Abstract
There have been no published reports of central respiratory control abnormalities in pediatric patients with UNC80 or KCNJ11 mutations which cause neurologic channelopathies. We describe an 8-year-old male with a pathogenic UNC80 mutation, intellectual disability, hypotonia and epilepsy with severe central sleep apnea (213.5 events/h) on polysomnography (PSG). We also describe a 20-month-old female with a KCNJ11 mutation, neonatal diabetes and developmental delay who had severe central sleep apnea (131.1 events/h). Both patients had irregular respiratory patterns during sleep and wakefulness and were placed on empiric bilevel positive airway pressure therapy, which was well tolerated with resolution of abnormal respiratory control and hypercapnia. Patients with UNC80 and KCNJ11 gene mutations may have abnormal respiratory rhythm during sleep and wakefulness, mirroring animal models. We recommend routine PSG tests and further investigation into the respiratory control of patients with pediatric channelopathies involved in chemoreceptor function or central integration of respiratory control.
Citation:
Hong H, Kamerman-Kretzmer R, Kato R, Rosser T, VanHirtum-Das M, Davidson Ward SL. Case report of pediatric channelopathies with UNC80 and KCNJ11 mutations having abnormal respiratory control treated with positive airway pressure therapy. J Clin Sleep Med. 2018;14(8):1419–1425.
Keywords: channelopathy, pediatrics, central apnea, respiratory control, positive airway pressure
INTRODUCTION
Channelopathies are a heterogeneous group of diseases due to defects in ion channels, either hereditary or acquired, many of which can cause neurologic disease. We describe two interesting cases of pediatric patients with neurologic channelopathies and abnormal respiratory control; one patient with a UNC80 gene mutation affecting a sodium leak channel who had developmental delay, intractable seizures, and prolonged episodes of periodic breathing; the other patient with a KCNJ11 gene mutation, encoding an ATP-sensitive potassium channel who had neonatal diabetes, developmental delay and bradypnea with frequent apneic events. Both patients were noted to have severe respiratory control abnormalities during sleep and wakefulness that were successfully treated with bilevel positive airway pressure (BPAP) therapy and resulted in significant clinical improvements.
The UNC80 gene encodes a large protein highly expressed in the brain and cerebellum that is necessary for the appropriate function of a sodium leak channel, non-selective (NALCN).1 The NALCN sodium leak channel is a voltage-independent, non-selective, non-inactivating cation channel which contributes to the control of neuronal excitability.1,2 Abnormalities in NALCN have been described in the literature to cause a syndrome characterized by congenital contractures of the limbs and face, hypotonia, and developmental delay.3 Mutations in UNC80 specifically have been described to be associated with a syndrome of hypotonia, growth retardation, intellectual disability, and a severe congenital encephalopathy.4
The KCNJ11 gene encodes for four of the eight subunits in the ATP-sensitive potassium (K-ATP) channel that is expressed in muscle, neurons and the pancreatic beta-cell. Abnormalities in the K-ATP channel are a common cause of neonatal diabetes with some cases presenting in syndromic form with developmental delay, hypotonia and epilepsy.5
Abnormal respiratory patterns and sleep disorders have been described in other hereditary neurologic disorders6; however, there is a dearth of information in pediatric channelopathies. There have been no descriptions in the literature of central respiratory control abnormalities or use of positive airway pressure (PAP) therapies in children with UNC80 or KCNJ11 gene mutations and thus, we present novel findings in patients with these two mutations.
