Abstract
Study Objectives:
Current pharmacological options for the treatment of insomnia insufficiently meet the needs of all insomnia patients. Approved treatments are not consistently effective in improving sleep onset and sleep maintenance, while also having complicated safety profiles. These limitations highlight the unmet need for additional medications and treatment strategies. Initial research suggests that the dual orexin receptor antagonists (DORAs) may offer an additional pharmaceutical option to treat insomnia in some patients.
Methods:
We reviewed the existing literature on dual orexin receptor antagonists in PubMed databases using the search terms “orexin receptor antagonist,” “almorexant” “filorexant,” “lembroexant” and “suvorexant”; searches were limited to English language primary research articles, clinical trials, and reviews.
Results:
Targeting the orexin receptor system for treatment of insomnia offers an additional and alternative pharmacological approach to more common gamma aminobutyric acid agonist sedative hypnotic treatment. Effectiveness is not well established in the current literature; however, the literature does suggest efficacy. Preclinical reports also suggest the potential for treatment in individuals with comorbid Alzheimer disease and insomnia.
Conclusions:
DORAs offer an additional treatment option for insomnia. More clinical trials are needed to robustly evaluate their safety and effectiveness in several subclasses of individuals with insomnia. Given the published literature, head-to-head comparisons to existing treatment for insomnia are warranted.
Citation:
Janto K, Prichard JR, Pusalavidyasagar S. An update on dual orexin receptor antagonists and their potential role in insomnia therapeutics. J Clin Sleep Med. 2018;14(8):1399–1408.
Keywords: Alzheimer disease, dual orexin receptor antagonists, geriatrics, insomnia
INTRODUCTION
Insomnia is one of the most prevalent sleep disorders that affects people across various age groups from childhood1 to old age, when it is the most common sleep disorder.2,3 Intermittent insomnia symptoms have been reported in at least 33% of adults, whereas chronic sleep difficulties have been reported in 10% to 22% of adults.4–8 The disorder is characterized by complaints of difficulty initiating sleep, difficulty staying asleep, frequent bouts of wakefulness throughout the night, early awakening with the inability to go back to sleep, and feelings of one's sleep being nonrestorative and is often comorbid with cardiovascular disease and mood disorders such as depression and anxiety.9–12 Patients with insomnia can experience deterioration in their general health because of associated symptoms of daytime fatigue and decreased cognitive, social, and physical functioning.8 Insomnia poses an enormous economic burden, costing the United States approximately $63 billion annually as a result of increases in work-related accidents, higher work absenteeism, decreased job performance, and increased use of health care resources.13,14 Insomnia has been implicated in higher risk of suicidal ideation15 and exacerbation of mood disorders,5,16 and is also implicated in the pathogenesis of Alzheimer disease (AD).17 As such, effective treatment for insomnia has long-term benefits for patients beyond improved sleep.
Cognitive behavioral therapy for insomnia (CBT-I) is the ethically preferred therapy for insomnia. CBT-I is a formal program offered by behavioral sleep specialists whose goal, among others, is to restructure the patient's maladaptive thoughts and practices about sleep, eliminate arousal factors that inhibit sleep, utilize relaxation techniques, and provide education about circadian rhythms. CBT-I procedures can often be effective when applied consistently, but patient adherence to these interventions can be impaired or limited.18,19 Some patients reject the CBT-I approach, wanting medications instead. Furthermore, efforts of CBT-I–like interventions in institutional populations may not be possible because of staffing limitations. Safe pharmacological approaches have relevance in such patient populations. Medications for sleep or agitation in institutional settings have long been an area for regulatory oversight because of the risk of using such psychotropic medications presents in such settings.20
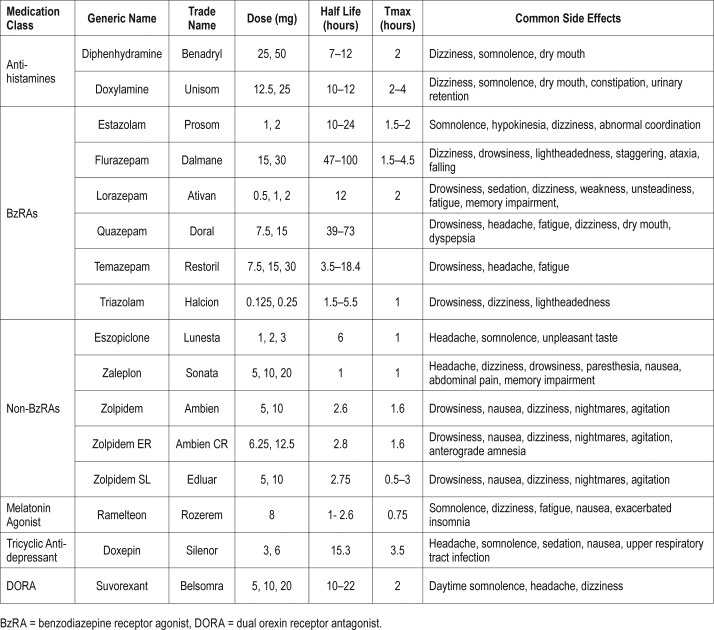
The United States Food and Drug Administration (FDA)-approved pharmacological treatment options for insomnia include over-the-counter antihistamines, benzodiazepines, nonbenzodiazepine receptor agonists (BzRAs), tricyclic antidepressants, and melatonin agonists21,22 (Table 1). Non-BzRAs, which are gamma amino butyric acid (GABA) acting hypnotics, remain common pharmacological treatment for the short-term and long-term management of both primary and secondary insomnia. The number of prescriptions filled for sedative/hypnotic agents have significantly increased in the past 20 years.23 Data from the National Center for Health Statistics 2005–2010 show that more than 4% of United States adults older than 19 years have reported prescription sleep aid use in the past month.24
Table 1.
Summary of FDA-approved insomnia treatment options.
The aforementioned pharmacological treatment options have been identified to have restricted efficacy profiles by the FDA.25 Not all of the non-BzRAs are effective at promoting sleep maintenance throughout the night in all patients with insomnia.18 The GABA-acting sedatives that target the major inhibitory neurotransmitter system in the central nervous system (CNS) are associated with several adverse side effects such as somnolence, grogginess, dizziness, ataxia, memory disturbances, and hallucinations.26 Hypnotics, particularly those with longer half-lives, are associated with increased risk of falls and motor vehicle accidents.27 Non-BzRAs, such as zolpidem and eszopiclone have increased risk for complex sleep-related behaviors such as sleepwalking and sleep eating.28 Further, the reappearance of insomnia with discontinuation, and rebound insomnia with discontinuation, along with development of bedtime physical and psychological dependence,29 draws into question the safety of unmindful and generalized chronic use.
