Abstract
Study Objectives:
To test whether a customized positional therapy device, PrenaBelt, would reduce time spent sleeping supine and evaluate any change in maternal or fetal parameters, in a group of healthy pregnant women in the third trimester of pregnancy.
Methods:
Participants underwent an in-home, overnight sleep study during late pregnancy (32–38 weeks). Participants were observed over 2 nights: 1 night when the PrenaBelt was not worn (nonintervention or control) and 1 night when it was (intervention). The intervention night was randomly allocated, and the study nights were consecutive. On the control night, participants were filmed using a night-capable (infrared) video camera, maternal sleep was measured by the Watch-PAT200, and the fetus was continuously monitored using the Monica AN24. On the intervention night, video, maternal, and fetal monitoring were repeated with the addition of the mother wearing the PrenaBelt.
Results:
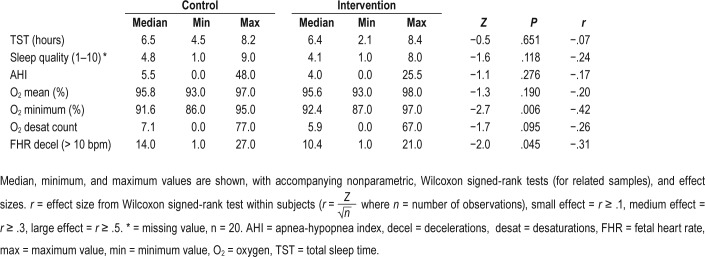
A total of 25 healthy pregnant women were studied. Four had missing data for the Watch-PAT or Monica, and eight had missing or disrupted video data. Video-determined time in bed was not significantly different during intervention and control nights (P = .196, r = −.23). Median time spent supine during the intervention night was reduced from 48.3 minutes, to 28.5 minutes during the control night (P = .064, r = −.33). The difference in the proportion of time spent supine was significant (P = .039). There was no significant difference in objectively estimated sleep time (P = .651, r = −.07). Improvement was observed in both maternal and fetal parameters during the intervention night with an increase in median minimum maternal oxygen saturations (control = 91.6%, intervention = 92.4%, P = .006, r = −.42), fewer maternal oxygen desaturations (control = 7.1, intervention = 5.9, P = .095, r = −.26), and fewer fetal heart rate decelerations (control = 14.0, intervention = 10.4, P = .045, r = −.31) compared to the control night.
Conclusions:
Results provide preliminary evidence that an intervention to reduce supine sleep in late pregnancy may provide maternal and fetal health benefits, with minimal effect on maternal perception of sleep quality and objectively estimated sleep time. Further research to explore relationships between objectively determined maternal sleep position, maternal respiratory indices, and fetal well-being is warranted.
Citation:
Warland J, Dorrian J, Kember AJ, Phillips C, Borazjani A, Morrison JL, O'Brien LM. Modifying maternal sleep position in late pregnancy through positional therapy: a feasibility study. J Clin Sleep Med. 2018;14(8):1387–1397.
Keywords: positional therapy, pregnancy, sleep position, stillbirth
BRIEF SUMMARY
Current Knowledge/Study Rationale: Research suggests that modifying maternal sleep position, especially the position in which pregnant women go to sleep, may be one way to reduce stillbirth. Positional therapy is a well-accepted means to reduce supine sleep time in the adult (nonpregnant) population with sleep-disordered breathing. However, it is not known whether positional therapy is similarly effective in the pregnant population.
Study Impact: The current study shows that wearing a customized positional therapy device significantly reduces time spent sleeping supine in late pregnancy, without significant effect on length and quality of sleep and with beneficial physiological effects for both the pregnant mother and her fetus. Future research is therefore needed to further explore the most effective means of promoting lengthy non-supine sleep in late pregnancy.
INTRODUCTION
In obstetrics, it is well known that when a pregnant woman assumes the supine position, whether during the day, during labor, or on the operating table, maternal cardiovascular parameters1–13 and/or fetal oxygenation4,11,12 are altered, occasionally causing substantial fetal heart rate changes.12,13 However, until recently, there has been little evidence on the effect of the supine position during sleep in pregnancy. Several epidemio-logical studies now suggest that maternal supine sleep position may be a risk factor for stillbirth14–19 and/or low birth weight/ fetal growth restriction (FGR); birth weight less than 2.5 kg or < 10th centile for gestational age.16,19 One suggested mechanism for the effect of supine sleep in late pregnancy focuses on occlusion of the inferior vena cava (running down the right side of the mother's body), and subsequent deprivation of oxygen to the fetus.9 It has also been suggested that supine sleep may influence maternal and fetal health via exacerbating symptoms of sleep-disordered breathing (SDB), including snoring, apnea, and hypopnea.20
This research is of interest because supine sleeping position is potentially modifiable. There have been many and varied methods proposed to avoid supine sleep in the general population. For example, in the late 18th century and early 20th century, soldiers in the American War of Independence and World War I were told to sleep with their rucksacks filled and strapped to their back in order to avoid supine-related snoring and thereby disclose their position to the enemy.21 In 1984, in a letter to the editor of Chest, a patient's wife explained how she cured her husband's snoring/apnea problem by sewing “a pocket into the back of a T-shirt” and inserting “a hollow, lightweight plastic ball (about the size of a tennis ball)” in order to prevent him from sleeping on his back.22 This form of intervention—preventing individuals from assuming the supine position during sleep—is now known as positional therapy (PT). PT is a noninvasive, inexpensive, long-established, safe, and effective treatment option for individuals with position-dependent snoring and mild to moderate obstructive sleep apnea (OSA).23–28 There are several ways PT may be used to alert the supine individual to change position, including pressure points, auditory alarms, and vibratory alarms. PT may also be used to restrict the individual's position on the sleeping surface and thus prevent him/her from adopting the supine position in the first place.
Given the emerging research indicating that supine sleep may have a negative effect on fetal well-being in late pregnancy and that this position may be relatively easy to modify in pregnancy, we conducted a study to determine the effect, on maternal and fetal parameters, of a PT device. In 2014, members of our team (AK, AB) developed a PT device specifically for use in pregnancy, called “PrenaBelt” (Global Innovations for Reproductive Health and Life, Cleveland, Ohio, United States) (Figure 1). The PrenaBelt is designed to cause subtle pressure points on the pregnant user's lower back and buttocks when she lies in the supine position and thereby activate her body's natural mechanism to spontaneously reposition to maintain comfort. We aimed to test the effectiveness of this device to reduce time spent sleeping supine in a group of healthy pregnant women in the third trimester of pregnancy, without negative effects on sleep duration or quality, and also to determine whether maternal and fetal indices of sleep quality and health improved as a result.
Figure 1. PrenaBelt: a positional therapy device designed specifically for pregnant women.