REPORT OF CASES
Case 1
An 8-year-old male with a pathogenic UNC80 mutation, intellectual disability, refractory epilepsy on ketogenic diet therapy, dysphagia with gastrostomy (G)-tube dependence, and a history of central sleep apnea on 1.25 L/min of supplemental oxygen by nasal cannula during sleep was admitted to the general pediatric inpatient service with “increased work of breathing” and possible aspiration pneumonia. At presentation, he was febrile to 39°C, tachypneic with a respiratory rate of 28 breaths/min, tachycardic (168 bpm), and hypoxemic, requiring supplemental oxygen by non-rebreather mask, though he was weaned to his baseline of 1.25 L/min nasal cannula within twelve hours of admission. He was well-nourished with a weight at approximately the 15th percentile for age and a body mass index at the 78th percentile for age. His chest radiograph demonstrated non-specific, bilateral opacities and a small left pleural effusion. His home medications included clonidine, divalproex sodium, leucovorin, levocarnitine and pyridoxine. It was noted that during his previous admission for initiation of a ketogenic diet, the patient's mother had noticed “pauses” in his breathing during sleep associated with desaturations. This was consistent during the current admission with his inpatient team noting adequate baseline oxygenation with intermittent desaturations to an SpO2 of approximately 80% while the patient was monitored on continuous pulse oximetry. Upon initial consultation by our pediatric pulmonology and sleep service, he was noted to have mild tachypnea with an irregular respiratory pattern observed during sleep and wakefulness. Capillary blood gases (CBG) revealed a respiratory acidosis with compensatory metabolic alkalosis with a PCO2 of 55–56 mmHg (both upon overnight and early morning samples) and a serum bicarbonate of 34 mEq/L.
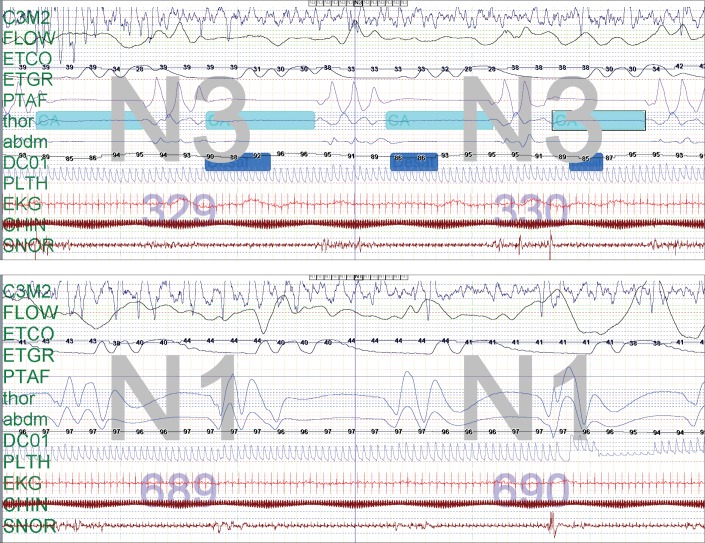
Further review of the chart revealed that he had a polysomnography (PSG) ordered by his primary care practitioner 5 years prior to presentation with a severely elevated central apnea index of 117.8 events/h. His obstructive apnea-hypopnea index was 1.5 events/h, and he was noted to have abnormal sleep architecture with no time in rapid eye movement (REM) sleep, with an increased arousal index. This study was completed prior to initiation of the ketogenic diet. An oxygen titration had been done during the night of the study up to 1.25 L/ min, which lessened the severity of the desaturations associated with these central apneas but did not affect the frequency of respiratory events (Figure 1). He was recommended at that time to be directly admitted into the hospital for initiation of positive airway pressure (PAP) therapy. However, his mother declined admission, and the patient continued to follow-up with the referring provider who initiated oxygen therapy during sleep. Of note, magnetic resonance imaging (MRI) of the brain at 24 months of age had been interpreted as normal; the patient was never able to cooperate with pulmonary function testing due to intellectual disabilities.
Figure 1. Case 1: 2 epochs from the first PSG at 3 years of age showing abnormal respiratory pattern control before and after supplemental oxygen.
Two epochs (60 seconds total) from the first PSG at 3 years of age showing abnormal respiratory pattern control before (top) and after supplemental oxygen at 1 L/min (bottom). Note that the central apneas are still present but desaturations are less severe with the supplemental oxygen administration. Only one electroencephalography lead tracing is presented in this figure as the quality of the other lead tracings were significantly limited by artifact. PetCO2 tracing may underestimate the true CO2 value. Transcutaneous CO2 monitoring was not used. PetCO2 = end-tidal carbon dioxide pressure, PSG = polysomnography.
