Abstract
Conventional treatments have failed to improve the prognosis of heart failure with preserved ejection fraction (HFpEF) patients. Thus, the purpose of this study was to determine the therapeutic efficacy of chronic interval exercise training (IT) on large-conductance Ca2+-activated K+ (BKCa) channel-mediated coronary vascular function in heart failure. We hypothesized that chronic interval exercise training would attenuate pressure overload-induced impairments to coronary BKCa channel-mediated function. A translational large-animal model with cardiac features of HFpEF was used to test this hypothesis. Specifically, male Yucatan miniswine were divided into three groups (n = 7/group): control (CON), aortic banded (AB)-heart failure (HF), and AB-interval trained (HF-IT). Coronary blood flow, vascular conductance, and vasodilatory capacity were measured after administration of the BKCa channel agonist NS-1619 both in vivo and in vitro in the left anterior descending coronary artery and isolated coronary arterioles, respectively. Skeletal muscle citrate synthase activity was decreased and left ventricular brain natriuretic peptide levels increased in HF vs. CON and HF-IT animals. A parallel decrease in NS-1619-dependent coronary vasodilatory reserve in vivo and isolated coronary arteriole vasodilatory responsiveness in vitro were observed in HF animals compared with CON, which was prevented in the HF-IT group. Although exercise training prevented BKCa channel-mediated coronary vascular dysfunction, it did not change BKCa channel α-subunit mRNA, protein, or cellular location (i.e., membrane vs. cytoplasm). In conclusion, these results demonstrate the viability of chronic interval exercise training as a therapy for central and peripheral adaptations of experimental heart failure, including BKCa channel-mediated coronary vascular dysfunction.
NEW & NOTEWORTHY Conventional treatments have failed to improve the prognosis of heart failure with preserved ejection fraction (HFpEF) patients. Our findings show that chronic interval exercise training can prevent BKCa channel-mediated coronary vascular dysfunction in a translational swine model of chronic pressure overload-induced heart failure with relevance to human HFpEF.
Keywords: BKCa, coronary vascular function, exercise, heart failure, pressure-volume analysis
INTRODUCTION
Exercise is a safe and effective therapeutic treatment to improve cardiorespiratory fitness and quality of life in patients with heart failure (10, 20, 22, 28, 39, 61, 65, 66). Whereas the general clinical efficacy of exercise treatment is becoming accepted, the underlying mechanisms and role of exercise therapy in the treatment of coronary vascular dysfunction in heart failure remain unresolved. Interestingly, under nonpathological conditions chronic aerobic exercise training appears to reduce receptor-mediated vasoconstriction through increases in Ca2+-activated K+ (KCa) channel activity, including the large-conductance Ca2+-activated K+ (BKCa) channel (1, 6, 43). Accordingly, we have shown in miniswine subjected to 6 mo of chronic cardiac pressure-overload that chronic interval exercise training prevents coronary vascular hypersensitivity to endothelin-1 (ET-1) and is associated with maintenance of normal coronary smooth muscle cell Ca2+-sensitive composite K+ current () (26).
Coronary vascular dysfunction significantly impacts left ventricular (LV) function in heart failure, partly via increased responsiveness to vascular vasoconstrictors such as ET-1 (9, 29, 51, 52, 75, 76). Although the molecular mechanisms underlying increased sensitivity to vasoconstrictors remain uncertain, evidence indicates that KCa channels play an important role in the regulation of arterial tone (3, 19, 34, 68) and by extension vascular resistance and coronary perfusion. Vascular smooth muscle ion channel dysfunction coincides with a cluster of heart failure-related comorbidities including hypertension and LV hypertrophy (26, 40, 49, 59). Specifically, BKCa channels are abundantly expressed in vascular smooth muscle and operate as a negative feedback mechanism in response to related increases in vascular smooth muscle intracellular Ca2+ and arterial tone (3, 34, 68). Thus, impaired coronary vascular BKCa channel function in the setting of heart failure may contribute to coronary vascular dysfunction by decreasing protection against excessive vasoconstriction. Consistent with this hypothesis, we have recently shown coronary vascular hypersensitivity to ET-1 (i.e., excessive vasoconstriction) that was associated with decreased coronary smooth muscle Ca2+-sensitive composite in aortic-banded Yucatan miniswine (26). Furthermore, our work has demonstrated an inability to increase coronary blood flow (CBF) in response to increasing myocardial workloads in the same animal model (53). Although evidence suggests that alterations in vascular smooth muscle cell electrophysiological phenotype are involved in the development of cardiovascular disease (40, 68, 78), it remains unknown whether specific changes in coronary vascular BKCa channel function are related to pathological alterations to cardiac function in heart failure.
The purpose of this study was to determine the therapeutic efficacy of chronic interval exercise training on BKCa channel-mediated coronary vascular function in heart failure. In vivo measures of CBF were matched with in vitro assessment of isolated coronary arteriole function in a Yucatan miniswine model of chronic pressure overload-induced heart failure with potential relevance to human heart failure with preserved ejection fraction (HFpEF). Our laboratory has shown that this translational model exhibits key characteristics of HFpEF including preserved ejection fraction at rest, diastolic dysfunction, cardiac hypertrophy, diminished LV contractile reserve, increased LV fibrosis, increased LV natriuretic peptide levels, and lung congestion (24, 26, 31–33, 53, 62–64). We hypothesized that chronic interval exercise training would attenuate pressure overload-induced impairments to coronary BKCa channel-mediated function.
METHODS
Aortic banding and exercise training.
Before aortic banding, 21 intact male Yucatan miniature swine (29–32 kg; 8 mo old) were assigned to three groups: nonsham sedentary control (CON), aortic-banded heart failure sedentary (HF), and aortic-banded heart failure interval exercise trained (HF-IT; n = 7 for all groups). Aortic banding was used to induce heart failure as previously described (31–33, 53, 62, 64). A transstenotic systolic gradient of ~70 mmHg [74 ± 2 and 74 ± 2 mmHg for HF and HF-IT, respectively; P = not significant (NS)] was achieved under anesthesia, using phenylephrine (1–3 μg·kg−1·min−1 iv) to maintain a distal peripheral vascular mean arterial pressure (MAP) of ~90 mmHg (90 ± 1 and 91 ± 2 mmHg for HF and HF-IT, respectively; P = NS) at a heart rate (HR) of 100 beats/min (104 ± 5 and 99 ± 8 beats/min for HF and HF-IT, respectively; P = NS). Two months after surgery, transthoracic echocardiography was performed under inhaled isoflurane anesthesia (0.5%) to measure LV end-diastolic dimension and LV diastolic wall thickness with M-mode recordings using a 1.5- to 4-MHz transducer on a GE Vivid i Ultrasound system as described previously (24, 33, 53). Aortic banding significantly increased LV diastolic wall thickness in HF animals (P < 0.05; 5.8 ± 0.5 and 7.6 ± 0.3 mm for CON and HF, respectively) but did not alter LV end-diastolic dimension (P = NS; 46.1 ± 0.7 and 44.6 ± 0.7 mm for CON and HF, respectively), indicating that concentric LV hypertrophy was present before the onset of exercise training. No differences in echocardiographic measures of morphology existed between HF and HF-IT groups at this time point; thus, data from all aortic-banded groups were combined before the start of exercise training. After the development of LV hypertrophy, animals began interval exercise training consisting of treadmill running 55 min/day, 3 days/wk, for 17 wk with gradually increasing intensity using the following protocol as previously published (24, 26, 53): 1) 5-min warm-up at 2 mph; 2) six 5-min intervals at 3 mph interspersed with five 3-min intervals at 4 mph; and 3) 5-min cool-down at 2 mph. Animals were fed a standard diet averaging 15–20 g/kg once daily, and water was provided ad libitum. Dissection of vital tissues and removal of skeletal muscle (deltoid and triceps) for analysis of citrate synthase activity (74) occurred at the time of death. All animal protocols were in accordance with the U. S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing Research and Training and were approved by the University of Missouri Animal Care and Use Committee.