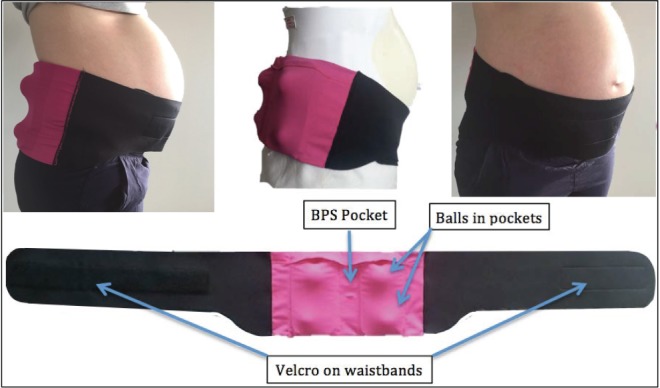
PrenaBelt (Global Innovations for Reproductive Health & Life, Cleveland, Ohio, USA Patent #WO2016176632A1). Device for investigational use only. BPS = body position sensor.
Specifically, we hypothesized that wearing a customized PT device during late pregnancy would be associated with:
A reduction in the amount of time spent sleeping supine;
No differences in sleep duration or sleep efficiency;
Lower maternal apnea-hypopnea index, and higher maternal oxygen saturations; and
Fewer fetal heart rate (FHR) decelerations.
In order to explore the effect of supine position-related SDB on these relationships, participants were also classified according to whether they met criteria for SDB (while not wearing the device). Analyses explored differences with and without the device as well as by SDB status.
METHODS
Sample and Procedure
Healthy pregnant women were recruited to the study via convenience29 and snowball sampling.30 Study flyers, with study details and eligibility information, were placed around the participating university's campuses and community notice boards. Potential participants who saw the flyer and expressed interest by contacting the researchers were sent the information sheet; if they were still interested, they were booked into the 2-night study. All women who expressed their interest in participating in the study were eligible and participated. Women who participated in the study were asked to share the study flyer with their pregnant friends. In this manner, we recruited healthy pregnant women (n = 25) for an in-home sleep study in late pregnancy (32–38 weeks).
The study was conducted over 2 nights—1 night with no intervention and 1 night with intervention—using participants as their own control. The intervention night was randomly allocated and the study nights were consecutive. On the control (nonintervention) night, participants were filmed using a nightcapable (infrared) video camera, maternal sleep was monitored using the Watch-PAT200 (Itamar Medical Ltd., Cesarea, Israel) (Watch-PAT), and the fetus was continuously monitored using the Monica AN24 (Monica Healthcare Ltd., Nottingham, United Kingdom). On the intervention night, video, maternal, and fetal monitoring were repeated with the addition of the PrenaBelt. On the intervention night, a body position sensor (BPS) (HOBO Pendant G Data Logger, UA-004-64, Onset Computer Corporation, Cape Cod, Massachusetts, United States), was also placed in a pocket on the posterior aspect of the belt.
A research assistant attended the home of the participant on the evening of both nights. She first gained written consent from the woman (and her bed partner) and then set up all equipment (as described in the next paragraphs). She returned the evening of the second night to collect the used equipment and replace with recharged equipment. Finally, she returned to the participant's home the morning after the second night of the study to collect all the equipment including the completed questionnaire. Participants were instructed to sleep as normal with regard to hours in bed, number of pillows used, and room lighting.
Questionnaire
All participants completed a short questionnaire regarding their usual sleep practices. We had used these questions previously.32 Questions included what position they usually slept in when not pregnant, and what position they had been sleeping in during the previous month. We also asked participants to estimate the time they spent sleeping supine on both study nights as well as a scaled question to determine potential effect of the study on sleep quality; this was “on a scale of 1–10 (1 restless and 10 sound) I would describe my quality of sleep last night as…” Finally, participants were asked to comment on the acceptability of wearing the PrenaBelt.
Infrared Video Camera
An infrared-capable handheld video camera was positioned on a tripod at the end of the participant's side of the bed. The participant was asked to turn it on at lights out and off at lights on. The camera was in the participant's complete control. A research assistant was employed to view and score sleep position from the video tape. Sleep position was scored as supine, left, or right in 1-minute epochs from the video camera recordings. This was conducted using the same protocol as a previous published study.32 Thus, time spent sleeping supine was calculated.
Watch-PAT
The Watch-PAT is a portable diagnostic device that uses finger-based plethysmography to record the peripheral arterial tonometry (PAT) signal, maternal heart rate, blood oxygen saturation (SpO2), and actigraphy from a built-in actigraph. The Watch-PAT proprietary software algorithm analyzes the PAT signal amplitude along with the heart rate and SpO2 to estimate the apnea-hypopnea index (AHI). O'Brien and colleagues reported that in a pregnant population, the Watch-PAT demonstrates excellent sensitivity and specificity for identification of obstructive sleep apnea, defined as AHI ≥ 5 events/h on polysomnography.33 However, they also found that the Watch-PAT may overestimate the respiratory disturbance index (RDI) somewhat, especially at high RDI values, and has poor correlation with sleep stages33; these indices were therefore not included in this study. The Watch-PAT measures that were analyzed for this study included total sleep time during the sleep period (minutes), maternal AHI, minimum SpO2 (%), and maternal SpO2 desaturation count. For analysis, participants were also classified into a group as being likely to have SDB if they exhibited an AHI ≥ 5 events/h33,34 during their control night—SDB versus no SDB.
Monica AN24
Overnight fetal and maternal heart rate were recorded using the Monica AN24 (Monica Healthcare, Nottingham, United Kingdom). The research assistant applied the monitor each evening following the manufacturer's instructions for skin preparation, electrode placement, and impedance testing. The device records a fetal electrocardiography with true beat-to-beat intervals being recorded in 1-minute epochs without autocorrelation as used in commercial cardiotocography machines. The software provides hourly numerical values of accelerations and decelerations, extracted from an automated Dawes-Redman analysis35 and then provides summary statistics including large (> 15 bpm) and small (> 10 bpm) deceleration counts. Monica AN24 and Watch-PAT records were aligned and data collected during the measured sleep period were isolated for analysis.
Body Position Sensor
The BPS is a battery-powered, commercially-available, three-axis accelerometer for measuring three-dimensional motion during sports or medical therapy (HOBO Pendant G Data Logger). The PrenaBelt contains a pocket on the posterior aspect of the belt that allows for placement of sensors or other electronic components. On the intervention night, the BPS was placed in this pocket as an additional measure to validate the device's recording of body position against the video recording. The BPS data were downloaded to a computer using HOBOware software (Onset Computer Corporation, Cape Cod, Massachusetts, United States). The participants' body position (left, right, supine, prone) throughout the intervention night (BPS-determined body position) was resolved by analyzing the data via a custom Java code.
