Abstract
Study Objectives:
To examine the presence of a first-night effect (FNE) and the level of internight variability in sleep bruxism (SB) activity when a self-applicable electrode set is used in home polysomnography (PSG) in a sample of subjects with possible SB.
Methods:
Fourteen females and two males aged 38.3 ± 9.1 years (mean ± standard deviation) with self-reported SB underwent home-PSG on three consecutive nights. The subjects applied PSG sensors themselves, including self-applicable electrode sets used to record sleep and masseter muscle activity. Repeated-measures analysis of variance was used to compare SB and sleep variables between the nights.
Results:
Surprisingly, there were statistically significant elevations in the rhythmic masticatory muscle activity (RMMA) episode index (P = .009), burst index (P = .016), and bruxism time index (P = .049) throughout the course of 3 nights. More bruxers were diagnosed on the second (6 bruxers, ≥ 2 episodes/h) and third night (7 bruxers) compared to the first night (2 bruxers). Most subjects (14/16) had their highest RMMA index on the second or third night. The mean coefficient of variation for RMMA episode index was 50.7%. No statistically significant differences were detected in other sleep variables.
Conclusions:
The results indicate that a FNE may be present in SB activity, possibly lasting several nights in some subjects. Furthermore, FNE appears to be combined with high internight variability of SB activity without indications of internight changes in sleep macrostructure. To confirm the level of ongoing SB activity, several nights of PSG may be required, especially in subjects with low first-night SB activity.
Commentary:
A commentary on this article appears in this issue on page 1281.
Citation:
Miettinen T, Myllymaa K, Hukkanen T, Töyräs J, Sipilä K, Myllymaa S. Home polysomnography reveals a first-night effect in patients with low sleep bruxism activity. J Clin Sleep Med. 2018;14(8):1377–1386.
Keywords: masticatory muscle, electromyography, electroencephalography, sleep disorder
BRIEF SUMMARY
Current Knowledge/Study Rationale: There are rather few reports assessing a first-night effect and internight variability in the activity of sleep bruxism (SB); this information is important because it would help to answer the question of how many nights of polysomnography (PSG) need to be recorded in order to reliably determine the level of ongoing SB activity.
Study Impact: The results of this study suggest that SB activity may have to be recorded for more than 1 night if one wishes to reliably diagnose patients in both clinical and research settings. In addition to influencing the methods for diagnosis in individual patients, this knowledge also has research implications (eg, in situations where the epidemiology of SB, the reliability of questionnaires or new treatment methods are being tested against PSG-quantified SB activity levels).
INTRODUCTION
Sleep bruxism (SB) is a sleep-related movement disorder, but the level of ongoing activity is generally considered challenging to quantify objectively and reliably.1 This involuntary sleep-time activity is conventionally associated with unwanted consequences as tooth wear, orofacial pain, headaches, temporomandibular disorders, and disturbances in the quality of the bed partner's sleep.2 The most recent exact definition of SB is “a repetitive jaw-muscle activity characterized by clenching or grinding of the teeth and/or by bracing or thrusting of the mandible” during sleep.3 The exact cause for SB is currently unknown. SB may be connected to sleep-disordered breathing in some patients when SB episodes follow a cortical arousal that has terminated an airway obstruction event. SB has been hypothesized to be an autonomic solution to keep airways open whenever breathing is compromised during the night; however, evidence for this proposal is lacking.4,5 Lobbezoo et al. listed three levels of certainty related to the SB diagnosis, based on the methods that have been utilized. A possible SB diagnosis is based solely on patient self-reports and/or questionnaires.3 A probable SB diagnosis includes also a clinical examination by a dentist.3 The definite assessment of SB diagnosis requires additional polysomnography (PSG) recordings in conjunction with application of all the aforementioned methods.3
In order to quantify SB activity in PSG, masseter and/or temporal electromyography (EMG) is recorded to assess the number of episodes and bursts of rhythmic masticatory muscle activity (RMMA) exhibiting the characteristic EMG patterns of SB.1 RMMA episodes can be divided to three distinct subtypes1: (1) Phasic RMMA episodes consisting of at least 3 EMG bursts lasting 0.25–2.00 seconds; (2) tonic RMMA episodes, which correspond to a single EMG burst that lasts over 2 seconds; and (3) mixed RMMA episodes that display the characteristics of both phasic and tonic RMMA. Phasic RMMA translates into grinding activity and tonic RMMA into clenching. Conventionally, RMMA episodes during sleep are estimated with respect to the total sleep time (TST) in order to form the RMMA episode index (EI), which is generally used to describe the ongoing activity of SB.1 Other similarly proportional parameters for SB activity are the burst index and the bruxism time index.1
PSG in a sleep laboratory is an accurate but unfortunately very expensive method for assessing SB activity in addition to being inconvenient and stressful to the patients. The availability of sleep laboratory resources is restricted and usually allocated for the diagnostics and research into sleep disorders with the most severe health consequences.6 Furthermore, because of the complexity and high costs of PSG, often only 1 night is assessed prior to making diagnostic decisions.7 Home-PSG (H-PSG) can be conducted in the patient's own home and it is significantly more cost-effective8 and with some limitations,1 represents a good alternative to laboratory PSG (L-PSG). However, the “first-night effect” (FNE) may weaken the reliability of all 1-night PSG recordings.