Terminal studies.
Animals were initially anesthetized with a Telazol (5 mg/kg)-xylazine (2.25 mg/kg) mix and maintained on propofol (6–10 mg·kg−1·min−1 with bolus as needed) as previously described (32, 33, 64). Heparin was given intravenously with an initial loading dose of 300 U/kg, followed by maintenance of 100 U/kg each hour. A median sternotomy was performed, and the pericardium was opened along the left anterior descending coronary artery (LAD) and near the apex for insertion of catheters and flow probes. Care was taken to cause minimal disruption to the pericardium. A 3PSB flow probe (Transonic Systems, Ithaca, NY) was placed around the LAD to measure CBF. Pressure-volume (P-V) loops were measured with a calibrated 5-F admittance-based ADVantage catheter (Transonic Systems) positioned in the LV via a small apical incision. A 14-F balloon occlusion catheter (Edward Life Sciences) was advanced to the inferior vena cava at the level of the apex of the heart via the deep femoral vein. Peripheral systemic MAP was measured via a fluid-filled 6-F LCB SH guide catheter (Boston Scientific) introduced through a 7-F sheath placed in the right femoral artery and positioned in the aorta distal to the aortic band. Catheter placement was visualized and confirmed with angiography (InfiMed software) and Visipaque contrast medium.
In vivo cardiovascular function.
Experiments were conducted as previously published (26, 32, 53). Briefly, after the insertion of catheters animals were allowed to stabilize for 10 min until a resting, baseline homeostasis was established (labeled “Baseline”). BKCa channel-mediated coronary vascular function was measured after intracoronary administration of NS-1619 (200 μg/min) alone and in the presence of penitrem A (Pen A; 10 μg/kg) (4, 30). The agonist action of NS-1619 on the BKCa channel α-subunit was used to identify BKCa channel-mediated aspects of CBF in vivo (19, 34, 35, 46). Pen A, an antagonist of the BKCa channel, was used to demonstrate that vascular responses to NS-1619 resulted from BKCa channel activation (41). Pilot experiments were conducted with 6, 60, 100, and 200 µg/min infusions of NS-1619. The 100 and 200 µg/min infusions provided similar coronary responses; thus, 200 µg/min was chosen to determine the functional response to NS-1619. Drug treatments were administered directly into the proximal LAD with an infusion catheter inserted via the guide catheter as previously reported (26). After insertion of the infusion catheter and placement of the flow probe, CBF was allowed to stabilize until a stable baseline was observed for ≈10 min, at which point baseline HR and MAP were measured before the intracoronary introduction of any treatments. Coronary function was assessed at Baseline and in response to three treatments applied individually in the following order: NS-1619 (1,000 μg total infused over 5 min), Pen A pretreatment (10 μg/kg infused over 5 min), and NS-1619 (1,000 μg total infused over 5 min) + Pen A (10 μg/kg infused over 5 min). Coronary vascular conductance (CVC) was calculated from MAP and CBF measurements (CVC = CBF/MAP). The difference in coronary vascular function between the NS-1619 dose and Baseline was used to evaluate BKCa channel-dependent vasodilatory capacity. CBF and conductance were normalized to the region of the heart perfused by the LAD, estimated to be 30% of total heart weight (27) and utilized in swine (5, 53).
P-V loops were recorded at Baseline under conditions of reducing preload, achieved through transient occlusion of the inferior vena cava via inflation of the balloon catheter. Indexes of LV function were generated using a minimum of 10 consecutive cardiac cycles with Laboratory Scribe software (iWorx, Dover, NH) including HR, LV end-systolic and -diastolic volume (LVESV and LVEDV, respectively), LV end-systolic and -diastolic pressure (LVESP and LVEDP, respectively), ejection fraction (EF%), and stroke volume (SV). Indexes of LV contractility and stiffness including the end-systolic P-V relationship (ESPVR), preload recruitable stroke work (PRSW), and the end-diastolic P-V relationship (EDPVR) were measured using at least 15 consecutive cardiac cycles of constantly reducing preload. A quadratic fit was used to determine ESPVR and PRSW, and an exponential fit was used to calculate EDPVR as previously reported (32).
Coronary resistance artery function.
After in vivo studies, hearts were rapidly excised and placed in cold (4°C) Krebs solution containing (in mM) 131.5 NaCl, 5.0 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 25.0 NaHCO3, 0.02 EDTA, and 11.2 glucose (pH 7.4) as previously described (58). Coronary resistance arteries (137 ± 39 µm) were isolated from the apex region of the LV, cannulated with glass micropipettes (75- to 80-μm OD, resistance = 150–250 kΩ), and maintained in physiological saline solution (PSS) containing (in mM) 3.0 MOPS, 145.0 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM NaH2PO4, 5.0 mM glucose, 2.0 pyruvate, and 0.02 EDTA with 10 mg/ml BSA. Arteries were viewed through an inverted microscope (Nikon Diaphot, ×20 magnification, spatial resolution <1 μm), with well-established methods from our laboratory (37, 42, 58). Intraluminal diameter was monitored continuously with a video tracking system (Microcirculation Research Institute, Texas A&M University) and recorded for the duration of the experiments with PowerLab data acquisition software (ADInstruments, Colorado Springs, CO). During the initial equilibration period (1 h), vessels were monitored for leaks and warmed gradually to 37°C. Micropipettes were connected to two independent fluid-filled reservoirs of similar height, and arteries were pressurized at 60 cmH2O.
During the initial equilibration, vasoreactivity was assessed in response to 80 mM KCl in PSS and vessels were monitored for development of spontaneous tone in the range of 20–40% relative to initial passive diameter. Vessels that spontaneously developed tone in this range were subsequently exposed to seven incremental logarithmic doses of the BKCa channel α-subunit agonist NS-1619 (Sigma-Aldrich, St. Louis, MO) ranging from 1e−10 to 1e−4. Vessels not developing spontaneous tone were preconstricted with U-46619 (Cayman, Ann Arbor, MI; a stable analog of prostaglandin H2), given in three log doses ranging from 1e−8 to 1e−6 to produce constriction to 20–40%. On reaching a steady level of tone, the dose response to NS-1619 was started. At the conclusion of each experiment, maximal dilation was determined by replacing the PSS with calcium-free buffer containing (in mM) 145.0 NaCl, 3.0 MOPS, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 4.7 KCl, 1.2 MgSO4, 0.0 CaCl2, 2.0 EDTA, and 0.1 sodium nitroprusside with 10 mg/ml BSA. There were no differences in the level of preconstriction among groups (CON = 23 ± 2%, HF = 24 ± 2%, and HF-IT = 28 ± 5%; P = NS), the required dose of U-46619 (CON = 1e−5 ± 0 M, HF = 1e−5 ± 0 M, HF-INT = 6e−6 ± 2e−6 M; P = NS), or the level of preconstriction obtained with U-46619 vs. spontaneous tone (U-46619 preconstriction = 25 ± 3%, spontaneous tone = 24 ± 2%; P = NS). There were also no differences in passive coronary arteriole diameters among groups (CON = 137 ± 11 µm, HF = 123 ± 8 µm, and HF-IT = 128 ± 9 µm; P = NS) or vasoreactivity to NS-1619 between vessels with spontaneous tone and those preconstricted with U-46619 (max NS-1619 dilation in vessels with tone = 60 ± 9% vs. preconstricted with U-46619 = 59 ± 7%; P = NS). Vasomotor responses to NS-1619 were expressed as percent possible dilation [i.e., the quotient of (∆NS-1619 dose diameter − Baseline diameter)/(∆maximal diameter − Baseline diameter), multiplied by 100].
qRT-PCR.
