Abstract
Inhaled cigarette smoke stimulated vagal bronchopulmonary C fibers via an action of nicotine on neuronal nicotinic acetylcholine receptor (nAChR). Recent studies have reported that nicotine at high concentrations can also activate the transient receptor potential ankyrin 1 receptor (TRPA1) expressed in these sensory nerves. This study was performed to characterize the airway response to inhaled nicotine aerosol and to investigate the relative roles of nAChR and TRPA1 in this response. Guinea pigs were anesthetized and mechanically ventilated; one tidal volume of nicotine aerosol (2% solution) was diluted by an equal volume of air and delivered directly into the lung via a tracheal cannula in a single breath. Our results showed the following: 1) Inhalation of nicotine aerosol triggered an immediate and pronounced bronchoconstriction; the increase in total pulmonary resistance reached a peak of 588 ± 205% (mean ± SE) in 10–40 s, which gradually returned to baseline after 1–5 min. 2) Pretreatment with either atropine (iv) or mecamylamine (aerosol) almost completely abolished the nicotine-induced bronchoconstriction; the mecamylamine pretreatment did not block the bronchoconstriction and bradycardia evoked by electrical stimulation of the distal end of one sectioned vagus nerve, indicating its minimal systemic effects. 3) Pretreatment with HC-030031, a selective TRPA1 antagonist, abolished the bronchoconstriction induced by allyl isothiocyanate, a selective TRPA1 agonist, but did not attenuate the nicotine-evoked bronchoconstriction. In conclusion, inhalation of a single breath of nicotine aerosol evoked acute bronchoconstriction mediated through the cholinergic reflex pathway. This reflex response was triggered by activation of nAChR, but not TRPA1, located in airway sensory nerves.
NEW & NOTEWORTHY Recent reports revealed that nicotine at high concentration activated transient receptor potential ankyrin 1 receptor (TRPA1) expressed in vagal bronchopulmonary sensory nerves. This study showed that inhalation of a single breath of nicotine aerosol consistently evoked acute bronchoconstriction that was mediated through the cholinergic reflex pathway and triggered by activation of nicotinic acetylcholine receptor, but not TRPA1, located in these nerves. This is new and important information considering the recent rapid and alarming rise in the prevalence of e-cigarette use for nicotine inhalation.
Keywords: airway, cigarette smoke, cough, TRPA1, vagus
INTRODUCTION
Cigarette smoke is a common inhaled irritant in human airways and can evoke acute bronchoconstriction (13, 20). Although the mechanisms underlying the bronchoconstrictive effect of smoke are not yet fully understood, inhaled cigarette smoke is known to directly stimulate sensory nerve endings in the respiratory tract, particularly C fiber endings and rapidly adapting receptors (RARs) located in the airways and lung (19, 22). These afferents upon stimulation may elicit reflex bronchoconstriction via the vagal efferent (cholinergic) pathway to the airway smooth muscles (6, 8, 23, 24). Studies performed in our laboratory have established the first evidence that nicotine contained in cigarette smoke is primarily responsible for generating the stimulatory effect on these sensory endings in the airways, and the action is mediated through activation of neuronal nicotinic acetylcholine receptor (neuronal nAChR) expressed in these sensory neurons (10, 17, 22, 36).
The transient receptor potential ankyrin 1 receptor (TRPA1), a ligand-gated nonselective cation channel expressed in a subset of vagal bronchopulmonary C fiber neurons, has been shown to be sensitive to a variety of inhaled irritants, including acrolein, reactive oxygen species, and ozone; some of these compounds are contained in cigarette smoke. Recent studies have further reported that nicotine at high concentrations also activated TRPA1 expressed in capsaicin-sensitive nociceptor neurons, including those innervating the airways (16, 34). As such, activation of TRPA1 by nicotine may also contribute to the airway responses evoked by cigarette smoke (1, 15).
It becomes even more important to gain a better understanding of the mechanisms underlying the nicotine-induced airway responses for the following reason: the prevalence of nicotine inhalation has risen rapidly and alarmingly in recent years because the electronic cigarette (e-cig), which delivers nicotine in vaporized and aerosolized form, has been advertised as a safe alternative in the replacement therapy for tobacco cigarette smokers (27, 29).
In the light of these new findings and unanswered questions, this study was performed to 1) characterize the airway response to inhaled nicotine aerosol, 2) test the hypothesis that the cholinergic reflex played a major role in this response, and 3) investigate the relative roles of nAChR and TRPA1 in generating the airway response to inhaled nicotine aerosol.
MATERIALS AND METHODS
The experimental procedures described below were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health, and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
Measurements of lung mechanics.
Young, male Hartley guinea pigs were anesthetized with intraperitoneal injections of chloralose (100 mg/kg) and urethane (500 mg/kg), and supplemental doses of the same anesthetics were administered intravenously (iv) whenever necessary to maintain abolition of the pain withdrawal reflexes. After the trachea was cannulated just below the larynx via a tracheotomy, the animal was placed in a supine position and ventilated with a respirator (Ugo Basile model 7025) at a constant frequency of 60 breaths/min and a tidal volume (VT) of ~8 ml/kg; the latter was adjusted in each animal to maintain the end-tidal CO2 concentration (Novametrix model 1260) between 4.5 and 5.5%. One jugular vein and one carotid artery were cannulated for intravenous injections and for arterial blood pressure (ABP) and heart rate (HR) measurements, respectively. A catheter for measuring pleural pressure (Ppl) was inserted into the right intrapleural cavity via a surgical incision between the fifth and sixth ribs, and the incision was subsequently sutured and further sealed to be airtight with silicone jelly. Pneumothorax was then corrected by briefly opening the intrapleural catheter to atmosphere when the lung was hyperinflated at 3 times VT. A heating pad was placed under the animal to maintain body temperature at ~36°C.