The pharmacokinetic profile of any therapeutic agent used for insomnia is a critical factor for potential adverse effects. An agent with too short a half-life will fail to promote sleep for the entire duration of the patient's sleep period, and thus will not be helpful in treating maintenance insomnia. However, an agent with too long a half-life poses risk of residual sleepiness and a hangover effect beyond the necessary sleep period.29 These specific requirements are often insufficiently met by current sedative/hypnotic agents, though they do help many patients. The current most common pharmacologic treatment options for insomnia do not uniformly treat both sleep onset and sleep maintenance across every patient, and too infrequently insufficiently treat the sleep needs of many patients. These agents have relevant safety concerns, highlighting a need for additional pharmacological options.
In this review article, the potential of orexin receptor antagonists—primarily dual orexin receptor antagonists (DORAs)— is discussed as an additional pharmacological option for treatment of insomnia. The neurobiology of the orexin system, the various DORAs, clinical trials, and the potential benefits and risks of the use of DORAs for the treatment of insomnia are reviewed. In addition, the theoretical basis for potential benefits of DORA treatment for individuals with comorbid insomnia and AD, a need presently unmet, is discussed.
OREXIN SIGNALING
In 1998, two independent research entities simultaneously identified the same novel neuropeptide. DeLecea et al. termed the molecule hypocretin,30 and Sakurai et al. named it orexin.31 The simultaneous discovery and naming of this molecule has resulted in the two names being used synonymously, but for the remainder of this review it will be referred to as orexin. Early indications of orexin's crucial role in the maintenance of arousal were revealed by animal studies showing that mice lacking a gene for the orexin peptide32 and dogs with orexin receptor mutations33 displayed symptoms comparable to those of humans with narcolepsy. In humans, narcolepsy is associated with a cell-type specific ∼90% reduction in orexinergic neurons,34 affecting both the degree of sleepiness and sleep-wake state stability.
There are two neurochemically distinct forms of orexin, orexin A and orexin B, which show only 46% sequence homology to one another. Orexin A consists of a peptide chain 33 amino acids in length, is cyclized at the N-terminal, contains two sets of disulfide bonds, and amidation of the C-terminal, whereas orexin B consists of 28 amino acids, contains an amide at the C-terminal, and forms its secondary protein structure with the help of hydrogen bonds of alpha helices.31 Orexin A and B neuropeptides are exclusively synthesized by orexinergic neurons located in the lateral and posterior hypothalamic areas of the diencephalon.31
The most prominent effect elicited by orexinergic signaling is the maintenance of wakefulness through continuous depolarizing effects in wake-promoting brain nuclei.31 The sleep-wake cycle is a complex system composed of reciprocally regulating neural systems operating under a feedback loop (the “flip-flop” cycle), which allows for stable transitions between states of wakefulness and sleep (see Fuller et al. for review).36 When one state is active, the other is inactive. The ascending reticular activating system (ARAS) promotes wakefulness, and the ventrolateral preoptic region (VLPO) promotes sleep. In brief, strong activation of the ARAS, involving the firing of cholinergic neurons, monoaminergic cell bundles, and orexin nuclei of the lateral hypothalamus, effectively inhibit VLPO during wakefulness, whereas activation of VLPO releases the inhibitory neurotransmitters GABA and galanin, which suppress neural actions of the ARAS. Orexinergic neurons innervate nearly all of the wake-promoting nuclei in the ARAS, including the locus coeruleus (LC), which contains noradrenergic neurons, lateral dorsal tegmentum/pedunculopontine tegmentum (LDT/PPT), which contains cholinergic neurons, dorsal raphe nucleus (DR), which contains serotonergic neurons, and tuberomammillary nucleus, which contains histaminergic neurons.35,36 Additionally, a second action of the hypocretin system is to stabilize the “flip-flop” switch, as its absence in narcolepsy can lead to unstable sleep-wake states, day and night.
Orexins A and B exert physiological effects by binding to specific G-protein coupled receptors—orexin receptor 1 (OX1R) and orexin receptor 2 (OX2R).37 OX1R shows specificity for orexin A as its ligand, whereas OX2R shows dual affinity for both orexin A and B. In a Sprague Dawley rat model, OX1R and OX2R are widely distributed throughout the CNS.38 Orexin signaling is implicated not only in the regulation of sleep and arousal states, but also other physiological functions such as memory, emotions, motivation, attention, autonomic control, feeding, and energy homeostasis.39–41 In rat models, some brain regions show specificity for one of the two receptor subtypes, whereas other regions show abundant expression of both receptor types.42 OX1R-specific regions include the prefrontal and infralimbic cortex, anterior hypothalamus, and LC, and OX2R-specific regions are more localized to hypothalamic regions including the arcuate nucleus, lateral hypothalamus, tuberomammillary nucleus, dorsomedial hypothalamic nucleus, paraventricular nucleus, and medial septal nucleus.38,42 Overlapping of both receptor types is observed in the hippocampus, amygdala, bed nucleus of the stria terminalis, paraventricular thalamic nucleus, DR, ventral tegmental area, and LDT/PPT.38,42 The increased concentration of orexin receptors in neural regions that regulate sleep-wake state, but not in cortical areas that underlie perception and cognition, create the opportunity for pharmaceutical agents to have a more focused effect on behavioral state regulation without direct, widespread alterations in cortical function. It is currently unknown what genetic variability may exist between individuals in these systems, or how they are epigenetically regulated.