Quantitative real-time PCR (qRT-PCR) was performed as previously described (25, 53). LV and arterioles (150–300 µm) isolated from the apex of the LV were quick frozen in liquid nitrogen and stored at −80°C until processed. Total RNA was isolated from pulverized LV tissue according to the manufacturer’s published protocol for TRIzol. Total RNA was isolated from arterioles with the Arcturus PicoPure RNA isolation kit (Applied Biosystems) per manufacturer’s instructions. cDNA was transcribed from total RNA with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) in a 20-μl reaction. A minus-reverse transcription reaction was also performed to ensure no genomic DNA contamination. qRT-PCR was performed on a Bio-Rad MyIQ2 cycler (model no. 170-9790). Each 25-μl reaction contained 1× SYBR Green Master Mix (Bio-Rad), 0.8 μM forward and reverse primers, and 1 μg of cDNA. The reaction conditions were optimized for each set of primers listed below. Target gene expression was normalized to 18S ribosomal RNA with the method, where Ct is threshold cycle (50). Linearity and efficiency of each PCR condition were verified by creating a standard curve plotting the critical threshold vs. log of the cDNA dilution. Analysis of 18S, brain natriuretic peptide (BNP), and BKCa channel α-subunit mRNA levels was performed with the following primers: 18S (F: 5′-CGG CTA CCA CAT CCA AGG AA-3′; R: 5′-AGC TGG AAT TAC CGC GGC-3′), BNP (F: 5′-GCA GCA GCC TCT ATC CTC TC-3′; R: 5′-TCC TGT ATC CCT GGC AGT TC-3′), and BKCa channel α-subunit (F: 5′-GGA ATG GGA GAC GCT TCA TA-3′; R: 5′ CCT GCA GCG AAG TAT CAT CA-3′).
Western blotting.
For each analysis, five to eight coronary resistance arterioles (150–300 µm) were dissected from the apex of the LV, transferred to a microcentrifuge tube, quickly frozen on dry ice, and stored at −80°C. Arterioles were solubilized in Laemmli buffer (62.5 mM Tris, pH 6.8, 6 M urea, 160 mM dithiothreitol, 2% SDS, and 0.0001% bromophenol blue), boiled, vortexed, and sonicated (36). Proteins were resolved by SDS-PAGE using 4–20% acrylamide, transferred onto PVDF membranes, and blotted with the following commercially available antibodies: BKCa channel α-subunit (110–130 kDa, 1:500; NeuroMab) and β-actin (42 kDa, 1:2,500; Sigma-Aldrich). Membranes were incubated with horseradish peroxidase (HRP)-linked secondary antibody (1:2,500–1:5,000) and incubated in Luminata Forte Western Chemiluminescent HRP substrate reagent (EMD Millipore). A Kodak image station (4000R) was used to visualize and quantify protein band densities.
Surface biotinylation of intact, endothelium-denuded coronary arteries.
A 5-mm sample of right coronary artery was dissected and denuded of endothelium. Loosely adherent perivascular fat and connective tissue were resected from the vessel’s adventitial surface before flash-freezing in liquid N2 for subsequent analysis of cell surface membrane and cytoplasmic protein levels of the BKCa channel α-subunit, as previously published (60). To obtain sufficient protein for the biotinylation analysis, studies were conducted with the right coronary artery. Arteries were incubated for 1 h in 1 mg/ml EZ-Link Sulfo-NHS-LC-LC-Biotin (Thermo Scientific) and EZ-Link Maleimide-PEG2-Biotin (Thermo Scientific) at 4°C while shaking. Unbound biotin was quenched by washing three times with 4°C 100 mM glycine-PBS, followed by one wash with 4°C PBS. Arteries were then minced and added to 4°C RIPA lysis buffer consisting of 150 mM NaCl, 50 mM Tris, 1% Triton X, 0.1% SDS, 5 mM EDTA, and 1 Complete EDTA-free Protease Inhibitor tablet (Roche). Arteries were homogenized by completing three cycles of 1 min of shaking (45 Hz; Qiagen) with Next Advance stainless steel beads, 1 min on ice, and a 5-s sonication. Samples were next incubated for 30 min at 4°C, followed by one final homogenization cycle, and then centrifuged (6,000g/1 min), after which supernatant containing protein was transferred to a clean tube. Total protein was quantified with the Thermo Fisher BCA Protein Assay to allow normalization for avidin pull-down of biotinylated surface proteins. Twenty-five micrograms of total protein of each sample was incubated with Monomeric Avidin Agarose beads (Pierce) for 1 h at 4°C. Samples were centrifuged (12,000g/3 min) at 4°C to pull down avidin-bound biotinylated surface proteins. The supernatant containing nonbiotinylated cytosolic proteins was removed and prepared for Western blot by adding Laemmli buffer + 5% (vol/vol) β-mercaptoethanol and boiling for 5 min before electrophoresis. Biotinylated surface proteins were eluted from the avidin beads by adding Laemmli buffer + 5% (vol/vol) β-mercaptoethanol, boiling for 5 min before electrophoresis, and centrifuging (6,000g/1 min) to pellet unbound beads. Supernatant containing biotinylated surface membrane proteins was removed and used for Western blotting. Membrane and cytosolic fractions of the BKCa channel α-subunit were quantified with Western blotting procedures outlined above, and membrane-to-cytosolic ratio was determined.
Statistical analysis.
Data analyses were performed with SPSS version 19.0 or SigmaStat version 3.5. Group comparisons for citrate synthase activity, BNP, heart and lung weight, P-V measures, BKCa channel-mediated vasodilatory capacity, and BKCa channel α-subunit mRNA/protein were made with a one-way ANOVA. Group comparisons for in vivo and in vitro coronary vascular function and systemic hemodynamics were made with a repeated-measures ANOVA (Group × Dose). Group differences revealed by ANOVA were found with Student-Newman-Keuls post hoc analysis. All data are presented as means ± SE, and significance is reported at the P < 0.10 and P < 0.05 levels (11, 80).
RESULTS
Citrate synthase activity, LV BNP mRNA levels, LV remodeling, and cardiac systolic/diastolic function.
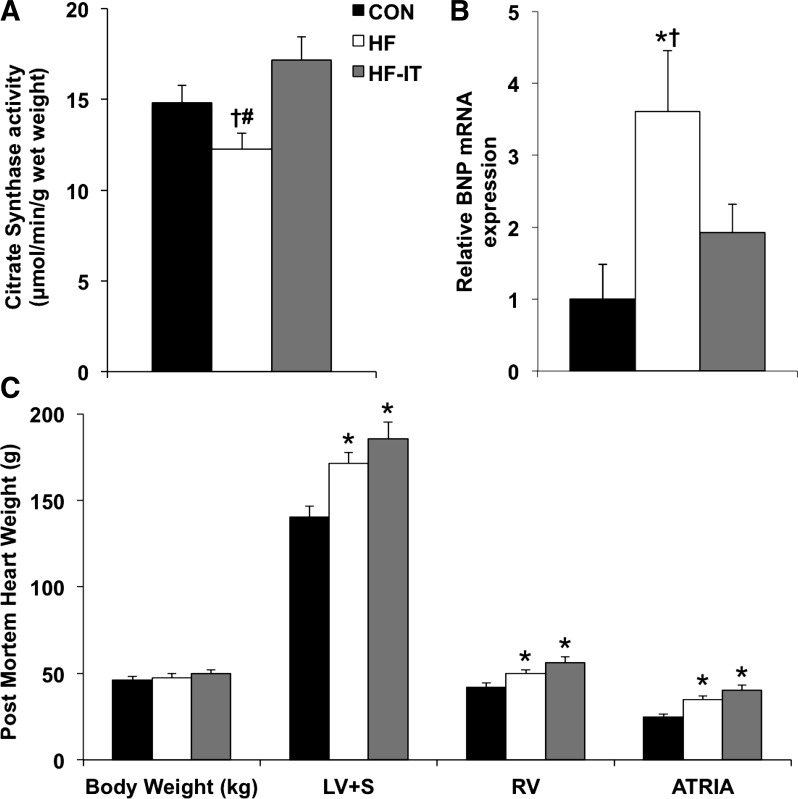
Skeletal muscle citrate synthase activity, a standard metabolic marker of exercise training, was decreased in the HF group compared with CON and HF-IT animals (Fig. 1A). Aortic banding increased LV BNP mRNA levels in HF relative to CON animals (Fig. 1B). This increase in ventricular BNP was attenuated in the HF-IT group. Lung weight was increased in both the HF and HF-IT groups compared with CON (P < 0.10; 258 ± 15, 317 ± 21, and 307 ± 16 g for CON, HF, and HF-IT, respectively). As previously shown by our laboratory (24, 53), aortic banding induced cardiac hypertrophy independent of exercise training status as indicated by postmortem evaluation (Fig. 1C). Increased LV + septum, right ventricle, and atrial weight were observed in HF and HF-IT animals compared with CON. Absolute heart and lung weights were used for analysis, given that body weights were the same between groups.