The transpulmonary pressure (Ptp) was measured by the difference between the tracheal pressure and Ppl with a differential pressure transducer (Validyne MP45-28). Respiratory flow was measured with a heated pneumotachograph and a differential pressure transducer (Validyne MP45-14); the pneumotachograph is made of stainless steel and has a linear flow-pressure relationship in the range of 0–20 ml/s and a flow resistance of 0.046 cmH2O·ml−1·s. All signals were recorded continuously (Biopac model MP150); total pulmonary resistance (RL) and dynamic lung compliance (Cdyn) were analyzed continuously by an online computer on a breath-by-breath basis using computer software (Biocybernetics TS-100).
Single-breath nicotine aerosol inhalation challenge.
Aerosol of nicotine solution (2% concentration in distilled water) was generated by a nebulizer (Aerogen Aeroneb) and mixed with room air in a 1:1 ratio. One single breath of this diluted nicotine aerosol at a volume of 2 times VT was immediately delivered into the lung via the trachea cannula, held in the lung for 2 s, and then exhaled. These parameters were chosen to simulate the general puffing profile in human e-cig users (33).
Experimental protocols.
Six series of studies were performed in this study. Study 1 was aimed to determine whether nicotine aerosol induced bronchoconstriction and, if so, whether the effect was reproducible. In each animal, RL, Cdyn, and ABP were measured continuously for 20 breaths during the baseline and for 10 min immediately after the challenge; at least 20 min elapsed between 2 tests for recovery. In study 2, to determine whether the nicotine aerosol-induced bronchoconstriction was concentration dependent, inhalation challenges of 0 (saline), 0.5, 1.0, and 2.0% nicotine aerosol were tested in the same animals, and the sequence of these challenges was alternated between animals to achieve a balanced design. In study 3, to determine whether the high pH in the 2% nicotine aerosol contributed to the bronchoconstriction, the responses to aerosols of 2% nicotine (pH = 10.5) and alkaline saline (with pH adjusted to 10.5 by adding NaOH) were compared in the same animals. In study 4, to determine the relative contributions of cholinergic mechanism and endogenous tachykinins, the bronchoconstrictions induced by a single breath of aerosolized nicotine (2%) and capsaicin (Cap, 85 µg/ml or 0.0085% wt/wt concentration), a selective stimulant of bronchopulmonary C fiber sensory endings, were both determined in the same animals before and after pretreatments with atropine sulfate (a muscarinic acetylcholine receptor antagonist; 0.05 mg/kg iv) alone, and then a combination of atropine and selective antagonists of neurokinin receptor type 1 (NK-1) and NK-2, L-732138 (1.5 mg/kg iv) and SR-48968 (0.15 mg/kg iv), respectively; for the combined treatment, atropine, SR-48968, and L-732138 were administered 15, 20, and 25 min before nicotine/Cap challenge, respectively. In study 5, to determine the role of nAChR, the responses to nicotine aerosol were compared before and after a pretreatment with aerosolized mecamylamine (solution concentration 2%; 4 breaths), a nonselective antagonist of the nAChR (2). In study 6, to determine whether an activation of TRPA1 receptor is involved in the nicotine-induced bronchoconstriction, responses to inhalations of nicotine and allyl isothiocyanate (AITC; 10 mg/ml or 1% wt/wt concentration, 30 breaths), a selective TRPA1 agonist, were compared before and after a pretreatment with HC-030031 (0.5 mg/kg iv), a selective antagonist of TRPA1.
Data analysis.
In each experiment, the baseline RL and Cdyn were averaged over 20 breaths before the aerosol challenge, and the peak responses were calculated by averaging 10 consecutive breaths of the highest RL and lowest Cdyn within 60 s after the challenge. A two-way analysis of variance (ANOVA) was used for the statistical analysis. When the two-way ANOVA showed a significant interaction, pairwise comparisons were made with a post hoc analysis (Fisher’s least significant difference). Data are reported as means ± SE. A P value <0.05 is considered significant.
Materials.
Nicotine (Sigma-Aldrich) and atropine sulfate (Sigma-Aldrich) were dissolved directly in isotonic saline. L-732138 (Tocris) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at a final concentration of 3.7 mg/ml. SR-48968 (Sanofi Recherche) was first dissolved in polyethylene glycol (average molecular weight: 200; Sigma-Aldrich) and then diluted in saline at a 1:1 ratio to a final concentration of 0.67 mg/ml. Mecamylamine hydrochloride was dissolved in saline at a concentration of 4% (wt/wt). Cap (250 µg/ml) and AITC (50 mg/ml) were prepared in 10% Tween 80, 10% ethanol, and 80% saline; HC-030031 was first dissolved in DMSO (30 mg/ml) and diluted to 2 mg/ml by a solution of 10% Tween 80, 10% ethanol, and 80% saline. All these stock solutions were then diluted with isotonic saline to desired concentrations before each experiment.
RESULTS
A total of 35 guinea pigs (body weight: 403 ± 6 g) were used in this study.
Study 1.