AN OVERVIEW OF OREXIN RECEPTOR ANTAGONISTS
Elucidation of orexin's role in the maintenance of arousal led to new pharmaceutical opportunities for treating various sleep disorders by targeting the orexin system.43 If the binding of orexin to either of the orexin receptors is a contributing factor to wakefulness, then an exogenous substance developed to act as an agonist to the orexin receptors could promote wakefulness, which could be helpful in patients with narcolepsy. Conversely, a substance designed to act as an antagonist to the orexin receptor could induce the opposite effect to promote sleep, which could be helpful to treat insomnia. The therapeutic strategy is that by blocking the orexin receptor through antagonistic action for an extended period of time, the typical wake-promoting actions of the orexin system will be reduced, resulting in subsequent sleepiness and longer sustained periods of sleep. In 2009, Dugovic et al. demonstrated that blocking OX2R with specially designed antagonists initiates and prolongs sleep in a Sprague Dawley rat model, confirming previous implications about the possibility of orexin system-targeted treatment options for insomnia.44
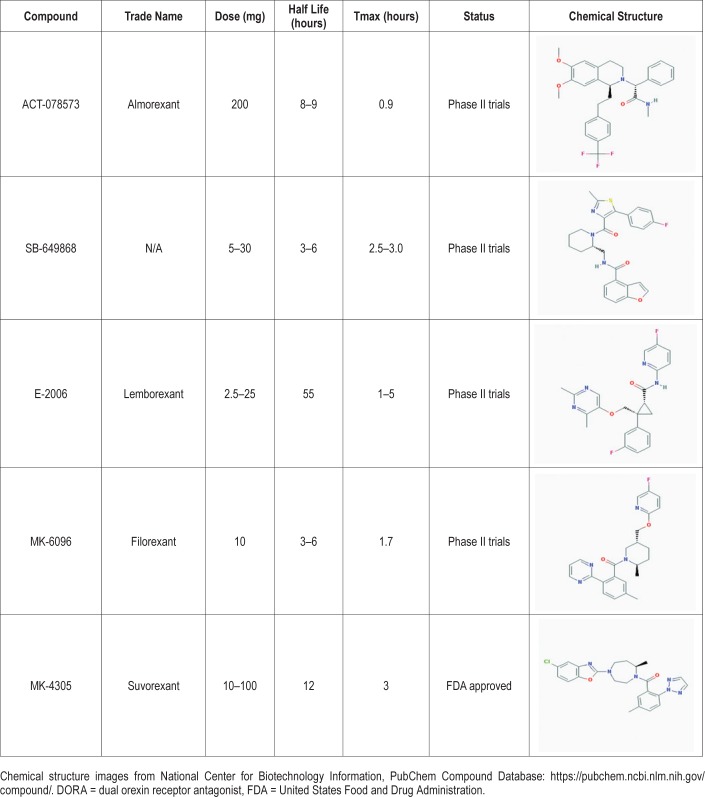
Since that time, two distinct classifications of orexin receptor antagonists have been developed: selective orexin receptor antagonists (SORAs) and dual orexin receptor antagonists (DORAs). As their name suggests, SORAs exhibit receptor-type selectivity, as they have a binding affinity for either OX1R or OX2R. Preliminary studies showed OX2R signaling primarily dictates arousal, but both OX1R and OX2R signaling is involved in shifting between sleep stages.45 DORAs offer a more holistic and systemic approach to the treatment of insomnia, acting in a nonspecific manner at both orexin receptor subtypes to stimulate sleep-promoting effects.46 Many DORAs have had success in their respective clinical trials, with one successfully obtaining FDA approval for treatment of insomnia in 2014.45 In our analysis we explore the safety, efficacy, risks, and benefits for treatment of insomnia associated with the extant orexin receptor antagonists (Table 2).
Table 2.
Overview of DORAs.
ALMOREX ANT
ACT-078573 (almorexant), developed by Acetelion and Glaxo-SmithKline in 2007, was the first DORA to reach phase III clinical trials. Preclinical trials of almorexant demonstrated that it has low to moderate bioavailability, easily crosses the blood-brain barrier, induces somnolence, and reduces loco-motor activity and muscle tone.47–50 As the preliminary testing of almorexant advanced to the clinical trial stage, studies indicated reduced locomotor activity, increases in sleep cataplectic episodes, improved sleep efficiency, increases in rapid eye movement (REM) sleep, and decreases in time to sleep initiation and time spent in slow wave sleep (SWS).50,51 With a relatively long half-life, almorexant has longer sustained effects compared to other DORAs. Although this pharmaceutical agent showed initial positive effects in the treatment of insomnia symptoms, clinical advancement of almorexant was ultimately discontinued in 2011 because of safety concerns related to abnormal elevated liver enzyme concentrations.52
SB- 649868
SB-649868 is an orally administered DORA developed by GlaxoSmithKline with a half-life of 3 to 6 hours. Preclinical studies show that in rats, doses of 10 and 30 mg/kg are associated with an increase in non-rapid eye movement (NREM) sleep and REM sleep, reduction of sleep latency, with motor impairments being completely absent.53 Clinical trials investigating the effects of SB-649868 have since shown improved sleep induction and sleep maintenance, reduced sleep latency, and increases in α, β, and Θ waves 2 hours after administration in men with primary insomnia.54 Minimal adverse effects of somnolence and fatigue have been reported throughout the clinical trial process, and the agent has generally been well tolerated with doses up to 80 mg.54
LEMBOREX ANT
Developed by Eisai, Inc., lemborexant was created from a parent compound that was previously shown in rats to decrease wakefulness and promote NREM sleep, with no effect on REM sleep.55 Phase II clinical trials have revealed lemborexant's ability to significantly improve mean sleep efficiency compared to placebo groups, including shortening sleep latency, and wake after sleep onset (WASO) in patients with insomnia.56 Adverse side effects such as somnolence, headache, and sleep paralysis have been reported.57 In August 2015, Eisai, Inc. and Purdue Pharma agreed to collaboratively develop lemborexant for the commercial market.58 As of 2018, phase III trials in patients with general insomnia are being conducted, along with a phase II study testing lemborexant in patients with irregular sleep-wake rhythm disorder and dementia.59
FILOREX ANT
Filorexant (MK-6096) is a DORA developed by Merck and Co. with a slightly different chemical composition from that of almorexant. Along with its treatment applications for insomnia,60 filorexant was originally investigated as a potential treatment option for episodic migraines61 and diabetic neuropathy.62 However, it was found to be ineffective for both afflictions. Preclinical studies have shown oral administration of 100 mg/kg doses of filorexant to be effective in decreasing locomotor activity in a dose-dependent manner, as well as increasing NREM sleep (+58.9%) and REM sleep (+122.2%) in mice for a 4-hour period.63 Furthermore, oral administration of a 3 mg/kg dose of filorexant elicited significantly reduced active wake time, and increased phase I and phase II SWS and REM sleep in male beagle dogs.63 It has thus far shown a favorable pharmacokinetic profile, indicating higher bioavailability and more rapid binding to orexin receptors than almorexant at a significantly smaller dose.63 In a double-blind, placebo-controlled, 51-site randomized study, filorexant was shown to significantly improve sleep efficiency in nonelderly patients with insomnia; dose-related improvements were observed in both sleep onset and maintenance outcomes.64 Filorexant allows for a favorable residual effect profile because of its short half-life (3 to 6 hours) relative to other DORAs.63 Somnolence was the most prominent residual effect, but was only significant at doses above 10 mg.64,65
SUVOREX ANT
Suvorexant, developed by Merck and Co., was approved by the FDA as a Schedule IV controlled substance in August 2014, making it the only DORA to be available to the public for treatment of insomnia.45,67 Preclinical trials showed suvorexant to be superior to almorexant across nearly all parameters in rats, monkeys, and dogs.43 Whereas almorexant primarily increases REM sleep, suvorexant showed a more balanced sleep architecture profile due to its promotion of both REM and NREM sleep. Suvorexant showed greater potency than almorexant, eliciting a peak bioavailable concentration (Cmax) at a dose of 100 mg/kg of 5.1 μM, whereas almorexant has a Cmax of 0.06 μM at a similar dose—a near 100-fold difference in potency.47
A sleep architecture analysis by Snyder et al. found that suvorexant reduced WASO and sleep latency, while increasing the time spent in each stage of sleep as well as the total sleep time (TST), as compared to placebo (P < .05).68 The percentage of TST spent in each stage of sleep upon administration of either 20/15 mg or 40/30 mg of suvorexant differed slightly as compared to placebo; stage N1 sleep (decrease of ≤ 1%), stage N2 sleep (decrease of ≤ 2.2%), stage N3 sleep (decrease of ≤ 0.8%), and stage R sleep (increase of ≤ 3.9%). The increase in amount of time spent in each sleep stage was consistent across each third of the night, with the exception of stage N2 sleep showing greater increases in the last two-thirds of the night and stage N3 sleep showing increases in the first one-third of the night. Power spectral analyses of NREM sleep in patients treated with suvorexant, as compared to placebo, revealed minimal effect on the power spectral sleep profile. One night of treatment showed slight decreases in the power of gamma and beta bands (3% to 6%) and a small increase in the power of delta band (4% to 8%), with no significant difference in power of these bands compared to placebo persisting after 1 and 3 months. Reduced WASO along with reduced sleep latency and increased TST were also confirmed with polysomnography.