Fig. 1.
Skeletal muscle citrate synthase activity, left ventricle (LV) brain natriuretic peptide (BNP) mRNA levels, body weight, and cardiac remodeling in nonsham sedentary control (CON), aortic-banded heart failure sedentary (HF), and aortic-banded heart failure interval exercise-trained (HF-IT) animals. A: exercise training prevents decreased skeletal muscle citrate synthase activity observed in HF animals (1-way ANOVA, P < 0.05). B: exercise training attenuates increased BNP mRNA levels in the LV of the HF group (1-way ANOVA, P < 0.05). C: aortic banding generates cardiac hypertrophy evident by increased postmortem LV + septum (LV+S), right ventricle (RV), and left + right atria (ATRIA) weight that is not inhibited by exercise training (1-way ANOVA, P < 0.05). Body weight was the same in all groups. *Post hoc vs. CON (P < 0.05); †post hoc vs. HF-IT (P < 0.05); #post hoc vs. CON (P < 0.10). n = 7, 7, and 7 for CON, HF, and HF-IT, respectively.
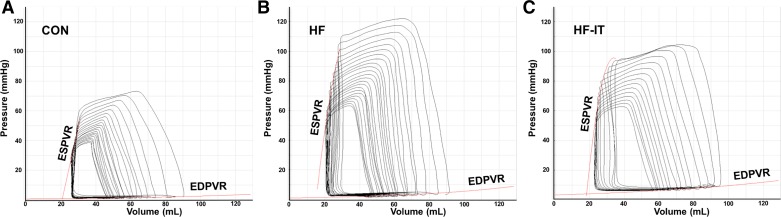
P-V analysis of Baseline LV function is presented in Table 1 (representative Baseline P-V loops are shown in Fig. 2). HR, end-systolic volume, EF%, and SV were the same in all groups, indicating that resting systolic function was generally normal in all aortic-banded animals. End-systolic pressure and contractility, as indicated by PRSW, were increased relative to CON in both the HF and HF-IT groups. Impaired diastolic function was present in both aortic-banded groups and indicated by an increase in the slope of the EDPVR, a gold standard measure for diastolic function. End-systolic and -diastolic volumes were the same between all groups. In total, cardiac pressure overload resulted in numerous characteristics consistent with heart failure and compensated resting systolic function (i.e., HFpEF) including LV hypertrophy, diastolic dysfunction, increased lung weight and LV natriuretic peptide levels, and normal resting EF%. Considered with previous reports from our laboratory showing increased LV fibrosis, reduced LV contractile reserve, altered LV mechanics, and impaired individual cardiomyocyte function (24, 26, 31–33, 53), these results demonstrate the relevance of this large-animal model to the pathophysiology of HFpEF.
Table 1.
Pressure-volume analysis of baseline systolic and diastolic function
| CON | HF | HF-IT | One-Way ANOVA | |
|---|---|---|---|---|
| Systolic function | ||||
| HR, beats/min | 113 ± 5 | 103 ± 8 | 106 ± 5 | NS |
| LVESV, ml | 39 ± 7 | 36 ± 6 | 32 ± 3 | NS |
| LVESP, mmHg | 78 ± 4 | 109 ± 6* | 106 ± 6* | P < 0.05 |
| LV EF, % | 64 ± 3 | 66 ± 3 | 65 ± 4 | NS |
| LV SV, ml | 66 ± 6 | 67 ± 4 | 74 ± 5 | NS |
| ESPVR, mmHg/ml | 6 ± 1 | 14 ± 3 | 11 ± 2 | NS |
| PRSW, mmHg | 65 ± 6 | 106 ± 15* | 103 ± 7* | P < 0.05 |
| Diastolic function | ||||
| LVEDV, ml | 105 ± 11 | 103 ± 9 | 103 ± 7 | NS |
| LVEDP, mmHg | 7 ± 1 | 9 ± 2 | 8 ± 1 | NS |
| EDPVR, mmHg/ml | 0.005 ± 0.001 | 0.012 ± 0.002* | 0.016 ± 0.003* | P < 0.05 |
Values are means ± SE; n = 5, 6, and 6 for CON, HF, and HF-IT, respectively. CON, nonsham sedentary control; HF, aortic-banded heart failure sedentary; HF-IT, aortic-banded heart failure interval exercise trained; HR, heart rate; LVESV, left ventricular (LV) end-systolic volume; LVESP, LV end-systolic pressure; EF, ejection fraction; SV, stroke volume; ESPVR, end-systolic pressure-volume relationship; PRSW, preload recruitable stroke work; LVEDV, LV end-diastolic volume; LVEDP, LV end-diastolic pressure; EDPVR, end-diastolic pressure-volume relationship; NS, not significant.
Post hoc, P < 0.05 vs. CON.
Fig. 2.
Representative pressure-volume (P-V) loops at Baseline from individual nonsham sedentary control (CON; A), aortic-banded heart failure sedentary (HF; B), and aortic-banded heart failure interval exercise-trained (HF-IT; C) animals. ESPVR, end-systolic P-V relationship; EDPVR, end-diastolic P-V relationship.
In vivo hemodynamics.