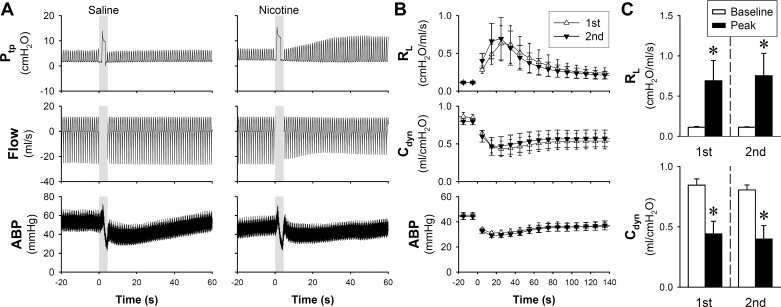
Within 10 s after a single breath of nicotine aerosol (generated from 2% nicotine solution and mixed with an equal volume of air to a total volume of 2 times VT) was delivered into the lungs, a pronounced and progressive increase in Ptp emerged in anesthetized guinea pigs (Fig. 1A). The increased Ptp resulted from a sharp increase in RL and decrease in Cdyn: RL and Cdyn reached the peak changes at 588 ± 205 and 53 ± 13% of their respective baselines in 10–40 s, and they gradually returned to baseline after 1–5 min (Fig. 1B). These responses were highly reproducible in the same animal when they were tested 20 min later (Fig. 1C). Inhalation challenges of both saline and nicotine aerosols caused a transient decrease in ABP, which returned to its baseline in 1–2 min (Fig. 1, A and B).
Fig. 1.
Airway constriction evoked by nicotine aerosol inhalation challenge in anesthetized guinea pigs. A: representative experimental records illustrating the responses of transpulmonary pressure (Ptp), flow rate, and arterial blood pressure (ABP) to inhalation challenges of single breaths of saline and nicotine aerosols. Shaded bars depict the delivery of air and nicotine aerosol (concentration of nicotine solution: 2%; aerosol mixed with air in 1:1 ratio; volume: 2 times tidal volume) held for 2 s. Line graph (B) and group data (C) of two consecutive nicotine aerosol inhalation challenges performed 20 min apart in the same guinea pigs (n = 7) to test the reproducibility of responses. The baseline responses of total pulmonary resistance (RL) and dynamic lung compliance (Cdyn) were averaged over 20 breaths before challenge, and the peak responses were calculated by averaging 10 consecutive breaths within 60 s after the challenge. Data represent means ± SE. *Significantly different from the corresponding baseline (P < 0.05).
Study 2.
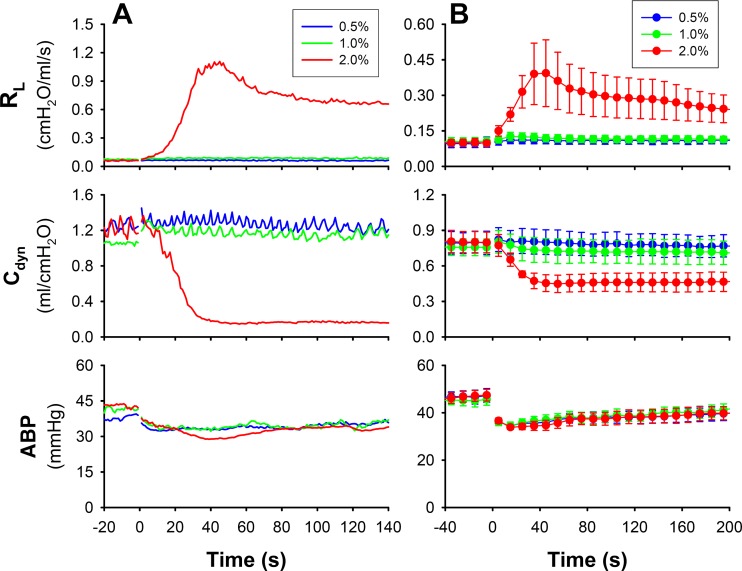
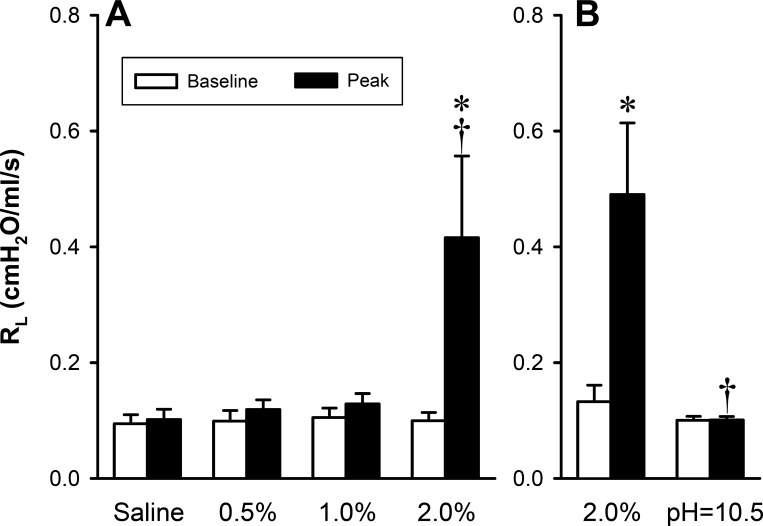
When the concentration of nicotine solution was lowered to 0 (saline), 0.5, and 1.0% and delivered in the same manner as in study 1, the responses of RL and Cdyn were almost completely absent in the same animals (Fig. 2), indicating that the bronchoconstrictive effect of nicotine was concentration dependent; the threshold concentration of nicotine solution was between 1.0 and 2.0% (Fig. 3A).
