Abstract
This study compares the effects of an 8-wk isocaloric high-protein (HP) diet versus a combination exercise (Comb-Ex) regimen on paralytic vastus lateralis (VL) and nonparalytic deltoid muscle in individuals with long-standing spinal cord injury (SCI). Fiber-type distribution, cross-sectional area (CSA), levels of translation initiation signaling proteins (Erk-1/2, Akt, p70S6K1, 4EBP1, RPS6, and FAK), and lean thigh mass were analyzed at baseline and after the 8-wk interventions. A total of 11 participants (C5-T12 levels, 21.8 ± 6.3 yr postinjury; 6 Comb-Ex and 5 HP diet) completed the study. Comb-Ex training occurred 3 days/wk and consisted of upper body resistance training (RT) in addition to neuromuscular electrical stimulation (NMES)-induced-RT for paralytic VL muscle. Strength training was combined with high-intensity arm-cranking exercises (1-min intervals at 85–90%, V̇o2peak) for improving cardiovascular endurance. For the HP diet intervention, protein and fat each comprised 30%, and carbohydrate comprised 40% of total energy. Clinical tests and muscle biopsies were performed 24 h before and after the last exercise or diet session. The Comb-Ex intervention increased Type IIa myofiber distribution and CSA in VL muscle and Type I and IIa myofiber CSA in deltoid muscle. In addition, Comb-Ex increased lean thigh mass, V̇o2peak, and upper body strength (P < 0.05). These results suggest that exercise training is required to promote favorable changes in paralytic and nonparalytic muscles in individuals with long-standing SCI, and adequate dietary protein consumption alone may not be sufficient to ameliorate debilitating effects of paralysis.
NEW & NOTEWORTHY This study is the first to directly compare the effects of an isocaloric high-protein diet and combination exercise training on clinical and molecular changes in paralytic and nonparalytic muscles of individuals with long-standing spinal cord injury. Our results demonstrated that muscle growth and fiber-type alterations can best be achieved when the paralyzed muscle is sufficiently loaded via neuromuscular electrical stimulation-induced resistance training.
Keywords: diet, exercise, fiber type, skeletal muscle, spinal cord injury
INTRODUCTION
Spinal cord injury (SCI) results in severe skeletal muscle atrophy below the level of injury. In general, paralyzed muscles undergo a conversion from a slower phenotype (expressing myosin heavy chain, regulatory proteins, and metabolic enzymes) to a faster phenotype arising from numerous changes in expression of metabolic enzymes (8, 15, 26, 38). Our previous findings (44) and those from other studies (34, 40) have repeatedly shown that fatigue-resistant and oxidative muscle fibers transform into highly fatigable and glycolytic muscle fibers with impaired oxidative capacity and mitochondrial function as early as several months after SCI. In addition, we demonstrated that chronically paralyzed muscle presents with muscle inflammation driven by the NF-κB signaling pathway, activation of which promotes atrophy, metabolic dysfunction, and fibrogenesis (42, 43). Even in the event that the ability to walk could be restored, the physiological consequences of these molecular adaptations are profound and could result in a nontreatable muscle tissue in the chronic stages of injury.
Fortunately, paralyzed skeletal muscle retains a great degree of plasticity after SCI (12, 15, 16, 21, 28). Among the available experimental strategies to reverse atrophy, there seems to be a consensus that muscle contraction via neuromuscular electrical stimulation (NMES) is the most potent approach. NMES-induced resistance training (RT) can prevent muscle atrophy and promote muscle hypertrophy (5, 16, 21). Dudley et al. extended these results by loading paralyzed muscles during static and dynamic contractions to mimic RT (16). They showed that loading the paralyzed muscle 48 wk postinjury reversed the process of skeletal muscle atrophy (16). The mechanisms by which this remarkable hypertrophy occurs in paralyzed muscle remain unclear. We recently reported (43) that one bout of NMES-RE markedly increased the activation of signaling proteins associated with initiation of translation (phospho-ribosomal protein S6 [RPS6] Ser-240/244 and phospho-p44/42 MAPK [Erk-1/2] Thr-202/Tyr-204) in chronically paralyzed muscle (∼22 yr postinjury). However, we do not know whether these changes in translation initiation signaling persist after NMES-RT. We also do not know whether long-term NMES-RT leads to changes in the fiber-type composition of chronically paralytic muscle (i.e., vastus lateralis, VL) or whether nonparalytic upper limb muscles (i.e., deltoid) respond to RT similarly to paralytic leg muscles. We designed a combination exercise (Comb-Ex) regimen to challenge strength, power, and endurance with utilization of both nonparalytic upper and paralytic lower limb muscles.
Although muscle contraction provides the most potent anabolic stimulus to date, contraction may not be feasible in situations in which the ability to move is severely restricted by SCI pathology, physical impairment, and/or environmental barriers (inadequate facilities, lack of transport). Consequently, alternative strategies, such as dietary manipulation, may provide an alternative approach to ameliorate the debilitating effects of inactivity. Adequate dietary protein has been shown to present the prerequisite framework required to protect muscle health during periods of physical inactivity (20). The anabolic effects of protein are principally driven by the transfer and incorporation of essential amino acids captured from dietary protein sources into skeletal muscle proteins (19, 33). However, the effect of a high-protein (HP) diet on stimulation of muscle protein synthesis via activation of translation initiation signaling proteins is unknown. Furthermore, it is unclear whether nonparalytic upper limb muscles respond to a HP diet similarly to paralytic leg muscles. A more detailed understanding of the individual effects of exercise or diet alone on nonparalytic and paralytic muscle responses is needed before designing and assessing a combined exercise + diet intervention in individuals with long-standing SCI. Therefore, to begin to address these questions, we compared the effects of an isocaloric HP diet versus a Comb-Ex regimen on myofiber growth and type distribution, lean thigh mass, and intracellular signaling pathways involved in translation initiation-induced muscle hypertrophy.
