Abstract
Study Objectives:
This study has as its primary objective to evaluate the quality and effectiveness of type II ambulatory polysomnography (Amb-PSG) versus type I attended laboratory polysomnography (Lab-PSG) in diagnosing obstructive sleep apnea (OSA). Its secondary objective is to evaluate the clinical efficacy, quality of life (QoL), and treatment adherence after diagnosis.
Methods:
An observational study of patients with OSA (n = 225) in whom diagnosis was made via Amb-PSG (n = 114) or Lab-PSG (n = 111). Patients' clinical data were retrospectively assessed (including general demographic and clinical data, Epworth Sleepiness Scale, blood pressure, indices from polysomnography, and treatment adherence. Cross-sectional assessment (patient questionnaire) was used to evaluate clinical efficacy indicators, comorbidities, current treatment, and QoL.
Results:
Polysomnography indices were comparable between Amb-PSG and Lab-PSG (apnea-hypopnea index: 38.9 ± 22.5 versus 35.8 ± 23.1 events/h; P > .05), except for an elevation of total sleep time (510 ± 54.7 versus 476.3 ± 79.4 minutes; P < .01) and loss of oximetry signal (9.8% versus 0.0%; P < .05). Based on polysomnography parameters, OSA was severe in 119 patients (52.9%), moderate in 88 (39.1%), and mild in 18 (8.0%). Diagnostic effect of Amb-PSG in clinical (body mass index, blood pressure, Epworth Sleepiness Scale) and treatment follow-up (CPAP adherence and QoL) indicators was comparable to that of Lab-PSG.
Conclusions:
Amb-PSG showed an OSA diagnostic capacity comparable to Lab-PSG. Secondary analyses (diagnostic quality, clinical efficacy, treatment compliance, QoL) underline the value of Amb-PSG as an emerging alternative to improve accessibility to care.
Citation:
Andrade L, Paiva T. Ambulatory versus laboratory polysomnography in obstructive sleep apnea: comparative assessment of quality, clinical efficacy, treatment compliance, and quality of life. J Clin Sleep Med. 2018;14(8):1323–1331.
Keywords: ambulatory polysomnography, CPAP adherence, laboratory polysomnography, obstructive sleep apnea, quality of life, sleep
BRIEF SUMMARY
Current Knowledge/Study Rationale: Ambulatory polysomnography (Amb-PSG) is often underestimated as a valuable alternative to laboratory polysomnography (Lab-PSG). This study aims to compare the quality and effectiveness of these two techniques in diagnosing obstructive sleep apnea.
Study Impact: Amb-PSG and Lab-PSG showed comparable diagnostic quality to Lab-PSG in diagnosing obstructive sleep apnea. Diagnostic effect of Amb-PSG in clinical (body mass index, blood pressure, Epworth Sleepiness Scale) and treatment follow-up (CPAP adherence and QoL) indicators was comparable to that of Lab-PSG. These data underline that Amb-PSG may have an important role in improving accessibility to testing.
INTRODUCTION
Obstructive sleep apnea (OSA) is increasingly prevalent in western populations,1–4 and is closely associated with several other conditions, such as hypertension, cardiovascular diseases, obesity, or diabetes mellitus.5–10 Still, despite its increasing effects, OSA is thought to remain undiagnosed in large proportions of patients.3
Attended laboratory polysomnography (Lab-PSG), or type I PSG, is considered the diagnostic gold standard for management of OSA.11 The capacity to perform Lab-PSG is below demand in most countries, contributing to long wait lists, high examination costs, and a high number of patients with suspected OSA without treatment.12
Unattended level III home assessments are commonly used to improve accessibility,13,14 but these have limitations inherent to the lack of sleep data.
Ambulatory polysomnography (Amb-PSG), or type II PSG, allows one to diagnose other sleep comorbidities with the advantage of characterizing sleep stages and measuring arousals, total sleep time (TST) and an accurate respiratory disturbance index (RDI).15 Because it is performed in an ambulatory setting, Amb-PSG requires more patient cooperation. Previous studies showed an increased number of errors in Amb-PSG compared to Lab-PSG16–19 that could be explained by the increased number of sensors and added complexity for the patient.
This study has the primary objective to evaluate the quality and effectiveness of Amb-PSG versus Lab-PSG in diagnosing OSA and the secondary objective to evaluate the clinical efficacy, quality of life (QoL), and treatment adherence after diagnosis.
METHODS
Study Design and Population
The study was conducted at a Lisbon Sleep Medicine Center (CENC), highly experienced in performing both Lab-PSG and Amb-PSG. The observational study was designed with: (1) a retrospective assessment to identify patients with OSA who underwent either Amb-PSG or Lab-PSG, and (2) a transversal cross-sectional assessment of patients to evaluate clinical indicators, comorbidities, current treatment, and QoL.
Patient inclusion criteria were to have a first consultation for sleep problems, a PSG with an RDI >5 events/h and follow-up consultations after treatment with noninvasive ventilation. Patient exclusion criteria were absence of clinical, demographic or treatment data.
From 2,158 PSG tests performed between 2007 and 2009, we found 914 (42.4%) with OSA (apnea-hypopnea index [AHI] > 5 events/h). From these, 420 (45.9%) were Lab-PSG and 494 (54.0%) were Amb-PSG. However, only 363 of these patients had complete medical records in the clinic, all the others were external referrals. In most cases these patients were referred to the sleep medicine center for PSG testing from other private practices. There are no indications that these patients present different levels of sleep study failure.