Fig. 2.
A: representative line graph illustrating the responses of total pulmonary resistance (RL) and dynamic lung compliance (Cdyn) to inhalation challenges of three increasing concentrations of nicotine in an anesthetized guinea pig (427 g). B: group data collected from six guinea pigs. Responses to inhalation of saline aerosol (0% nicotine) were also measured, but the data are not shown here to avoid clustering in the graph. ABP, arterial blood pressure. Data represent means ± SE (n = 6).
Fig. 3.
Changes in total pulmonary resistance (RL) in response to increasing the concentration of nicotine solution (A) and saline with the same pH (10.5) as that in the 2% nicotine solution (B). For the calculation of baseline and peak responses, see the legend of Fig. 1. A: group data (n = 6) showing the concentration-dependent effect of nicotine aerosol inhalation challenges; baseline and peak responses were calculated from the data in Fig. 2. *Significantly different from the corresponding baseline. †Significantly different from the corresponding data point in the saline group. B: group data (n = 4) indicating a lack of bronchoconstrictive effect of alkaline saline aerosol inhalation challenge. *Significantly different from the corresponding baseline. †Significantly different from the corresponding data point in the 2% nicotine group.
Study 3.
When the pH of isotonic saline (solvent of nicotine solution) was raised to 10.5, the same as that in the 2% nicotine solution by adding NaOH (1.0 N), the inhalation challenge of this alkaline saline aerosol did not cause any change in RL (Fig. 3B), indicating that the nicotine aerosol-triggered airway constriction was not caused by its alkaline property.
Study 4.
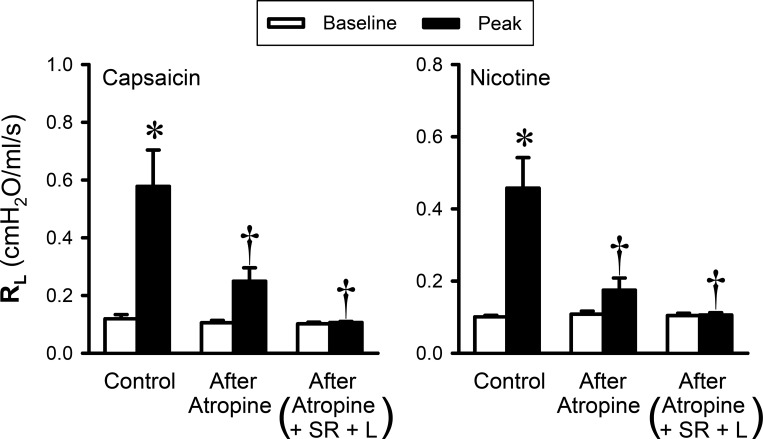
A pretreatment with atropine sulfate (0.05 mg/kg iv) alone did not cause any change in the baseline RL, Cdyn, HR, or ABP but significantly diminished the increase in RL produced by Cap aerosol inhalation challenge: Cap increased RL to 491.6 ± 110.8 and 235.4 ± 40.7% of their respective baselines before and after the atropine pretreatment, respectively (P < 0.05, n = 6; Fig. 4, left); the remaining bronchoconstriction was subsequently blocked by the pretreatment with a combination of atropine (0.05 mg/kg iv), SR-48968 (0.15 mg/kg iv), and L-732138 (1.5 mg/kg iv) (Fig. 4, left). In the same group of guinea pigs, pretreatments with the same dose of atropine almost completely abolished the bronchoconstriction evoked by nicotine aerosol (2%): nicotine increased RL to 461.0 ± 95.1 and 157.4 ± 17.8% of their respective baselines before and after the atropine pretreatment, respectively (P < 0.05, n = 6; Fig. 4, right). The remaining nicotine-induced bronchoconstriction was also completely prevented by the pretreatment with atropine + SR-48968 + L-732138 (Fig. 4, right).
Fig. 4.
Effect of pretreatment with atropine, SR-48968 (SR), and L-732138 (L) on the responses of total pulmonary resistance (RL) to inhalation challenges of aerosolized nicotine (2%) and capsaicin (0.0085%) in anesthetized guinea pigs (n = 6). Atropine (0.05 mg/kg iv), SR-48968 (0.15 mg/kg iv), and L-732138 (1.5 mg/kg iv) were administered 15, 20, and 25 min before nicotine/capsaicin challenges, respectively. *Significantly different from the corresponding baseline (P < 0.05). †Significantly different from the corresponding data point in control group (P < 0.05).
Study 5.
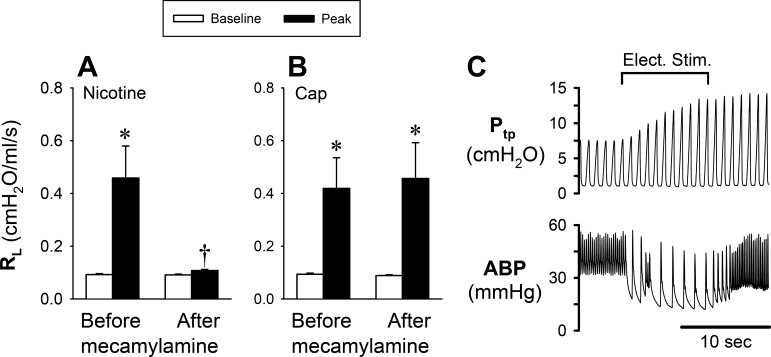
A pretreatment with mecamylamine aerosol (2%, 4 breaths) did not cause any change in the baseline RL, HR, or ABP and only a small decrease in Cdyn (∆ = ~5.5 ± 1.3%; P < 0.05) but completely abolished the increase in RL evoked by inhaled nicotine aerosol (2%) (P < 0.05, n = 11; Fig. 5A). In 5 of these 11 animals, the response to Cap (0.75–1.0 µg/kg iv) was also tested before and after the pretreatment with the same dose of mecamylamine. However, in a sharp contrast, the mecamylamine pretreatment did not alter the increase in RL evoked by Cap (P > 0.05, n = 5; Fig. 5B). In addition, the same mecamylamine pretreatment did not block the bronchoconstriction and bradycardia evoked by electrical stimulation of the distal end of one sectioned cervical vagus nerve in all seven animals tested; an example is shown in Fig. 5C.
