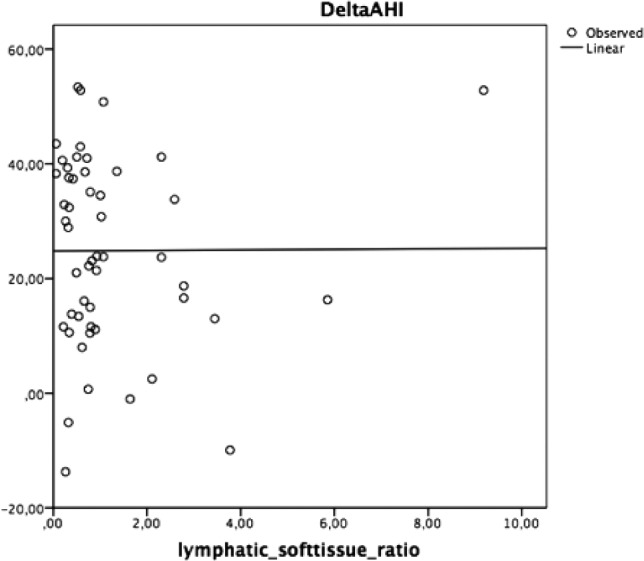
AHI = apnea-hypopnea index.
Figure 4. Univariate linear regression test correlating delta AHI to BOT total thickness.
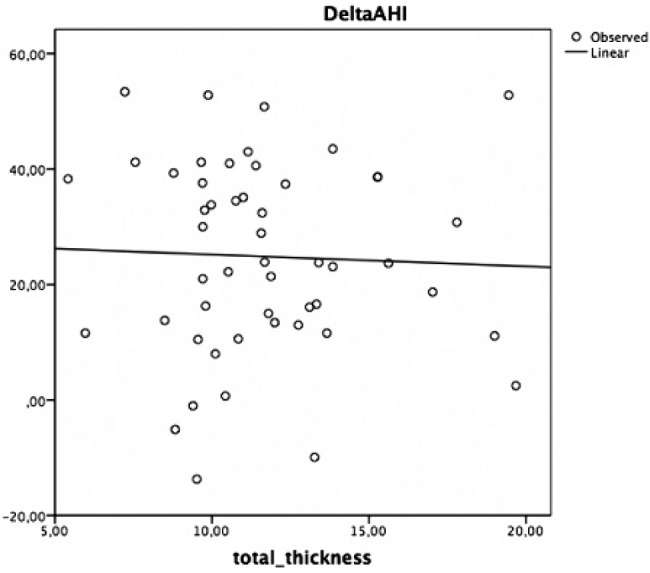
AHI = apnea-hypopnea index, BOT = base of tongue.
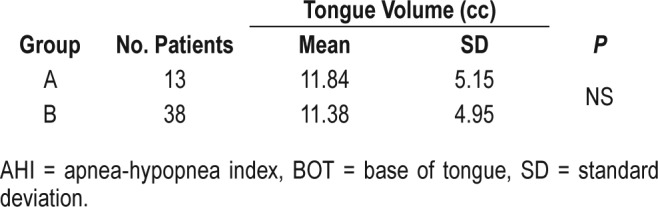
Moreover, no significant difference of excised BOT volume between group A and group B was found (Table 3).
Table 3.
Comparison of values of excised BOT volume between group A (postoperative AHI < 20 events/h) and group B (postoperative AHI > 20 events/h).
DISCUSSION
Several factors seem to influence the surgical outcome in patients undergoing TORS for OSA. In particular, patients presenting with BMI < 30 kg/m2, AHI < 60 events/h, Friedman stage II-III, and absence of lateral pharyngeal wall collapse generally benefit more from TORS than other phenotypes.16,26
However, few studies have focused their attention on the correlation between anatomic measures and surgical success. Chiffer et al. found that MRI-measured changes in the volume of the pharyngeal lateral walls correlated with changes in AHI.27
In the past decade, the use of drug-induced sleep endoscopy has allowed detection of BOT collapses in patients affected by OSA more precisely.28 Considering the importance of retrolingual collapse, the aim of our work was to evaluate the role of the BOT and its histologic composition in the prediction of success in patients treated with trans-oral robotic tongue reduction for OSA. In our series, a surgical success rate of 74.5% was seen, in line with other authors' experiences.14
Despite the retrospective nature of our study and the relatively small sample, taking into consideration that TORS was part of a multilevel surgical approach, and considering the limits of histological measures techniques, the following considerations can be made.
From our results, it seems that both the total volume of the resected BOT and its lymphatic/muscular percentages did not influence the clinical outcomes 1 year after surgery. Moreover, no significant difference of excised BOT volume between patients with successful outcomes and failures was found.
Furthermore, histologic examination of our samples did not highlight a macroscopic distribution of fat within the resected BOT. This evidence appears in contrast with other authors' experience that underlines the influence of tongue fat on the severity of OSA in obese patients.29
However, a recent study by Chen et al. demonstrated that tongue base thickness, measured by ultrasound monitoring during natural sleep, does not correlate with the severity of OSA.30 These findings reinforce the general opinion that OSA is not only influenced by anatomic factors but other causes may play a fundamental role in its genesis. For instance, low responsiveness of the upper airway dilator muscles during sleep,4,5 low respiratory arousal threshold,6–10 and the presence of high loop gain10–12 may influence the surgical outcome and therefore must be considered during patient selection process.
However, several surgical techniques can be performed on BOT by means of TORS. In this study, all patients underwent a BOT reduction method by Vicini et al.23 The resection of BOT performed with midline or other extended techniques may potentially lead to different surgical outcomes and for this reason further studies are warranted to validate our findings.15
Taking this into account and considering the heterogeneous patterns of OSA patients, a deeper understanding of the OSA pathogenesis is needed to tailor an individual treatment strategy that could lead to a more effective therapy.