These 363 patients were invited to participate in the study, and an informed consent form and patient questionnaire were sent to them via postal mail with a prepaid return envelope. Patients were also contacted by email or telephone to ask for their participation. Technicians of ventilation treatment companies helped to collect continuous positive airway pressure (CPAP) readings to establish treatment adherence when existing readings were older than 12 months. The response rate was 62%. The final sample included 225 patients.
The study protocol, data collection forms, and informed consent form were approved by the Ethics Committee of Faculdade de Medicina de Lisboa, University of Lisbon, Portugal. The informed consent form constituted the first page of the documentation received by patients invited to participate in the study. Only patients who signed their informed consent form were enrolled in the study.
Assessments
Retrospective Component (PSG and Clinical Data)
Demographic, anthropometric, and clinical data were collected from patient files available at the center. Data collected included symptoms, Epworth Sleepiness Scale (ESS) scores, blood pressure (BP), comorbidities, ongoing therapy, and PSG results.
For patients enrolled in the study, Lab-PSG had been carried out using Alice 5 (Philips Respironics, Murrysville, Pennsylvania, United States) in 27.0% of cases, Domino (SOMNOmedics GmbH, Randersacker, Germany) in 19.5%, Embla 7000 (Embla Systems, Inc., Broomfield, Colorado, United States) in 26.4%, and Nicolet (Vyassis Healthcare, San Diego, California, United States) in 27.1%. Amb-PSG had been carried out using Domino in 87.4% of cases and Embla 7000 in 12.6%. The main criteria the sleep center used when assigning patients to Lab-PSG or Amb-PSG were: (1) clinical history suggestive of other sleep pathologies, (2) severity of cardiovascular disease or other comorbidities, and (3) patient preference, especially for patients living in other cities.
All PSG tests were carried out according to standardized techniques with digital data recording. Preparation of examinations and placement of electrodes lasted approximately 50 to 60 minutes. For Lab-PSG, the examinations took place under the vigilance of specialized technicians with the supervision of a physician, in a room with appropriate technical conditions. The study center is equipped with four bedrooms in which these examinations are performed.
Amb-PSG used the same sensors, with the same care and time taken by the technician to place the electrodes, which were placed at the end of the day in the clinic, with a few additional recommendations: not to sleep with electric blankets or over electric mattresses, and to avoid using mobile phones or microwaves at least 2 hours prior to recording. Time to go to bed and to get up was agreed upon by the technician and the patient. On the following morning, the patient returned the equipment, and a brief questionnaire informing the exact time the patient went to bed.
PSGs were staged according to the American Academy of Sleep Medicine 2007 recommendations, with automatic staging followed by manual staging in phases of 30 seconds, according to modified Rechtschaffen and Kales criteria, carried out by experienced technicians and analyzed by sleep specialists. Data were obtained from two central derivations (C4-A1, C3-A2), two occipital derivations (O2-A1, O1-A2), two derivations for the detection of ocular movement (ROC-A1, LOC-A2), one electromyogram (EMG) derivation, two electrocardiogram electrodes, a nasal pressure sensor, nasal thermistor, piezoelectric snore sensor, respiratory movement sensors with thoracic and abdominal piezoelectric bands, pulse oximetry, EMG of the right and left tibialis muscle, and body position.
Hypopnea was defined as a decrease in amplitude of the thermistor ≥ 30%, with desaturation ≥ 4% compared to the prior baseline value, over at least 90% of the duration of an event ≥ 10 seconds. OSA was classified as light (AHI 5.1–15 events/h), moderate (AHI 15.1–30 events/h), and severe (AHI > 30 events/h).20
Clinical data were retrieved from clinical records. Hypertension was assumed for patients with systolic BP > 135 mmHg and/or diastolic BP > 85 mmHg in clinical records and also patients treated with antihypertensive agents irrespective of recorded BP. Patients with body mass index (BMI) values between 25 and 30 kg/m2 were considered overweight, and patients with BMI values > 30 kg/m2 were considered obese. Previous BP and previous BMI were based on clinical records from the first clinical consultation, and BP and BMI after CPAP treatment based on the last clinical consultation if they had been measured in the previous 12 months. For registries older than 12 months, technicians of ventilation companies were asked to measure BP; if not possible, BP reported in the patient questionnaire was used.