Inclusion Criteria
Self-declared healthy pregnancy (ie, no current medical or obstetric complications); self-declared gestational age between 32–38 weeks; singleton.
Exclusion Criteria
Body mass index of ≥ 35 kg/m2 at the beginning of the pregnancy; multiple pregnancy; and known fetal abnormality.
Ethics Approval
Ethics approval was gained from the UniSA Human Research Ethics committee, approval number 0000033795. All participants (and their bed partners) signed a consent form that included all the usual assurances of privacy and confidentiality and participant anonymity.
Analyses
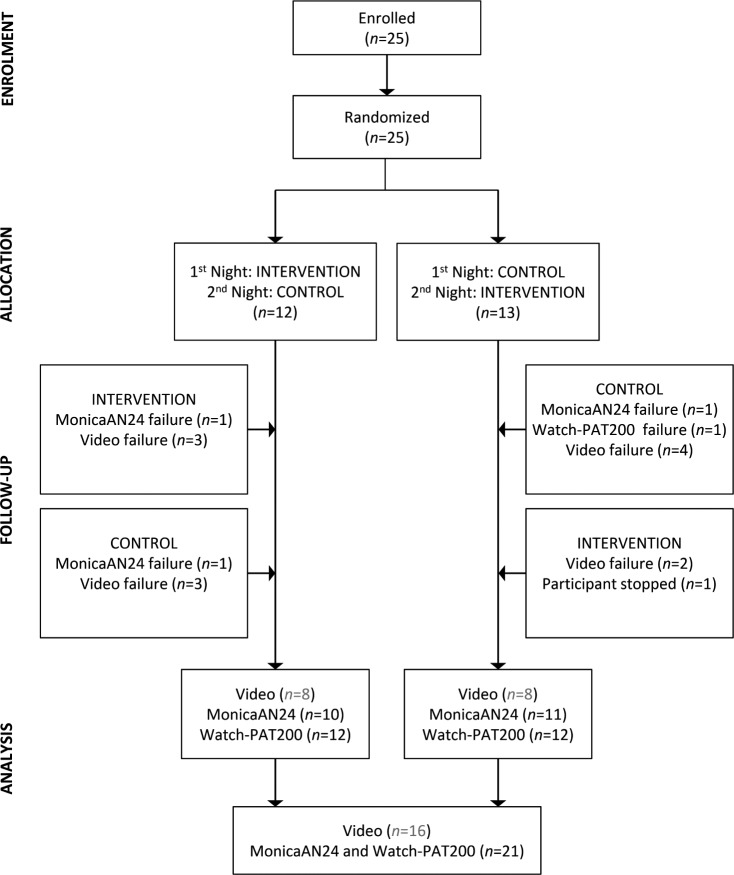
Twenty-five participants were recruited into the study, agreeing to be recorded for 2 nights each (Figure 2).
Figure 2. CONSORT diagram.
There were 25 participants enrolled into this repeated-measures study. Participants were randomized to completed either the intervention or the control night first. Equipment failures for Monica AN24, Watch-PAT, and video recordings are shown, leading to final complete datasets for both intervention and control nights of n = 18 for the primary video analyses, and n = 21 for the primary Monica AN24 and Watch-PAT analyses.
Video-Determined Time in Bed and Sleep Position
There were errors in the video recording for 7 participants, leaving 16 with video data for both nights (Figure 2). Wilcoxon Signed Rank Tests (and associated effect sizes) were used to examine within-subject differences in video-determined time in bed (TIB), time spent supine (minutes), and percentage of TIB spent supine on control compared to intervention nights. To visualize the effect of the intervention on position, lateral/supine position was plotted across elapsed TIB for 16 participants, who spent at least 5 hours in bed on both control and intervention nights. The number of participants on their back across elapsed TIB was also plotted on control compared to intervention nights. For proportion of time in bed spent supine, a generalized estimating equations (GEE) model for binomial distributions with a logit link function (appropriate for proportions) was also conducted, specifying a predictor of condition (control/intervention).
Sleep Length, Quality, Sleep Position, Respiration and Heart Rate
On 2 of the nights, the Monica AN24 failed to record, and on 1 night, because of dropout, the recording was not sufficient for analysis. Data for these two participants was excluded. Data from a third participant was excluded because on 1 night their Watch-PAT recording failed, and on the other night they withdrew partway through the night because of false labor pains. This left full Watch-PAT and Monica AN24 datasets for analysis (both nights) for 21 participants (Figure 2). Of these, 1 participant did not complete their sleep quality ratings. Wilcoxon signed-rank tests (and associated effect sizes) were used to examine within-subject differences between control and intervention nights in total sleep time (TST), TIB, sleep quality ratings, maternal respiratory indicators, and FHR.
In order to explore the potential influence of SDB status on the control night (yes/no), a series of models were conducted. For continuous dependent variables maternal AHI, minimum and mean maternal blood oxygen saturation: minimum SPO2, average FHR, mixed-effects regression specified fixed effects of condition (control/intervention), SDB group (SDB/no SDB) and the condition × SDB group interaction, with a random effect of subject on the intercept. For parsimony, nonsignificant interaction effects were dropped in final reported models. To control for night (order) effects, order was entered as an additional predictor variable. However, it was not significant, nor did it influence the effect of the other predictors on the dependent variable, and therefore it was dropped from final models. This type of model allows for appropriate treatment of within-and between-subject variance.36 For count variables (maternal oxygen desaturation count, FHR deceleration count), GEE for Poisson distributions with a log link function (appropriate for count variables) specified a repeated effect of study night with an exchangeable covariance structure, and predictors of condition (control/intervention), SDB group (SDB/no SDB), and the condition × SDB group interaction. Nonsignificant interaction effects were dropped in final reported models.
Body Position Sensor
Of the available 25 BPS/PrenaBelt nights total, we had 6 BPS/PrenaBelt nights with no video, leaving 19 for analysis. The ability of the BPS to correctly classify the participant's position (supine versus lateral) in comparison with the gold standard (infrared video) was evaluated via 2 × 2 contingency table and the sensitivity and specificity for the supine position classification were calculated.