METHODS
Study Participants
This study's protocol was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board. All participants provided written informed consent after receiving a thorough explanation of study procedures and risks and having an opportunity to ask questions. Twenty individuals with SCI were randomly assigned to the Comb-Ex (n = 10) or HP-diet (n = 10) group. Eleven individuals (6 Comb-Ex and 5 HP diet) completed the study. Participants who completed the study had injuries ranging from the C5–T12 levels and were classified according to the American Spinal Injury Association Impairment Scale as class A and B. The mean age of participants who completed the study was 46.0 ± 7.8 yr, and the mean time postinjury was 21.8 ± 6.3 yr (means ± SD). Participant characteristics may be found in Table 1. Subjects were excluded for any medical or other health conditions that would be expected to affect testing (e.g., muscle contractures) or for which testing might be contraindicated. Medications were recorded; the only medication used in either group (Comb-Ex and HP diet) was an antispasticity medication (Baclofen) in three of the SCI individuals.
Table 1.
Clinical characteristics of study participants
| Subject | Sex | Age | Level and AIS | Years Postinjury | Lean Mass, kg |
|---|---|---|---|---|---|
| Exercise | |||||
| SCI 01 | M | 36 | C6/B | 10 | 57 |
| SCI 02 | M | 56 | T5-6/A | 25 | 58 |
| SCI 03 | M | 50 | T4/B | 23 | 46 |
| SCI 04 | M | 49 | T6/B | 31 | 45 |
| SCI 05 | M | 50 | T2/A | 24 | 56 |
| SCI 06 | M | 50 | C7/B | 30 | 51 |
| Mean ± SD | 48.5 ± 6.6 | 23.8 ± 7.5 | 52.1 ± 5.7 | ||
| Diet | |||||
| SCI 07 | F | 38 | C5-6/B | 15 | 50 |
| SCI 08 | M | 50 | T2/B | 25 | 59 |
| SCI 09 | M | 46 | T5/B | 20 | 47 |
| SCI 10 | M | 37 | T12/A | 16 | 48 |
| SCI 11 | M | 44 | C5-6/B | 21 | 69 |
| Mean ± SD | 43.0 ± 5.4 | 19.4 ± 4.0 | 54.6 ± 9.3 |
SCI, spinal cord injury; AIS, American Spinal Injury Association Impairment Scale; C, cervical SCI; T, thoracic SCI.
Interventions
Combined exercise.
All sessions were supervised by an experienced trainer certified by the American College of Sports Medicine or the National Strength and Conditioning Association. Heart rate (HR) was recorded throughout each session using a Polar HR monitor.
Supervised combined exercise training was completed 3 days/wk in the UAB Center for Exercise Medicine facility. Combined exercise (Comb-Ex) consisted of a combination of exercises designed to challenge strength, power, and endurance with utilization of both upper and lower limb muscles. The full volume prescription consisted of 1) six movements to improve strength and muscle mass (knee extension via neuromuscular electrical stimulation, chest press, overhead press, pull-down, biceps curl, and triceps extension), each performed for three sets of 8–12 repetitions (~30 total repetitions) and 2) arm-cranking exercises (1-min high-intensity intervals at 80–90% V̇o2peak) to improve endurance. With respect to arm cranking, the goal was to accumulate 15 1-min bouts of exercise in between the 18 sets of six strength exercises.
A quantifiable form of knee resistance training using NMES, developed by Gary Dudley (6), was employed for training the paralyzed knee extensors muscle group. The protocol was implemented using surface NMES and ankle weights, and it involved concentric/eccentric contractions from a seated position. Two surface electrodes (7.6 × 12.7 cm; Axelgaard Manufacturing, Fallbrook, CA) were placed proximally over the VL and distally over the vastus medialis. Electrodes were replaced every week. A Theratouch Mini 2.0 NMES unit (Richmar; Chattanooga, TN) supplied current, according to the following parameters: 35-Hz frequency and 250/50-ms pulse duration/interval. NMES current was increased gradually over ~5 s until a full leg extension was achieved, followed by a gradual decrease in current. For the first 2 wk (6 sessions), no ankle weights were used to ensure achievement of full-knee extension against gravity and to protect against skeletal muscle damage. Ankle weights were progressively increased by 1–2 lbs on a weekly basis if a participant achieved 40 repetitions of full-knee extensions in a session. In case of failure to attain full-knee extension for four sets of 10 repetitions, the same load was maintained until the desired number of repetitions was met. NMES current amplitudes varied between participants (45–160 mA). However, the current amplitude was monitored for each repetition and for each participant to ensure that the increase in weight lifted was a result of muscle adaptation, not increased electrical stimulation. Leg training sets were separated by 1-min arm-cranking intervals.
High-protein diet.
The controlled daily diet was designed to fall within the Acceptable Macronutrient Distribution Range established by the Institute of Medicine and met the recommended daily intake for fiber, vitamins, and minerals for adults aged 19–60 yr. Protein (1.6 g·kg−1·day−1) and fat each made up ~30% of total energy intake, and the carbohydrate-to-protein ratio was maintained at less than 1.5. Dietary fat sources primarily consisted of monounsaturated and polyunsaturated fats (i.e., plant oils and nuts); dietary carbohydrate sources emphasized whole grains, fruits, vegetables, and legumes; and dietary protein sources included lean meats, fish, chicken, eggs, and nonfat dairy foods (i.e., fat-free milk and low-fat cheese).
All food was provided by the UAB Clinical Research Unit (CRU) Bionutrition Unit. The overall energy amount was determined based on the resting energy expenditure, as assessed via indirect calorimetry and multiplied by an activity factor. Throughout the 8-wk intervention, participants or their caregivers reported to the UAB CRU Bionutrition Unit twice a week, where they picked up their weekly food to consume at home. Once a week, participants were weighed to ensure weight stability. If weight changed more than 2 kg from baseline, the overall energy amount was modified to maintain participants’ weight. To improve both dietary compliance and study retention, participants assigned to the HP diet were contacted by phone 3 days/wk. Each participant completed a daily self-report form to assess diet adherence. Participants recorded missed study foods and nonstudy foods eaten, including beverages containing caffeine and alcohol. When the participants deviated from the diet, the types of foods consumed and not consumed, as well as the reasons for noncompliance were assessed.