FNE is associated with abnormally poor sleep on the first night of PSG and characterized by lower sleep efficiency, shorter sleep time, longer sleep latency, and rapid eye movement (REM) sleep latency and a decreased amount of REM sleep.9–13 The FNE has been proposed to be caused by several different psychological and physical factors, including the anxiety related to the change in sleeping environment, the discomfort caused by electrodes and other measurement devices, movement limitations because of cables, and the psychological consequences of being under scrutiny.9,14 The severity of the FNE is affected by the setting where the recording takes place; the quality of first night of sleep is more similar to following nights in H-PSG as compared to L-PSG, where FNE is more pronounced.14,15 Furthermore, there is also evidence that the FNE may actually last for more than 1 night.9 The existence of an FNE results in the need for several nights of recording, because the first recorded night may not be truly representative.
The FNE in patients with SB has not been studied extensively. Hasegawa et al.16 retrospectively studied SB and sleep variables in 16 young and otherwise healthy patients with SB over 2 consecutive nights of L-PSG. They concluded that 1 night of PSG would be sufficient for patients with SB and with high SB activity; this would be evident in the first night but confirmation in patients with low SB activity would require an assessment also on the second night of PSG because of the high variability in the SB activity. Other previous studies have examined mostly the variance in SB activity over different time spans and found that internight variance is higher in H-PSG than in LPSG.17–19 Based on the conflicting results in studies conducted with portable EMG devices, there is some uncertainty about the existence of an FNE in the home environment.20–22 This is partially because of a certain degree of unreliability associated with using only EMG to record SB activity, substantially limiting the reliability of these published findings.
Currently, the evidence does not permit one to draw definitive conclusions about the number of consecutive nights of recording needed for accurate estimation of SB activity. This knowledge is nevertheless crucial not only in diagnosing conditions in patients but also in research settings where the epidemiology of SB, the reliability of questionnaires, or new treatment methods will be tested against the PSG-quantified SB activity levels.
Because no systematic studies concerning the FNE of SB in H-PSG in consecutive nights exist, our aim in this study was to examine the presence of an FNE and to assess the variability of SB activity in H-PSG over the course of 3 consecutive nights. Our working hypothesis was that there would be no FNE and that the internight variance of SB activity would be high in H-PSG.
METHODS
Subjects
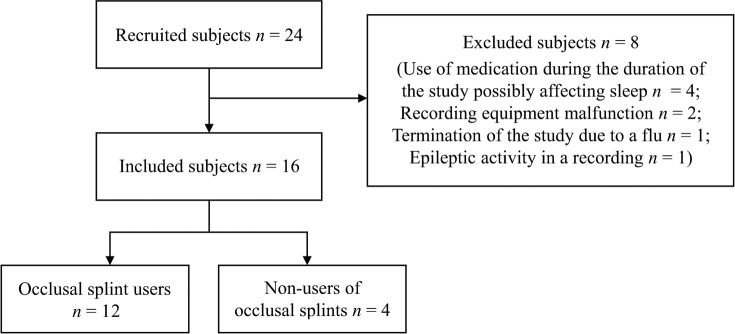
Forty-eight recordings in sets of 3 consecutive nights from 16 subjects (14 females, 2 males) aged 38.3 ± 9.1 years (mean ± standard deviation) were included from the recordings of the initially recruited 24 subjects (Figure 1). All subjects were recruited either with an open call for volunteers, posted in the intrawebs of Kuopio University Hospital and University of Eastern Finland or by a recommendation to participate given by a dentist working in the dental clinic of the city of Kuopio.
Figure 1. Subject flow chart.
There were 24 initially recruited subjects but 8 had to be excluded from this study. Of the 16 included subjects, 12 were long-term occlusal splint users (> 3 months) and the other 4 had never used an occlusal splint.
The inclusion criterion for all recruited subjects was self-reported SB, assessed with the first question of the Finnish version of Oral Behaviors Checklist (OBC-FIN)23 in the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) by Schiffman et al.24 (“How often do you clench or grind your teeth when asleep based on any information you have in the last month?”). A subject could be included in the study if he/ she answered that clenching or grinding occurred at least 1 night every week in the past month. According to the discussions with the subjects, the most common reasons for subjects to suspect sleep-time clenching or grinding of the teeth included (not mutually exclusive): (1) the subject had pain in the teeth, jaw, or neck area in the morning; (2) the subject had unexplained headaches in the morning; and (3) the subject's bed partner reported grinding sounds during the night. Twelve of the included subjects also had been informed by their dentist that they were displaying the characteristic signs of dental erosion reflecting the existence of bruxism in the past (ie, they were long-term users of occlusal splints for at least 3 months). The exclusion criteria based on an interview were: (1) any ongoing illness, disorder or medical condition in addition to SB; (2) use of any medication that possibly could affect sleep or motor behavior; (3) smoking or excessive use of alcohol; and (4) missing more than one posterior tooth (excluding third molars). All recruited subjects had daytime jobs with regular working hours and had a regular sleep schedule.
Eight of the recruited subjects were excluded from this study (Figure 1). Four subjects were excluded because of the reported use of a medication possibly affecting sleep during the study. Two subjects were lost because of a recording equipment malfunction leading to a loss of one of three consecutive recordings. One subject was lost because of a termination of the study as the subject caught a flu during the study. One subject was lost because of epileptic sleep activity detected in a recording.
The study protocol was reviewed by the Research Ethics Committee of the Hospital District of Northern Savo, Kuopio, Finland (favorable opinion: 34/2013) and permission was obtained from the National Supervisory Authority for Welfare and Health (Valvira, 220/2013). Written informed consent was obtained from all subjects.