RESULTS
Participants
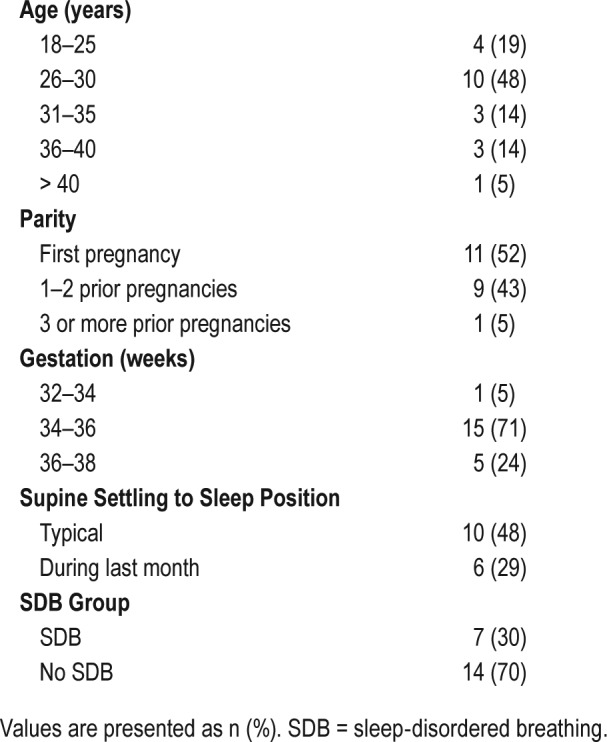
Table 1 shows the age and parity distribution for study participants. Although approximately 50% (n = 10) reported typically sleeping supine, self-reported frequency reduced to 30% during the month immediately before the study nights. Thirty percent (n = 7) met the cutoff for SDB during the control night.
Table 1.
Participant demographic and descriptive information (n = 21).
Video-Determined Sleep Position (n = 16)
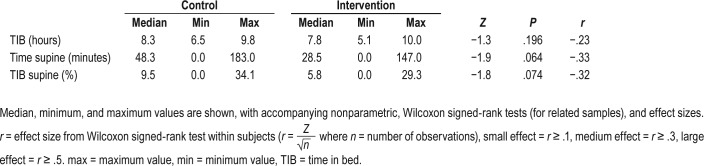
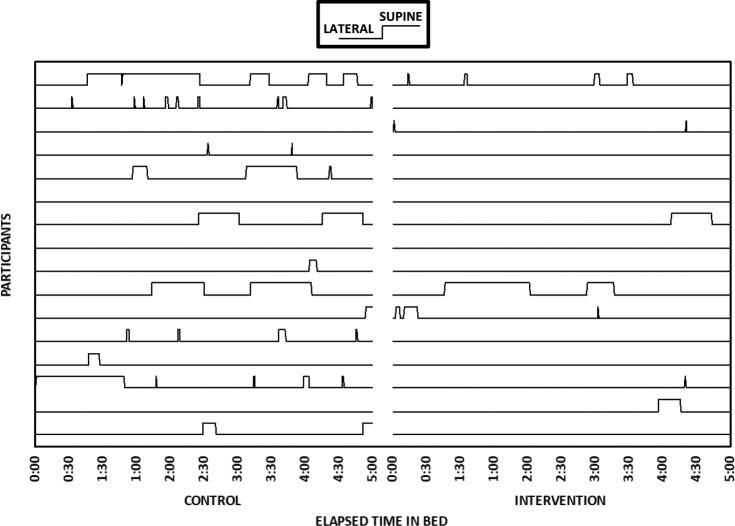
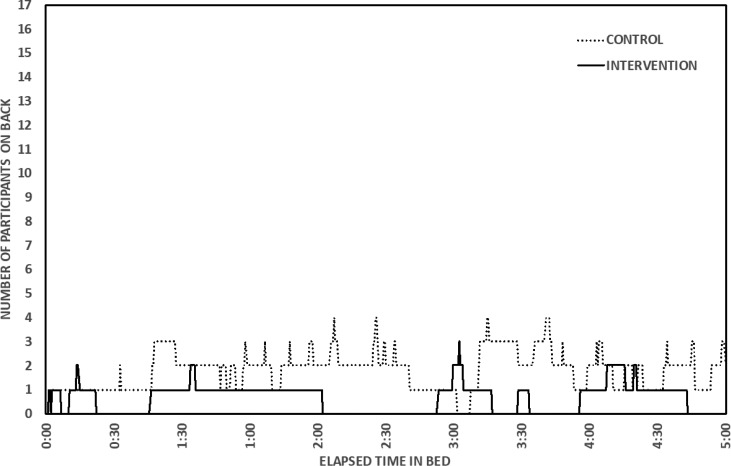
The effect size for differences in video-determined TIB on control and intervention nights was small to medium (r = .23, P = .196, Table 2). Effect sizes for differences in time spent supine (P = .064) and percentage of TIB spent supine (P = .074) were medium (Table 2). GEE (for proportions) yielded a significant difference in video-determined proportion of sleep time spent supine, indicating that it was significantly lower on the intervention night (B = 1.38, χ12 = 4.24, P = .039). Figure 3 shows the patterns in lateral/supine sleep by elapsed time in bed on control and intervention nights for each participant. Most participants showed reduced or eliminated supine sleep during the intervention night; however, 2 participants spent no time in the supine position during the first 5 hours of their TIB, and 1 participant did sleep in the supine position for more than 1 hour during the intervention night. Figure 4 provides a summary of number of participants on their back across elapsed TIB on the control compared to intervention nights.
Table 2.
Video-determined maternal sleep measures (n = 16) during the control night and the intervention (PrenaBelt) nights.
Figure 3. Sleep position for each participant (lines) on control (left) and intervention (right) nights by elapsed time in bed.
n = 16 participants are shown. These participants have complete video records for both nights. The first 5 hours are shown, because these participants were in bed for at least 5 hours on both nights.
Figure 4. Number of participants on their back on control (dotted line) and intervention (solid line) nights by elapsed time in bed.
n = 16 participants are shown. These participants have complete video records for at least 5 hours on both nights.
Sleep Length and Self-Rated Sleep Quality
TST was not significantly different on control and intervention nights (P = .651), with a very small effect size (Table 3). The effect size for the difference in sleep quality ratings between control and intervention nights (P = .118) was small to medium (Table 3). We also asked all our participants to comment on the acceptability of wearing the PrenaBelt, and 22 (88%) agreed that they would participate in such a study again.
Table 3.
Maternal sleep and respiratory measures (from the Watch-PAT) and fetal heart rate measures (from the Monica AN24) during the control night and the intervention (PrenaBelt) nights (n = 21).
Maternal Respiratory Indicators and Fetal Heart Rate
Initial nonparametric comparisons between control and intervention nights revealed a small, nonsignificant effect for AHI (P = .276), small to medium effects for mean maternal oxygen desaturation (P = .190) and desaturation count (P = .095), and medium to large effects for minimum maternal oxygen desatu-ration (P = .006) and FHR deceleration count (P = .045).