Clinical Tests
Before and after the 8-wk exercise training and diet program, individuals completed a battery of clinical assessments, including a dual-energy X-ray absorptiometry (DXA) scan, peak oxygen consumption (V̇o2peak) assessment, and maximum voluntary upper body strength assessment. For each subject, all pretraining and posttraining assessments were conducted by the same trained member of the research team. Posttraining V̇o2peak assessments, upper body strength assessments, and DXA scans were completed within 48–72 h after the final exercise training or diet session.
DXA scans.
Regional body composition, including thigh lean mass, was measured using DXA (Lunar Prodigy; GE Healthcare Lunar; Madison, WI). Participants were scanned in light clothing while lying flat on their backs with their arms at their sides. The scans were analyzed using ADULT software version 1.33 (Lunar Radiation, Madison, WI).
Peak oxygen consumption assessment.
All subjects underwent a progressive peak oxygen assessment in the UAB Center for Exercise Medicine Cardiorespiratory Testing Laboratory to ascertain peak physical work capacity. Participants were instructed to crank an arm ergometer (Monark, Vansbro, Sweden) at 30 W for 2 min. Every 2 min thereafter, the power output was increased by 30 W until voluntary fatigue. Peak aerobic power was defined as peak oxygen consumption (V̇o2) at the point of failure to maintain 60–65 rotations per minute (18). Minute ventilation, oxygen uptake, and carbon dioxide production were continuously analyzed and recorded using an open-circuit system (CareFusion VMax system, BD, Sydney, Australia). The physiological data obtained from the V̇o2 peak test was used to calculate the exercise intensity corresponding to 80–90% V̇o2peak.
Maximum voluntary upper body strength.
Voluntary strength of the upper body (e.g., bench press, overhead press) was assessed by one-repetition maximum (1RM) strength assessment using our well-established protocols (30). Before undergoing the assessment, subjects attended a familiarization session that included an introduction designed to familiarize participants with the bilateral and unilateral movements tested (e.g., chest press and bicep curl). During this session, each machine was configured for a given subject, and the configuration settings were recorded. Subjects then completed several full range-of-motion repetitions with minimal loads and completed practice 1RM strength tests. This session served to both familiarize subjects with 1RM testing procedures and to instruct and encourage subjects to exert maximal voluntary effort. Participants returned to the laboratory 2–3 days later for 1RM assessments. Before testing, subjects warmed up for 5 min on an arm ergometer or low row set at a low intensity. For each movement, the test protocol was preceded by a set of 8–12 repetitions with a light load. Thereafter, attempts of one repetition with progressively increasing load were performed. Attempts were separated by 90–120-s rest intervals. 1RM was defined as the highest load lifted through a full range of motion before two failed attempts at a given load.
Tissue Collection and Laboratory Analyses
Muscle biopsy.
Muscle samples were collected from the VL and mid-deltoid 24 h before and after the last exercise or diet session via our established percutaneous needle biopsy procedure (2). Briefly, biopsies were performed under local anesthetic (1% lidocaine) using a 5-mm Bergstrom-type biopsy needle under suction, as previously described. Approximately 50–70 mg of muscle collected for immunohistochemistry was mounted cross-sectionally and frozen in liquid nitrogen-cooled isopentane. The remaining tissue was snap frozen in ~30-mg portions for biochemical assays. Sufficient muscle biopsy samples could not be obtained from two participants (1 Comb-Ex and 1 HP diet participant); therefore, muscle protein and histology analysis was performed on a total of nine samples (5 Comb-Ex and 4 HP diet).
Muscle histology.
Myofiber type distribution and type-specific myofiber cross-sectional area (CSA) were assessed by myosin heavy chain isoform immunohistochemistry using established methods, as previously described (23, 24). All preinterevention and postintervention histological assays for the same subject were performed together by the same technician, and all image analyses were conducted in a blinded fashion.
Determination of type-specific muscle fiber size.
For CSA measurements, a minimum of 50 myofibers of each type were randomly selected and manually traced along their laminin-stained borders. SCI samples contained an insufficient number of type I myofibers for accurate fiber size determination.
Determination of myofiber type distribution.
Hybrid IIax fibers were pooled with type IIx fibers. This step was deemed necessary due to the remarkably high percentage of IIax myofibers noted in the SCI muscle samples. For the Comb-Ex group, myofiber type distribution was determined for 808 mid-deltoid and 874 VL myofibers pretraining and for 783 mid-deltoid and 698 VL myofibers posttraining. Myofiber-type distribution was determined for 921 mid-deltoid and 893 VL myofibers pretraining and 873 mid-deltoid and 823 VL myofibers posttraining for the HP diet group.
Immunoblotting.
Mixed muscle protein lysate was prepared using established methods in our laboratory. Briefly, muscle samples (~30 mg) were homogenized after a 15-min preincubation in 6 µl of ice-cold lysis buffer (150 mM NaCl; 50 mM Tris·HCl, pH 7.4; 0.5% Nonidet P-40; 1% deoxycholate; 0.1% SDS; 1% Triton X-100; 5 mM EDTA) containing protease and phosphatase inhibitors (P2714 and P0044; Sigma-Aldrich, St. Louis, MO) per milligram muscle. The samples were then centrifuged at 15,000 g for 40 min at 4°C. The supernatants were stored at −80°C before assaying to measure protein concentrations using the bicinchoninic acid technique using BSA as a standard.