Fig. 5.
Effect of pretreatment with aerosolized mecamylamine on the airway response to nicotine aerosol (n = 11) and capsaicin (Cap; n = 5) challenges. Group results showing the effect of mecamylamine (2% for 4 breaths) pretreatment on the responses of total pulmonary resistance (RL) to nicotine aerosol (2%) inhalation challenge (A) and Cap (0.75–1.0 µg/kg iv; B). C: experimental record illustrating the electrical stimulation (Elect. Stim.; 5 V, 10 Hz, 1-ms duration for 10 s) on one sectioned cervical vagus nerve in an anesthetized guinea pig (395 g) after pretreatment with mecamylamine. ABP, arterial blood pressure; Ptp, transpulmonary pressure. *Significantly different from the corresponding baseline (P < 0.05). †Significantly different from the corresponding data point before mecamylamine (P < 0.05).
Study 6.
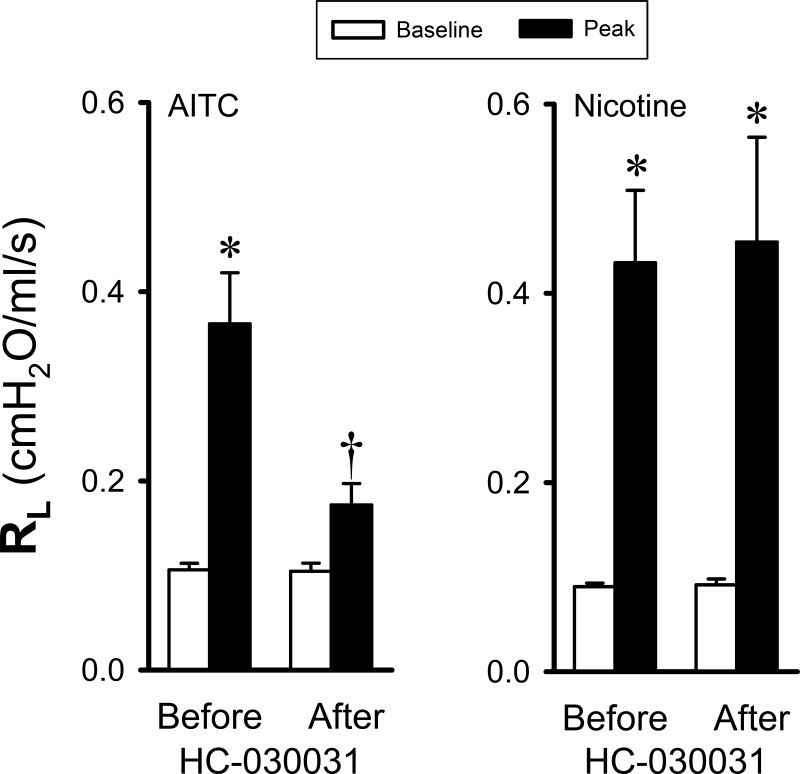
A pretreatment with HC-030031 (0.5 mg/kg iv) did not cause any change in the baseline RL (Fig. 6), Cdyn, or HR and caused only a small decrease in ABP (∆ = 7.5 ± 2.5 mmHg; P < 0.05). The HC-030031 pretreatment significantly diminished the bronchoconstriction induced by inhalation challenge of AITC aerosol: AITC increased RL to 351.4 ± 61.7 and 166.7 ± 16.2% of their respective baselines before and after HC-030031 pretreatment, respectively (P < 0.05, n = 7; Fig. 6, left). However, in a distinct contrast, the same HC-030031 pretreatment did not cause any change in the bronchoconstriction evoked by the inhaled nicotine aerosol (2%; P > 0.05, n = 7; Fig. 6, right) in the same animals: nicotine increased RL to 501.1 ± 103.2 and 565.5 ± 173.2% of their respective baselines before and after HC-030031 pretreatment, respectively (P > 0.05, n = 7; Fig. 6, right). Similar results were also found in six additional animals when AITC was delivered by intravenous injection (1.7 mg/kg). The same HC-030031 pretreatment almost completely abolished the increase in RL triggered by AITC but had no effect on the airway constriction caused by nicotine, indicating a minimal involvement of TRPA1 activation in the bronchoconstrictive effect of inhaled nicotine at the dose delivered in this study.
Fig. 6.
Effect of pretreatment with HC-030031 (0.5 mg/kg iv) on the responses of total pulmonary resistance (RL) to inhalation challenges of aerosolized allyl isothiocyanate (AITC; 1%, 30 breaths) and nicotine (2%, 1 breath) in guinea pigs (n = 7). HC-030031 was given 10 min before AITC and nicotine challenges. *Significantly different from the corresponding baseline (P < 0.05). †Significantly different from the corresponding data point before the HC-030031 pretreatment (P < 0.05).