CONCLUSIONS
Our results suggest that the volume of the resection and the histologic composition of the removed BOT do not influence the outcome of TORS in patients affected by OSA. A deeper understanding of the OSA pathogenesis is needed to tailor an individual treatment strategy that could lead to a more effective therapy.
DISCLOSURE STATEMENT
Work for this study was performed at the ENT unit of Ospedale Morgagni Pierantoni, Forlì, Italy. All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- BOT
base of the tongue
- CPAP
continuous positive airway pressure
- OSA
obstructive sleep apnea
- Pcrit
passive critical closing pressure of the upper airway
- TORS
trans-oral robotic surgery
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 3.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143(6):1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 4.Jordan AS, Wellman A, Heinzer RC, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62(10):861–867. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loewen AH, Ostrowski M, Laprairie J, Maturino F, Hanly PJ, Younes M. Response of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patients. Sleep. 2011;34(8):1061–1073. doi: 10.5665/SLEEP.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci. 2011;120(12):505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169(5):623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 8.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103(6):1929–1941. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 9.Longobardo GS, Evangelisti CJ, Cherniack NS. Analysis of the interplay between neurochemical control of respiration and upper airway mechanics producing upper airway obstruction during sleep in humans. Exp Physiol. 2008;93(2):271–287. doi: 10.1113/expphysiol.2007.039917. [DOI] [PubMed] [Google Scholar]
- 10.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105(5):1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 11.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163(5):1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 12.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(11):1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 14.Meccariello G, Cammaroto G, Montevecchi F, et al. Transoral robotic surgery (TORS) for the management of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2016;274(2):647–653. doi: 10.1007/s00405-016-4113-3. [DOI] [PubMed] [Google Scholar]
- 15.Cammaroto G, Montevecchi F, D'Agostino G, et al. Tongue reduction for OSAHS: TORSs vs coblations, technologies vs techniques, apples vs oranges. Eur Arch Otorhinolaryngol. 2016;274(2):637–645. doi: 10.1007/s00405-016-4112-4. [DOI] [PubMed] [Google Scholar]
- 16.Hoff PT, Glazer TA, Spector ME. Body mass index predicts success in patients undergoing transoral robotic surgery for obstructive sleep apnea. ORL J Otorhinolaryngol Relat Spec. 2014;76(5):266–272. doi: 10.1159/000368415. [DOI] [PubMed] [Google Scholar]
- 17.Shigeta Y, Ogawa T, Ando E, Clark GT, Enciso R. Influence of tongue/mandible volume ratio on oropharyngeal airway in Japanese male patients with obstructive sleep apnea. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(2):239–243. doi: 10.1016/j.tripleo.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welch KC, Foster GD, Ritter CT, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002;25(5):532–542. [PubMed] [Google Scholar]
- 19.Lowe AA, Gionhaku N, Takeuchi K, Fleetham JA. Three-dimensional CT reconstructions of tongue and airway in adult subjects with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1986;90(5):364–374. doi: 10.1016/0889-5406(86)90002-8. [DOI] [PubMed] [Google Scholar]
- 20.Chi L, Comyn FL, Mitra N, et al. Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. Eur Respir J. 2011;38(2):348–358. doi: 10.1183/09031936.00119210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168(5):522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 22.Ahn SH, Kim J, Min HJ, et al. Tongue volume influences lowest oxygen saturation but not apnea-hypopnea index in obstructive sleep apnea. PLoS One. 2015;10(8):e0135796. doi: 10.1371/journal.pone.0135796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicini C, Dallan I, Canzi P, Frassineti S, La Pietra MG, Montevecchi F. Transoral robotic tongue base resection in obstructive sleep apnoea hypopnoea syndrome: a preliminary report. ORL J Otorhinolaryngol Relat Spec. 2010;72(1):22–27. doi: 10.1159/000284352. [DOI] [PubMed] [Google Scholar]
- 24.Pang KP, Woodson BT. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007;137(1):110–114. doi: 10.1016/j.otohns.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 26.Lin HS, Rowley JA, Folbe AJ, Yoo GH, Badr MS, Chen W. Transoral robotic surgery for treatment of obstructive sleep apnea: factors predicting surgical response. Laryngoscope. 2015;125(4):1013–1020. doi: 10.1002/lary.24970. [DOI] [PubMed] [Google Scholar]
- 27.Chiffer RC, Schwab RJ, Keenan BT, Borek RC, Thaler ER. Volumetric MRI analysis pre- and post-transoral robotic surgery for obstructive sleep apnea. Laryngoscope. 2015;125(8):1988–1995. doi: 10.1002/lary.25270. [DOI] [PubMed] [Google Scholar]
- 28.De Vito A, Carrasco Llatas M, Vanni A, et al. European position paper on drug-induced sedation endoscopy (DISE) Sleep Breath. 2010;18(3):453–465. doi: 10.1007/s11325-014-0989-6. [DOI] [PubMed] [Google Scholar]
- 29.Turnbull CD, Wang SH, Manuel AR, et al. Relationships between MRI fat distributions and sleep apnea and obesity hypoventilation syndrome in very obese patients. Sleep Breath. 2017 Dec 2; doi: 10.1007/s11325-017-1599-x. . [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JW, Huang CC, Weng CK, Chang CH, Wang SJ. Simultaneous recording of ultrasound and polysomnography during natural sleep in patients with obstructive sleep apnea: a pilot study. J Sleep Res. 2017;26(4):481–486. doi: 10.1111/jsr.12515. [DOI] [PubMed] [Google Scholar]