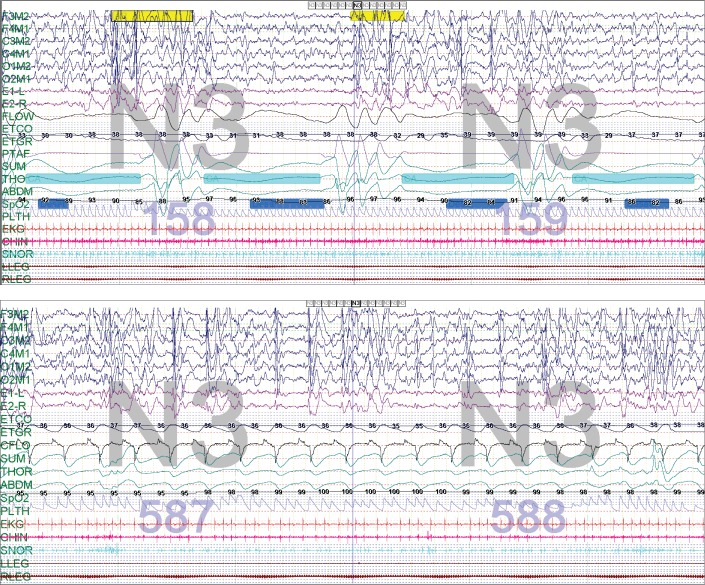
After the inpatient consultation, he was initiated on BPAP therapy as an inpatient. He was started on empiric pressures with a backup rate appropriate for his age. He tolerated the therapy well, and his PCO2 rapidly corrected to 44 mmHg. He had a follow-up PSG shortly after discharge, which included a BPAP titration portion. The baseline portion revealed persistent central apnea similar to his PSG 5 years prior with a central apnea index of 213.5 events/h. The obstructive apneahypopnea index was 1.5 events/h. The sleep architecture was abnormal with no REM sleep; however, there was an increase in stage N3 stage with an elevated arousal index. BPAP therapy was initiated with dramatic improvement, and it was titrated to achieve successful resolution of central apnea and desaturations (Figure 2). Recommended BPAP settings from his study were spontaneous/timed mode, inspiratory positive airway pressure (IPAP) 16 cmH2O, expiratory positive airway pressure (EPAP) 6 cmH2O, with a backup rate of 20 breaths/min and 1 L/min supplemental oxygen through the circuit (a small nasal mask was used.) Given the patient's age and ability to generate some spontaneous breaths, spontaneous/timed mode was used in an effort to promote patient-PAP synchrony. Upon outpatient follow-up 2.5 months after admission, his mother reported clinical improvement with increased alertness and a more regular breathing pattern during wakefulness. Serum bicarbonate values normalized to 24 mEq/L by one week following discharge and remained in the 24–27 mEq/L range at 3-month and 4-month follow-up. The family reported good adherence to PAP therapy, and this was confirmed with BPAP machine usage data.
Figure 2. Case 1: 2 epochs from the second PSG at 8 years of age showing abnormal respiratory pattern control before and after initiation of BPAP therapy.
Two epochs (60 seconds total) from second PSG at 8 years of age showing abnormal respiratory pattern control before (top) and after initiation of BPAP therapy (bottom) at IPAP 15 cmH2O, EPAP 5 cmH2O, 20 breaths/min, with 1 L/min oxygen. Note that the regular breathing pattern and rate of 20 breaths/min demonstrate that the breaths are BPAP initiated. BPAP = bilevel positive airway pressure, IPAP = inspiratory positive airway pressure, PSG = polysomnography.