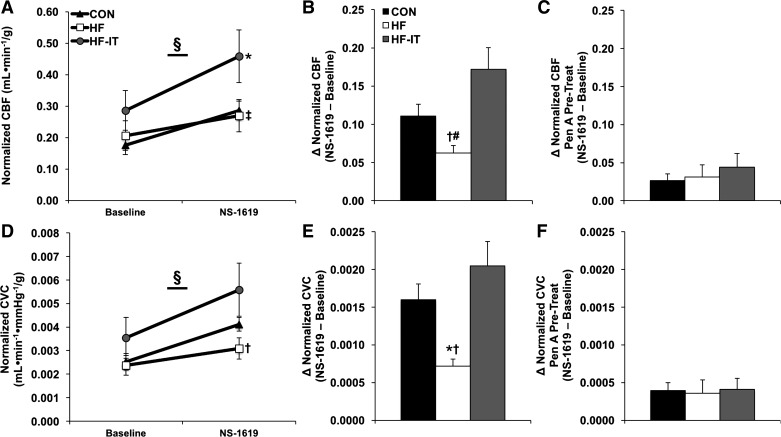
Changes in CBF following BKCa channel activation by NS-1619 were dependent on experimental group (Group × Dose interaction; Fig. 3A). Specifically, absolute CBF normalized to heart size was greatest in HF-IT animals compared with CON and HF during NS-1619 infusion. The relative CBF response to BKCa channel activation, measured as the difference in CBF following infusion of NS-1619 minus Baseline, was significantly decreased in HF animals compared with CON and HF-IT groups (Fig. 3B). Pretreatment of the coronary vasculature with Pen A, an antagonist of the BKCa channel, blocked the NS-1619 response in all groups, supporting the role of the BKCa channel in these responses (Fig. 3C). To examine the vasodilatory response to BKCa activation, we also examined CVC during NS-1619 infusion. These results showed that absolute CVC was greatest in HF-IT animals compared with CON and HF during NS-1619 infusion (Group × Dose interaction; Fig. 3D). Again, relative coronary vasodilation in response to BKCa channel activation, measured as the difference in CVC following infusion of NS-1619 minus Baseline, was significantly decreased in the HF group compared with CON and heart failure-induced reductions were restored by exercise training (Fig. 3E). Vasodilation to NS-1619 was inhibited by pretreatment with Pen A (Fig. 3F). The CBF (13–44 ml/min before normalization to heart weight) and vascular conductance values in this study match previous work from our laboratory (26, 53), and are consistent (albeit slightly reduced because of measurement under sedation) with published studies in conscious swine (14–16, 55). MAP was lower in the CON group over the experimental protocol (Group × Dose interaction; Table 2), and no group differences in HR were observed.
Fig. 3.
In vivo normalized coronary blood flow (CBF) and vascular conductance responses to large-conductance Ca2+-activated K+ (BKCa) channel α-subunit activation in nonsham sedentary control (CON), aortic-banded heart failure sedentary (HF), and aortic-banded heart failure interval exercise-trained (HF-IT) animals. A: CBF response [relative to left ventricle (LV) weight] following BKCa channel α-subunit activation by NS-1619 is dependent on group (repeated-measures ANOVA, P < 0.05). B: exercise training prevents decreased BKCa channel-mediated increases in CBF observed in HF animals, measured as the difference (∆) in CBF following infusion of NS-1619 minus Baseline (1-way ANOVA, P < 0.05). C: NS-1619-induced increases in CBF are attenuated by pretreatment with penitrem A (Pen A) [P = not significant (NS)]. D: increased coronary vascular conductance (CVC; relative to LV weight) following BKCa channel α-subunit activation by NS-1619 is dependent on group (repeated-measures ANOVA, P < 0.05). E: again, exercise training prevents decreased BKCa channel-mediated vasodilatory capacity observed in HF animals, measured as the difference in CVC following infusion of NS-1619 minus Baseline (1-way ANOVA, P < 0.05). F: NS-1619-induced increases in CVC are attenuated by pretreatment with Pen A (P = NS). §Interaction effect: Group × Dose (P < 0.05); *post hoc vs. CON (P < 0.05), †post hoc vs. HF-IT (P < 0.05); #post hoc vs. CON (P < 0.10); ‡post hoc HF vs. HF-IT (P < 0.10). n = 5, 6, and 6 for CON, HF, and HF-IT, respectively.
Table 2.
Hemodynamic variables at baseline and during drug infusions
| Baseline | NS-1619 | Pen A | Pen A + NS-1619 | |
|---|---|---|---|---|
| MAP, mmHg§ | ||||
| CON | 69 ± 6 | 69 ± 4 | 71 ± 4 | 69 ± 4 |
| HF | 98 ± 6* | 85 ± 6 | 80 ± 6 | 82 ± 6 |
| HF-IT | 91 ± 7* | 83 ± 7 | 90 ± 8 | 90 ± 9# |
| HR, beats/min | ||||
| CON | 113 ± 5 | 102 ± 5 | 100 ± 5 | 102 ± 4 |
| HF | 103 ± 8 | 94 ± 8 | 92 ± 7 | 90 ± 7 |
| HF-IT | 106 ± 5 | 100 ± 4 | 96 ± 4 | 94 ± 4 |
| CBF, ml·min−1·g−1 | ||||
| CON | 0.18 ± 0.03 | 0.29 ± 0.03 | 0.22 ± 0.02 | 0.21 ± 0.03 |
| HF | 0.21 ± 0.05 | 0.27 ± 0.05 | 0.28 ± 0.05 | 0.29 ± 0.06 |
| HF-IT | 0.29 ± 0.06 | 0.46 ± 0.08 | 0.28 ± 0.06 | 0.27 ± 0.03 |
Values are means ± SE; n = 5, 6, and 6 for CON, HF, and HF-IT, respectively. Pen A, penitrem A; CON, nonsham sedentary control; HF, aortic-banded heart failure sedentary; HF-IT, aortic-banded heart failure interval exercise trained; MAP, mean arterial pressure; HR, heart rate; CBF, coronary blood flow. Significance is indicated at
P < 0.10, 2-way repeated-measures ANOVA, Group × Dose interaction;
post hoc, P < 0.05 vs. CON;
post hoc, P < 0.10 vs. CON.
In vitro coronary arteriole function and mRNA, protein, and cellular distribution of BKCa α-subunit protein.
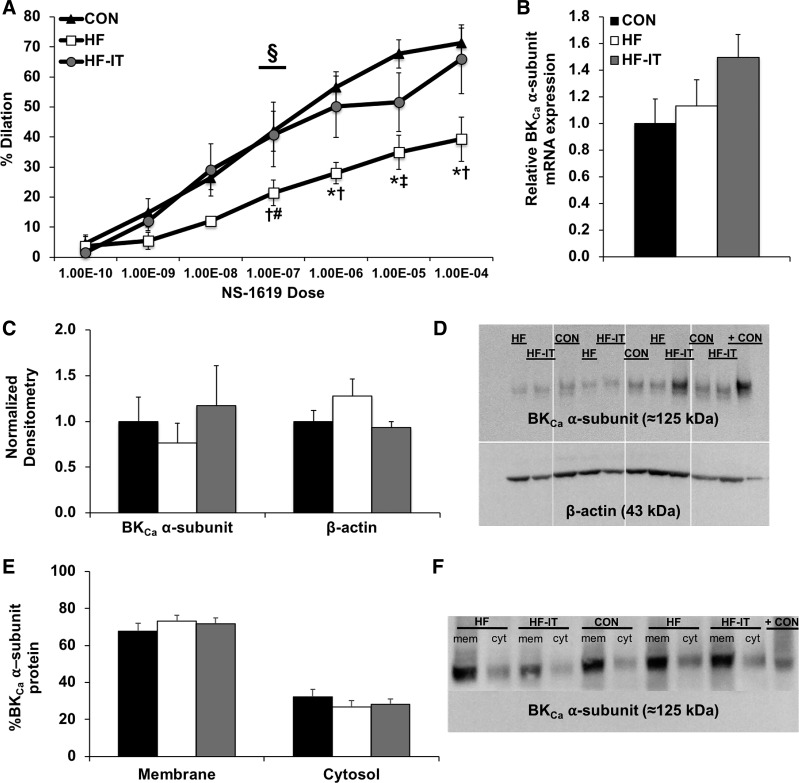
Vasodilation of isolated coronary arterioles to NS-1619 supported the in vivo findings. The vasodilatory response to activation of the α-subunit of the BKCa channel by NS-1619 was dependent on experimental group (Group × Dose interaction; Fig. 4A). Specifically, arteriole vasodilation was decreased in HF animals compared with the CON and HF-IT groups. Preservation of normal BKCa-dependent vasodilatory function after exercise training was not associated with any changes in LV arteriole BKCa channel α-subunit mRNA (Fig. 4B) or protein (Fig. 4, C and D) levels. Arteriolar β-actin (loading control) protein levels were determined to be the same between groups; therefore, BKCa channel α-subunit protein level was analyzed relative to CON. Similarly, there were no measurable differences in the cellular location of endogenous BKCa channel α-subunit protein between the basal membrane and cytosol, or membrane-to-cytosolic ratio (P > 0.59; 2.4 ± 0.4, 3.0 ± 0.5, and 2.8 ± 0.4 for CON, HF, and HF-IT, respectively) in samples from the right coronary artery (Fig. 4, E and F).