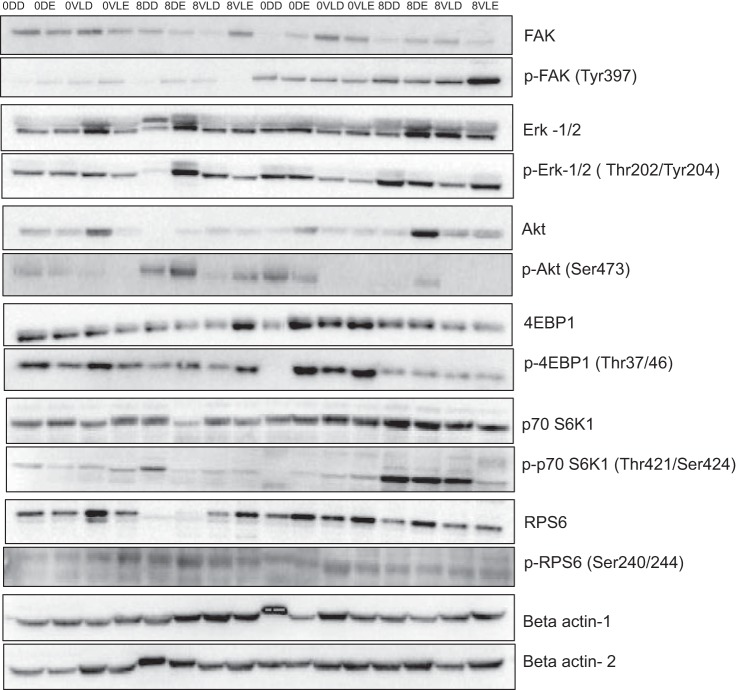
To assess the content and phosphorylation of signaling proteins associated with initiation of skeletal muscle translation, 25-µg samples of skeletal muscle mixed-protein lysate were resolved on 4–12% SDS-PAGE gels and transferred to polyvinylidene fluoride membranes, as previously described (29, 41). Proper transfer was verified by Ponceau S staining before antibody (Ab) incubations. All gels contained samples from both exercise and diet group participants loaded in series (Fig. 3). Primary Abs [p70 S6K1 (Thr-421/Ser-424); RPS6 (Ser-240/244); 4EBP1 (Thr-37/46); FAK (Tyr-397); Akt (Ser-473), and Erk-1/2 (Thr-202/Tyr-204)] were purchased from Cell Signaling Technologies (Danvers, MA) and were diluted 1:1,000 in 5% goat serum (monoclonal Abs) or 2% milk + 2% BSA (polyclonal Abs). HRP-conjugated secondary Abs were diluted 1:50,000. Bound Abs were visualized by chemiluminescent detection (SuperSignal West Dura Chemiluminescent Substrate; Thermo Scientific; Rockford, IL) using a Bio-Rad ChemiDoc imaging system. Parameters for image acquisition using the ChemiDoc were consistent across all membranes and used predefined saturation criteria for the CCD camera, as previously described (3). Band densitometry was performed using Bio-Rad Image Laboratory (version 5.2.1).
Fig. 3.
Representative immunoblots for 12 studied proteins and loading control (β-actin) in nonparalytic deltoid and paralytic vastus lateralis (VL) in combined exercise (Comb-Ex) vs. high-protein (HP)-diet group. The samples were loaded in the order as shown. 0DD = week 0, deltoid-diet; 0DE = week 0, deltoid-exercise; 0VLD = week 0, VL-diet; 0VLE: week 0, VL-exercise; 8DD = week 8 deltoid-diet; 8DE = week 8 deltoid-exercise; 8VLD = week 8, VL-diet; 8VLE = week 8, VL-exercise. Total and phosphorylated proteins were blotted in two separate membranes.
Statistical Analyses
Descriptive statistics, including means and standard deviations, were calculated for all continuous variables, including both clinical and laboratory measurements. Distributions of these variables were examined using stem-and-leaf plots, box plots, normal probability plots, and the Kolmogorov-Smirnov test. None of the distributions were found to deviate substantially from a normal distribution. Statistical analyses were performed using mixed models repeated-measures analysis. An unstructured covariance matrix was assumed. The Tukey-Kramer multiple-comparisons test was then used to identify specific pairs with significantly different means. These models included terms for group, time, extremity (arm or leg), and group-by-time, or extremity-by-time interactions. The two-group Studentʼs t-test was used to determine whether the mean ages, sex, injury level and duration, lean mass, fiber-type distribution and CSA of the groups were significantly different. All statistical tests were two-sided and were conducted at a 5% level of significance. SAS software (version 9.4; SAS Institute; Cary, NC) was used to conduct statistical analyses.
RESULTS
Intervention Adherence
Exercise.
Adherence to the exercise regimen was determined to be ~96% (23 of 24 sessions) for the six Comb-Ex participants over the 8-wk period. In cases in which two consecutive exercise sessions were missed (e.g., due to travel or illness), make-up sessions were scheduled, extending a given participant’s exercise training program to ~9 wk.
Dietary intake.
Four out of five participants in the HP group (80%) consumed all of the meals. One participant did not consume entire portions of study foods during the first week because of reported dislike for the foods. (The participant did not eat fish and olives.) When converted into mean calories per day, deviations for this participant were considered small and did not affect total energy. There were no reports of nonstudy food consumption.
Clinical results.
No differences were found in age, sex, injury level and duration, and lean mass between groups at baseline.
Thigh lean mass.
There was a nonsignificant increase in total lean (+0.8 kg) and thigh lean mass (+480 g) at week 8 versus baseline in the Comb-Ex group (Table 2).
Table 2.
Clinical results before and after 8-wk exercise (Comb-Ex) or dietary (HP diet) interventions
| Comb-Ex |
HP Diet |
|||
|---|---|---|---|---|
| Week 0 | Week 8 | W0 | W8 | |
| Lean mass, kg | 52.0 ± 5.7 | 52.8 ± 5.6 | 54.8 ± 9.6 | 53.3 ± 8.8* |
| Thigh lean tissue, kg | 10.1 ± 1.7 | 10.9 ± 2.2 | 9.6 ± 0.8 | 9.4 ± 1.1 |
| V̇o2peak, kg·ml−1·l−1·min−1 | 13.2 ± 3.4 | 14.6 ± 3.3* | 17.7 ± 3.2 | 17.0 ± 3.5 |
| Arm curl, kg | 21.9 ± 8.3 | 37.5 ± 7.2* | 28.6 ± 13.0 | 28.1 ± 11.8 |
| Overhead press, kg | 53 ± 13.9 | 68.2 ± 14.0* | 49.1 ± 16.4 | 49.1 ± 17 |
| Tricep extension, kg | 19.1 ± 6.1 | 23.5 ± 7.7 | 20 ± 4.8 | 19.7 ± 3.6 |
| Chest fly, kg | 22.7 ± 8.3 | 32.9 ± 9.2* | 25.2 ± 9.4 | 27.7 ± 12.4 |
| Lat pull-down, kg | 49.6 ± 8.9 | 73.5 ± 15.9* | 59.3 ± 19.4 | 57.6 ± 20.2 |
Results are presented as means ± SD. Comb-Ex, combination exercise; HP, high protein.