CPAP prescription was made by a senior sleep specialist with the following protocol: the PSG results were shown and explained to each patient, specifically the presence of snoring and apnea, and their effect on O2 saturation, heart rate, and arousals; furthermore, the long-term risks of OSA were explained, namely cardiovascular and cerebrovascular risks, accident risks, cognitive, and sexual effects; patients had the CPAP machine on the same day of prescription or within a few days; a second visit would occur within 3 to 4 weeks in order to assess adaptation difficulties. Adherence to CPAP treatment was established based on clinical file registers. If the last results were older than 12 months, the companies providing home care ventilation were asked to read the memory card of the ventilation equipment. Good compliance with positive pressure therapy was defined as more than 4 h/night in more than 70% of days.21 A residual AHI < 5 events/h was considered a treatment efficacy indicator.22
Cross-Sectional Component (Patient Questionnaire)
Patient questionnaires included questions regarding: (1) PSG procedure (date, anthropometric information, type of PSG, quality of sleep on the night of the examination, preference on PSG location study and if they had to repeat it); (2) frequency of symptoms before PSG; (3) comorbidities (4) current treatments; (5) previous and current BP; (6) previous and current ESS13; and (7) Medical Outcomes Study 36-Item Short Form Health Survey (SF-36).23
For the purposes of this study the Portuguese validated versions of ESS (Santos et al.13) and SF-36 (Ferreira and Santana23) were used. The SF-36 survey is composed of 36 items, which evaluate 8 health domains: physical functioning, physical problems, emotional problems, social functioning, mental health, energy and vitality, pain, perception of general health. The score is coded and is transformed in a scale that varies between 0 (minimum) and 100 (maximum). The SF-36 QoL survey was used reliably in OSA.24
A score less than 10 in the ESS scale was considered within normal limits. Light excessive sleepiness was defined as scores between 10 and 12, moderate excessive sleepiness as scores between 13 and 17, and severe excessive sleepiness as scores between 18 and 24.25 For the purpose of analysis, previous ESS scores were retrieved for the clinical files.
Data Analysis and Statistical Methods
Data analysis was divided into four main areas: quality, clinical efficacy, treatment efficacy, and QoL. Quality indicators in PSG diagnosis included PSG indexes, percentage of studies with insufficient registered time (< 240 minutes),18,26 and percentage of studies with absence of sensors recording.18 Clinical efficacy indicators included subjective sleep quality and preferred PSG location,18,26 ESS,13 BMI, and prior and current BP.27 Treatment efficacy indicators included adherence to ventilation treatment (patients who discontinued treatment were not considered) and residual AHI.22,27 QoL was evaluated according to SF-36 results.23
Nominal variables were summarized using frequency distributions and proportions and quantitative variables by using means and standards deviation or medians and ranges. The t, Wilcoxon, Mann-Whitney U, or chi-square tests were used as appropriate; parametric approaches were preferred whenever suitable. Statistical analyses were performed using SPSS version 20.0 for Windows (SPSS Inc, Chicago, Illinois, United States), with significance at P < .05.
RESULTS
Study Population
A total of 225 patients participated in the study. Table 1 shows the demographic and clinical characteristics of the study population. A total of 114 patients had Amb-PSG and 111 Lab-PSG performed, at a median age of 55.9 ± 10.9 versus 57.6 ± 10.8, (P = .266). There was male predominance in both groups (82.5% Amb-PSG versus 59.5% Lab-PSG).
Table 1.
Demographic and clinical characteristics of the study population.
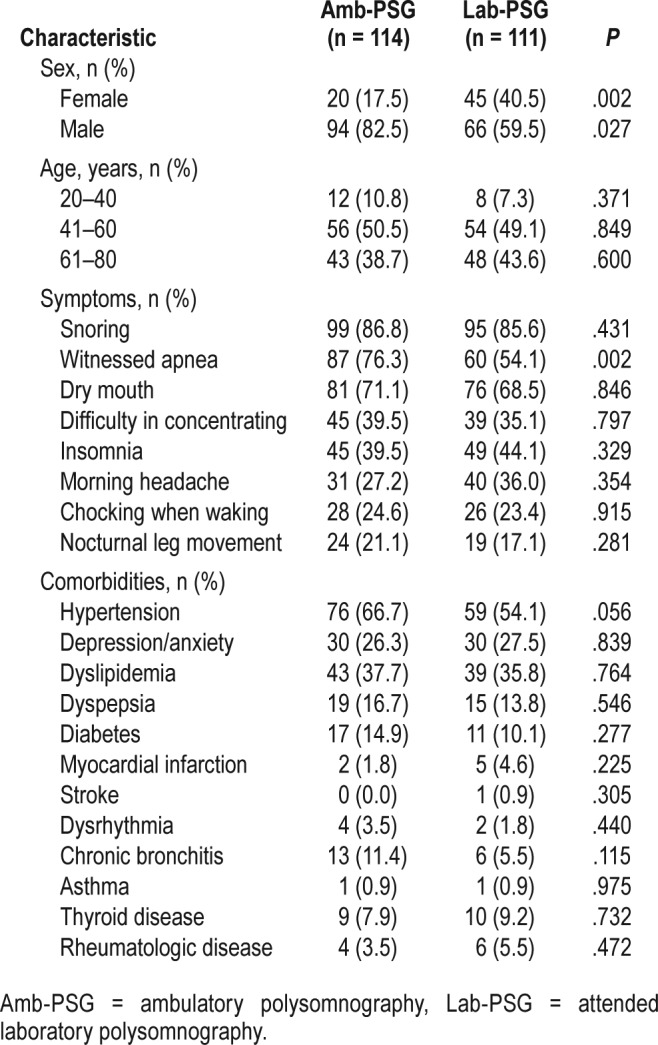
The symptoms that most frequently led patients to seek a medical consultation were snoring, witnessed apnea, dry mouth, and difficulty with concentration (Table 1). No signifi-cant differences in reported symptoms between the two groups were found, except for witnessed apnea (P = .002) in the Amb-PSG group. The most frequent comorbidities were hypertension, lipid metabolism changes, and depression/anxiety, with no significant differences between the two study groups.