In a randomized, double-blind phase II clinical trial for primary insomnia with two 4-week periods of oral administration of suvorexant at increasing doses (10 mg, 20 mg, 40 mg, and 80 mg),69 results showed suvorexant significantly improved in a dose-dependent manner. In two phase III trials, one lasting 3 months and the other lasting 1 year, suvorexant proved effective at improving sleep onset and maintenance in adult patients with insomnia through nightly administration (20/15 mg and 40/30 mg) of suvorexant.70,71
Existing data available on the safety profile of suvorexant is limited because the sample sizes from published studies are still relatively small and include mostly healthy volunteers. Thus far, the medication has been well tolerated by elderly (age 65 years and older)71 and nonelderly (age 18–64 years) men and women with insomnia at doses up to 20 mg.72 Several studies report somnolence as the most frequent adverse event.69,71,72 Excessive sedation and falls are a risk for all sedative hypnotics,73–75 and few data are currently available to assess these risks in suvorexant. There was no reported difference in falls for patients receiving suvorexant compared to placebo.76 Using an on-the-road driving performance assessment, there was no residual impairment detected 9 hours after bedtime dosing of healthy volunteers.77 However, further studies with larger sample sizes are needed to better assess both the risk of falls and accidents related to somnolence.
Headaches, abnormal dreams, dry mouth, cough, diarrhea, and upper respiratory tract infection were all reported at the 20-mg dose in healthy volunteers.69 Doses of 40 mg and higher had higher prevalence of adverse effects, such as mild somnolence, headaches, dizziness, and abnormal dreams whereas doses of 10 and 20 mg showed adverse events similar to those of the placebo group.69 Even after continual use for 4 weeks, administration of suvorexant was not associated with next-day hangover effects, rebound insomnia, complex sleep-related behaviors, or withdrawal effects.70 Importantly, cognitive and motor impairments, next-day hangover, anterograde amnesia, rebound insomnia, and withdrawal effects were all absent.70,72
Suvorexant reduces REM sleep latency and increases the duration of REM sleep in mice.78,79 This effect can potentially exacerbate certain sleep disorders including obstructive sleep apnea (OSA), REM sleep behavior disorder, or isolated sleep paralysis. In a randomized placebo-controlled crossover study in patients with mild to moderate OSA, neither a single dose (40 mg) of suvoxerant nor multiple doses resulted in clinically meaningful respiratory effects during sleep compared to placebo. However, the study participants did not have evidence of hypoxemia/hypoventilation on their baseline polysomnography. Effects of suvorexant in patients with severe OSA have not been studied. Until further studies are completed, the medication must be used cautiously at lower doses in patients with OSA.80 Reductions in orexin levels can result in cataplexy, sleep paralysis, and both hypnogogic and hypnopompic hallucinations, all of which have been reported in phase 3 clinical trials.76 Suvorexant is a Schedule IV medication and, like most other benzodiazepines and BzRAs hypnotics, there is addiction potential that will need to be estimated over time.
DOR As IN COMPARISON WITH OTHER CURRENTLY AVAILABLE SEDATIVE HYPNOTICS
Without head-to-head trials, comparisons between DORAs and other currently available sedative hypnotics are speculative. However, some inferences about efficacy and safety can be made and underlying mechanisms of action can be compared. Benzodiazepines and non-BzRAs, enhance the functioning of the brain's primary inhibitory neurotransmitter at higher levels in the limbic system and the cortex, which contributes to their risk profile, including impaired motor coordination, lethargy, slurred speech, dizziness, intense mood swings, and fatigue.81 Animal studies demonstrated that prolonged use of benzodiazepines leads to adaptations of GABA receptors, resulting in tolerance and withdrawal symptoms.82 In clinical observational samples, long-term use of traditional sedative hypnotics is associated with dose tolerance, tachyphylaxis, and dependence for sleeping. Studies also suggest increased all-cause mortality.29 Although in these studies, confounding by comorbidity limits certainty about causal relationships to medications used for sleep.