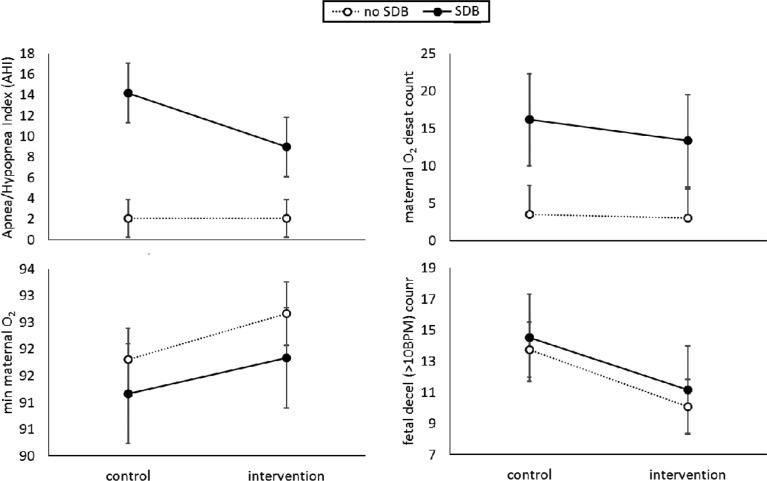
Analyses exploring the potential influence of SDB at baseline suggested that for those in the SDB group, there was a reduction in maternal AHI on the intervention, compared to control nights, whereas for others, maternal AHI was stable across both conditions (Figure 5). There was a significant main effect of SDB group for maternal AHI (F1,19.0 = 9.11, P = .007), with the effect of condition (F1,19.0 = 4.03, P = .059) and the SDB group × condition interaction effect (F1,19.0 = 3.95, P = .061) approaching significance (Figure 5). Maternal oxygen desaturation count was significantly lower during the intervention night (B = 0.18, χ12 = 5.28, P = .022), and for those without SDB (B = −1.56, χ12 = 4.58, P = .032). Minimum maternal oxygen saturation was significantly higher during the intervention night (F1,20.0 = 10.90, P = .004), but was not significantly different between SDB groups (F1,19.0 = 0.469, P = .502; Figure 2). There were no significant differences in mean maternal oxygen saturation by condition (F1,20.0 = 1.72, P = .204) or SDB group (F1,19.0 = 1.28, P = .271). FHR decelerations > 10 bpm were significantly lower during the intervention night (B = 0.296, χ12 = 5.24, P = .022), but were not significantly different between SDB groups (B = −0.073, χ12 = 0.12, P = .734).
Figure 5. Maternal AHI, maternal oxygen desaturations, minimum maternal oxygen saturation, and FHR decelerations during the control and intervention nights by SDB group.
n = 21 participants are shown (no SDB = 14, SDB = 7). AHI = apnea-hypopnea index, FHR = fetal heart rate, SDB = sleep-disordered breathing.
Illustrative Case Example
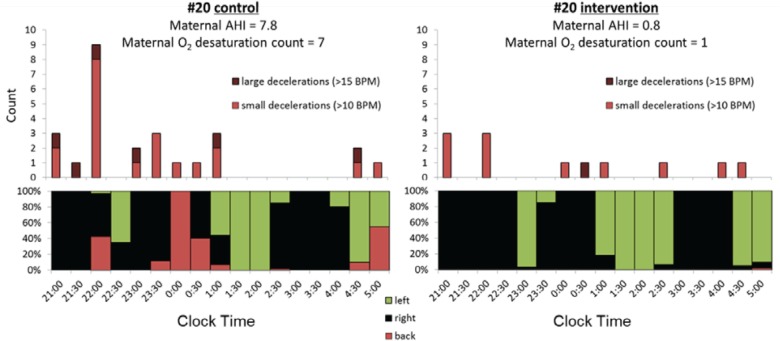
Results from a single case, participant number 20, are shown in Figure 6 for the control (left) and intervention (right) nights. This case was chosen because there were noticeably more large (> 15 bpm) fetal heart rate decelerations (dark red) and small (10–15 bpm) decelerations (light red) relative to supine sleep position (lower section) on the control night relative to the intervention night. Maternal AHI and SPO2 desaturation count were also improved on the intervention night. Following the study, the patient was advised by the midwife on the research team (JW) to avoid supine sleep position for the remaining weeks of the pregnancy (she reported to us that her infant was born alive and well 2 weeks after the study nights).
Figure 6. Example results from individual number 20 during the control (left) and intervention (right) nights.
Large (>15 bpm, dark red) and small (>10 bpm, light red) FHR decelerations are shown (upper) relative to maternal sleep position (lower). Proportion of sleep time spent on back (red), left (green) and right (black) is shown across clock time. Maternal AHI and desaturation count for each night are also indicated. AHI = apnea-hypopnea index, FHR = fetal heart rate.
Body Position Sensor
The video recording was not available for 6 participants; therefore, these participants were excluded from the sensitivity and specificity analysis. From the remaining 19 participants, we analyzed 14,838 30-second epochs of BPS-determined body position in comparison to the reference body position as determined by infrared video. The BPS identified supine sleeping position with a sensitivity of 48% and specificity of 93%. As would be expected in late pregnancy, no prone position was detected by either the video or BPS.
DISCUSSION
Our findings indicate that reducing time spent in the supine maternal sleep position may confer benefits to the mother-baby dyad during late pregnancy. Although the exact mechanism for why the supine sleeping position is detrimental to the fetus is still not known, this study indicates that maternal oxygen desaturations and increased symptoms of SDB and FHR decelerations are more common when the mother is sleeping supine. The fetal response may be an adaptive response to either inferior vena cava (IVC) compression or maternal deoxygenation, or both.
Our findings are supported by the earlier epidemiological studies14–19 with support from recent physiological work by Stone et al. who demonstrated that when women lie supine, whether that is during the day37 or during sleep,38 that the fetus is more likely to enter fetal behavioral state “1F.” This is a quiescent state, demonstrated by stable HR with little FHR variability (less than 5 bpm), and is thought to be oxygen/energy conserving.39 Interestingly, Stone et al. did not describe observing FHR decelerations when their pregnant participants assumed the supine position in either their daytime or overnight study. However, as reduction in FHR variability often occurs prior to any noticeable change in the HR itself,40 we surmise that most fetuses cope with the supine stressor by entering 1F, and only certain fetuses respond with significantly more FHR decelerations when the mother is supine, as occurred in our participant number 20 (Figure 3).
Most pregnant women spend about 25% of their sleep time supine.41 If women are asked to sleep on their left without using PT the percentage of left-sided sleep typically increases, but this may come at a cost of a slightly reduced sleep duration— perhaps because the women feel they need to make a conscious effort to maintain sleep position.32 The current study showed that when wearing the PrenaBelt, the proportion of time participants spent sleeping supine was reduced without the apparent cost of loss of self-reported sleep quality and objectively measured sleep duration. As sleep duration and quality are also important to avert poor pregnancy outcomes in late pregnancy,42,43 we suggest that it may be better to use PT to reduce supine sleep time in late pregnancy rather than simply asking the woman herself to try to maintain or avoid certain sleep positions. However, more research is warranted to confirm this finding and to further investigate the most efficient means to promote comfortable lengthy nonsupine sleep in late pregnancy.