Recordings
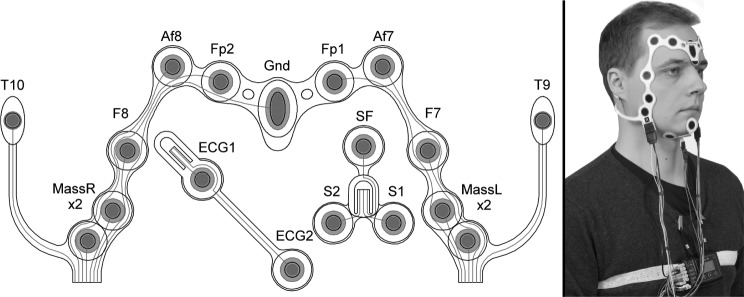
H-PSG recordings were conducted on 3 consecutive nights for each participant, in order to take into account the possibility that FNE would last for more than 2 nights. These sets of three recordings took place between Monday and Thursday or between Tuesday and Friday in the middle of a normal working week when the subjects had a regular sleep schedule. The subjects were given oral and written instructions with clear illustrations for using the PSG equipment at home. The subjects were instructed not to use oral splints during the course of the study. The Nox A1 portable H-PSG system (Nox Medical, Reykjavík, Iceland) together with an ambulatory electrode set (Figure 2) were used to record masseter EMG bilaterally (MassL, MassR) to detect RMMA events. In our previous studies, the electrode set has shown good performance in H-PSG and high accuracy when quantifying the SB activity compared to L-PSG.25–27 Nox A1 recorded true audio, so that RMMA could be distinguished from other orofacial or movement events by listening to the recording. In addition, electroencephalography (Af8-T9, Af7-T10, Fp2-T9, Fp1-T10), electrooculography (F8-T9, F7-T10), chin EMG (1-F, 2-F) and electrocardiography were recorded in order to determine sleep stages, TST, and cortical arousals during sleep. The subjects applied all of the equipment by themselves before going to sleep, and recording started automatically at a predetermined time point.
Figure 2. The self-applicable electrode set used in this study.
The electrode set, worn by one of the authors (T.M.) in the image to the right, consists of two sets of bipolar masseter electromyography electrodes (MassR x2, MassL x2), four electroencephalography electrodes (Af8, Fp2, Fp1, Af7), two electrooculography electrodes (F8, F7), three chin electromyography electrodes (SF, S1, S2), two electrocardiography electrodes (ECG1, ECG2), and two reference electrodes (T10, T9).
Data Analysis
All 48 anonymized recordings from 16 included subjects were analyzed with Noxturnal software (version 4.4.2, Nox Medical) in randomized order. The first author (T.M.) analyzed RMMA events in masseter EMG signals according to the American Academy of Sleep Medicine (AASM) criteria.28 A burst event was scored whenever there was an elevation in masseter EMG activity that was at least twice the amplitude of background EMG and having duration of at least 0.25 seconds. A phasic RMMA event consisted of at least three bursts, each lasting 0.25 to 2.00 seconds and with maximum of 3 seconds in between each consecutive burst. A tonic RMMA event consisted of one sustained burst lasting longer than 2 seconds. A mixed RMMA event had a minimum of three bursts with any duration within 3 seconds of each other. RMMA events were distinguished from other orofacial or muscular activities such as swallowing and sleep talking by listening to the concomitant audio recordings. RMMA events occurring during wakefulness were excluded from the analysis. Sleep stages and cortical arousals were scored in accordance with AASM guidelines28 by an expert technologist (Seppo Silvennoinen, Oivauni Oy, Kuopio, Finland). We used the rule for scoring N1 sleep in subjects who do not create an alpha rhythm28 because the recording montage did not include occipital electrodes that are necessary for the recognition of an alpha rhythm.
All event indices were calculated as the number of events per TST. The bruxism time index was determined as the total duration of RMMA episodes divided with TST. Total recording time (TRT) was defined as the time between the subject going to bed (BT) and the time of final awakening in the morning (WT). BT was set as the earliest time point when the continuous movement activity in the recording ceased before falling asleep close to the subject's reported time of going to sleep. Sleep efficiency was defined as TST divided by TRT. Sleep latency was calculated between BT and beginning of the first 30 second epoch scored as sleep (ST). Similarly, REM latency was calculated between ST and first epoch scored as REM sleep. Wake after sleep onset represented the intermittent time spent awake between ST and WT.
Statistical Analysis
One-way repeated-measures analysis of variance (RMANOVA) was used for internight comparison of all SB and sleep-related variables among all subjects. Two-way RMANOVA was used to investigate whether there were any differences in SB variables between occlusal splint users and nonusers, and in addition between subjects in whom bruxism was diagnosed (based on RMMA index, in any of the 3 nights) and those without bruxism. The normality of the data distribution was verified by using Shapiro-Wilk test. In the case of data being non-normally distributed, logarithmic transformation was performed before conducting the comparisons. The coefficient of variation (CV) was used to estimate night-to-night variability of the RMMA episode indices. Threshold for statistical significance was P = .05. All statistical analyses were conducted with SPSS software (version 21.0; SPSS, Chicago, Illinois, United States).