DISCUSSION
Results of this study showed that inhalation of a single breath of nicotine aerosol evoked a pronounced bronchoconstriction in a concentration-dependent manner. This response was reversible in 1–5 min and reproducible in the same animals. After a pretreatment with either intravenous injection of atropine or inhalation of mecamylamine aerosol, the nicotine-induced airway constriction was almost completely blocked. In contrast, a pretreatment with the TRPA1 antagonist had no effect on this acute bronchoconstrictive response. On the basis of these results, we suggest that the bronchoconstriction was mediated through the cholinergic reflex pathway and triggered by activation of nAChR, but not TRPA1, located in airway sensory nerves.
A potent stimulatory effect of nicotine on both C fibers and RARs in the lung and airways has been reported in various animal species (17–19, 22). Previous studies conducted in healthy nonsmokers further confirmed that the irritant effect of inhaled cigarette smoke in the human respiratory tract was generated by the action of nicotine (20). Furthermore, in isolated pulmonary sensory neurons, both nicotine and acetylcholine can depolarize the membrane potential, generate action potentials, and evoke inward current in a concentration-dependent manner (10, 21). The irritant effect of nicotine is believed to result from an activation of nAChRs expressed on the sensory terminals located in the airway mucosa as both cough reflex in humans and electrophysiological responses in sensory nerves and isolated neurons can be prevented by a pretreatment with hexamethonium, a nonselective antagonist of nAChR (10, 18, 20, 22, 36). Indeed, reverse transcription polymerase chain reaction analysis demonstrated the expression of mRNA encoding the nAChR subunits α4, α5, α6, α7, β2, β3, and β4 in pulmonary sensory neurons, adding further support to our conclusion (10).
In this study, the pretreatment with mecamylamine completely abolished the bronchoconstriction evoked by inhaled nicotine aerosol but did not cause any change in the airway response to Cap in the same animals (Fig. 5), indicating the specific antagonistic effect of mecamylamine on nAChRs. In addition, the same dose of mecamylamine did not block the airway constriction and bradycardia evoked by electrical stimulation of the distal end of the sectioned vagus nerve (Fig. 5C). Despite the fact that mecamylamine at high dose can completely block the nAChRs involved in the synaptic transmission at autonomic ganglia, the persistence of airway constriction and bradycardia after the mecamylamine pretreatment indicated that the neural transmissions at the airway and cardiac cholinergic ganglia, respectively, were not blocked. Thus, this observation suggested that minimal systemic effects were induced by the aerosolized mecamylamine administered at this dose in these animals.
In addition, it is possible that absorbed nicotine can act on other target cells in addition to the airway ganglia and contributed to the airway constriction; for example, absorbed nicotine can also stimulate central chemoreceptors located in the brain stem and cause bronchoconstriction via the cholinergic pathway (11, 12). However, the lack of systemic effect of mecamylamine described above strongly argues against any involvement of these possibilities in this study.
TRPA1, a member of the superfamily of TRP channels (30), is known as a nociceptive chemical transducer and expressed abundantly in a subset of bronchopulmonary C fiber sensory nerves (28). TRPA1 can be activated by a number of airborne chemical irritants (e.g., acrolein, formaldehyde, ozone, etc.), endogenous inflammatory mediators (e.g., bradykinin, etc.), and reactive oxygen species (1, 4, 5, 25, 35) and is believed to be responsible for the neurogenic inflammation in the airways caused by sustained and/or intense exposure to inhaled irritants, including tobacco smoke (1). Talavera et al. recently reported that nicotine at concentrations of 0.1–1.0 mM activated TRPA1 expressed in mouse trigeminal neurons (34). In addition, their study showed that nicotine also activated heterologously expressed mouse and human TRPA1. These results revealed that TRPA1 activation may account for the local irritation caused by the nicotine skin patch or gum that delivers high concentrations of nicotine (>10 mM). It should be noted, however, that more than 100-fold higher concentrations of nicotine were required to activate TRPA1 than were required for nAChR (34). This distinct difference in the threshold concentrations of nicotine required to activate TRPA1 and nAChR raised the question as to whether activation of TRPA1 is involved in the airway irritation evoked by inhaled cigarette smoke, which contains nicotine in a substantially lower concentration (34). More recently, Kichko and colleagues have also challenged the role of TRPA1 in the stimulatory effect of nicotine (<0.1 mM) on the sensory nerves innervating the trachea in their studies using isolated mouse trachea and larynx preparations (15, 16). In the present study, we have established definitive evidence demonstrating a minimal involvement of TRPA1 activation in the acute reflex bronchoconstriction evoked by inhalation of a single breath of nicotine aerosol in guinea pigs.
The entire respiratory tract is innervated by vagal C fiber (nonmyelinated, small-diameter) sensory nerves, which constitute the dominant type of bronchopulmonary afferents (14). Pronounced physiological effects elicited by stimulation of these afferents have been extensively studied under both physiological and pathophysiological conditions (8, 24, 26). Stimulation of bronchopulmonary C fiber endings by chemical irritants can elicit powerful reflex responses, including bronchoconstriction and mucus hypersecretion, mediated through the cholinergic pathway, accompanied by the sensation of airway irritation and urge to cough (8, 23, 24). Intense and/or sustained stimulation can further trigger the local release of tachykinins and calcitonin gene-related peptides from the sensory terminals; these sensory neuropeptides can act on a number of effector cells in the airways (e.g., smooth muscles, mucous glands, and immune cells) and elicit local “axon reflexes” such as bronchoconstriction, protein extravasation, and inflammatory cell chemotaxis (9).