ESS was used to characterize previous excessive daytime sleepiness, which was elevated in 138 out of 219 participants (63.0%) (67.3% of Amb-PSG versus 58.7% Lab-PSG; P = .190), with mild intensity in 36 (16.4%) participants (15.5% of Amb-PSG versus 17.6% of Lab-PSG), moderate intensity in 63 (28.8%) participants (30.0% of Amb-PSG versus 27.8% of Lab-PSG), and severe intensity in 39 (17.8%) participants (21.8% of Amb-PSG versus 13.9% of Lab-PSG). Excessive daytime sleepiness intensity was not significantly different between Amb-PSG and Lab-PSG groups (P = .375).
Most patients were overweight prior to PSG; 65 (31.6%) had BMI 25–30 kg/m2 and 107 (51.9%) had BMI > 30 kg/m2. Mean baseline BP was within the normal range, with mean systolic BP of 133.4 ± 16.3 mmHg and mean diastolic BP of 81.1 ± 10.8 mmHg), but 72.8% of participants had hypertension. There were no significant differences between groups in mean baseline BMI or BP.
Most participants (57.2%) lived in Lisbon (ie, close to the laboratory), with only 19.7% in the Amb-PSG group and 21.3% in the Lab-PSG group living more than 40 km from the laboratory (P = .484). Participants in the Lab-PSG group attended more medical consultations associated with the procedure, with an average of 1.80 ± 1.21 consultations compared to 1.46 ± 0.92 consultations in the Amb-PSG group (P = .02).
Primary Analysis: Diagnostic Quality
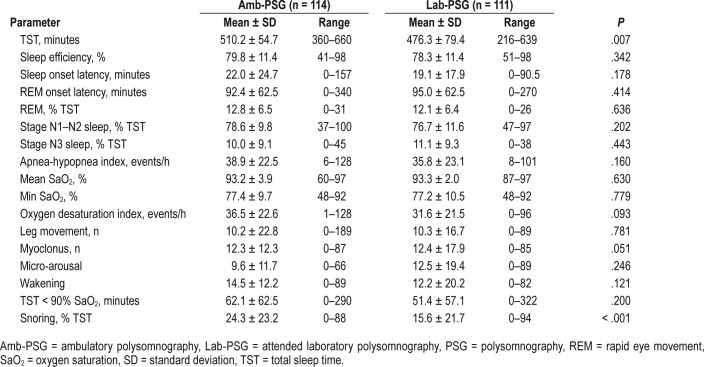
PSG indexes (Table 2) were comparable between groups, with two exceptions: TST was significantly longer for Amb-PSG (P = .007), and snoring (measured as percentage of total sleep time) significantly lower for the Lab-PSG group (P < .001).
Table 2.
PSG indexes in patients who underwent Amb-PSG versus Lab-PSG.
Based on PSG parameters, severe OSA was diagnosed in a total of 119 patients (52.9%), moderate OSA in 88 (39.1%), and 8 mild OSA in 18 (8.0%); patients who underwent Amb-PSG showed overall similar OSA severity distributions compared to Lab-PSG (P = .305). Interestingly, for both groups, mean AHI was > 30 events/h, which highlights disease severity in this population.
The type of PSG examination (ie, ambulatory versus laboratory) did not interfere with the diagnosis of other pathologies. As such, movement disorders were diagnosed in 19.6% of patients, insomnia in 2.7%, and arrhythmias in 4.9%, but there was no significant difference in the diagnosis of these conditions between the Amb-PSG group and the Lab-PSG group.
There was no need to repeat Amb-PSG because of insufficient recording time. We found a larger proportion of Amb-PSG patients with lack of nocturnal oximetry data (9.8% versus 0.0% in Lab-PSG; P = .010). No data loss was detected for other sensors. In any case, data losses did not prevent OSA diagnosis, although in some studies, the AHI was changed to apnea indexes.
Figure 1 presents the self-reported quality of sleep on the night of PSG and shows that the location of PSG (ambulatory versus laboratory) did not significantly influence self-reported quality of sleep (P = .763). When patients were questioned on the preferred location if there was a hypothetical need to repeat the examination,most would prefer to repeat the examination in the format that had been previously used. Interestingly, those who would prefer Amb-PSG also showed significantly increased sleep efficiency (P = .036).
Figure 1. Self-reported quality of sleep on PSG night and preferred location for hypothetically repeated PSG.