RESULTS
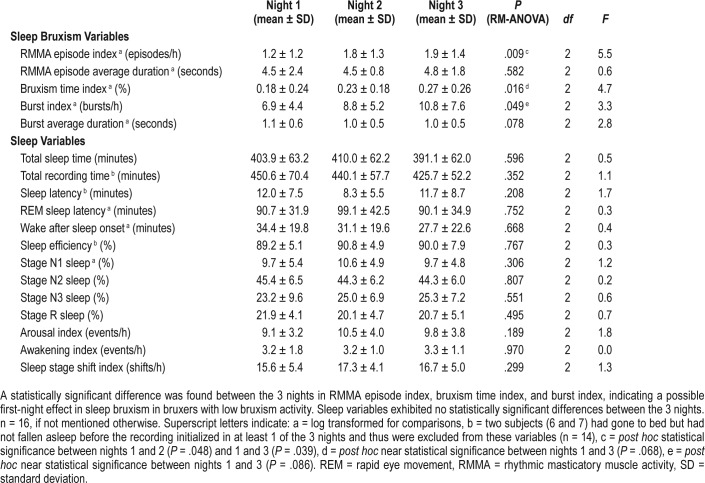
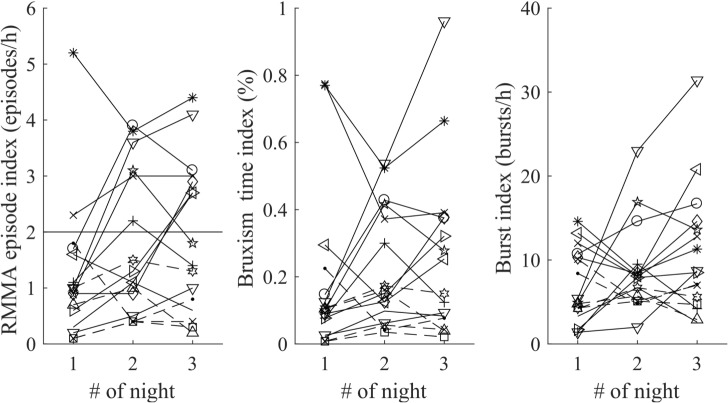
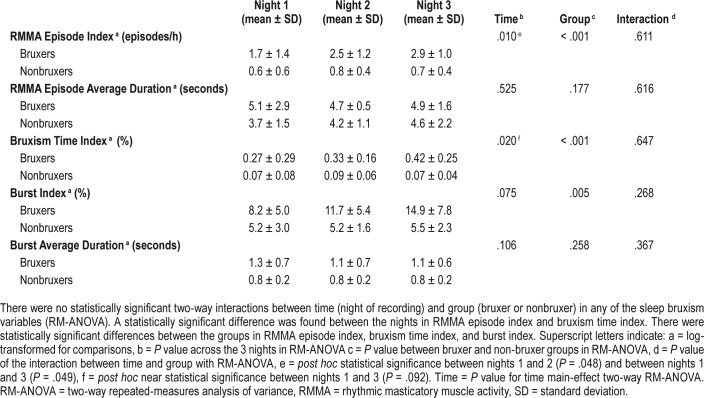
RM-ANOVA detected a statistically significant difference in the three SB variables between the three recorded nights (Table 1): RMMA EI (P = .009), bruxism time index (P = .016), and burst index (P = .049). Most of the subjects in this study had a low RMMA index on the first night of the study (1.2 ± 1.2 episodes/h, mean ± standard deviation) and post hoc pairwise Bonferroni test revealed significantly higher RMMA episode indices on the second night (1.8 ± 1.3 episodes/h, P = .048) and on the third night (1.9 ± 1.4 episodes/h, P = .039) (Table 1). Bruxism time index and the burst index displayed nonsignifi-cant differences between the nights in pairwise post hoc tests. No statistically significant differences between nights were found with respect to the sleep variables.
Table 1.
Sleep bruxism and other sleep variables in the 3 consecutive nights.
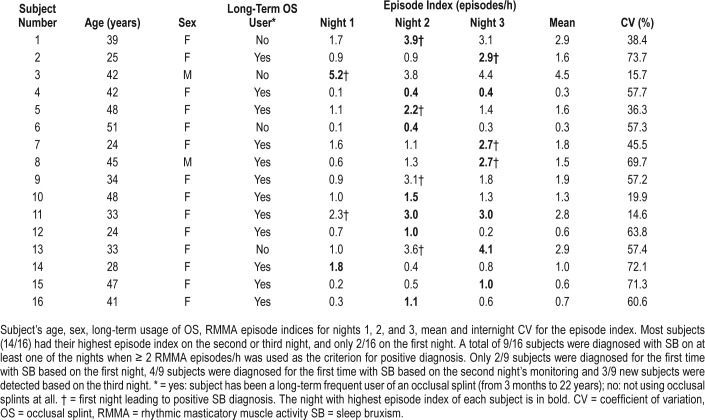
Intrasubject variability of RMMA EI ranged from 14.6% to 73.7%, the mean CV being 50.7% (Table 2). Most subjects (14/16) had their highest RMMA EI on the second (9/16) or third night (7/16) of the study (Table 2, Figure 3, two subjects had the same EI on both nights). Most subjects (11/16) had their lowest RMMA EI in the first night. In 75% of the subjects the RMMA EI was higher on the second night and in 81% of the subjects on the third night compared to the first night.
Table 2.
Subject demographics, episode indices, and internight coefficient of variations for the episode indices.
Figure 3. Rhythmic masticatory muscle activity (RMMA) episode indices, bruxism time indices and burst indices, of each subject during the 3 nights.
The threshold value for low-frequency sleep bruxism diagnosis (2 episodes/h) is illustrated as a horizontal line in the first graph. There is extensive variance in episode indices between the nights in the individual subjects (mean of individual coefficient of variance (CV) 50.7 %, min 14.6 %, max 73.7 %).
In all, 9/16 subjects met criteria for SB on at least 1 of the nights (Table 2) when ≥ 2 and < 4 episodes/h were used as the criteria for low-frequency and ≥ 4 episodes/h as that for high-frequency SB diagnosis.1 Only two of the bruxers met criteria on the first night (Figure 4, Table 2), whereas six bruxers did on night 2 and seven bruxers did on night 3 (two of the bruxers had positive results on every night and another two on both of the last two nights). Only 2/9 subjects met criteria for high-frequency SB (both of them also for low-frequency SB on another night) and 7/9 met criteria for low-frequency SB (but not high-frequency SB) on at least 1 of the nights.