The supine sleep position may be more important to avoid in some pregnancies than in others. This may be because the fetus who is vulnerable to stillbirth44 may be less able to mount an adequate response to regular nightly insults because it has already adapted its cardiovascular system to survive.45–48 The growth restricted fetus16,19 may be particularly vulnerable. Thus if FGR is known, it may be especially important for the maternity care provider to recommend the mother avoids the supine sleep position using PT. Although there has been some progress in improved detection rates of FGR during pregnancy, there is still room for improvement in detecting all FGR fetuses and significant debate as to the most effective means to determine FGR.45 Also, McCowan et al.18 have recently reported a substantial increase in stillbirth associated with supine settling to sleep position in term (adjusted odds ratio 10.26, 3.00–35.04) versus preterm (adjusted odds ratio 3.12, 0.97–10.05) pregnancy. Together, this information suggests that the best approach to mitigate the effect of supine sleep on poor pregnancy outcome may be to simply adopt a universal precaution and advise all women to settle to sleep on their side from 28 weeks onward.
Our findings indicate that there was significant SDB group × condition interaction such that, for those with an AHI ≥ 5 events/h on the control night, AHI was significantly reduced during the intervention night, whereas for others who did not meet this threshold there was no significant effect. This suggests that the supine sleeping position exacerbates SDB in women who had an AHI ≥ 5 events/h on the control night. There is a biologically plausible interplay between regular nocturnal oxygen desaturations in the mother and the fetus' adaptive response to reduction in oxygen delivery46,47 seen in reduced fetal growth or movements and/or fetal heart rate decelerations. However, none of the case-control studies that have examined maternal sleep practices and stillbirth have reported statistically significant association between indicators of SDB such as self-reported snoring, choking, gasping, and/or a high score on the Epworth Sleepiness Scale and stillbirth rate.14,16,18,19 Nevertheless, a study by Blyton et al.49 saw improvement in fetal movement activity in a group of 10 pregnant women undergoing a study using continuous positive airway pressure (CPAP) for obstructive sleep apnea (OSA). They reported the average number of fetal movements on the night without CPAP treatment was 319 (standard deviation 32). On the subsequent night with CPAP treatment, the number of fetal movements increased to 592 (standard deviation 48; P < .0001). This, along with our findings suggests that objective rather than subjective measures are needed to assess the association between OSA and stillbirth. Therefore, further research is called for using PT as well as objective measures for OSA powered to detect effect on stillbirth rate.
The BPS identified supine sleeping position with a sensitivity of 48% and specificity of 93%. As configured in the Prena-Belt and per our custom Java code for resolving body position from the BPS data, the BPS is less sensitive for the supine position than lateral positions and more specific for the supine position than lateral positions. Thus, the BPS, as configured in the current study, should not be relied on for supine time determination. We surmise that if the PrenaBelt was not snuggly fit to the participant's body, its configuration to the participant's body may have been altered during the night as the participant changed positions or got up to void, which would introduce error into the BPS-determined position. We also noted that despite being snug when donned in the standing position, the PrenaBelt loosened slightly when the participant laid flat on her bed, sometimes requiring tightening and readjustment.
Relevance and Guidelines
Recommendations based on conclusions from this relatively small feasibility study should be cautious. However, our study lends weight to previous physiological37,38 and epidemiological14–19 studies and also signals that supine sleep position is potentially detrimental to both maternal and fetal well-being. We have also shown that PT may be of merit in late pregnancy as it appears to promote longer nonsupine sleep without effect on sleep quality and duration.
Although we are the first to report the benefits of using a specific PT device in late pregnancy, comfort measures such as maternal body pillows, regular pillows (used between legs, under bump or behind back), and pelvic belts (lumbar support) have been regularly used by pregnant women during sleep.50 It is not yet known if pregnant women would be prepared to wear such a device for more than 1 night. Members of our group (AK, AB, LO) are currently investigating compliance in a Ghanaian population with the most commonly cited reason for nonadherence was “feeling hot.” None of our participants made any comments about feeling hot while wearing the PrenaBelt; however, the study was mainly conducted in the Australian winter and spring, and participants were only asked to wear the device on 1 night, whereas the Ghanaian cohort were asked to wear the device for the entire third trimester.
It is apparent that avoiding supine sleep in late pregnancy through use of PT may have substantial benefits for particular mother-fetus dyads. For example, in the case of participant number 20, the difference for both mother and baby was measurably and significantly different on the intervention to control night (Figure 3). Other participants' responses on the intervention night were less profound but still likely to be of potential benefit to both mother and fetus. Maternal benefits were in reducing symptoms of SDB, improving oxygen saturation, and reducing numbers of oxygen desaturations. These benefits are likely interrelated (ie, if SDB is supine position dependent, then lowering the mother's AHI naturally improves her oxygenation). Benefits for the fetus were in fewer heart rate decelerations. We surmise that these fetal benefits are likely to be related to improvement in maternal oxygenation and/or through reduction in IVC compression which may, in turn, lead to improved maternal blood flow and thus oxygen delivery to the placenta for transfer to the fetus. There is currently no method for identifying fetuses vulnerable to stillbirth or knowing which will respond poorly to maternal supine sleep position. However, it is plausible that adopting a universal approach for the use of PT in late pregnancy may protect all fetuses in a similar manner to how the universal adoption of infant safe sleep does little harm to all babies but tends to protect the vulnerable baby from sudden unexpected infant death (SUID).
Limitations
The control condition night of 10% spent sleeping supine was very low compared with other studies.18,32,41 We propose that this may be because the participants were aware that the aim of the study was to investigate the effectiveness of a PT device that has been designed to assist pregnant women to avoid sleeping on their back. However, because we still found a significant reduction in the percentage of the night spent sleeping supine we surmise that the use of PT may be a useful aid for women to maintain lengthy nonsupine sleep position and this may be particularly important because it is yet unknown how much supine sleep time might be detrimental to fetal well-being.
We conducted this study with a small group (n = 25) of healthy, nonobese women. Therefore, results may not be generalizable to women with body mass index > 35 kg/m2 or those with moderate-severe SDB. We cannot know the effect of obesity or other medical conditions such as hypertension on sleep position or even if the PrenaBelt will be effective in these populations. However, because we found statistically significant results in this small population we surmise that later studies that include these higher risk populations may well find greater response to PT than we were able to detect in this small feasibility study of healthy low-risk pregnant women.
The PrenaBelt did not prevent supine sleep entirely in our participants, and we hypothesize that this may have been because of softness of the sleeping surface. Body weight may also be a factor and as obese pregnant women are at increased risk of a range of poor pregnancy outcomes including stillbirth,51 it may be particularly important to determine which PT devices, such as the PrenaBelt or one that vibrates when the user lies supine,27 are most effective in this group.