P < 0.05, different from week 0.
Peak oxygen consumption and maximum voluntary upper body strength.
Peak oxygen consumption was significantly higher in the Comb-Ex group at week 8 versus baseline (P < 0.05; pre: 13.2 versus post: 14.6 kg·ml·l−1·min−1; Table 2). A significant intervention group × time interaction (P < 0.001) was observed for maximum voluntary upper body strength (arm curl, overhead press, chest fly, and lat pull) with Comb-Ex exercise. Participants in the Comb-Ex group experienced a significant increase in upper body strength at week 8 compared with baseline (P < 0.05; Table 2). No effects were observed in the HP group.
Molecular Analyses
Myofiber size and type distribution.
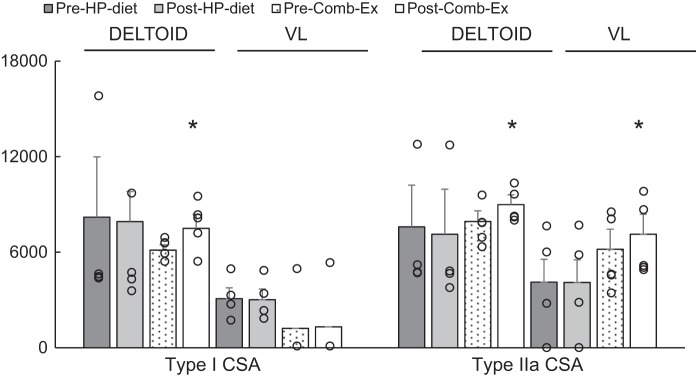
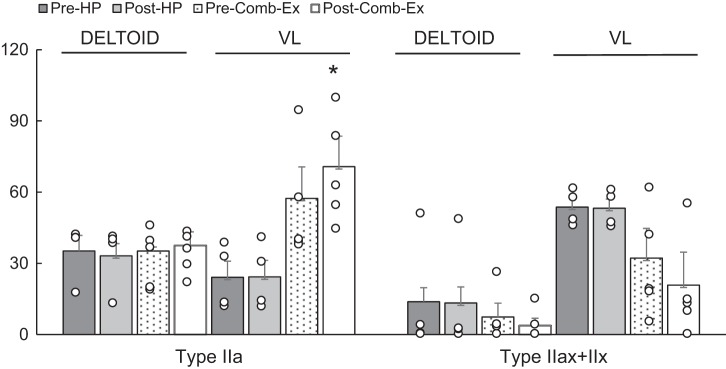
Overall (diet and Comb-Ex group combined) type I distribution was lower (16%, VL versus 54%, deltoid; P < 0.001), and Type IIx distribution was higher (43%, VL vs. 10%, deltoid; P < 0.05) at baseline in the VL versus deltoid muscle. In addition, Type I CSA was lower (1913 μm2 vs. 6671 μm2; P < 0.05) at baseline in the VL versus deltoid muscle. As shown in Fig. 1, Comb-Ex training induced significant hypertrophy of both type I and type IIa myofibers in the deltoid muscle and hypertrophy of type IIa myofibers in the VL muscle (P < 0.05). Comb-Ex training also promoted a shift in myofiber type distribution from IIx to IIa (P < 0.05; Fig. 2) in VL muscle, similar to changes consistently shown with resistance training in healthy adults and individuals with SCI. We observed no difference in myofiber size or type distribution in HP diet group.
Fig. 1.
Effects of high-protein (HP) diet and combination exercise (Comb-Ex) training on muscle fiber cross-sectional area (CSA; μm2) in nonparalytic deltoid and paralytic vastus lateralis (VL) muscle in individuals with long-standing spinal cord injury (SCI). Values are expressed as means ± SE *P < 0.05, different from Pre-Comb-Ex. Dot plots show the complete data set.
Fig. 2.
Effects of high-protein (HP) diet and combined exercise (Comb-Ex) training on muscle fiber-type distribution (%) in nonparalytic deltoid and paralytic vastus lateralis (VL) muscle in individuals with long-standing spinal cord injury (SCI). Values are expressed as means ± SE *P < 0.05, different from Pre-Comb-Ex. Dot plots represent the complete data set.
Markers of translation initiation signaling.
We assessed the expression of candidate skeletal muscle signaling proteins known to mediate translation initiation signaling in resting deltoid and VL muscle biopsy samples. In contrast to the changes observed in myofiber size and distribution with 8 wk of Comb-Ex training, we did not observe increased levels of phosphorylated or total levels of translation initiation signaling proteins in the VL or deltoid muscle after Comb-Ex or HP diet (representative blots presented in Fig. 3). Overall phosphorylated and total levels of 4EBP1 were higher (P < 0.05) in deltoid muscle versus VL muscle in the Comb-Ex group (figure not shown) at baseline.
DISCUSSION
To our knowledge, this study is the first to directly compare the effects of an isocaloric HP diet and combination exercise training on the assessed clinical and molecular changes in paralytic and nonparalytic muscle. Specifically, we compared muscle fiber size and type, thigh mass, and intracellular signaling pathways involved in translation initiation that are associated with induced muscle hypertrophy. Results from this study demonstrate that Type IIa fiber distribution, myofiber CSA are markedly increased in paralytic VL muscle following an 8-wk Comb-Ex training regimen. Furthermore, 8 wk of Comb-Ex increased Type I myofiber CSA in nonparalytic deltoid muscle in individuals with long-standing SCI. These results attest to the robust response of chronically paralytic muscle to NMES-RT. A mere 40 actions per day (3–4 min of actual contraction time) three times per week was sufficient to induce a shift in fiber type from IIx to IIa and a concomitant increase in IIa myofiber size of ~2% per week. In contrast, the HP diet did not increase muscle size or alter fiber-type distribution in the VL muscle. These results demonstrate that muscle growth and fiber-type alterations can best be achieved when the paralyzed muscle is sufficiently loaded, as has been repeatedly shown in nonparalyzed muscle (2, 22, 23).