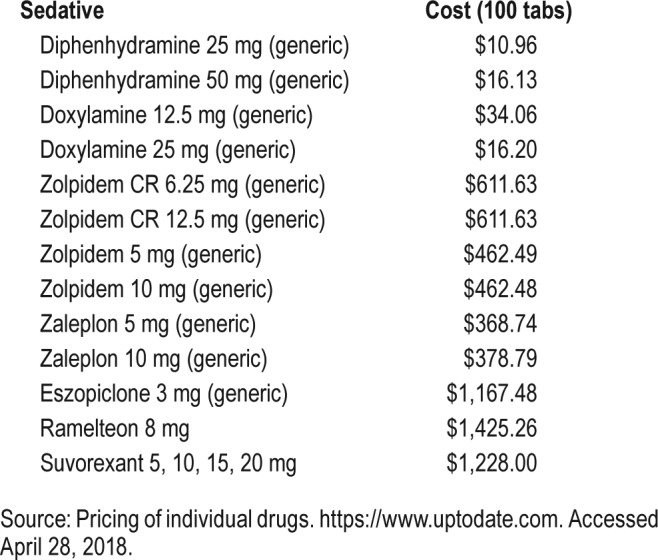
DORAs offer an alternative approach in promoting and maintaining sleep by targeting the orexin system. The scope of orexin signaling in the brain is much more targeted that that of the whole-brain population of GABA neurons, which may result in a more favorable side-effect profile for some patients. Initial research suggests that DORAs promote not only NREM sleep but REM sleep as well, unlike the GABA-mediating agents and SORAs.69,82 Because of the relatively modest sample sizes from the extant suvorexant trials, the prevalence of rarer side effects might not yet be detected. Also, there is currently a knowledge gap about potential drug interactions with DORAs, including with other first-line insomnia treatments. For all sedative hypnotics, side effects of sedation and risk for falls are an important consideration. Whether DORAs pose less of a risk for falls for vulnerable populations (including some geriatric patients, those on multiple polypharmacy) than traditional hypnotics remains to be seen. The American Geriatric Society recommended avoiding use of benzodiazepine hypnotics for treatment of insomnia in the geriatric population because of an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle crashes.83 Minimally this suggests a need for additional insomnia treatments in the elderly. The increased cost of suvorexant, the only FDA-approved DORA, in comparison with other generic hypnotics, is another major concern (Table 3). Finally, drug interactions with CYP3A inhibitors and suvorexant could pose challenges to clinicians.84 In comparison, non-BzRAs and other sedative hypnotics have a low to moderate potential for adverse drug interaction.
Table 3.
Cost of sedative/hypnotics in US dollars.
THEORETIC BASIS FOR A POTENTIAL ROLE OF DOR As IN TREATMENT OF INSOMNIA IN PATIENTS WITH AD
Current scientific exploration has discovered strong evidence for a bidirectional relationship between sleep and AD85,86 (Figure 1). The pathogenesis of AD is initiated by aggregation of amyloid β (Aβ), leading to formation of amyloid plaques, neurofibrillary tau tangles, and cerebral neuronal deficits (see Sanabria-Castro87 for review). AD is preceded by mild cognitive impairment (MCI), a preclinical stage of dementia with both subjective and objective impairment in cognition that is not severe enough to interfere with usual activities of daily living.
Figure 1. Conceptual model of the relationship between sleep and pathogenesis of Alzheimer disease.
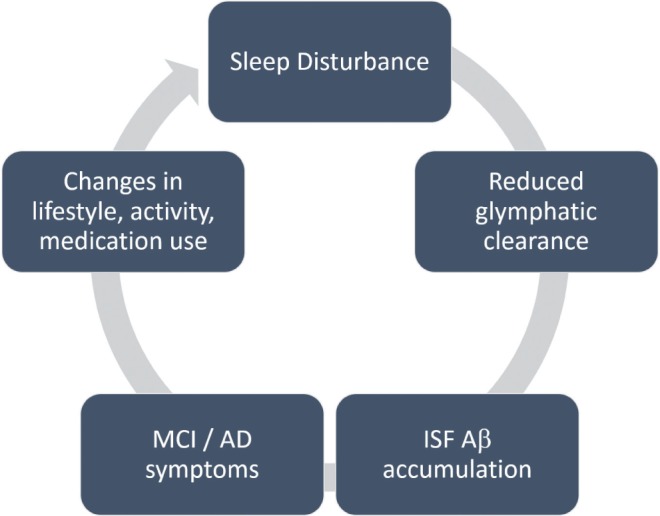
Proposed model of the mechanistic connections between sleep disturbance and the pathogenesis of Alzheimer disease (AD). Disturbances in sleep, particularly slow wave sleep, reduce glymphatic clearance, leading to accumulation of beta amyloid (Aβ). Greater Aβ aggregation accelerates the pathophysiological progression of AD via elevated Aβ burden, which in turn, accelerates mild cognitive impairment (MCI), lifestyle changes, and sleep disruption.
In general, aging results in a decline of the amount of sleep, and this decline is greater in individuals with AD.20 Patients with AD and MCI report insomnia and excessive daytime sleepiness.88,89 Sleep architecture in these patients is remarkable for increased WASO and the reduced duration of stage N3 sleep and REM sleep,90–92 which play major roles in declarative memory (NREM), non-declarative memory (REM), and emotion regulation (REM).93
Sleep deprivation studies using animal models have shown an increase in levels of Aβ output and Aβ aggregation, thereby increasing the risk for amyloid plaque formation.94 This subsequently alters the neural circuitry underlying control of sleep and circadian rhythms, thus exacerbating the sleep disruption.17,95 Cognitive decline can actually expedite the cascade of events via reduced lifestyle regularity, leading to advancement of the AD.17 At the cellular level, fluctuation of Aβ levels in the interstitial fluid (ISF) of the brain follows sleep/wake rhythms. High neuronal activity during wakefulness and REM sleep is associated with increased levels of ISF Aβ,94 whereas Aβ and other ISF metabolic waste is cleared via the glymphatic system during SWS.96 The orexin system likely plays a significant role in modulating the relationship between sleep disturbance and AD pathology. Patients with MCI and AD with subjective sleep problems had higher cerebrospinal fluid orexin levels than patients with MCI without sleep disturbances and controls who had similar subjective sleep problems.97 The association between orexin and AD is strengthened by studies demonstrating significant increase in brain interstitial fluid (ISF) Aβ levels with infusion of orexin to the hippocampus of in Tg2576 mice, an AD disease model.94 Similarly, an orexin knockout mouse strain, APPswe/PS1dE9/ OR-/-, showed increased amounts of sleep as well as decreased amounts of Aβ.95 Animal studies also indicate that DORAs may offer a mechanism for both enhancing sleep and reducing Aβ levels, which has implications for the progression of the AD.98 In Tg2576 mice, intracerebroventricular infusion of almorexant suppressed ISF Aβ.94 Similarly, enhancing sleep in Tg2576 mice through treatment with once-daily almorexant for 8 weeks showed significant declines in Aβ plaque formation.94 These studies strengthen the evidence that orexin potentially mediates the relationship between disturbed sleep and the pathogenesis of AD via modulation of ISF Aβ concentrations. It is plausible that administration of DORAs to treat insomnia in patients with MCI in the early stages of AD during the MCI period could not only treat the insomnia, but could also mitigate cognitive deficits of the disease progression via their effect on Aβ reduction.83 Further studies are needed to evaluate the therapeutic potential of DORAs in managing insomnia symptoms in patients with AD/MCI. A recent randomized controlled trial found that for elderly patients newly admitted to emergency care, 3 days of acute treatment with 15 mg of suvorexant reduced the likelihood of the development of delirium while in the hospital, as compared to placebo.99 The authors speculate this effect could be mediated by the improvement of the sleep-wake cycle via the orexin system.