Fig. 4.
In vitro coronary arteriole functional responses to large-conductance Ca2+-activated K+ (BKCa) channel α-subunit activation in nonsham sedentary control (CON), aortic-banded heart failure sedentary (HF), and aortic-banded heart failure interval exercise-trained (HF-IT) animals. A: exercise training prevents the decreased vasodilation in isolated coronary arterioles observed in HF animals after activation of the BKCa channel α-subunit by NS-1619 (repeated-measures ANOVA, P < 0.05). B and C: biochemical analysis of isolated coronary arterioles show no differences in BKCa channel α-subunit mRNA levels (B) or protein (C). D: representative Western blots of BKCa channel α-subunit and β-actin (loading control) arteriole protein levels. + CON, positive control – rat brain. A separate group of animals not included in the analysis of the present report were run on the original gel. Since these samples are not relevant to the present study, they have been removed from the gel as indicated by white dividing lines. E: biotinylation analysis reveals no differences in the cellular distribution of the BKCa channel α-subunit in denuded right coronary artery samples. F: representative Western blot of membrane (mem) and cytosolic (cyt) BKCa channel α-subunit protein levels in the right coronary artery. + CON, positive control – CON arteriole sample from D. §Interaction effect: Group × Dose (P < 0.05); *post hoc vs. CON (P < 0.05); †post hoc vs. HF-IT (P < 0.05); #post hoc vs. CON (P < 0.10); ‡post hoc vs. HF-IT (P < 0.10). n = 6, 5, and 6 for CON, HF, and HF-IT, respectively, for A; n = 6, 6, and 7 for CON, HF, and HF-IT, respectively, for B–F.
DISCUSSION
In this study, we examined the therapeutic efficacy of chronic interval exercise training on BKCa channel-mediated coronary vascular function in heart failure. Several novel findings were revealed from these experiments, including that 1) pressure overload-induced heart failure is associated with BKCa channel-mediated coronary vascular dysfunction; 2) chronic interval exercise training prevents coronary BKCa channel-mediated dysfunction observed in the HF group; and 3) chronic interval exercise training is effective at limiting central and peripheral adaptations of heart failure.
Our experiments, conducted in a translational model of chronic pressure overload-induced heart failure, indicate that chronic interval exercise training is therapeutically effective in preserving normal BKCa channel-mediated coronary vascular function. Our findings are consistent with exercise preserving this important ion channel-mediated mechanism of vascular dilation that has previously been shown to be necessary for preventing imbalances between constricting and dilating factors. This imbalance is thought to underlie an impaired ability to regulate vascular tone in cardiovascular disease (73). Under physiological conditions, the beneficial effects of exercise on coronary vascular function are well known (7, 21, 44) and include both increases in smooth muscle cell K+ channel activity (7) and reductions in coronary artery sensitivity to ET-1 (38). Work in nonpathological swine models has shown that exercise training impacts coronary smooth muscle electrophysiological contributions to coronary basal tone, including BKCa currents (6). Chronic endurance exercise training has also been shown to positively impact factors influencing KCa channel function and functional coupling in swine models of diabetes and metabolic syndrome (56, 57, 81). However, it is important to note that the studies cited above demonstrated modulation of BKCa channel function by exercise using training protocols of an intensity generally considered to be intolerable for heart failure patients. Physiological swine training protocols are typically 45–60 min in duration at 4–6 mph, 5 days/wk, and often preceded by a 15-min 6–8 mph sprint phase (45). In contrast, our maximum training treadmill speed was 4 mph, applied in 3-min intervals at a frequency of 3 days/wk, demonstrating that modulation of BKCa channel-mediated coronary vascular function is possible with an exercise intensity tolerable to heart failure patients. Interestingly, Albarwani et al. (1) reported that low-intensity exercise attenuated decreases in both coronary artery smooth muscle BKCa current density and protein expression of the BKCa channel α- and β1-subunits in a rat model of aging. Collectively, our findings indicate that chronic exercise training positively impacts BKCa channel-mediated coronary vascular function with intensities likely tolerated by heart failure patients in a clinical setting.
A strength of the present study was that impaired coronary vasodilatory capacity to pharmacological activation of the pore-forming BKCa channel α-subunit by NS-1619 was demonstrated both in vivo and in vitro in a large-animal model reminiscent of HFpEF. These observations importantly integrate with previous work showing enhanced coronary vasoconstriction to ET-1, as well as augmented cerebral vasoconstriction to neuropeptide Y in this animal model (26, 63). In the latter study, enhanced cerebral vasoconstriction to neuropeptide Y occurred alongside depressed indexes of nitric oxide signaling and impaired BKCa channel α-subunit function, further highlighting the important concept that fundamental to impaired vasomotor control in cardiovascular disease is an imbalance between constricting and dilating factors. It is also important to note that similar coronary vascular dysfunction was reported in the Ossabaw swine model of metabolic syndrome, where a diminished increase in CBF in vivo following acute administration of NS-1619 and reduced BKCa channel-dependent vasodilation to adenosine in isolated coronary arterioles were observed (4). These similarities in results from the different models carry added clinical relevance given that obesity, metabolic syndrome, and type 2 diabetes are common comorbidities in HFpEF and suggest a potential therapeutic target for treatment of these patients.
Although our physiological data are consistent with an exercise-induced increase in BKCa channel α-subunit protein level, we did not observe such a change nor did we observe any shift in cellular location of the pore-forming unit of the channel (as reflected by the cell membrane-to-cytosol protein ratio). Interestingly, this outcome adds to a substantial list of studies demonstrating an uncoupling between vascular BKCa channel expression and function (68). Indeed, swine models of diabetes, metabolic syndrome, and cardiogenic dementia have shown both impaired BKCa channel function in the presence of increased α-subunit protein level (4, 63) and increased BKCa current with no difference in channel expression (57). Given that multiple animal models and disease states have now demonstrated this disconnect (68), perhaps it is not surprising to see another instance in which BKCa channel function and protein level do not directly parallel one another. Reasonable speculation for these discrepancies includes functional changes in coronary vascular smooth muscle IKCa or specifically IBK, which our laboratory (26) and others (4, 57, 81) have shown to be altered in swine models of disease. A recent study in healthy cerebral vessels showed that angiotensin II, a powerful neurohumoral factor upregulated in heart failure, promotes trafficking of the BKCa channel α-subunit from the cell membrane to the cytosol via a PKC-mediated mechanism (47). Although the proportion of membrane-bound BKCa channel α-subunit was unchanged in this study, it does not preclude the possibility that PKC-mediated BKCa channel α-subunit trafficking may be altered in this model at different time points or under different experimental conditions. Interestingly, we found that the membrane-to-cytosolic ratio of the BKCa channel α-subunit was ~2.5:1 in CON animals, which differs from the ~15: 1 seen in cerebral and cremaster arteries from rats (60), perhaps highlighting important distinctions between vascular beds and animal species.