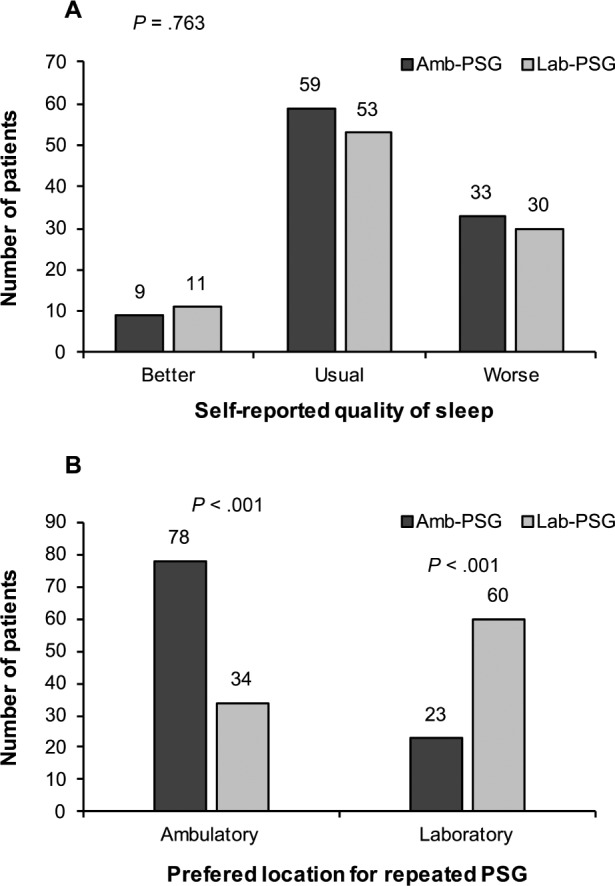
Amb-PSG = ambulatory polysomnography, Lab-PSG = attended laboratory polysomnography, PSG = polysomnography.
Secondary Analysis
Clinical Efficacy
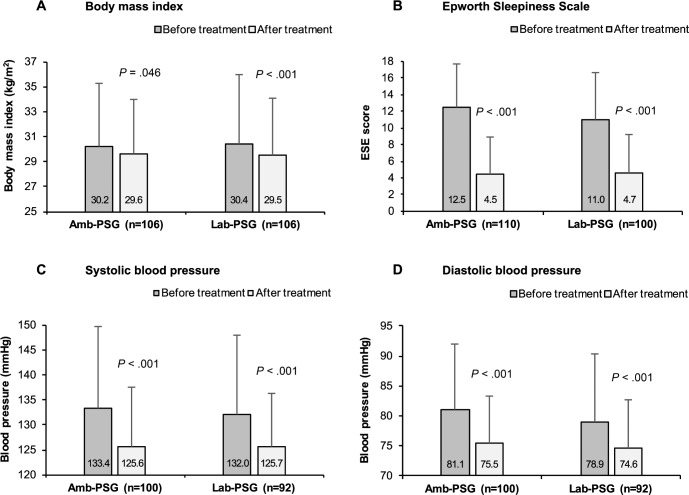
Both patient groups showed similar clinical efficacy results after OSA treatment with CPAP, with no significant differences in BMI (P = .756), mean systolic BP (P = .733), mean diastolic BP (P = .451), and mean ESS score (P = .764). Mean BMI was significantly decreased for 32.4% Amb-PSG (P = .046) and 44% Lab-PSG (P < .001) groups after treatment, although the magnitude of BMI changes was small, despite being statistically significant (Figure 2A).
Figure 2. Change in clinical variables before PSG and after OSA diagnosis and CPAP treatment.
Amb-PSG = ambulatory polysomnography, CPAP = continuous positive airway pressure, Lab-PSG = attended laboratory polysomnography, OSA = obstructive sleep apnea, PSG = polysomnography.
Daytime sleepiness significantly decreased in 120 patients after treatment; only 9.1% of patients (n = 9 Amb-PSG versus n = 9 Lab-PSG; P = .946) showed residual daytime sleepiness, despite treatment. Overall, ESS scores were reduced by a mean of 7.4 ± 6.1 points, with no significant difference between Amb-PSG and Lab-PSG (P = .111) (Figure 2B).
There was a substantial reduction in the overall prevalence of hypertension according to measured BP, from 45.8% to 29.7% (Figure 2C and Figure 2D). Amb-PSG and Lab-PSG groups showed comparable hypertension patterns (P = .721).
Treatment Compliance
It was feasible to evaluate compliance with CPAP treatment in 182 patients (80.8%); 31 patients (17%) abandoned CPAP (16 in Amb-PSG and 15 in Lab-PSG), and 12 patients were rapidly directed to surgery (11 otorhinolaryngology surgery and 1 thyroid surgery; 5 in Amb-PSG and 7 in Lab-PSG). The main equipment type was auto- CPAP, used by 62.1% patients (59 Amb-PSG and 54 Lab-PSG), (P = .701). The type of equipment was not associated with abandonment: 15 patients (8.2%) abandoned CPAP and 14 (7.7%) auto-CPAP treatment (P = .095).
Mean CPAP use was over 5 h/night (5.8 ± 1.4 hours in Amb-PSG versus 5.6 ± 1.3 hours in Lab-PSG, P = .884), in more than 70% of days (79% ± 25.4% in Amb-PSG versus 77.6% ± 25.3% in Lab-PSG, P = .193), with mean residual AHI < 5 events/h (3.6 ± 2.3 events/h in Am-PSG versus 4.1 ± 3.0 events/h in Lab-PSG, P = .995), which is considered within clinically efficient limits.21 Overall, 88.8% of patients used ventilation treatment for more than 4 hours in more than 70% of nights, with similar compliance between groups (P = .915).
CPAP compliance at approximately 1-year follow-up was independent of OSA severity. Duration of OSA treatment was also not associated with compliance, when comparing patients under CPAP treatment for less than 1 year to those under treatment for more than 1 year.