Under normal conditions, human skeletal muscles are composed of a mixture of fast- and slow-twitch muscle fibers. Evoked muscle activity has previously been reported to play a major role in controlling the phenotypic expression of muscle properties (7). This observation is consistent with muscle adaptations that take place soon after SCI. Paralyzed muscle displays a dramatic decrease in muscle fiber size and shifts toward an increase in IIx fibers soon after injury (8, 9, 15). At a mean of 22 yr after injury, study participants in the HP diet and Comb-Ex groups combined presented with 16% Type I fibers, 40% Type IIa fibers, and 43% Type IIx fibers in the paralytic VL muscle versus 54% Type I fibers, 35% Type IIa fibers, and 10% Type IIx fibers in the nonparalytic deltoid muscle at baseline. While no changes were detected after 8 wk of HP diet, 8 wk of Comb-Ex significantly increased the proportion of type IIa fibers from 57% to 70% (P < 0.05) and decreased the proportion of type IIx fibers from 32% to 20% (not significant) in the VL muscle. To the best of our knowledge, this is the first study to analyze changes in the fiber-type distribution in the SCI population after NMES-RT using the Dudley Protocol (6).
Previous studies (10–12, 31, 36) reported varied responses with respect to fiber-type changes in individuals with long-standing SCI after static (isometric) and dynamic (cycling) NMES. The greatest alterations in fiber-type composition in the VL muscle were observed after 10 wk of NMES-induced static resistance training, with significantly higher proportions of Type I (2% pre- vs. 27% post-NMES) and Type IIa fibers (6% pre- vs. 23% post-NMES) and lower proportions of Type IIx fibers (88% pre- vs. 18% post-NMES) in individuals with complete injuries (11). Our results provide additional support for previous studies showing similar increases in Type IIa fibers posttraining; however, no changes were observed in Type I fibers in the present study. Because of the remarkable loss of Type I myofibers among individuals with long-standing spinal cord injury, results regarding fiber-type shifts are often limited to type II versus type I myofibers (since the number of type I fibers pretraining and posttraining is often too small to reliably assess fiber type changes).
A significant increase (13%) in fiber CSA was observed in Type IIa fibers of the VL muscle and in Type I (19%) fibers of the deltoid muscle after 8 wk of Comb-Ex training compared with baseline. Although our results are in agreement with previous studies in able-bodied individuals and individuals with SCI (11, 12, 28, 39), the observed changes in myofiber CSA in the present study were smaller than those observed in previous SCI studies, which reported 20–80% increases via MRI with the Dudley protocol (16, 39) or a 100% increase with static NMES-RT (11). Regulation of myofiber CSA can be influenced by mode, intensity, and volume of muscle contraction. Therefore, when considering the findings of other studies, it is possible that the amount of force generated during static NMES-RT is higher. In addition, a longer duration of training and differences between completeness of injury could also explain the disparities among these studies. Individuals with complete injuries were included in the previous study that reported a 100% increase in myofiber CSA. We argue that the preservation of functional neural tissue (i.e., skeletal muscle) in incomplete injuries is greater. Thus, the improvements may be more limited because the contractions induced via NMES-RT may represent a smaller fraction of daily muscle activity in individuals with incomplete SCI compared with individuals with complete SCI. In addition to differences in myofiber CSA, an increase of 2.5–3% per week in muscle size was reported via magnetic resonance imaging (MRI) in previous studies (16, 39) using Dudley protocol, whereas, here, we report a 2% increase per week via muscle histology. This small difference in results may be due to differences in measurement techniques. Similarly, myofiber CSAs obtained from biopsy samples may be difficult to reconcile with whole muscle MRI measurements, as regional differences in hypertrophic responses could significantly confound localized biopsy sampling (1). Previous studies (39) using the Dudley protocol also reported a 10% increase in thigh lean mass, and here, we report a similar increase in thigh lean mass (7.5%, Table 2) following 8-wk training. Thus, the minor differences between our results and those reported in previous studies may largely be reflective of differences in measurement methods and study populations.
We observed a significant increase (19%, P < 0.05) in Type I myofiber CSA in the deltoid muscle following 8 wk of Comb-Ex training. RT-induced hypertrophy has been found to occur preferentially in Type II myofibers (4, 24). This preference is thought to result from greater Type II motor unit activation during intensive RT. On the other hand, RT has also been shown to result in marked hypertrophy of both Type I and II myofibers in younger adults (1). We carefully designed an exercise prescription that combines key features of both resistance and aerobic exercise, allowing for the ability to simultaneously challenge both strength and endurance. The full-volume Comb-Ex regimen consisted of strength training for the upper extremity (via voluntary contraction) and lower extremity (via NMES) combined with 15 min of an aerobic, intermittent, high-intensity arm cranking exercise (1-min intervals at 80–90% V̇o2 peak), which resulted in a significant increase in upper body strength and peak V̇o2 (P < 0.05, Table 2). We hypothesize that the greater increase in Type I myofiber CSA in Comb-Ex participants may be due to greater involvement of Type I fibers during high-intensity arm-cranking intervals. Along with increases in deltoid type I myofiber CSA, the Comb-Ex group exhibited a nonsignificant 15% increase in Type IIa myofiber CSA in deltoid muscle, consistent with previous studies in human elbow flexors and extensors with long-term RT (27, 37).
Net protein synthesis required for muscle hypertrophy is regulated primarily by initiation of translation (1, 30, 32, 35). Intracellular signaling pathways that modulate translation initiation are sensitive to many stimuli, including the mechanical forces of muscle contraction. We recently reported increased phosphorylation of p70 S6K1 (Thr-421/Ser-424) and RPS6 (Ser-240/244) following an acute bout of NMES static-RT of the VL muscle (43). Despite acute changes following NMES-RT in our previous study, to our surprise, no significant change was noted in the levels of total or phosphorylated translation initiation signaling proteins in the present study. A number of animal and human studies have reported an acute increase in p70 S6K1 and RPS6 phosphorylation at various residues following resistance exercise that may persist for several hours (13, 14). In the present study, muscle samples were collected from the VL and deltoid muscles at least 24 h before and after the last exercise or diet session; thus, it is certainly possible that the timing of the biopsy was a confounding factor for observing changes in levels of phosphorylation of p70 S6K1 or RPS6 in response to Comb-Ex training. Many pathways and a multitude of cellular processes are required for hypertrophy to occur in vivo; however, our results are admittedly limited to the proteins that we analyzed. Therefore, it is possible that the increase in the activity and total expression of translation initiation signaling proteins that we and others (29, 43) have observed after an acute exercise bout was simply not recapitulated in the trained state in paralytic and nonparalytic muscles of SCI individuals.