Figure 4. Number of subjects in whom a diagnosis was made on each recorded night.
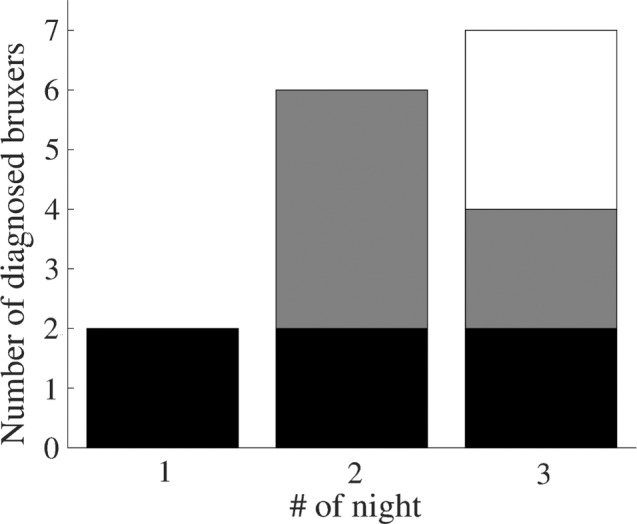
On the first night, only two subjects met criteria for sleep bruxism (> 2 RMMA episodes/h). Six subjects met criteria for sleep bruxism on the second night and seven subjects on the third night. Both of the subjects that met criteria on the first night, also met criteria on nights 2 and 3 (illustrated with the black bar). Two of the four subjects that met criteria for the first time on the second night also met criteria on the third night (gray bar).
No statistically significant two-way interactions were found between the night of recording and groups of bruxers and nonbruxers in any of the SB variables (Table 3). A statistically significant difference between the two groups was found in RMMA EI (P < .001), bruxism time index (P < .001) and burst index (P = .005).
Table 3.
Sleep bruxism variables over 3 consecutive nights in the bruxer group (n = 9) and the nonbruxer group (n = 7).
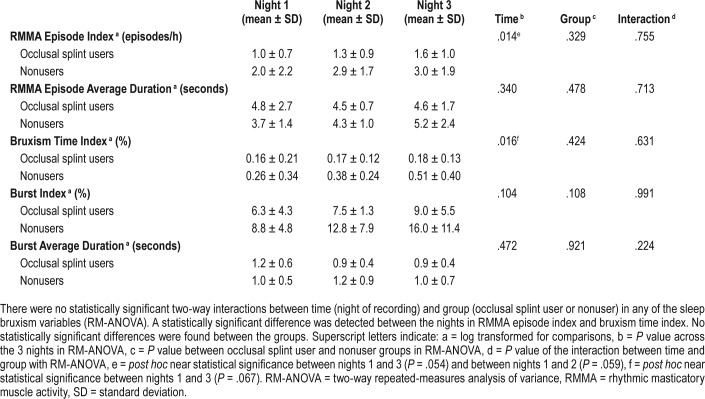
No two-way interactions between the night of recording and the groups of occlusal splint users and non-users were found (Table 4), nor were any statistically significant group differences detected.
Table 4.
Sleep bruxism variables over 3 consecutive nights in the occlusal splint user group (n = 12) and the nonuser group (n = 4).
Based on the diagnostic grading system devised by Lobbezoo et al.3 and the OBC-FIN questionnaire,23 all 16 included subjects fulfilled the criteria of a “possible” bruxer. Of these 16 “possible” bruxers, 9 would be classified as “definite” bruxers based on PSG results on at least 1 of the nights if a clinical examination by a dentist would yield positive results in regard to SB.
DISCUSSION
Our results provide evidence that an FNE might exist in SB activity with patients having low SB activity level. Not only is there a statistically significant elevation in the RMMA EI on the course of 3 nights, but also the fact that most subjects (14/16) displayed their highest RMMA EI in the second or third night supports the existence of an FNE in SB activity. However, no observable FNE was found in other sleep variables. This may indicate that the physiological mechanisms behind SB activity are more sensitive than those behind actual sleep with respect to the physical and/or psychological changes in the sleeping environment. However, the number of subjects was rather low, making it difficult to draw definite conclusions, and the outcomes need to be viewed with caution.
Internight variability of SB activity was extensive, as hypothesized. The higher mean CV of 50.7% compared to the earlier reported CVs of 30%,16 25%,17 and 22%18 in L-PSG and 37%19 in H-PSG may be partially explained by the higher variability in the domestic setting, but it should be noted that CV is also affected by low level of SB activity. If the mean value of the variable is low, then the CV tends to be high with the same standard deviation, because CV = standard deviation / mean.
Our hypothesis that there would be no FNE in SB subjects in H-PSG seems to be incorrect. Apparently, the familiarity of the domestic environment may not be enough to prevent the occurrence of an FNE in subjects with low SB activity. This result is in line with the study of Ikeda et al.,20 in which patients used an EMG-based device in their home and a steady increase was observed in the RMMA EI over the course of 4 nights. The results are also in line with the report of Hasegawa et al.16 in the case of subjects with low-frequency SB. In that study, six out of eight (75%) subject with low-frequency SB had a higher RMMA EI on night 2 as compared to night 1. However, our results suggest that the FNE affects not only low-frequency bruxers but also nonbruxers, because no two-way interactions were found between the recorded nights and the two groups (bruxers and nonbruxers), and also because most nonbruxers (5/7) had their lowest RMMA EI on the first night. The main difference between the groups is that the effect of FNE was significant enough in the bruxer group so that in new subjects the diagnosis was made also on the second and third nights.