Quality of Life
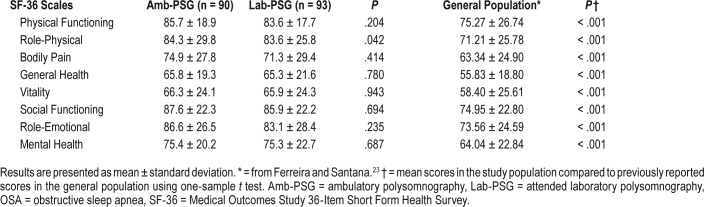
A total of 148 patients answered the QoL SF-36 survey, 70 in the Amb-PSG group (47.3%) and 78 in Lab-PSG group (70.2%). For the first question on the SF-36, 110 patients (74.3%) stated to have a better QoL compared with the previous year. These patients also showed significantly higher SF-36 global score (P = .001) than those reporting similar or worse QoL in the previous year. Among patients reporting better QoL, most (104, or 74.8%) had good adherence to CPAP. Actually, nonadherence to CPAP treatment was significantly associated with worsening QoL (odds ratio: 5.4, 95% confidence interval: 1.9–15.8, P = .002).
Table 3 shows mean scores for each specific scale of the SF-36 survey. There were no significant differences in mean scores among patients in the Amb-PSG and the Lab-PSG groups, except for an increase in the Role-Physical scale in the Amb-PSG group (P = .042). Importantly, SF-36 scores in this study population were significantly increased compared to that of the general Portuguese population23 as found by other authors.24
Table 3.
Mean SF-36 scores reported by patients after OSA diagnosis and noninvasive ventilation treatment.
The subpopulation of patients considered treatment compliant (as previously mentioned) showed significantly higher scores than noncompliant patients in Role-Emotional (P = .024), Role-Physical (P = .041), Mental Health (P = .024), Vitality (P = .039), and Social Functioning (P = .007) scales. However, when further stratifying for Amb-PSG and Lab-PSG groups, only compliant patients in Amb-PSG group showed significantly higher scores in Vitality (P = .046) and Mental Health (P = .004) scales; compliant patients in the Lab-PSG group showed no significantly different SF-36 scores compared to noncompliant counterparts.
This study included examinations that were carried out over a period of 3 years: 16.8% 1 year prior to patients' survey, 28.3% 2 years prior to patients' survey, and 54.8% 3 years prior to patients' survey. No dependencies were found between QoL in the first (subjective) question and the number of years of treatment (P = .772) nor with excessive daytime sleepiness (ESS score > 10) (P = .757).
DISCUSSION
We compared two groups of patients with clinical homogenous features to assess the overall effectiveness of PSG performed in ambulatory and laboratory settings. We found 98% prevalence of moderate and severe OSA. Both groups had comparable PSG characteristics and diagnostic capacities for other sleep pathologies, which is not the case for type III PSG.
Snoring (79.1%) and witnessed apnea (65.3%) prevalence in this study were comparable to the population studied by Masa et al.28 Nonetheless, snoring was more frequent than what was reported by Casale et al.29 which may be because our sample had already been screened and referenced by an assistant physician, as mentioned by Ramsey et al.30
Amb-PSG in this study maintained reliability and diagnostic precision, with a mean total sleep time (TST) over 240 minutes, in fact significantly higher in the Amb-PSG versus Lab-PSG groups. These findings are consistent with those of Iber et al.,26 and further contradict the earlier results of Fry et al.19 Longer total sleep time in an ambulatory setting could be due to the existence of a waking time in the laboratory setting,31 which would shorten sleep time, or be due to the existence of agreed sleeping time in Amb-PSG.
Subjective evaluation of quality of sleep on the night of the examination was considerably better in this study than in previous ones,15 with fewer patients reporting having slept worse than usual (50% previously versus 15% here).15 In previous studies most patients preferred to repeat the examination in a laboratory context,15,16,19 but in our sample most preferred to repeat the examination in the known format, which could indicate a higher degree of satisfaction with both procedures.
Obesity was highly prevalent in the study population (51.9%), which is in line with previous findings.32,33 After treatment, obese patients proportion was reduced, which can be explained by healthier lifestyles, either in response to physician advice or due to apnea and nocturnal desaturations corrections with CPAP treatment as suggested by other authors.10
Treatment compliance was within clinical efficient boundaries as stated by Park et al.21 or Torre-Bouscoulet et al.,27 with no significant difference between both groups.
This study has 98% of patients with moderate and severe OSA and 68.3% of symptomatic sleepy patients, with a mean reduction of 7.4 points in ESS with CPAP treatment, which is in accordance with the results of Masa et al.28 or Mulgrew et al.12 or Lewis et al.34 who found a mean reduction of 5.8 points.
McEvoy et al.35 found no benefit in cardiovascular outcomes with a median CPAP adherence of 3.3 ± 2.3 h/night. This multicenter randomized controlled trial selected patients with previous acute cardiovascular events and minimally symptomatic OSA (median ESS score of 7.3 ± 3.6), which contrasts with our population, where 68.3% had an ESS score > 10 and only 8 patients (3.5%) had such a major previous cardiovascular event. Although clinical guidelines indicate that adherence over 4 h/night is clinically efficient to control sleepiness, Weaver et al.36 found a positive association between ESS reduction and more CPAP nighttime adherence and also a greater improvement in visual memory tasks in patients who had an adherence over 6 h/night,37 suggesting better clinical outcomes with higher adherence rates. Also, Barbé et al.38 found that blood pressure is only significantly lowered with CPAP adherence over 5.6 h/night and McArdle et al.39 found an association of long-term adherence in sleepy snorers (ESS score > 10) with severe OSA.