Although it is clear that muscle contraction provides a potent anabolic stimulus, it may not be feasible for individuals with SCI due to SCI pathology and environmental constraints. Therefore, we also analyzed whether adequate dietary protein support could stimulate protein synthesis via increased phosphorylation and expression of translation initiation proteins in individuals with long-standing SCI. Previous studies in young and older adults (19, 33) have shown that ingestion of essential amino acids, as little as 2–5 g leucine, stimulates muscle protein synthesis via activation of S6K1 and 4EBP-1 and can offset the catabolic response to prolonged inactivity in able-bodied individuals (3, 25). The average leucine intake was 10–13 g (for an 1,800–2,000 kcal diet) in the HP-diet group; however, no changes in translation initiation signaling were noted in response to the HP diet in individuals with SCI. These results demonstrate that high-protein ingestion alone is not sufficient to promote hypertrophy in individuals with long-standing SCI.
In summary, our results confirm an increase in Type I myofiber CSA in nonparalytic deltoid muscle and upper body strength and demonstrate an increase in Type IIa myofiber CSA and distribution in paralytic VL muscle following an 8-wk Comb-Ex training regimen. We observed no differences in muscle signaling responses induced by NMES-RT on paralytic VL muscle or RT on nonparalytic deltoid muscle. Consuming a HP diet alone had no effect on any of the outcome measures. These results suggest exercise training is required to promote favorable changes in muscle and pave the way for determining whether combining consumption of adequate amounts of dietary protein with RT can additively promote favorable changes in muscles of individuals with long-standing SCI. Limitations of the current study include lack of functional muscle measures (strength and fatigue), information regarding dietary intake of the Comb-ex group, and a small sample size. Further work is needed to more effectively examine the combined effects of HP diet and Comb-Ex on paralytic and nonparalytic muscles of individuals with SCI.
GRANTS
This work was supported by: National Institute on Disability, Independent Living and Rehabilitation Research (NIDILRR) Mary Switzer Fellowship Award (to C. Yarar-Fisher), VA Merit Award (to M. M. Bamman), UAB Center for Exercise Medicine, Department of Physical Medicine and Rehabilitation (to A. B. McLain), and National Institutes of Health 5T32 DK-62710.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.Y.-F. and M.M.B. conceived and designed research; C.Y.-F., K.F.P., M.E., K.Y.H., G.I.K., and S.T.W. performed experiments; C.Y.-F., M.E., G.I.K., and R.A.O. analyzed data; C.Y.-F., K.F.P., C.S.B., A.B.M., R.A.O., and M.M.B. interpreted results of experiments; C.Y.-F. prepared figures; C.Y.-F. drafted manuscript; C.Y.-F. and M.M.B. edited and revised manuscript; C.Y.-F. and M.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors sincerely thank the participants for their tireless dedication and S. C. Tuggle for assistance with project coordination.
REFERENCES
- 1.Adams GR, Bamman MM. Characterization and regulation of mechanical loading-induced compensatory muscle hypertrophy. Compr Physiol 2: 2829–2870, 2012. doi: 10.1002/cphy.c110066. [DOI] [PubMed] [Google Scholar]
- 2.Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol (1985) 97: 1329–1337, 2004. doi: 10.1152/japplphysiol.01387.2003. [DOI] [PubMed] [Google Scholar]
- 3.Batt J, Bain J, Goncalves J, Michalski B, Plant P, Fahnestock M, Woodgett J. Differential gene expression profiling of short- and long-term denervated muscle. FASEB J 20: 115–117, 2006. doi: 10.1096/fj.04-3640fje. [DOI] [PubMed] [Google Scholar]
- 4.Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc 43: 1177–1187, 2011. doi: 10.1249/MSS.0b013e318207c15d. [DOI] [PubMed] [Google Scholar]
- 5.Bickel CS, Slade JM, Haddad F, Adams GR, Dudley GA. Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol (1985) 94: 2255–2262, 2003. doi: 10.1152/japplphysiol.00014.2003. [DOI] [PubMed] [Google Scholar]
- 6.Bickel CS, Yarar-Fisher C, Mahoney ET, McCully KK. Neuromuscular electrical stimulation-induced resistance training after SCI: a review of the Dudley protocol. Top Spinal Cord Inj Rehabil 21: 294–302, 2015. doi: 10.1310/sci2104-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaauw B, Schiaffino S, Reggiani C. Mechanisms modulating skeletal muscle phenotype. Compr Physiol 3: 1645–1687, 2013. doi: 10.1002/cphy.c130009. [DOI] [PubMed] [Google Scholar]
- 8.Burnham R, Martin T, Stein R, Bell G, MacLean I, Steadward R. Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord 35: 86–91, 1997. doi: 10.1038/sj.sc.3100364. [DOI] [PubMed] [Google Scholar]
- 9.Castro MJ, Apple DF Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol 80: 373–378, 1999. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- 10.Chilibeck PD, Jeon J, Weiss C, Bell G, Burnham R. Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord 37: 264–268, 1999. doi: 10.1038/sj.sc.3100785. [DOI] [PubMed] [Google Scholar]
- 11.Crameri RM, Cooper P, Sinclair PJ, Bryant G, Weston A. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve 29: 104–111, 2004. doi: 10.1002/mus.10522. [DOI] [PubMed] [Google Scholar]
- 12.Crameri RM, Weston A, Climstein M, Davis GM, Sutton JR. Effects of electrical stimulation-induced leg training on skeletal muscle adaptability in spinal cord injury. Scand J Med Sci Sports 12: 316–322, 2002. doi: 10.1034/j.1600-0838.2002.20106.x. [DOI] [PubMed] [Google Scholar]
- 13.Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. Eur J Appl Physiol 104: 57–65, 2008. doi: 10.1007/s00421-008-0786-7. [DOI] [PubMed] [Google Scholar]