Complexities in studying the BKCa channel also stem from observations that genetic knockout of the channel can increase blood pressure (8, 67, 69) and that both function and transcription of the channel can be modulated by estrogen (18, 48, 77, 79). These ideas are particularly important in terms of heart failure, given that there is a disproportionate sex component to HFpEF (the prevalence being ∼2 times greater in women compared with men) (12, 70, 82) and ~80% of HFpEF patients are hypertensive (13, 17, 72). Recent work from our laboratory has begun to examine these important concepts in female aortic-banded Yucatan pigs that were either left intact or ovariectomized (63). This study showed that both BKCa channel function and protein were altered in the cerebral vasculature in the absence of any increases in systemic blood pressure, demonstrating that some disease-mediated changes to the BKCa channel may be heart failure specific (as opposed to simply a function of hypertension). While we are currently unable to completely elucidate the cellular mechanism underlying our functional findings in this particular study, the combination of significant number of factors that can influence how the BKCa channel functions (including amino acid oxidation and phosphorylation, steroid hormones, gasses such as CO and NO, iron, H+, reactive oxygen species, and lipids) considered with our present findings indicate that a number of potential avenues for future examination of BKCa channel function are warranted in this translational model of chronic pressure overload-induced heart failure, some of which may include using patch-clamp techniques including voltage- and calcium-dependent protocols (35, 71).
Perspectives.
Our data suggest that interval exercise is effective in preventing both central and peripheral adaptations of heart failure. Recent paradigms have characterized HFpEF as a whole body syndrome incorporating multiple organ systems (72), and elucidating the mechanistic impact of exercise may be even more important regarding therapeutic strategies for HFpEF patients specifically, given the lack of established effective treatments for this heart failure subtype (54). In this regard, interval exercise training was found to be beneficial in terms of exercise capacity and diastolic function for patients with HFpEF (2) but not heart failure with reduced ejection fraction (23). As exercise therapy is fine-tuned to treat specific subtypes of heart failure, it will be important to keep in mind the integrative impact of exercise systemically and how this may be best utilized to maximize therapeutic value for the heart failure patient both centrally and peripherally.
Although considerable progress has been made, disease heterogeneity has made development of a universal exercise prescription for heart failure patients difficult. A great deal remains unknown with respect to the optimal dose of exercise, i.e., frequency, duration, and intensity, for heart failure patients and the cellular mechanisms by which improvements in health are realized. The results of this study support our hypothesis that the vascular smooth muscle BKCa channel is an important mechanism underlying coronary vascular dysfunction in heart failure. Our data show the efficacy of chronic interval exercise training as a therapeutic option for treating coronary vascular dysfunction in a setting of pressure overload-induced heart failure, using an intensity tolerable to heart failure patients. They also demonstrate the ability of exercise to limit central and peripheral adaptations of heart failure and physical inactivity. In conclusion, these findings highlight the potential of the vascular smooth muscle BKCa channel as a molecular therapeutic target for heart failure patients.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant RO1 HL112998 (principal investigator: C.A. Emter). T. D. Olver was supported by American Heart Association Postdoctoral Fellowship Award 16POST27760052 (principal investigator: T. D. Olver).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.D.O., J.A.H., M.A.H., M.H.L., and C.A.E. conceived and designed research; T.D.O., J.C.E., B.S.F., J.A.H., P.K.T., and C.A.E. performed experiments; T.D.O., J.C.E., B.S.F., J.A.H., P.K.T., and C.A.E. analyzed data; T.D.O., J.C.E., B.S.F., P.K.T., M.A.H., M.H.L., and C.A.E. interpreted results of experiments; T.D.O., J.C.E., B.S.F., and C.A.E. prepared figures; T.D.O., J.C.E., P.K.T., M.A.H., M.H.L., and C.A.E. drafted manuscript; T.D.O., B.S.F., J.A.H., P.K.T., M.A.H., M.H.L., and C.A.E. edited and revised manuscript; T.D.O., J.C.E., B.S.F., J.A.H., P.K.T., M.A.H., M.H.L., and C.A.E. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jan Ivey, Melissa Cobb, Zahra Nourian, and Daniel G. Dozier for considerable technical contributions, which were essential to the successful completion of the study, and Gore for the generous gift of vascular Gore-Tex sleeves used for aortic banding.
REFERENCES
- 1.Albarwani S, Al-Siyabi S, Baomar H, Hassan MO. Exercise training attenuates ageing-induced BKCa channel downregulation in rat coronary arteries. Exp Physiol 95: 746–755, 2010. doi: 10.1113/expphysiol.2009.051250. [DOI] [PubMed] [Google Scholar]
- 2.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol (1985) 119: 753–758, 2015. doi: 10.1152/japplphysiol.00518.2014. [DOI] [PubMed] [Google Scholar]
- 3.Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 90: 1437–1459, 2010. doi: 10.1152/physrev.00049.2009. [DOI] [PubMed] [Google Scholar]
- 4.Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Neeb ZP, Bratz IN, Sturek M, Tune JD. Impaired function of coronary BKCa channels in metabolic syndrome. Am J Physiol Heart Circ Physiol 297: H1629–H1637, 2009. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Contribution of BKCa channels to local metabolic coronary vasodilation: Effects of metabolic syndrome. Am J Physiol Heart Circ Physiol 298: H966–H973, 2010. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowles DK, Laughlin MH, Sturek M. Exercise training increases K+-channel contribution to regulation of coronary arterial tone. J Appl Physiol (1985) 84: 1225–1233, 1998. doi: 10.1152/jappl.1998.84.4.1225. [DOI] [PubMed] [Google Scholar]
- 7.Bowles DK, Woodman CR, Laughlin MH. Coronary smooth muscle and endothelial adaptations to exercise training. Exerc Sport Sci Rev 28: 57–62, 2000. [PubMed] [Google Scholar]
- 8.Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 9.Cannan CR, Burnett JC Jr, Lerman A. Enhanced coronary vasoconstriction to endothelin-B-receptor activation in experimental congestive heart failure. Circulation 93: 646–651, 1996. doi: 10.1161/01.CIR.93.4.646. [DOI] [PubMed] [Google Scholar]
- 10.Crimi E, Ignarro LJ, Cacciatore F, Napoli C. Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol 6: 292–300, 2009. doi: 10.1038/nrcardio.2009.8. [DOI] [PubMed] [Google Scholar]
- 11.Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Regul Integr Comp Physiol 287: R247–R249, 2004. doi: 10.1152/ajpregu.00346.2004. [DOI] [PubMed] [Google Scholar]
- 12.Czubryt MP, Espira L, Lamoureux L, Abrenica B. The role of sex in cardiac function and disease. Can J Physiol Pharmacol 84: 93–109, 2006. doi: 10.1139/y05-151. [DOI] [PubMed] [Google Scholar]
- 13.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S; ALLHAT Collaborative Research Group . Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation 118: 2259–2267, 2008. doi: 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Beer VJ, Bender SB, Taverne YJ, Gao F, Duncker DJ, Laughlin MH, Merkus D. Exercise limits the production of endothelin in the coronary vasculature. Am J Physiol Heart Circ Physiol 300: H1950–H1959, 2011. doi: 10.1152/ajpheart.00954.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Beer VJ, Sorop O, Pijnappels DA, Dekkers DH, Boomsma F, Lamers JM, Duncker DJ, Merkus D. Integrative control of coronary resistance vessel tone by endothelin and angiotensin II is altered in swine with a recent myocardial infarction. Am J Physiol Heart Circ Physiol 294: H2069–H2077, 2008. doi: 10.1152/ajpheart.01163.2007. [DOI] [PubMed] [Google Scholar]