All the results concerning the subjective sleep parameters and the acceptance of the device are based on a single night of testing. Because it has been recommended that pregnant women should avoid settling in the supine position prior to sleep from 28 weeks,15 studies will be needed to determine if sleep parameters, acceptance, and adherence to a PT device changes over many weeks of use.
All of our participants declared that they were in a healthy singleton pregnancy; however, we did not have access to their medical records to confirm this, and we also were not able to gather their pregnancy outcome/delivery data. We therefore cannot be sure that the pregnancies were not complicated by obstetric or medical complication, and this may explain why we observed FHR decelerations associated with supine position in our study whereas Stone et al.37,38 did not.
CONCLUSIONS
This study showed that it is feasible to use PT in late pregnancy to reduce the amount of time women spend sleeping supine. Demonstrating that maternal sleep position can be simply modified through use of PT may be one of the keys to achieving significant reduction in late stillbirth rates.
Further research is needed to further explore the risks and benefits of PT in late pregnancy, including the potential effect of wearing the device on maternal anxiety and if it confers benefits for other poor pregnancy outcomes such as low birth weight and FGR.
DISCLOSURE STATEMENT
Work for this study was performed at University of South Australia. All authors have seen and approved the manuscript. AK and AB, are officers at GIRHL, which has a patent application for the PrenaBelt (#WO2016176632A1) on which they are listed as inventors. The other authors report no conflicts of interest. JLM was funded by a NHMRC Career Development Fellowship (APP1066916). LMO was funded by the American Sleep Medicine Foundation. The study was supported by UniSA: School of Nursing and Midwifery ‘Pathfinder’ grant.
ACKNOWLEDGMENTS
The authors acknowledge Mr. Kevin Lee for his expertise in writing a custom Java code to process the BPS raw data for the correlational analysis and thank Professor Kurt Lushington for his ongoing interest and support of this project.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- BPS
body position sensor
- CPAP
continuous positive airway pressure
- FGR
fetal growth restriction
- FHR
fetal heart rate
- GEE
generalized estimating equations
- IVC
inferior vena cava
- OSA
obstructive sleep apnea
- PT
positional therapy
- RDI
respiratory disturbance index
- SDB
sleep-disordered breathing
- SUID
sudden unexpected infant death
REFERENCES
- 1.Holmes F. The supine hypotensive syndrome. Anaesthesia. 1960;50(11):972–977. doi: 10.1111/j.1365-2044.1995.tb05931.x. [DOI] [PubMed] [Google Scholar]
- 2.Jeffreys RM, Stepanchak W, Lopez B, Hardis J, Clapp JF. Uterine blood flow during supine rest and exercise after 28 weeks of gestation. BJOG. 2006;113(11):1239–1247. doi: 10.1111/j.1471-0528.2006.01056.x. [DOI] [PubMed] [Google Scholar]
- 3.Kauppila A, Koskinen M, Puolakka J, Tuimala R, Kuikka J. Decreased intervillous and unchanged myometrial blood flow in supine recumbency. Obstet Gynecol. 1980;55(2):203–205. [PubMed] [Google Scholar]
- 4.Kinsella SM, Lee A, Spencer JA. Maternal and fetal effects of the supine and pelvic tilt positions in late pregnancy. Eur J Obstet Gynecol Reprod Biol. 1990;36(1-2):11–17. doi: 10.1016/0028-2243(90)90044-2. [DOI] [PubMed] [Google Scholar]
- 5.Kinsella SM, Lohmann G. Supine hypotensive syndrome. Obstet Gynecol. 1994;83(5 Pt 1):774–788. [PubMed] [Google Scholar]
- 6.Lee SW, Khaw KS, Ngan Kee WD, Leung TY, Critchley LA. Haemodynamic effects from aortocaval compression at different angles of lateral tilt in nonlabouring term pregnant women. Br J Anaesth. 2012;109(6):950–956. doi: 10.1093/bja/aes349. [DOI] [PubMed] [Google Scholar]
- 7.Lees MM, Scott DB, Kerr MG, Taylor SH. The circulatory effects of recumbent postural change in late pregnancy. Clin Sci. 1967;32(3):453–465. [PubMed] [Google Scholar]
- 8.Lees MM, Taylor SH, Scott DB, Kerr MG. A study of cardiac output at rest throughout pregnancy. J Obstet Gynaecol Br Commonw. 1967;74(3):319–328. doi: 10.1111/j.1471-0528.1967.tb03956.x. [DOI] [PubMed] [Google Scholar]
- 9.Milsom I, Forssman L. Factors influencing aortocaval compression in late pregnancy. Am J Obstet Gynecol. 1984;148(6):764–771. doi: 10.1016/0002-9378(84)90563-5. [DOI] [PubMed] [Google Scholar]
- 10.Rossi A, Cornette J, Johnson MR, et al. Quantitative cardiovascular magnetic resonance in pregnant women: cross-sectional analysis of physiological parameters throughout pregnancy and the impact of the supine position. J Cardiovasc Magn Reson. 2011;13(1):31. doi: 10.1186/1532-429X-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khatib N, Haberman S, Belooseski R, Vitner D, Weiner Z, Thaler I. Maternal supine recumbency leads to brain auto-regulation in the fetus and elicit the brain sparing effect in low risk pregnancies. Am J Obstet Gynecol. 2011;204(1):S278. [Google Scholar]
- 12.Carbonne B, Benachi A, Lévèque ML, Cabrol D, Papiernik E. Maternal position during labor: effects on fetal oxygen saturation measured by pulse oximetry. Obstet Gynecol. 1996;88(5):797–800. doi: 10.1016/0029-7844(96)00298-0. [DOI] [PubMed] [Google Scholar]
- 13.Thurlow I, Kinsella SM. Intrauterine resuscitation: active management of fetal distress. Int J Obstet Anesth. 2002;11(2):105–116. doi: 10.1054/ijoa.2001.0933. [DOI] [PubMed] [Google Scholar]
- 14.Stacey T, Thompson JD, Mitchell EA, Ekeroma AJ, Zuccollo JM, McCowan LM. Association between maternal sleep practices and risk of late stillbirth: a case-control study. BMJ. 2011;342:d3403. doi: 10.1136/bmj.d3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heazell AE, Li M, Budd J, et al. Association between maternal sleep practices and late stillbirth-findings from a stillbirth case‐control study. BJOG. 2018;125(2):254–262. doi: 10.1111/1471-0528.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon A, Raynes-Greenow C, Bond D, Morris J, Rawlinson W, Jeffery H. Sleep position, fetal growth restriction, and late-pregnancy stillbirth: the Sydney stillbirth study. Obstet Gynecol. 2015;125(2):347–355. doi: 10.1097/AOG.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 17.Lakshmi ST, Thankam U, Jagadhamma P, Ushakumari A, Chellamma N, Hariharan SV. Risk factors for stillbirth: a hospital based case control study. Int J Reprod Contracept Obstet Gynecol. 2017;6(3):970–974. [Google Scholar]