- 14.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576: 613–624, 2006. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley-Javoroski S, Shields RK. Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev 45: 283–296, 2008. doi: 10.1682/JRRD.2007.02.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley GA, Castro MJ, Rogers S, Apple DF Jr. A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 80: 394–396, 1999. doi: 10.1007/s004210050609. [DOI] [PubMed] [Google Scholar]
- 18.El-Sayed MS, Younesian A. Lipid profiles are influenced by arm cranking exercise and training in individuals with spinal cord injury. Spinal Cord 43: 299–305, 2005. doi: 10.1038/sj.sc.3101698. [DOI] [PubMed] [Google Scholar]
- 19.English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, Ploutz-Snyder R, Sheffield-Moore M, Paddon-Jones D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr 103: 465–473, 2016. doi: 10.3945/ajcn.115.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13: 34–39, 2010. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorgey AS, Shepherd C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: case report. J Spinal Cord Med 33: 90–95, 2010. doi: 10.1080/10790268.2010.11689681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288: E1110–E1119, 2005. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol (1985) 99: 2149–2158, 2005. doi: 10.1152/japplphysiol.00513.2005. [DOI] [PubMed] [Google Scholar]
- 24.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 101: 531–544, 2006. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 25.Léger B, Senese R, Al-Khodairy AW, Dériaz O, Gobelet C, Giacobino JP, Russell AP. Atrogin-1, MuRF1, and FoXO, as well as phosphorylated GSK-3β and 4E-BP1 are reduced in skeletal muscle of chronic spinal cord-injured patients. Muscle Nerve 40: 69–78, 2009. doi: 10.1002/mus.21293. [DOI] [PubMed] [Google Scholar]
- 26.Lotta S, Scelsi R, Alfonsi E, Saitta A, Nicolotti D, Epifani P, Carraro U. Morphometric and neurophysiological analysis of skeletal muscle in paraplegic patients with traumatic cord lesion. Paraplegia 29: 247–252, 1991. [DOI] [PubMed] [Google Scholar]
- 27.MacDougall JD, Elder GC, Sale DG, Moroz JR, Sutton JR. Effects of strength training and immobilization on human muscle fibres. Eur J Appl Physiol Occup Physiol 43: 25–34, 1980. doi: 10.1007/BF00421352. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney ET, Bickel CS, Elder C, Black C, Slade JM, Apple D Jr, Dudley GA. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil 86: 1502–1504, 2005. doi: 10.1016/j.apmr.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Mayhew DL, Hornberger TA, Lincoln HC, Bamman MM. Eukaryotic initiation factor 2B epsilon induces cap-dependent translation and skeletal muscle hypertrophy. J Physiol 589: 3023–3037, 2011. doi: 10.1113/jphysiol.2010.202432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol (1985) 107: 1655–1662, 2009. doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohr T, Andersen JL, Biering-Sørensen F, Galbo H, Bangsbo J, Wagner A, Kjaer M. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord 35: 1–16, 1997. doi: 10.1038/sj.sc.3100343. [DOI] [PubMed] [Google Scholar]
- 32.Nader GA. Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol 37: 1985–1996, 2005. doi: 10.1016/j.biocel.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab 89: 4351–4358, 2004. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- 34.Petrie M, Suneja M, Shields RK. Low-frequency stimulation regulates metabolic gene expression in paralyzed muscle. J Appl Physiol (1985) 118: 723–731, 2015. doi: 10.1152/japplphysiol.00628.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 66: 799–828, 2004. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- 36.Rochester L, Barron MJ, Chandler CS, Sutton RA, Miller S, Johnson MA. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 2. Morphological and histochemical properties. Paraplegia 33: 514–522, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Roman WJ, Fleckenstein J, Stray-Gundersen J, Alway SE, Peshock R, Gonyea WJ. Adaptations in the elbow flexors of elderly males after heavy-resistance training. J Appl Physiol (1985) 74: 750–754, 1993. doi: 10.1152/jappl.1993.74.2.750. [DOI] [PubMed] [Google Scholar]
- 38.Round JM, Barr FM, Moffat B, Jones DA. Fibre areas and histochemical fibre types in the quadriceps muscle of paraplegic subjects. J Neurol Sci 116: 207–211, 1993. doi: 10.1016/0022-510X(93)90327-U. [DOI] [PubMed] [Google Scholar]
- 39.Ryan TE, Brizendine JT, Backus D, McCully KK. Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch Phys Med Rehabil 94: 2166–2173, 2013. doi: 10.1016/j.apmr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Ryan TE, Erickson ML, Young HJ, McCully KK. Case report: endurance electrical stimulation training improves skeletal muscle oxidative capacity in chronic spinal cord injury. Arch Phys Med Rehabil 94: 2559–2561, 2013. doi: 10.1016/j.apmr.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Thalacker-Mercer AE, Dell’Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 40: 141–149, 2010. doi: 10.1152/physiolgenomics.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarar-Fisher C, Bickel CS, Kelly NA, Stec MJ, Windham ST, McLain AB, Oster RA, Bamman MM. Heightened TWEAK-NF-κB signaling and inflammation-associated fibrosis in paralyzed muscles of men with chronic spinal cord injury. Am J Physiol Endocrinol Metab 310: E754–E761, 2016. doi: 10.1152/ajpendo.00240.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yarar-Fisher C, Bickel CS, Kelly NA, Windham ST, Mclain AB, Bamman MM. Mechanosensitivity may be enhanced in skeletal muscles of spinal cord-injured versus able-bodied men. Muscle Nerve 50: 599–601, 2014. doi: 10.1002/mus.24248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yarar-Fisher C, Bickel CS, Windham ST, McLain AB, Bamman MM. Skeletal muscle signaling associated with impaired glucose tolerance in spinal cord-injured men and the effects of contractile activity. J Appl Physiol (1985) 115: 756–764, 2013. doi: 10.1152/japplphysiol.00122.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]