Cap, a potent and selective chemical stimulant of bronchopulmonary C fiber afferents, is known to generate bronchoconstriction in guinea pigs mediated through both the cholinergic reflex mechanism and local releases of tachykinins (3, 23). In this study, the pretreatment with atropine blocked ~65% of the Cap-evoked increase in RL (∆RL), and the remainder was completely abolished by additional pretreatments with selective antagonists of tachykinin receptors NK-1 and NK-2 (L-732138 and SR-48968, respectively). In comparison, the same dose of atropine blocked ~84% of the increase in RL (∆RL) caused by inhalation of nicotine aerosol in the same animals (Fig. 4). The more dominant contribution of the cholinergic mechanism in the bronchoconstriction evoked by nicotine compared with that evoked by Cap might suggest an involvement of stimulation of RARs in the airways; stimulation of RARs may also elicit the bronchoconstriction via the cholinergic reflex mechanism (6), but not the tachykinergic mechanism, because these sensory terminals, unlike the C fiber endings, do not usually contain or release tachykinins (32). Indeed, a direct intense stimulatory effect of nicotine contained in inhaled cigarette smoke on RARs in the lungs of anesthetized dogs has been demonstrated (18, 19). On the other hand, an absence of the bronchoconstriction generated by RAR stimulation has also been reported in anesthetized rabbits (37). Therefore, a possible contribution of RAR stimulation to the reflex bronchoconstriction elicited by inhaled nicotine aerosol could not be evaluated in this study.
Inhalation of nicotine aerosol also caused an abrupt and transient decrease in ABP, which gradually recovered in 1–2 min (Fig. 1, A and B). However, this change in ABP was not caused by nicotine since a similar change was also observed in the response to saline (0% nicotine) aerosol (Fig. 1A); furthermore, there was no difference in the degree of ABP depression between different concentrations of nicotine aerosol (Fig. 2B). We believe that this transient hypotension was generated by the hyperinflation of lung (2 times VT for 2 s) during the aerosol delivery (saline or nicotine) as the sustained positive alveolar air pressure may compress alveolar capillaries and render a transient reduction in cardiac output and ABP.
It has been extensively documented that the number of e-cig users has risen at an alarming rate in the United States during the last decade (27, 29). This is, in part, related to the fact that e-cigs have been promoted as a safer replacement for tobacco cigarettes for beginning smokers and for smokers who undergo smoking cessation therapy (7, 31). Because aerosolized and vaporized nicotine is the primary constituent generated from e-cigs and inhaled into the lungs, it is important to recognize this potent bronchoactive effect of inhaled nicotine and the mechanisms involved. Its potential health hazardous consequence and chronic effects should merit further investigation.
GRANTS
This study was supported in part by NIH Grants AI-123832 (L.-Y. Lee), HL-96914 (L.-Y. Lee), UL1-TR-001998 (M. Khosravi and L.-Y. Lee), and HL-107462 (F. Xu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.-Y.L., R.-L.L., M.K., and F.X. conceived and designed research; L.-Y.L., R.-L.L., and M.K. performed experiments; L.-Y.L., R.-L.L., and M.K. analyzed data; L.-Y.L., R.-L.L., M.K., and F.X. interpreted results of experiments; L.-Y.L. and R.-L.L. prepared figures; L.-Y.L., R.-L.L., M.K., and F.X. drafted manuscript; L.-Y.L., R.-L.L., M.K., and F.X. edited and revised manuscript; L.-Y.L., R.-L.L., M.K., and F.X. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ashami Athukorala for technical assistance.
REFERENCES
- 1.Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118: 2574–2582, 2008. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacher I, Wu B, Shytle DR, George TP. Mecamylamine: a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders. Expert Opin Pharmacother 10: 2709–2721, 2009. doi: 10.1517/14656560903329102. [DOI] [PubMed] [Google Scholar]
- 3.Ballati L, Maggi CA, Evangelista S. Effect of ruthenium red on the bronchoconstriction induced by capsaicin and by selective tachykinin receptor agonists in anaesthetized guinea-pig. J Auton Pharmacol 12: 369–375, 1992. doi: 10.1111/j.1474-8673.1992.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 4.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23: 360–370, 2008. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118: 1899–1910, 2008. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol (1985) 101: 971–985, 2006. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- 7.Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R. Efficiency and Safety of an Electronic Cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One 8: e66317, 2013. [Erratum in PLoS One 9: 10.1371/annotation/e12c22d3-a42b-455d-9100-6c7ee45d58d0.] 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- 9.De Swert KO, Joos GF. Extending the understanding of sensory neuropeptides. Eur J Pharmacol 533: 171–181, 2006. doi: 10.1016/j.ejphar.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 10.Gu Q, Ni D, Lee LY. Expression of neuronal nicotinic acetylcholine receptors in rat vagal pulmonary sensory neurons. Respir Physiol Neurobiol 161: 87–91, 2008. doi: 10.1016/j.resp.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartiala J, Mapp C, Mitchell RA, Shields RL, Gold WM. Cigarette smoke-induced bronchoconstriction in dogs: vagal and extravagal mechanisms. J Appl Physiol Respir Environ Exerc Physiol 57: 1261–1270, 1984. doi: 10.1152/jappl.1984.57.4.1261. [DOI] [PubMed] [Google Scholar]