In our study, both groups (Amb-PSG 5.8 ± 1.4 h/night and Lab-PSG 5.6 ± 1.3 h/night) showed higher time of CPAP use than McEvoy et al.35 (3.3 ± 2.3 h/night), Tsara et al.40 (4.8 ± 2.4 h/night), or Lewis et al.34 (5.2 ± 2.0 h/night) study populations, but closer to the population of Masa et al.28 (5.2 ± 2.0 h/night). The relevance of this OSA sleepy phenotype in CPAP adherence was also underlined by Saaresranta et al.41
SF-36 scores were significantly higher among treatment compliant patients and comparable between the Amb-PSG and Lab-PSG groups, which is consistent with previous findings.42 The increased SF-36 scores in comparison with those of the general population are in accordance with the results of Siccoli et al.24 QoL improvement in our sample may be related to weight loss,31 reduction of excessive daytime sleepiness,43 and good treatment compliance (88.8%) with the expected secondary improvement in cognitive capacities and control of cardiovascular risk factors. Because not all patients completed the survey, there might be a bias due to higher response rate among satisfied patients.
Few studies compared PSG conducted in ambulatory and laboratory settings. Some authors mention difficulties such as technicians' visits to patients' homes or patient travel to sleep clinics,15,16,19 quality control procedures or certification to ensure reliability, and a low failure rate with few repetitions. In previous comparative studies, considerable proportions of patients were excluded due to data loss, poor data quality, or insufficient registering times. Portier et al.15 had to exclude 20% of patients in the Amb-PSG group and 5% in the Lab-PSG group, whereas Gagnadoux et al.16 had to exclude 23.4% of patients in the Amb-PSG group and 11.2% in the Lab-PSG groups. In this study we had lower rates of data loss and there was no need to repeat PSG or to exclude patients. These success rates could be explained by the fact that technicians were highly experienced in the placement of electrodes sensors, using the same methodology in Amb-PSG and Lab-PSG. Patient education also has a central role, with patients being thoroughly informed about proper handling of equipment prior to and during the examination. In our experience, Amb-PSG only presents about two-thirds of the cost of Lab-PSG, which further emphasizes the potential of high-quality home studies, particularly in contexts of unmet needs and resource constrains.
This study had limitations mainly associated with its design. The study was based on a single sleep pathology center, using retrospective data complemented by patient questionnaires, with a potential for selection and recall bias. This center is used by patients from other centers as a diagnostic facility, in which case only basic identifying information is registered. This explains the lack of clinical and demographic data of 60% of OSA PSGs performed in the same period as the study. Although there are no indications that those patients present different levels of sleep study failure or different outcomes, given this context, we were not able to systematically validate these assumptions due to the retrospective nature of the study and, thus, the potential for selection bias should be considered. Nonetheless, the remaining patients with demographic and clinical data available had a fairly high response rate and most measures were comparable to previous studies, which in our view contributes to minimize the potential for selection bias.
In a study with these characteristics there is also a potential for selection bias based on the selected outcomes. Patients with better outcomes could be more prone to participate and, thus, affect the results toward better outcomes. Furthermore, in a study assessing the diagnostic quality of PSG methodologies it would be important to consider the effect of sleep position. Although data on positional apnea are systematically collected in the center, because of the severity of AHI and the increased weight, the number of patients with positional apnea was small in both groups, preventing statistical analysis. Thus, we were not able to consider these effects in the study. This study was conducted in a private certified sleep reference center, locally known to perform type I and II PSG, which may contribute to some of our population characteristics, with high prevalence of snorers and sleepy patients with median to high levels of education, that could partially explain the good compliance.
In conclusion, Amb-PSG showed an OSA diagnostic capacity comparable to Lab-PSG as assessed in our primary analysis. Technician training and patient education should be emphasized to improve reliability and effectiveness of Amb-PSG. Secondary analyses (diagnostic quality, clinical efficacy, treatment compliance, and QoL) underline the value of Amb-PSG as an emerging alternative to improve accessibility and to tackle unmet Lab-PSG needs.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank laboratory technicians Sofia Rebocho and Ana Viegas for scoring PSG tests, as well as the ventilation equipment technicians for CPAP compliance reports. The authors also thank Tiago Campos, MSc (ARC Publishing) for providing medical writing and editorial assistance.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- Amb-PSG
ambulatory polysomnography
- BMI
body mass index
- BP
blood pressure
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- Lab-PSG
attended laboratory polysomnography
- OSA
obstructive sleep apnea
- QoL
quality of life
- RDI
respiratory disturbance index
REFERENCES