CONCLUSIONS
Current pharmacological options for the treatment of insomnia insufficiently meet the needs of all patients with insomnia, especially in more medically complex populations, such as the elderly. Traditional therapeutic options for patients with insomnia are complicated by their safety profiles and are not always effective for improving both sleep onset and maintenance. From the initial published clinical trials, targeting the orexin receptor system offers an additional pharmaceutical option to treat insomnia.100,101
Some mild to moderate side effects have been reported for suvorexant, but the sample sizes are likely insufficient to detect all possible side effects. Participants in the studies were mostly healthy volunteers with primary insomnia. Somnolence and risk for falls is a consideration with all sedative hypnotics, including DORAs. Suvorexant is expensive in comparison with many generic sedatives, an important consideration. The potential for adverse drug interactions is likely higher for suvorexant in comparison with non-BzRAs.
Importantly, because of the newly established role of the orexin system in AD, and the bidirectional relationship between disturbed sleep and AD progression,85,97,98 DORAs may offer a potential treatment of comorbid insomnia in patients with AD. More clinical trials are needed to evaluate the effectiveness of DORAs for the treatment of not only primary insomnia, but also in secondary insomnia with other medical comorbidities. Head-to-head trials between DORAs and traditional sedatives and hypnotics and studies evaluating long-term efficacy of the medications are warranted.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Off-label or investigational use is included. The authors report no conflicts of interest.
ABBREVIATIONS
- Aβ
amyloid β
- AD
Alzheimer disease
- ARAS
ascending reticular activating system
- BzRA
benzodiazepine receptor agonist
- CBT-I
cognitive behavioral therapy for insomnia
- CNS
central nervous system
- DORA
dual orexin receptor antagonist
- DR
dorsal raphe nucleus
- FDA
United States Food and Drug Administration
- GABA
gamma amino butyric acid
- ISF
interstitial fluid
- LC
locus coeruleus
- LDT/PPT
lateral dorsal tegmentum/pedunculopontine tegmentum
- MCI
mild cognitive impairment
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- OX1R
orexin receptor 1
- OX2R
orexin receptor 2
- REM
rapid eye movement
- SORA
selective orexin receptor antagonist
- SWS
slow wave sleep
- TST
total sleep time
- VLPO
ventrolateral preoptic region
- WASO
wake after sleep onset
REFERENCES
- 1.Calhoun SL, Fernandez-Mendoza J, Vgontazas AN, Liao D, Bixler EO. Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects. Sleep Med. 2014;15(1):91–95. doi: 10.1016/j.sleep.2013.08.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Family Med Prim Care. 2016;5(4):780–784. doi: 10.4103/2249-4863.201153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 4.Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment. Prevalence and correlates. Arch Gen Psychiatry. 1985;42(3):225–232. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- 5.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 6.Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997;31(3):333–346. doi: 10.1016/s0022-3956(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 7.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69(6):592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. doi: 10.1016/S0140-6736(11)60750-2. [DOI] [PubMed] [Google Scholar]
- 10.Jaussent I, Bouyer J, Ancelin ML, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011;34(8):1103–1110. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blachier M, Dauvilliers Y, Jaussent I, et al. Excessive daytime sleepiness and vascular events: the Three City Study. Ann Neurol. 2012;71(5):661–667. doi: 10.1002/ana.22656. [DOI] [PubMed] [Google Scholar]
- 12.Jaussent I, Empana JP, Ancelin ML, et al. Insomnia, daytime sleepiness and cardio-cerebrovascular diseases in the elderly: a 6-year prospective study. PLoS One. 2013;8(2):e56048. doi: 10.1371/journal.pone.0056048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillman DR, Lack LC. Public health implications of sleep loss: the community burden. Med J Aust. 2013;199(8):S7–S10. doi: 10.5694/mja13.10620. [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 2011;34(9):1161–1171. doi: 10.5665/SLEEP.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pigeon WR, Woosley JA, Lichstein KL. Insomnia and hypnotic medications are associated with suicidal ideation in a community population. Arch Suicide Res. 2014;8(2):170–180. doi: 10.1080/13811118.2013.824837. [DOI] [PubMed] [Google Scholar]
- 16.Soehner AM, Harvey AG. Prevalence and functional consequences of severe insomnia symptoms in mood and anxiety disorders: results from a nationally representative sample. Sleep. 2012;35(10):1367–1375. doi: 10.5665/sleep.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 19.Ong JC, Kuo TF, Manber R. Who is at risk for dropout from group cognitive-behavior therapy for insomnia? J Psychosom Res. 2008;64(4):419–425. doi: 10.1016/j.jpsychores.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gooneratne NS, Vitiello MV. Sleep in older adults: normative changes, sleep disorders, and treatment options. Clin Geriatr Med. 2014;30(3):591–627. doi: 10.1016/j.cger.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 22.Pinto LR, Jr, Bittencourt LR, Treptow EC, Braga LR, Tufik S. Eszopiclone versus zopiclone in the treatment of insomnia. Clinics (Sao Paulo) 2016;71(1):5–9. doi: 10.6061/clinics/2016(01)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann CN, Spira AP, Depp CA, Mojtabia R. Long-term use of Benzodiazepines and Nonbenzodiazepine Hypnotics, 1999-2014. Psychiatr Serv. 2018;69(2):235–238. doi: 10.1176/appi.ps.201700095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prescription Sleep Aid Use Among Adults: United States, 2005-2010; National Center for Health Statistics. [Accessed August 29, 2016]. https://www.cdc.gov/nchs/data/databriefs/db127.htm. Published August 2013.
- 25.FDA. Advisory committee briefing document: suvorexant (NDA 204569) [Accessed April 6, 2016]. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/peripheralandcentralnervoussystemdrugsadvisorycommittee/ucm352970.pdf. Published 2013.