Case 2
A 20-month-old female with a known KCNJ11 mutation, neonatal diabetes on an insulin pump (hemoglobin A1c ranged 7.1% to 8.3%) and developmental delay was admitted to the hospital for emesis and hypoglycemia presumed to be from a viral illness. At admission, her parent had not reported any respiratory concerns or abnormalities. However, on hospital day 1, her pediatrics team had noted episodic bradypnea (to a nadir of 9 breaths/min) with associated desaturations, which would self-resolve to a normal respiratory rate for age, observed during sleep and especially during wakefulness. On hospital day 3, she had a significant observed apnea with perioral cyanosis and desaturation to 62% on pulse oximetry. She was stimulated and given supplemental oxygen temporarily for resolution of the event, but it was noted that she had snoring, hypotonia, and intermittent desaturations throughout her hospitalization. While the initial apneas were during sleep, her breathing pattern was also episodically irregular during wakefulness. On hospital day 4, she had a recurrent apnea lasting 10–30 seconds with perioral cyanosis and was transferred to the pediatric intensive care unit for closer monitoring. Her chest x-ray was reported as normal. Her initial CBG had a normal pH of 7.41, PCO2 of 46 mmHg, and serum bicarbonate of 27 mEq/L. She was noted to have bradypnea to 7–8 breaths/min, and she was intubated on hospital day 6 for persistent central hypoventilation and apnea. She had continuous electroencephalography monitoring which was consistent with a mild diffuse encephalopathy but showed no epileptiform abnormalities. She also had a normal brain MRI. She was extubated 4 days later but was still noted to have apnea with a respiratory rate of 7–10 breaths/min and associated bradycardia to 60 bpm. She was started on a trial of caffeine with no improvement in respiratory rate, though her heart rate improved. She had a normal echocardiogram, and the patient never had a documented cardiac arrhythmia.
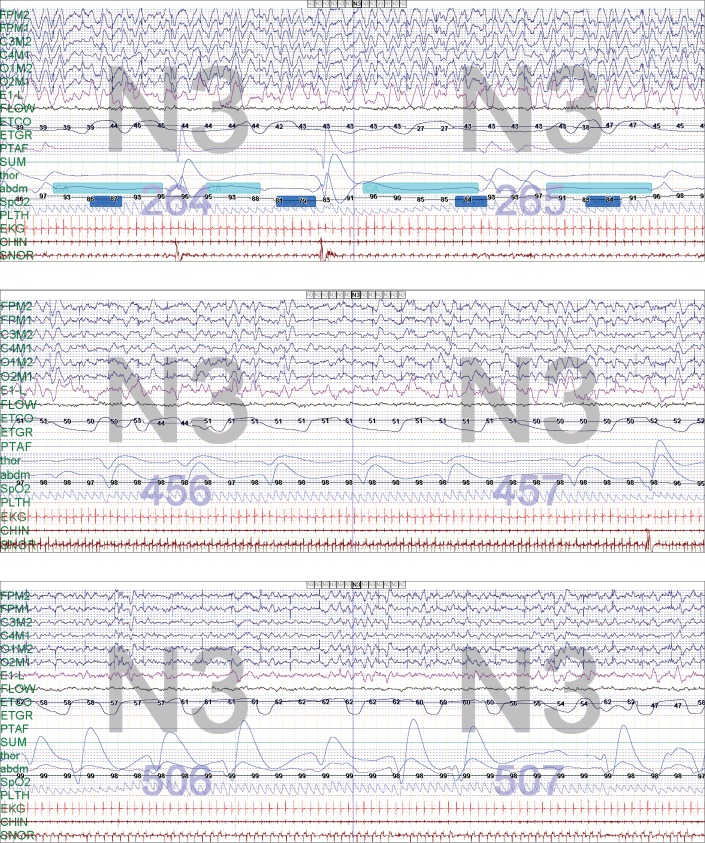
She had an inpatient overnight PSG done (total sleep time 292 minutes, sleep efficiency 98%, REM sleep was not achieved), which revealed an elevated central sleep apnea index of 131.1 events/h with mild obstructive sleep apnea (apnea-hypopnea index 3.1 events/h), prolonged persistent periodic breathing and bradypnea of 6–12 breaths/min. She had an oxygen titration during the night due to frequent desaturations (lowest 74%); however, with the addition of supplemental oxygen, hypoventilation ensued with PetCO2 recordings ranging from 50–60 mmHg (Figure 3). There were no arrhythmias captured on the electrocardiogram lead of her PSG.