- 1.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LRA. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 7.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology foundation scientific statement from the American Heart Association council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing. In collaboration with the National Heart, Lung, and Blood Institute national center on sleep disorders research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 8.Guardiano SA, Scott JA, Ware JC, Schechner SA. The long-term results of gastric bypass on indexes of sleep apnea. Chest. 2003;124(4):1615–1619. doi: 10.1378/chest.124.4.1615. [DOI] [PubMed] [Google Scholar]
- 9.Netzer NC, Hoegel JJ, Loube D, et al. Prevalence of symptoms and risk of sleep apnea in primary care. Chest. 2003;124(4):1406–1414. doi: 10.1378/chest.124.4.1406. [DOI] [PubMed] [Google Scholar]
- 10.Tuomilehto HP, Seppä JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179(4):320–327. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 11.Van de Water AT, Holmes A, Hurley DA. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography--a systematic review. J Sleep Res. 2011;20(1 Pt 2):183–200. doi: 10.1111/j.1365-2869.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 12.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146(3):157–166. doi: 10.7326/0003-4819-146-3-200702060-00004. [DOI] [PubMed] [Google Scholar]
- 13.Santos-Silva R, Sartori DE, Truksinas V, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32(5):629–636. doi: 10.1093/sleep/32.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 15.Portier F, Portmann A, Czernichow P, et al. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162(3 Pt 1):814–818. doi: 10.1164/ajrccm.162.3.9908002. [DOI] [PubMed] [Google Scholar]
- 16.Gagnadoux F, Pelletier-Fleury N, Philippe C, Rakotonanahary D, Fleury B. Home unattended vs hospital telemonitored polysomnography in suspected obstructive sleep apnea syndrome: a randomized crossover trial. Chest. 2002;121(3):753–758. doi: 10.1378/chest.121.3.753. [DOI] [PubMed] [Google Scholar]
- 17.Kapur VK, Rapoport DM, Sanders MH, et al. Rates of sensor loss in unattended home polysomnography: the influence of age, gender, obesity, and sleep-disordered breathing. Sleep. 2000;23(5):682–688. [PubMed] [Google Scholar]
- 18.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 19.Fry JM, DiPhillipo MA, Curran K, Goldberg R, Baran AS. Full polysomnography in the home. Sleep. 1998;21(6):635–642. doi: 10.1093/sleep/21.6.635. [DOI] [PubMed] [Google Scholar]
- 20.Cao MT, Guilleminault CK. Clinical features and evaluation of OSA and UARS. In: Kryger MH, Roth T, editors. Principles and Practice of Sleep Medicine. 5th ed. St Louis, MO: Elsevier, Saunders; 2011. [Google Scholar]
- 21.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86(6):549–554. doi: 10.4065/mcp.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira PL, Santana P. Percepção de estado de saúde e de qualidade de vida da população activa: contributo para a definição de normas portuguesas. Rev Port Saude Publica. 2003;21(2):15–30. [Google Scholar]
- 24.Siccoli MM, Pepperell JC, Kohler M, Craig SE, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2008;31(11):1551–1558. doi: 10.1093/sleep/31.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paiva T, Penzel T. Centro de Medicina do Sono: manual prático. 2011 Lidel -Edições Técnicas. [Google Scholar]
- 26.Iber C, Redline S, Kaplan Gilpin AM, et al. Polysomnography performed in the unattended home versus the attended laboratory setting--Sleep Heart Health Study methodology. Sleep. 2004;27(3):536–540. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 27.Torre-Bouscoulet L, Meza-Vargas MS, Castorena-Maldonado A, Reyes-Zúñeiga M, Pérez-Padilla R. Autoadjusting positive pressure trial in adults with sleep apnea assessed by a simplified diagnostic approach. J Clin Sleep Med. 2008;4(4):341–347. [PMC free article] [PubMed] [Google Scholar]
- 28.Masa JF, Jiménez A, Durán J, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004;170(11):1218–1224. doi: 10.1164/rccm.200312-1787OC. [DOI] [PubMed] [Google Scholar]
- 29.Casale M, Pappacena M, Rinaldi V, Bressi F, Baptista P, Salvinelli F. Obstructive sleep apnea syndrome: from phenotype to genetic basis. Curr Genomics. 2009;10(2):119–26. doi: 10.2174/138920209787846998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsey R, Hhanna A, Strhol K. History and Physical Examination. In: Kushida C, editor. Obstructive Sleep Apnea: Diagnosis and Treatment. 1st ed. New York, NY: Informa HealthCare USA, Inc; 2007. [Google Scholar]
- 31.Mokhlesi B, Punjabi NM. “REM-related” obstructive sleep apnea: an epiphenomenon or a clinically important entity? Sleep. 2012;35(1):5–7. doi: 10.5665/sleep.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez PP, Stefan B, Schulman CI, Byers PM. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008;74(9):834–838. [PubMed] [Google Scholar]
- 33.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154(15):1705–1711. [PubMed] [Google Scholar]
- 34.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27(1):134–138. doi: 10.1093/sleep/27.1.134. [DOI] [PubMed] [Google Scholar]
- 35.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 36.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181(7):718–726. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 39.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 40.Tsara V, Kaimakamis E, Serasli E, Katsarou Z, Christaki P. Health related quality of life in Greek patients with sleep apnea-hypopnea syndrome treated with continuous positive airway pressure. Sleep Med. 2009;10(2):217–225. doi: 10.1016/j.sleep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Saaresranta T, Hedner J, Bonsignore MR, et al. Clinical phenotypes and comorbidity in european sleep apnoea patients. PLoS One. 2016;11(10):e0163439. doi: 10.1371/journal.pone.0163439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sforza E, Janssens JP, Rochat T, Ibanez V. Determinants of altered quality of life in patients with sleep-related breathing disorders. Eur Respir J. 2003;21(4):682–687. doi: 10.1183/09031936.03.00087303. [DOI] [PubMed] [Google Scholar]