- 12.Hartiala JJ, Mapp C, Mitchell RA, Gold WM. Nicotine-induced respiratory effects of cigarette smoke in dogs. J Appl Physiol (1985) 59: 64–71, 1985. doi: 10.1152/jappl.1985.59.1.64. [DOI] [PubMed] [Google Scholar]
- 13.Hong JL, Rodger IW, Lee LY. Cigarette smoke-induced bronchoconstriction: cholinergic mechanisms, tachykinins, and cyclooxygenase products. J Appl Physiol (1985) 78: 2260–2266, 1995. doi: 10.1152/jappl.1995.78.6.2260. [DOI] [PubMed] [Google Scholar]
- 14.Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst 5: 165–176, 1982. doi: 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- 15.Kichko TI, Kobal G, Reeh PW. Cigarette smoke has sensory effects through nicotinic and TRPA1 but not TRPV1 receptors on the isolated mouse trachea and larynx. Am J Physiol Lung Cell Mol Physiol 309: L812–L820, 2015. doi: 10.1152/ajplung.00164.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kichko TI, Lennerz J, Eberhardt M, Babes RM, Neuhuber W, Kobal G, Reeh PW. Bimodal concentration-response of nicotine involves the nicotinic acetylcholine receptor, transient receptor potential vanilloid type 1, and transient receptor potential ankyrin 1 channels in mouse trachea and sensory neurons. J Pharmacol Exp Ther 347: 529–539, 2013. doi: 10.1124/jpet.113.205971. [DOI] [PubMed] [Google Scholar]
- 17.Kou YR, Frazier DT, Lee LY. The stimulatory effect of nicotine on vagal pulmonary C-fibers in dogs. Respir Physiol 76: 347–356, 1989. doi: 10.1016/0034-5687(89)90075-3. [DOI] [PubMed] [Google Scholar]
- 18.Kou YR, Lee LY. Mechanisms of cigarette smoke-induced stimulation of rapidly adapting receptors in canine lungs. Respir Physiol 83: 61–75, 1991. doi: 10.1016/0034-5687(91)90093-X. [DOI] [PubMed] [Google Scholar]
- 19.Kou YR, Lee LY. Stimulation of rapidly adapting receptors in canine lungs by a single breath of cigarette smoke. J Appl Physiol (1985) 68: 1203–1210, 1990. doi: 10.1152/jappl.1990.68.3.1203. [DOI] [PubMed] [Google Scholar]
- 20.Lee LY, Gerhardstein DC, Wang AL, Burki NK. Nicotine is responsible for airway irritation evoked by cigarette smoke inhalation in men. J Appl Physiol (1985) 75: 1955–1961, 1993. doi: 10.1152/jappl.1993.75.5.1955. [DOI] [PubMed] [Google Scholar]
- 21.Lee LY, Gu Q. Cough sensors. IV. Nicotinic membrane receptors on cough sensors. Handb Exp Pharmacol 187: 77–98, 2009. doi: 10.1007/978-3-540-79842-2_5. [DOI] [PubMed] [Google Scholar]
- 22.Lee LY, Kou YR, Frazier DT, Beck ER, Pisarri TE, Coleridge HM, Coleridge JC. Stimulation of vagal pulmonary C-fibers by a single breath of cigarette smoke in dogs. J Appl Physiol (1985) 66: 2032–2038, 1989. doi: 10.1152/jappl.1989.66.5.2032. [DOI] [PubMed] [Google Scholar]
- 23.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001. doi: 10.1016/S0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 24.Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol 4: 287–324, 2014. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 25.Lin YS, Hsu CC, Bien MY, Hsu HC, Weng HT, Kou YR. Activations of TRPA1 and P2X receptors are important in ROS-mediated stimulation of capsaicin-sensitive lung vagal afferents by cigarette smoke in rats. J Appl Physiol (1985) 108: 1293–1303, 2010. doi: 10.1152/japplphysiol.01048.2009. [DOI] [PubMed] [Google Scholar]
- 26.Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev 96: 975–1024, 2016. doi: 10.1152/physrev.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 17: 1195–1202, 2015. doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- 28.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586: 1595–1604, 2008. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenborn CA, Gindi RM. Electronic Cigarette Use Among Adults: United States, 2014. National Center for Health Statistics No. 217, 2015. https://www.cdc.gov/nchs/products/databriefs/db217.htm. [PubMed] [Google Scholar]
- 30.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 31.Polosa R, Morjaria JB, Caponnetto P, Prosperini U, Russo C, Pennisi A, Bruno CM. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respir Res 17: 166, 2016. doi: 10.1186/s12931-016-0481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricco MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol 496: 521–530, 1996. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson RJ, Hensel EC, Morabito PN, Roundtree KA. Electronic Cigarette Topography in the Natural Environment. PLoS One 10: e0129296, 2015. doi: 10.1371/journal.pone.0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci 12: 1293–1299, 2009. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- 35.Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respir Physiol Neurobiol 178: 406–413, 2011. doi: 10.1016/j.resp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Yang W, Zhang G, Gu Q, Lee LY. Calcium transient evoked by nicotine in isolated rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 292: L54–L61, 2007. doi: 10.1152/ajplung.00182.2006. [DOI] [PubMed] [Google Scholar]
- 37.Yu J, Zhang JF, Roberts AM, Collins LC, Fletcher EC. Pulmonary rapidly adapting receptor stimulation does not increase airway resistance in anesthetized rabbits. Am J Respir Crit Care Med 160: 906–912, 1999. doi: 10.1164/ajrccm.160.3.9809085. [DOI] [PubMed] [Google Scholar]