Figure 3. Case 2: 2 epochs demonstrating the patient at 20 months of age, baseline on room air and on supplemental oxygen.
Two epochs (60 seconds total) demonstrating the patient at 20 months of age, baseline on room air (top) and then on 0.5 L/min supplemental oxygen (middle). Note that the central apneas are still present but the desaturations are not severe, and events are unable to be scored. There was mild hypoventilation with the addition of oxygen at 0.5 L/min, and with increasing oxygen to 0.75 L/min the patient developed worsening hypoventilation with PetCO2 ranging 50–60 mmHg (bottom). PetCO2 = end-tidal carbon dioxide pressure.
Because of the results of her PSG, this patient was started on BPAP therapy, used during sleep only, at settings of: timed mode, IPAP 8 cmH2O, EPAP 4 cmH2O, back up rate of 20 breaths/min, I-time 0.8 seconds and room air (a small nasal interface was used). Given the patient's young age, small size, and abnormal respiratory pattern, timed mode was most appropriate as she was less likely to effectively trigger spontaneous breaths. Her CBG on these settings was: pH of 7.46, PCO2 41 mmHg, and bicarbonate of 29 mEq/L. She was noted to tolerate the BPAP well with improvement in her respiratory status and was discharged. She followed-up in pulmonary clinic one month after discharge with continued good tolerance of the BPAP therapy and was noted to have a respiratory rate appropriate for age during the visit when awake. She was ordered to have a formal BPAP titration PSG; however, she died at home (details unknown) prior to any further follow-up care at our institution.
DISCUSSION
The understanding of respiratory control mechanisms and the control of breathing during sleep has advanced in the last century. The neural control of breathing during sleep is mediated by medullary respiratory neurons, which include the dorsal respiratory groups located in the dorsomedial medulla in the ventrolateral nucleus of the solitary tract and the ventral respiratory group, including the nucleus ambiguous, expiratory and inspiratory neurons.7 The center for respiratory rhythmic activity or rhythmogenesis has been identified as the pre-Bötzinger complex (preBötC) and the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG), a cluster of neurons in the brain stem region that generate normal respiratory patterns.8–10
NALCN is found in the RTN, and in mouse knock out models there was in vivo attenuation of CO2-induced activation of RTN neurons, blunting respiratory responses to CO2.11 UNC80, the gene affected in our patient, codes for a protein that forms a complex with NALCN and is required for control of NALCN. This may explain the similarities between the respiratory phenotype produced by pathogenic mutations in UNC80 and the syndromes associated with NALCN mutations.1 Mutations in NALCN in mouse models have demonstrated that the NALCN is an essential ion channel, and mutant mouse pups have severely disrupted respiratory rhythm with a breathing pattern characterized by 5 seconds of apnea followed by 5 seconds of breathing.2,12 UNC80 and the associated NALCN gene mutations have been described phenotypically to include intellectual disability, hypotonia, and failure to thrive.1 Stray-Pedersen et al. described 4 individuals with UNC80 mutations with a similar phenotype, and two patients reported sleep disturbances, with one individual having obstructive sleep apnea.13 However, there have been no clinical cases reporting the abnormal respiratory pattern of pediatric patients with UNC80 mutations.
Our patient with a UNC80 gene mutation also had an abnormal respiratory rhythm with frequent central apnea both while asleep and awake. We speculate that periodic breathing during wakefulness was a manifestation of the degree of severity of his abnormal respiratory control. During the entire monitored duration of the PSG, he had repetitive episodes of periodic breathing, even during wakefulness. He had a quick response to BPAP therapy, requiring only low pressures and benefiting from the back up rate to treat his centrally mediated apnea. There was concern that, due to his developmental delay, he would be unable to tolerate mask therapy. However, he adapted well with successful resolution of central apnea.