- 26.Toner LC, Tsambiras BM, Catalano G, Catalano MC, Cooper DS. Central nervous system side effects associated with zolpidem treatment. Clin Neuropharmacol. 2000;23(1):54–58. doi: 10.1097/00002826-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs. 2004;18(5):297–328. doi: 10.2165/00023210-200418050-00003. [DOI] [PubMed] [Google Scholar]
- 28.Dolder CR, Nelson MH. Hypnosedative-induced complex behaviours: incidence, mechanisms and management. CNS Drugs. 2008;22(12):1021–1036. doi: 10.2165/0023210-200822120-00005. [DOI] [PubMed] [Google Scholar]
- 29.Wilson SJ, Nutt DJ, Alford C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24(11):1577–1601. doi: 10.1177/0269881110379307. [DOI] [PubMed] [Google Scholar]
- 30.DeLecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 32.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 33.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 34.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian, regulation, and regulatory feedback. J Biol Rhythms. 2006;21(6):482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- 37.Nixon JP, Kotz CM, Novak CM, Billington CJ, Teske JA. Neuropeptides controlling energy balance: orexins and neuromedins. Handb Exp Pharmacol. 2012;(209):77–109. doi: 10.1007/978-3-642-24716-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav. 2000;37(4):335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- 39.Nattie E, Li A. Respiration and autonomic regulation and orexin. Prog Brain Res. 2012;198:25–46. doi: 10.1016/B978-0-444-59489-1.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahler SV, Moorman DE, Smith RJ, James MG, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17(10):1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villano I, Messina A, Valenzano A, et al. Basal forebrain cholinergic system and orexin neurons: effects on attention. Front Behav Neurosci. 2017;11:10. doi: 10.3389/fnbeh.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 43.Kodadek T, Cai D. Chemistry and biology of orexin signaling. Mol Biosyst. 2010;6(8):1366–1375. doi: 10.1039/c003468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dugovic C, Shelton JE, Aluisio LE, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330(1):142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- 45.Kumar A, Chanana P, Choudhary S. Emerging role of orexin antagonists in insomnia therapeutics: an update on SORAs and DORAs. Pharmacol Rep. 2016;68(2):231–242. doi: 10.1016/j.pharep.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Morairty SR, Revel FG, Malherbe P, et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One. 2012;7(7):e39131. doi: 10.1371/journal.pone.0039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palasz A, Lapray D, Peyron C, et al. Dual orexin receptor antagonists—promising agents in the treatment of sleep disorders. Int J Neuropsychopharmacol. 2014;17(1):157–168. doi: 10.1017/S1461145713000552. [DOI] [PubMed] [Google Scholar]
- 48.Malherbe P, Borroni E, Pinard E, Wettstein JG, Knoflach F. Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol Pharmacol. 2009;76(3):618–631. doi: 10.1124/mol.109.055152. [DOI] [PubMed] [Google Scholar]
- 49.Neubauer DN. Almorexant, a dual orexin receptor antagonist for the treatment of insomnia. Curr Opin Investig Drugs, 2010;11(1):101–110. [PubMed] [Google Scholar]
- 50.Mang GM, Durst T, Burki H, et al. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep. 2012;35(12):1625–1635. doi: 10.5665/sleep.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoever P, de Haas SL, Dorffner G, Chiossi E, van Gerven JM, Dingemanse J. Orexin receptor antagonism: an ascending multiple-dose study with almorexant. J Psychopharmacol. 2012;26(8):1071–1080. doi: 10.1177/0269881112448946. [DOI] [PubMed] [Google Scholar]
- 52.Roecker AJ, Cox CD, Coleman PJ. Orexin receptor antagonists: New therapeutic agents for the treatment of insomnia. J Med Chem. 2016;59(2):504–530. doi: 10.1021/acs.jmedchem.5b00832. [DOI] [PubMed] [Google Scholar]
- 53.Dugovic C, Shelton JE, Yun S, Bonaventure P, Shireman BT, Lovenberg TW. Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Front Neurosci. 2014;8:28. doi: 10.3389/fnins.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bettica P, Squassante L, Zamuner S, Nucci G, Danker-Hopfe H, Ratti E. The orexin antagonist SB-649868 promotes and maintains sleep in men with primary insomnia. Sleep. 2012;32(8):1097–1104. doi: 10.5665/sleep.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshida Y, Terauchi T, Naoe Y, et al. Design, synthesis, and structure—activity relationships of a series of novel N-aryl-2-phenylcyclopropanecarboxamide that are potent and orally active orexin receptor antagonists. Bioorg Med Chem. 2014;22(21):6071–6088. doi: 10.1016/j.bmc.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 56.Murphy P, Moline M, Mayleben D, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299. doi: 10.5664/jcsm.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eisai Demonstrates Efficacy of Investigational Dual Orexin Receptor Antagonist e2006 in Sleep Initiation and Maintenance. [Accessed April 25, 2017]. http://www.eisai.com/news/news201477.htm. Published December 10, 2014.
- 58.Eisai Inc. and Purdue Pharma Enter Worldwide Collaboration to Develop and Commercialize Lemborexant. [Accessed April 25, 2017]. http://www.eisai.com/news/news201558.html. Published August 31, 2015.
- 59.Long-term Study of Lemborexant in Insomnia Disorder. [Accessed January 15, 2018]. https://clinicaltrials.gov/ct2/show/NCT02952820. Published November 2, 2016.
- 60.To Evaluate The Effects Of SB-649868 (10, 30 Mg and 60 Mg) On Subjects With Primary Insomnia. [Accessed April 28, 2016]. https://clinicaltrials.gov/ct2/show/NCT00426816. Published January 23, 2007. Updated August 9, 2012.
- 61.Chabi A, Zhang Y, Jackson S, et al. Randomized controlled trial of the orexin receptor antagonist filorexant for migraine prophylaxis. Cephalalgia. 2015;35(5):379–388. doi: 10.1177/0333102414544979. [DOI] [PubMed] [Google Scholar]
- 62.Herring WJ, Ge JY, Jackson S, Assaid C, Connor KM, Michelson D. Orexin receptor antagonism in painful diabetic neuropathy: a phase 2 trial with filorexant. Clin J Pain. 2018;34(1):37–43. doi: 10.1097/AJP.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 63.Winrow CJ, Gotter AL, Cox CD, et al. Pharmacological characterization of MK-6096—A dual orexin receptor antagonist for insomnia. Neuropharmacology. 2012;62(2):978–987. doi: 10.1016/j.neuropharm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Connor KM, Mahoney E, Jackson S, et al. A phase II dose-ranging study evaluating the efficacy and safety of the orexin receptor antagonist filorexant (MK-6096) in patients with primary insomnia. Int J Neuropsychopharmacol. 2016;19(8) doi: 10.1093/ijnp/pyw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun H, Kennedy WD, Laethem T, et al. The single dose pharmacokinetic (PK) and pharmacodynamic (PD) profiles of suvorexant (MK-4305), a dual orexin receptor antagonist, in health male subjects. Sleep Biol Rhythms. 2011;9:332. [Google Scholar]
- 66.Connor KM, Ceesay P, Hutzelmann J, et al. Phase II proof-of-concept trial of the orexin receptor antagonist filorexant (MK-6096) in patients with major depressive disorder. Int J Neuropsychopharmacol. 2017;20(8):613–618. doi: 10.1093/ijnp/pyx033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.FDA approves new type of sleep drug, Belsomra. [Accessed April 25, 2017]. https://wayback.archive-it.org/7993/20161022200044/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm409950.htm. Published August 13, 2014. Updated August 18, 2014.