Hasegawa et al.16 recommended that in clinical practice when the first night of PSG revealed signs of low frequency SB, the recording should be repeated to confirm the level of SB activity. However, instead of the repetition being only because of the high intrinsic variability of SB activity, our findings seem to support the concept that the repetition of recordings for at least 3 nights might actually be needed because of a delayed FNE in some patients. Recording should be repeated in patients diagnosed with low-frequency SB and also in those patients who show nonbruxer levels of bruxism activity on the first night. In concordance with Hasegawa et al.,16 our results indicate that there might exist a subgroup of patients with SB who are more susceptible to FNE and are at an increased risk of misdiagnosis if only a single night of PSG is recorded. This should be taken into account when making diagnostic decisions based on PSG.
The implications of our findings could be even more crucial in research settings. False conclusions may be drawn if one only has a single night of PSG recording because it is possible that this will not represent the subjects' levels of RMMA during normal nights of sleep. Thus here, if only 1 night of PSG had been utilized for assessing the SB activity of the subjects, most subjects (11/16) would have had their lowest RMMA index on that night. With those patients who are more susceptible for disturbances in the sleeping environment and suffer from a delayed FNE or have higher variability of SB activity, several nights of PSG invariably will be needed to allow acclimation to the PSG setting and in that way, to diminish the effect of these disturbances on the results. It should be noted that the existence of a delayed FNE in some subjects might even explain some recent findings that self-reported SB and many clinical signs and symptoms correspond poorly with the results of PSG.29,30 This poor correspondence was demonstrated in our study as only 2 of the 16 “possible” bruxer subjects were diagnosed (for the first time) on the first night, whereas 4 more of them were diagnosed on the second night and an additional 3 on the third night.
One factor that may explain why indications a of FNE were observed in this study, even though H-PSG should show less FNE, is that the subjects were rather old (eg, significantly older than in the study by Hasegawa et al.16). Generally, the severity of FNE increases with older age.31 Different medical conditions may also affect the severity of FNE.7 Because medical conditions were currently controlled only by the means of interview, it is possible that some factors (eg, concomitant conditions) may have been missed. In the literature, there are no indications suggesting that the SB activity1,32 or FNE severity9,33 might be affected by sex. Nevertheless, the results presented here concerning FNE in SB activity should be confirmed in a population with a more representative sex distribution before generalizing the results to both sexes, as our study population consisted of only 2 males and 14 females.
There are a few other drawbacks and limitations in this study. We did not control for caffeine or alcohol intake of the subjects, which may have affected the results. Although we used audio recordings to distinguish RMMA from other nocturnal activities, we did not record video in H-PSG to confirm the SB episodes. Furthermore, the microphone in the Nox A1 PSG system is placed inside the recording unit, which is positioned on the chest during the recording. This causes the microphone occasionally to be covered by a blanket when the subject is sleeping. This may have affected the accuracy of RMMA scoring if audible teeth grinding or other orofacial activities such as swallowing would have been missed because of the suppressing effect of the blanket. Twelve subjects were long-term (> 3 months) users of occlusal splints and stopped their usage only for the duration of this study. Although it is recognized that occlusal splints inhibit SB activity over the short term, their effect usually diminishes after 2 weeks of use.34 Therefore, it is possible, but unlikely, that this may have affected the results, because it is also known that at least after the short-term use of occlusal splints RMMA activity returns to normal levels immediately when their use is discontinued.35–37 However, no statistically significant differences were found between the groups of occlusal splint users and nonusers in the current study. Although the nonusers showed a trend toward generally higher values in the SB variables, no difference was detected with respect to FNE. Nevertheless, this confounding factor could have been avoided by including the use of occlusal appliances in the exclusion criteria or implementing a washout period for the users before the initiation of the 3-night study period.
Because the FNE severity may depend on the subjects, recording environment, and devices that are used, more research will be required to gain more in-depth knowledge of FNE in all settings where diagnostics or research are being conducted. The recording environments and devices should be separately tested for the FNE and for a duration longer than 2 nights due to the possibility of a delayed FNE. Furthermore, it would be beneficial if one could identify the characteristics of those possible individuals who are more susceptible to an FNE; in that way, one could predict beforehand the need for several recording nights. It has been proposed that one can take better account of the variability of SB in diagnostics by the implementation of cutoff bands for SB diagnosis.19 If such cutoff bands are introduced, we would like to emphasize that a comprehensive study not only of the variability of SB but also of the FNE should be conducted and taken into account in every device and environment separately. In order to overcome the problem of the FNE and internight variability, comfortable self-applicable devices could be worn for several nights with the more cost-efficient H-PSG, even though it may not fully prevent the occurrence of an FNE. In the future, psychological approaches to overcome FNE should also be investigated because FNE seems to exist to a certain extent even when simple and more comfortable EMG devices are used.20 One possibility to overcome the problem of numerous nights of consecutive PSG because of FNE would be to use “dummy” electrodes and devices for the preceding nights in the home environment to allow the patient to become accustomed to the devices before the actual recording.