KCNJ11 encodes for subunits of a K-ATP channel that is expressed in the neurons of the pFRG.14 In mouse models, inserting K-ATP channel activators into the pFRG region while measuring the discharges of the hypoglossal rootlets resulted in prolonged burst duration, prolonged burst interval, and decreased burst frequency, indicating that the K-ATP channels in the pFRG neurons may be involved in central regulation of the respiratory rhythm.15 Mutations in KCNJ11 are a common cause of neonatal diabetes, and many of these patients can have neurologic dysfunction including developmental delay, hypotonia and epilepsy.16,17 In a survey study of 30 subjects with KCNJ11-related neonatal diabetes mellitus and 25 unaffected sibling controls, Landmeier et al. found that 47% of these patients had sleep disturbances, and there was a significant difference compared to their sibling controls.18 Our patient with a KCNJ11 mutation and neonatal diabetes mellitus had frequent apnea while asleep and awake, which we speculate to be the clinical manifestation of abnormal central respiratory control mediated through the pFRG neurons. This patient also had a positive response to BPAP therapy, requiring only low pressures and a physiologic back up rate to treat her centrally mediated apnea with resolution of desaturations.
While it is possible that other disorders (such as PHOX2B mutations causing congenital central hypoventilation syndrome [CCHS]) could account for abnormal control of ventilation, patients with CCHS typically present with respiratory failure during sleep from their first day of life (and most, though not all, have relatively normal ventilation during wakefulness). Additionally, the ventilatory pattern is usually characterized by a diminutive tidal volume rather than periodic breathing and central apnea. Neither of our patients' abnormalities in oxygenation and ventilation could be entirely explained by any other lung pathology given their relatively unremarkable pulmonary history. While seizures could explain occasional apneic episodes, they do not explain the persistent central apneas and periodic breathing on PSG without concurrent electroencephalographic evidence of seizures on PSG.
CONCLUSIONS
In pediatric patients with pathogenic UNC80 or KCNJ11 gene mutations, sleep-disordered breathing may manifest as a disrupted respiratory rhythm with severe central apnea, periodic breathing and hypercapnia, progressing to abnormal respiratory rhythms even during wakefulness. These patients can be supported with BPAP therapy to treat their bradypnea, hypoxemia and hypercapnia. As genetic testing has become more available, further investigation into the respiratory control of patients with neurologic channelopathies is warranted, and we recommend a low threshold for evaluating the respiratory pattern and gas exchange of patients in this group.
DISCLOSURE STATEMENT
The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Thomas Keens for providing pulmonary care and contributing clinical information for one of the presented cases.
Author contributions: Dr. Hong conceptualized and drafted the initial manuscript and approved the final manuscript as submitted; Dr. Kamerman-Kretzmer conceptualized and drafted the initial manuscript and approved the final manuscript as submitted; Dr. Kato reviewed the manuscript and approved the final manuscript as submitted; Dr. Rosser reviewed and revised the manuscript and approved the final manuscript as submitted; Dr. VanHirtum-Das reviewed and revised the manuscript and approved the final manuscript as submitted; Dr. Davidson Ward conceptualized and reviewed and revised the manuscript, and approved the final manuscript as submitted.