- 68.Snyder E, Ma J, Svetnik V, et al. Effects of suvorexant on sleep architecture and power spectral profile in patients with insomnia: analysis of pooled phase 3 data. Sleep Med. 2016;19:93–100. doi: 10.1016/j.sleep.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: A randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–2274. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- 70.Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant, an orexin receptor antagonist, during 1-year treatment of insomnia followed by abrupt discontinuation of treatment: a randomized, double-blind, placebo-controlled clinical trial. Lancet Neurol. 2014;13(5):461–471. doi: 10.1016/S1474-4422(14)70053-5. [DOI] [PubMed] [Google Scholar]
- 71.Herring WJ, Connor KM, Synder E, et al. Suvorexant in elderly patients with insomnia: Pooled analyses of data from phase III randomized controlled clinical trials. Am J Geriatr Psychiatry. 2017;25(7):791–802. doi: 10.1016/j.jagp.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Herring WJ, Connor KM, Snyder E, et al. Clinical profile of suvorexant for the treatment of insomnia over 3 months in women and men: subgroup analysis of pooled phase-3 data. Psychopharmacology. 2017;234(11):1703–1711. doi: 10.1007/s00213-017-4573-1. [DOI] [PubMed] [Google Scholar]
- 73.Kolla BP, Lovely JK, Mansukhani MP, et al. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1–6. doi: 10.1002/jhm.1985. [DOI] [PubMed] [Google Scholar]
- 74.Berry SD, Lee Y, Cai S, et al. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med. 2013;173(9):754–761. doi: 10.1001/jamainternmed.2013.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diem SJ, Ewing SK, Stone KL, et al. Use of non-benzodiazepine sedative hypnotics and risk of falls in older men. J Gerontol Geriatr Res. 2014;3(3):158. doi: 10.4172/2167-7182.1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farkas RH, Katz R, Illoh K, et al. Application Number 204569Orig1s000: Medical Review(s) [Accessed April 25, 2017]. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/204569Orig1s000MedR.pdf. Published 2013.
- 77.Vermeeren A, Vets E, Vuurman EF, et al. On-the-road driving performance the morning after bedtime use of suvorexant 15 and 30 mg in healthy elderly. Psychopharmacology. 2016;233(18):3341–3351. doi: 10.1007/s00213-016-4375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Betschart C, Hintermann S, Behnke D, et al. Identification of a novel series of orexin receptor antagonists with a distinct effect on sleep architecture for the treatment of insomnia. J Med Chem. 2013;56(19):7590–7607. doi: 10.1021/jm4007627. [DOI] [PubMed] [Google Scholar]
- 79.Etori K, Saito YC, Tsujino N, Sakurai T. Effects of a newly developed potent orexin-2 receptor-selective antagonist, compound 1 m, on sleep/wakefulness states in mice. Front Neurosci. 2014;8:8. doi: 10.3389/fnins.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun H, Palcza J, Card D, et al. Effects of suvorexant, an orexin receptor antagonist, on respiration during sleep in patients with obstructive sleep apnea. J Clin Sleep Med: 2016;12(1):9–17. doi: 10.5664/jcsm.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Griffin CE, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214–223. [PMC free article] [PubMed] [Google Scholar]
- 82.Brisbare-Roch C, Dingemanse J, Koberstein R, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med, 2007;13(2):150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 83.American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merck Sharp & Dohme Corp. Whitehouse Station, NJ: Merck Sharp & Dohme; 2014. BELSOMRA Prescribing Information. [Google Scholar]
- 85.Cedernaes J, Osorio RS, Varga AW, Kam K, Schiöth HB, Benedict C. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer's disease. Sleep Med Rev. 2017;31:102–111. doi: 10.1016/j.smrv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kent BA, Mistlberger RE. Sleep and hippocampal neurogenesis: implications for Alzheimer's disease. Front Neuroendocrinol. 2017;45:35–52. doi: 10.1016/j.yfrne.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Sanabria-Castro A, Alvarado-Echeverría I, Monge-Bonilla C. Molecular pathogenesis of Alzheimer's Disease: an update. Ann Neurosci. 2017;24(1):46–54. doi: 10.1159/000464422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer's disease. Sleep Med. 2005;6(4):347–352. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 89.Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr. 2009;21(4):654–666. doi: 10.1017/S1041610209009120. [DOI] [PubMed] [Google Scholar]
- 90.Hita-Yañez E, Atienza M, Cantero JL. Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep. 2013;36(9):1327–1334. doi: 10.5665/sleep.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naismith SL, Hickie IB, Terpening Z, et al. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimers Dis. 2014;38(4):857–866. doi: 10.3233/JAD-131217. [DOI] [PubMed] [Google Scholar]
- 92.Holth J, Patel T, Holtzman DM. Sleep in Alzheimer's Disease - beyond amyloid. Neurobiol Sleep Circadian Rhythms. 2017;2:4–14. doi: 10.1016/j.nbscr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 2016;352(6287):812–816. doi: 10.1126/science.aad5252. [DOI] [PubMed] [Google Scholar]
- 94.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roh JH, Huang Y, Bero AW, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med. 2012;4(150):150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xie L, Kang H, Xu Q, et al. Sleep drives metabolic clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liguori C, Nuccetelli M, Izzi F, et al. Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinalfluid levels in mild cognitive impairment due to Alzheimer's disease. Neurobiol Aging. 2016;40:120–126. doi: 10.1016/j.neurobiolaging.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 98.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci. 2016;39(8):552–566. doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hatta K, Kishi Y, Wada K, et al. J Clin Psychiatry. 2017;78(8):e970–e979. doi: 10.4088/JCP.16m11194. [DOI] [PubMed] [Google Scholar]
- 10.Herring WJ, Connor K, Snyder E, et al. Suvorexant in elderly patients with insomnia: pooled analyses of data from phase III randomized controlled clinical trials. Am J Geriatr Psychiatry. 2017;25(7):791–802. doi: 10.1016/j.jagp.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 101.Roth T, Black J, Cluydts R, et al. Dual orexin receptor antagonist, almorexant, in elderly patients with primary insomnia: a randomized, controlled study. Sleep. 2017;40(2) doi: 10.1093/sleep/zsw034. [DOI] [PubMed] [Google Scholar]