Because we found indications of FNE and high variability of RMMA activity in SB subjects with low SB activity, we agree with Hasegawa et al.16 that PSG of patients with low-frequency SB should be repeated because of the possible underestimation of the activity levels. Based on our results, this should be done also in the case of a patient displaying sub-threshold level of SB activity. The sleep itself appears to be unaffected by the FNE and only SB activity seems to be significantly lower on the first night—or the first 2 nights—in subjects with low SB activity. On the basis of these results, we would like to emphasize the need for at least 3 consecutive nights of PSG in any research or diagnostic setting in order to take into account both the FNE and the varying nature of SB and thus achieve a “super-definite” diagnosis. By taking FNE better into account in the future, subgroups suffering from FNE and the number of recorded nights needed for accurate estimation of SB activity should be identified and recording procedures should be further refined. If one wishes to minimize the FNE and to enable several nights of recordings, we suggest that PSG could be conducted with the more cost-efficient H-PSG with self-applicable unobtrusive and comfortable sensors.
DISCLOSURE STATEMENT
Work was performed in the Department of Applied Physics, University of Eastern Finland and in the Department of Clinical Neurophysiology, Kuopio University Hospital. All authors have seen and approved the manuscript.
This work was financially supported by The Finnish Funding Agency for Technology and Innovation project of 40047/14 (‘Bruxism’), and by grants from the Research Committee of Kuopio University Hospital Catchment Area for the State Research Funding (projects 5041739, 5041748 and 5041761), Research Foundation of the Pulmonary Diseases and Ulla Tuominen Foundation. T. Miettinen was supported by grants from Instrumentarium Science Foundation and North Savo Regional fund of Finnish Cultural Foundation. Authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The technologist staff of Department of Clinical Neurophysiology in Diagnostic Imaging Center of Kuopio University Hospital are acknowledged for instructing subjects on how to use the self-applicable polysomnographic set and for managing the recordings. Seppo Silvennoinen (Oivauni Oy, Kuopio, Finland) is acknowledged for sleep stage scoring. The authors also thank Screentec Oy (Oulu, Finland) for manufacturing the ambulatory electrode sets and Kuopio Academy of Design (Savonia University of Applied Sciences, Kuopio, Finland) for collaboration in creating illustrated instructions to instruct the subject on how to undertake the PSG study at home.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- BT
time of going to bed
- CV
coefficient of variation
- DC/TMD
diagnostic criteria for temporomandibular disorders
- EI
episode index
- EMG
electromyography
- FNE
first-night effect
- H-PSG
home polysomnography
- L-PSG
laboratory polysomnography
- OBC
oral behavior checklist
- PSG
polysomnography
- REM
rapid eye movement
- RM-ANOVA
repeated measures analysis of variance
- RMMA
rhythmic masticatory muscle activity
- SB
sleep bruxism
- ST
time of the beginning of the first 30-second epoch scored as sleep
- TRT
total recording time
- TST
total sleep time
- WT
time of final awakening in the morning
REFERENCES
- 1.Carra MC, Huynh N, Lavigne G. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am. 2012;56(2):387–413. doi: 10.1016/j.cden.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Carra MC, Huynh N, Fleury B, Lavigne G. Overview on Sleep Bruxism for Sleep Medicine Clinicians. Sleep Med Clin. 2015;10(3):375–384. xvi. doi: 10.1016/j.jsmc.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Lobbezoo F, Ahlberg J, Glaros AG, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil. 2013;40(1):2–4. doi: 10.1111/joor.12011. [DOI] [PubMed] [Google Scholar]
- 4.Jokubauskas L, Baltrušaitytė A. Relationship between obstructive sleep apnoea syndrome and sleep bruxism: a systematic review. J Oral Rehabil. 2017;44(2):144–153. doi: 10.1111/joor.12468. [DOI] [PubMed] [Google Scholar]
- 5.Manfredini D, Guarda-Nardini L, Marchese-Ragona R, Lobbezoo F. Theories on possible temporal relationships between sleep bruxism and obstructive sleep apnea events. An expert opinion. Sleep Breath. 2015;19(4):1459–1465. doi: 10.1007/s11325-015-1163-5. [DOI] [PubMed] [Google Scholar]
- 6.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 7.Newell J, Mairesse O, Verbanck P, Neu D. Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples. Psychiatry Res. 2012;200(2):795–801. doi: 10.1016/j.psychres.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 8.Bruyneel M, Ninane V. Unattended home-based polysomnography for sleep disordered breathing: current concepts and perspectives. Sleep Med Rev. 2014;18(4):341–347. doi: 10.1016/j.smrv.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Le Bon O, Staner L, Hoffmann G, et al. The first-night effect may last more than one night. J Psychiatr Res. 2001;35(3):165–172. doi: 10.1016/s0022-3956(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H, Sowers M, Buysse DJ, et al. Sources of variability in epidemiological studies of sleep using repeated nights of in-home polysomnography: SWAN Sleep Study. J Clin Sleep Med. 2012;8(1):87–96. doi: 10.5664/jcsm.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toussaint M, Luthringer R, Schaltenbrand N, et al. First-night effect in normal subjects and psychiatric inpatients. Sleep. 1995;18(6):463–469. doi: 10.1093/sleep/18.6.463. [DOI] [PubMed] [Google Scholar]