- 18.McCowan LM, Thompson JM, Cronin RS, et al. Going to sleep in the supine position is a modifiable risk factor for late pregnancy stillbirth; findings from the New Zealand multicentre stillbirth case-control study. PLoS One. 2017;12(6):e0179396. doi: 10.1371/journal.pone.0179396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owusu JT, Anderson FJ, Coleman J, et al. Association of maternal sleep practices with pre-eclampsia, low birth weight, and stillbirth among Ghanaian women. Int J Gynaecol Obstet. 2013;121(3):261–265. doi: 10.1016/j.ijgo.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morong S, Hermsen B, de Vries N. Sleep-disordered breathing in pregnancy: a review of the physiology and potential role for positional therapy. Sleep Breath. 2014;18(1):31–37. doi: 10.1007/s11325-013-0849-9. [DOI] [PubMed] [Google Scholar]
- 21.Ravesloot MJL, van Maanen JP, Dun L, de Vries N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea-a review of the literature. Sleep Breath. 2013;17(1):39–49. doi: 10.1007/s11325-012-0683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patient's wife cures his snoring. Chest. 1984;85(4):582. [Google Scholar]
- 23.Oksenberg A, Gadoth N. Are we missing a simple treatment for most adult sleep apnea patients? The avoidance of the supine sleep position. J Sleep Res. 2014;23(2):204–210. doi: 10.1111/jsr.12097. [DOI] [PubMed] [Google Scholar]
- 24.Sunnergren O, Broström A, Svanborg E. Positional sensitivity as a confounder in diagnosis of severity of obstructive sleep apnea. Sleep Breath. 2013;17(1):173–179. doi: 10.1007/s11325-012-0666-6. [DOI] [PubMed] [Google Scholar]
- 25.Jokic R, Klimaszewski A, Crossley A, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115(3):771–781. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 26.Skinner MA, Kingshott RN, Filsell S, Taylor DR. Efficacy of the tennis ball technique versus nCPAP in the management of position-dependent obstructive sleep apnoea syndrome. Respirology. 2008;13(5):708–715. doi: 10.1111/j.1440-1843.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- 27.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6(3):238–243. [PMC free article] [PubMed] [Google Scholar]
- 28.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011;7(4):376–383. doi: 10.5664/JCSM.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson OC. Sampling in interview-based qualitative research: a theoretical and practical guide. Qual Res Psychol. 2014;11(1):25–41. [Google Scholar]
- 30.Cohen N, Arieli T. Field research in conflict environments: Methodological challenges and snowball sampling. J Peace Res. 2011;48(4):423–435. [Google Scholar]
- 31.Vienna, Austria: R Foundation for Statistical Computing; 2016. R: A language and environment for statistical computing. [Google Scholar]
- 32.Warland J, Dorrian J. Accuracy of self-reported sleep position in late pregnancy. PLoS One. 2014;9(12):e115760. doi: 10.1371/journal.pone.0115760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien LM, Bullough AS, Shelgikar AV, Chames MC, Armitage R, Chervin RD. Validation of Watch-Pat-200 against polysomnography during pregnancy. J Clin Sleep Med. 2012;8(3):287–294. doi: 10.5664/jcsm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry RB, Brooks R, Gamaldo CE, et al. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2015. Version 2.2. [Google Scholar]
- 35.Dawes GS, Visser GH, Goodman JD, Redman CW. Numerical analysis of the human fetal heart rate: the quality of ultrasound records. Am J Obstet Gynecol. 1981;141(1):43–52. doi: 10.1016/0002-9378(81)90673-6. [DOI] [PubMed] [Google Scholar]
- 36.Van Dongen HP, Olofsen E, Dinges DF, Maislin G. Mixed-model regression analysis and dealing with interindividual differences. Methods Enzymol. 2004;384:139–171. doi: 10.1016/S0076-6879(04)84010-2. [DOI] [PubMed] [Google Scholar]
- 37.Stone PR, Burgess W, McIntyre JP, et al. Effect of maternal position on fetal behavioural state and heart rate variability in healthy late gestation pregnancy. J Physiol. 2017;595(4):1213–1221. doi: 10.1113/JP273201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone PR, Burgess W, McIntyre J, et al. An investigation of fetal behavioural states during maternal sleep in healthy late gestation pregnancy: an observational study. J Physiol. 2017;595(24):7441–7450. doi: 10.1113/JP275084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pillai M, James D. Behavioural states in normal mature human fetuses. Arch Dis Child. 1990;65:39–43. doi: 10.1136/adc.65.1_spec_no.39. 1 Spec No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalton KJ, Dawes GS, Patrick JE. The autonomic nervous system and fetal heart rate variability. Am J Obstet Gynecol. 1983;146(4):456–462. doi: 10.1016/0002-9378(83)90828-1. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien LM, Warland J. Typical sleep positions in pregnant women. Early Hum Dev. 2014;90(6):315–317. doi: 10.1016/j.earlhumdev.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang JJ, Pien GW, Duntley SP, Macones GA. Sleep deprivation during pregnancy and maternal and fetal outcomes: is there a relationship? Sleep Med Rev. 2010;14(2):107–114. doi: 10.1016/j.smrv.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warland J, Dorrian J, Morrison JL, O'Brien LM. Maternal sleep during pregnancy and poor fetal outcomes: a scoping review of the literature with meta-analysis. Sleep Med Rev. 2018 Mar 27; doi: 10.1016/j.smrv.2018.03.004. . [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Warland J, Mitchell EA. A triple risk model for unexplained late stillbirth. BMC Pregnancy Childbirth. 2014;14(1):142. doi: 10.1186/1471-2393-14-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Figueras F, Gratacos E. An integrated approach to fetal growth restriction. Best Pract Res Clin Obstet Gynaecol. 2017;38:48–58. doi: 10.1016/j.bpobgyn.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol. 2008;35(7):730–743. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- 47.Danielson L, McMillen IC, Dyer JL, Morrison JL. Restriction of placental growth results in greater hypotensive response to alpha-adrenergic blockade in fetal sheep during late gestation. J Physiol. 2005;563(Pt 2):611–620. doi: 10.1113/jphysiol.2004.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giussani DA. The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol. 2016;594(5):1215–1230. doi: 10.1113/JP271099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blyton DM, Skilton MR, Edwards N, Hennessy A, Celermajer DS, Sullivan CE. Treatment of sleep disordered breathing reverses low fetal activity levels in preeclampsia. Sleep. 2013;36(1):15–21. doi: 10.5665/sleep.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pennick VE, Young G. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2007;2:CD001139. doi: 10.1002/14651858.CD001139.pub2. [DOI] [PubMed] [Google Scholar]
- 51.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16(8):621–638. doi: 10.1111/obr.12288. [DOI] [PubMed] [Google Scholar]