ABBREVIATIONS
- BPAP
bilevel positive airway pressure
- CBG
capillary blood gas
- CO2
carbon dioxide
- EPAP
expiratory positive airway pressure
- IPAP
inspiratory positive airway pressure
- K-ATP
ATP-sensitive potassium channel
- mmHg
millimeters of mercury
- NALCN
sodium (Na+) leak channel, non-selective
- PAP
positive airway pressure
- PCO2
partial pressure of carbon dioxide
- PetCO2
end-tidal carbon dioxide pressure
- pFRG
parafacial respiratory group
- preBötC
pre-Bötzinger complex
- PSG
polysomnography
- REM
rapid eye movement
- RTN
retrotrapezoid nucleus
REFERENCES:
- 1.Perez Y, Kadir R, Volodarsky M, et al. UNC80 mutation causes a syndrome of hypotonia, severe intellectual disability, dyskinesia and dysmorphism, similar to that caused by mutations in its interacting cation channel NALCN. J Med Genet. 2016;53(6):397–402. doi: 10.1136/jmedgenet-2015-103352. [DOI] [PubMed] [Google Scholar]
- 2.Lu B, Su Y, Das S, Liu J, Xia J, Ren D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129(2):371–383. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Chong JX, McMillin MJ, Shively KM, et al. De novo mutations in NALCN cause a syndrome characterized by congenital contractures of the limbs and face, hypotonia, and developmental delay. Am J Hum Genet. 2015;96(3):462–473. doi: 10.1016/j.ajhg.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shamseldin HE, Faqeih E, Alasmari A, Zaki MS, Gleeson JG, Alkuraya FS. Mutations in UNC80, encoding part of the UNC79-UNC80-NALCN channel complex, cause autosomal-recessive severe infantile encephalopathy. Am J Hum Genet. 2016;98(1):210–215. doi: 10.1016/j.ajhg.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350(18):1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 6.Gadoth N, Oksenberg A. Sleep and sleep disorders in rare hereditary diseases: a reminder for the pediatrician, pediatric and adult neurologist, general practitioner, and sleep specialist. Front Neurol. 2014;5:133. doi: 10.3389/fneur.2014.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orem J, Kubin L. Respiratory Physiology: Central Neural Control. Philadelphia, PA: Saunders; 2000. [Google Scholar]
- 8.Wilmott R, Boat T, Bush A, Chernick V, Deterding R, Ratjen F. Kendig and Chernick's Disorders of the Respiratory Tract in Children. 8th ed. Philadelphia, PA: Elsevier; 2012. [Google Scholar]
- 9.Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol. 2010;173(3):244–255. doi: 10.1016/j.resp.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Abe C, Holloway BB, et al. NALCN is a “leak” sodium channel that regulates excitability of brainstem chemosensory neurons and breathing. J Neurosci. 2016;36(31):8174–8187. doi: 10.1523/JNEUROSCI.1096-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren D. Sodium leak channels in neuronal excitability and rhythmic behaviors. Neuron. 2011;72(6):899–911. doi: 10.1016/j.neuron.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stray-Pedersen A, Cobben JM, Prescott TE, et al. Biallelic mutations in UNC80 cause persistent hypotonia, encephalopathy, growth retardation, and severe intellectual disability. Am J Hum Genet. 2016;98(1):202–209. doi: 10.1016/j.ajhg.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onimaru H, Ikeda K, Kawakami K. Phox2b, RTN/pFRG neurons and respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168(1-2):13–18. doi: 10.1016/j.resp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Zhang J, Ding Y, et al. K(ATP) channels of parafacial respiratory group (pFRG) neurons are involved in H2S-mediated central inhibition of respiratory rhythm in medullary slices of neonatal rats. Brain Res. 2013;1527:141–148. doi: 10.1016/j.brainres.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto Y, Dateki S, Hirose M, et al. Molecular and clinical features of KATP -channel neonatal diabetes mellitus in Japan. Pediatr Diabetes. 2017;18(7):532–539. doi: 10.1111/pedi.12447. [DOI] [PubMed] [Google Scholar]
- 17.Gloyn AL, Diatloff-Zito C, Edghill EL, et al. KCNJ11 activating mutations are associated with developmental delay, epilepsy and neonatal diabetes syndrome and other neurological features. Eur J Hum Genet. 2006;14(7):824–830. doi: 10.1038/sj.ejhg.5201629. [DOI] [PubMed] [Google Scholar]
- 18.Landmeier KA, Lanning M, Carmody D, Greeley SA, Msall ME. ADHD, learning difficulties and sleep disturbances associated with KCNJ11-related neonatal diabetes. Pediatr Diabetes. 2017;18(7):518–523. doi: 10.1111/pedi.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]