- 12.Curcio G, Ferrara M, Piergianni A, Fratello F, De Gennaro L. Paradoxes of the first-night effect: a quantitative analysis of antero-posterior EEG topography. Clin Neurophysiol. 2004;115(5):1178–1188. doi: 10.1016/j.clinph.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Virtanen I, Kalleinen N, Urrila AS, Polo-Kantola P. First-night effect on sleep in different female reproductive states. Behav Sleep Med. 2016;4:1–13. doi: 10.1080/15402002.2016.1228646. [DOI] [PubMed] [Google Scholar]
- 14.Herbst E, Metzler TJ, Lenoci M, et al. Adaptation effects to sleep studies in participants with and without chronic posttraumatic stress disorder. Psychophysiology. 2010;47(6):1127–1133. doi: 10.1111/j.1469-8986.2010.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edinger JD, Fins AI, Sullivan RJ, Jr, et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20(12):1119–1126. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa Y, Lavigne G, Rompré P, Kato T, Urade M, Huynh N. Is there a first night effect on sleep bruxism? A sleep laboratory study. J Clin Sleep Med. 2013;9(11):1139–4115. doi: 10.5664/jcsm.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavigne GJ, Guitard F, Rompre PH, Montplaisir JY. Variability in sleep bruxism activity over time. J Sleep Res. 2001;10(3):237–244. doi: 10.1046/j.1365-2869.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- 18.Dal Fabbro C, Tufik S. A linear study of a man with sleep bruxism for 30 consecutive nights-preliminary reports. J Sleep Res. 1996;5(Suppl 1):41–82. [Google Scholar]
- 19.Van Der Zaag J, Lobbezoo F, Visscher C, Hamburger H, Naeije M. Time-variant nature of sleep bruxism outcome variables using ambulatory polysomnography: implications for recognition and therapy evaluation. J Oral Rehabil. 2008;35(8):577–584. doi: 10.1111/j.1365-2842.2008.01893.x. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda T, Nishigawa K, Kondo K, Takeuchi H, Clark GT. Criteria for the detection of sleep-associated bruxism in humans. J Orofac Pain. 1996;10(3):270–282. [PubMed] [Google Scholar]
- 21.Minakuchi H, Sakaguchi C, Hara ES, et al. Multiple sleep bruxism data collected using a self-contained EMG detector/analyzer system in asymptomatic healthy subjects. Sleep Breath. 2012;16(4):1069–1072. doi: 10.1007/s11325-011-0602-1. [DOI] [PubMed] [Google Scholar]
- 22.Stuginski-Barbosa J, Porporatti AL, Costa YM, Svensson P, Conti PCR. Diagnostic validity of the use of a portable single-channel electromyography device for sleep bruxism. Sleep Breath. 2016;20(2):695–702. doi: 10.1007/s11325-015-1283-y. [DOI] [PubMed] [Google Scholar]
- 23.Ohrbach R, editor. Oral Behaviors Checklist. Diagnostic Criteria for Temporomandibular Disorders: Assessment Instruments. [Accessed February 1, 2018]. https://ubwp.buffalo.edu/rdc-tmdinternational/.2016.
- 24.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28(1):6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myllymaa S, Muraja-Murro A, Westeren-Punnonen S, et al. Assessment of the suitability of using a forehead EEG electrode set and chin EMG electrodes for sleep staging in polysomnography. J Sleep Res. 2016;25(6):636–645. doi: 10.1111/jsr.12425. [DOI] [PubMed] [Google Scholar]
- 26.Miettinen T, Myllymaa K, Westeren-Punnonen S, et al. Success rate and technical quality of home polysomnography with self-applicable electrode set in subjects with possible sleep bruxism. IEEE J Biomed Health Inform. 2017;99 doi: 10.1109/JBHI.2017.2741522. Epub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen T, Myllymaa K, Muraja-Murro A, et al. Screen-printed ambulatory electrode set enables accurate diagnostics of sleep bruxism. J Sleep Res. 2018;27(1):103–112. doi: 10.1111/jsr.12536. [DOI] [PubMed] [Google Scholar]
- 28.Berry RB, Brooks R, Gamaldo CE, et al. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2012. Version 2.0. [Google Scholar]
- 29.Palinkas M, De Luca Canto G, Rodrigues LA, et al. Comparative capabilities of clinical assessment, diagnostic criteria, and polysomnography in detecting sleep bruxism. J Clin Sleep Med. 2015;11(11):1319–1325. doi: 10.5664/jcsm.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raphael KG, Janal MN, Sirois DA, et al. Validity of self-reported sleep bruxism among myofascial temporomandibular disorder patients and controls. J Oral Rehabil. 2015;42(10):751–758. doi: 10.1111/joor.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb WB, Campbell SS. The first night effect revisited with age as a variable. Waking Sleeping. 1979;3(4):319–324. [PubMed] [Google Scholar]
- 32.Khoury S, Carra MC, Huynh N, Montplasir J, Lavigne GJ. Sleep bruxism-tooth grinding prevalence, characteristics and familial aggregation: a large cross-sectional survey and polysomnographic validation. Sleep. 2016;39(11):2049–2056. doi: 10.5665/sleep.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goel N, Kim H, Lao RP. Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiol Int. 2005;22(5):905–915. doi: 10.1080/07420520500263235. [DOI] [PubMed] [Google Scholar]
- 34.Harada T, Ichiki R, Tsukiyama Y, Koyano K. The effect of oral splint devices on sleep bruxism: a 6-week observation with an ambulatory electromyographic recording device. J Oral Rehabil. 2006;33(7):482–488. doi: 10.1111/j.1365-2842.2005.01576.x. [DOI] [PubMed] [Google Scholar]
- 35.Solberg WK, Clark GT, Rugh JD. Nocturnal electromyographic evaluation of bruxism patients undergoing short term splint therapy. J Oral Rehabil. 1975;2(3):215–223. doi: 10.1111/j.1365-2842.1975.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 36.Rugh J, Solberg W. Electromyographic studies of bruxist behavior before and during treatment. J Calif Dent Assoc. 1975;3(9):56–59. [PubMed] [Google Scholar]
- 37.Pierce CJ, Gale EN. A comparison of different treatments for nocturnal bruxism. J Dent Res. 1988;67(3):597–601. doi: 10.1177/00220345880670031501. [DOI] [PubMed] [Google Scholar]