- 16.de Beer VJ, Taverne YJ, Kuster DW, Najafi A, Duncker DJ, Merkus D. Prostanoids suppress the coronary vasoconstrictor influence of endothelin after myocardial infarction. Am J Physiol Heart Circ Physiol 301: H1080–H1089, 2011. doi: 10.1152/ajpheart.01307.2010. [DOI] [PubMed] [Google Scholar]
- 17.Dhingra A, Garg A, Kaur S, Chopra S, Batra JS, Pandey A, Chaanine AH, Agarwal SK. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep 11: 354–365, 2014. doi: 10.1007/s11897-014-0223-7. [DOI] [PubMed] [Google Scholar]
- 18.Dick GM, Sanders KM. (Xeno)estrogen sensitivity of smooth muscle BK channels conferred by the regulatory beta1 subunit: a study of beta1 knockout mice. J Biol Chem 276: 44835–44840, 2001. doi: 10.1074/jbc.M106851200. [DOI] [PubMed] [Google Scholar]
- 19.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood) 235: 10–22, 2010. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 20.Dieberg G, Ismail H, Giallauria F, Smart NA. Clinical outcomes and cardiovascular responses to exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta-analysis. J Appl Physiol (1985) 119: 726–733, 2015. doi: 10.1152/japplphysiol.00904.2014. [DOI] [PubMed] [Google Scholar]
- 21.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 22.Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 58: 1780–1791, 2011. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Ellingsen Ø, Halle M, Conraads V, Støylen A, Dalen H, Delagardelle C, Larsen AI, Hole T, Mezzani A, Van Craenenbroeck EM, Videm V, Beckers P, Christle JW, Winzer E, Mangner N, Woitek F, Höllriegel R, Pressler A, Monk-Hansen T, Snoer M, Feiereisen P, Valborgland T, Kjekshus J, Hambrecht R, Gielen S, Karlsen T, Prescott E, Linke A; SMARTEX Heart Failure Study (Study of Myocardial Recovery After Exercise Training in Heart Failure) Group . High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation 135: 839–849, 2017. doi: 10.1161/CIRCULATIONAHA.116.022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emter CA, Baines CP. Low-intensity aerobic interval training attenuates pathological left ventricular remodeling and mitochondrial dysfunction in aortic-banded miniature swine. Am J Physiol Heart Circ Physiol 299: H1348–H1356, 2010. doi: 10.1152/ajpheart.00578.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emter CA, Bowles DK. Store-operated Ca2+ entry is not essential for PDGF-BB induced phenotype modulation in rat aortic smooth muscle. Cell Calcium 48: 10–18, 2010. doi: 10.1016/j.ceca.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emter CA, Tharp DL, Ivey JR, Ganjam VK, Bowles DK. Low-intensity interval exercise training attenuates coronary vascular dysfunction and preserves Ca2+-sensitive K+ current in miniature swine with LV hypertrophy. Am J Physiol Heart Circ Physiol 301: H1687–H1694, 2011. doi: 10.1152/ajpheart.00610.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feigl EO, Neat GW, Huang AH. Interrelations between coronary artery pressure, myocardial metabolism and coronary blood flow. J Mol Cell Cardiol 22: 375–390, 1990. doi: 10.1016/0022-2828(90)91474-L. [DOI] [PubMed] [Google Scholar]
- 28.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP; HF-ACTION Investigators . Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1451–1459, 2009. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu LX, Sun XY, Hedner T, Feng QP, Liang QM, Hoebeke J, Hjalmarson A. Decreased density of mesenteric arteries but not of myocardial endothelin receptors and function in rats with chronic ischemic heart failure. J Cardiovasc Pharmacol 22: 177–182, 1993. doi: 10.1097/00005344-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Haitsma DB, Merkus D, Vermeulen J, Verdouw PD, Duncker DJ. Nitric oxide production is maintained in exercising swine with chronic left ventricular dysfunction. Am J Physiol Heart Circ Physiol 282: H2198–H2209, 2002. doi: 10.1152/ajpheart.00834.2001. [DOI] [PubMed] [Google Scholar]
- 31.Hiemstra JA, Gutiérrez-Aguilar M, Marshall KD, McCommis KS, Zgoda PJ, Cruz-Rivera N, Jenkins NT, Krenz M, Domeier TL, Baines CP, Emter CA. A new twist on an old idea part 2: cyclosporine preserves normal mitochondrial but not cardiomyocyte function in mini-swine with compensated heart failure. Physiol Rep 2: e12050, 2014. doi: 10.14814/phy2.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiemstra JA, Lee DI, Chakir K, Gutiérrez-Aguilar M, Marshall KD, Zgoda PJ, Cruz Rivera N, Dozier DG, Ferguson BS, Heublein DM, Burnett JC, Scherf C, Ivey JR, Minervini G, McDonald KS, Baines CP, Krenz M, Domeier TL, Emter CA. Saxagliptin and tadalafil differentially alter cyclic guanosine monophosphate (cGMP) signaling and left ventricular function in aortic-banded mini-swine. J Am Heart Assoc 5: e003277, 2016. doi: 10.1161/JAHA.116.003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiemstra JA, Liu S, Ahlman MA, Schuleri KH, Lardo AC, Baines CP, Dellsperger KC, Bluemke DA, Emter CA. A new twist on an old idea: a two-dimensional speckle tracking assessment of cyclosporine as a therapeutic alternative for heart failure with preserved ejection fraction. Physiol Rep 1: e00174, 2013. doi: 10.1002/phy2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett 584: 2033–2042, 2010. doi: 10.1016/j.febslet.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou S, Heinemann SH, Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda) 24: 26–35, 2009. doi: 10.1152/physiol.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingram DG, Newcomer SC, Price EM, Eklund KE, McAllister RM, Laughlin MH. Chronic nitric oxide synthase inhibition blunts endothelium-dependent function of conduit coronary arteries, not arterioles. Am J Physiol Heart Circ Physiol 292: H2798–H2808, 2007. doi: 10.1152/ajpheart.00899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jasperse JL, Laughlin MH. Flow-induced dilation of rat soleus feed arteries. Am J Physiol Heart Circ Physiol 273: H2423–H2427, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Jones AW, Rubin LJ, Magliola L. Endothelin-1 sensitivity of porcine coronary arteries is reduced by exercise training and is gender dependent. J Appl Physiol (1985) 87: 1172–1177, 1999. doi: 10.1152/jappl.1999.87.3.1172. [DOI] [PubMed] [Google Scholar]
- 39.Keteyian SJ, Piña IL, Hibner BA, Fleg JL. Clinical role of exercise training in the management of patients with chronic heart failure. J Cardiopulm Rehabil Prev 30: 67–76, 2010. doi: 10.1097/HCR.0b013e3181d0c1c1. [DOI] [PubMed] [Google Scholar]
- 40.Kim N, Chung J, Kim E, Han J. Changes in the Ca2+-activated K+ channels of the coronary artery during left ventricular hypertrophy. Circ Res 93: 541–547, 2003. doi: 10.1161/01.RES.0000090087.66390.F2. [DOI] [PubMed] [Google Scholar]
- 41.Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB 3rd, Kaczorowski GJ, Garcia ML. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 33: 5819–5828, 1994. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- 42.Korzick DH, Laughlin MH, Bowles DK. Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. J Appl Physiol (1985) 96: 1425–1432, 2004. doi: 10.1152/japplphysiol.01077.2003. [DOI] [PubMed] [Google Scholar]
- 43.Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol 302: H10–H23, 2012. doi: 10.1152/ajpheart.00574.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laughlin MH, McAllister RM. Exercise training-induced coronary vascular adaptation. J Appl Physiol (1985) 73: 2209–2225, 1992. doi: 10.1152/jappl.1992.73.6.2209. [DOI] [PubMed] [Google Scholar]
- 45.Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol (1985) 67: 1140–1149, 1989. doi: 10.1152/jappl.1989.67.3.1140. [DOI] [PubMed] [Google Scholar]
- 46.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78, 2006. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 47.Leo MD, Bulley S, Bannister JP, Kuruvilla KP, Narayanan D, Jaggar JH. Angiotensin II stimulates internalization and degradation of arterial myocyte plasma membrane BK channels to induce vasoconstriction. Am J Physiol Cell Physiol 309: C392–C402, 2015. doi: 10.1152/ajpcell.00127.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li XT, Qiu XY. 17β-estradiol upregulated expression of α and β subunits of larger-conductance calcium-activated K+ channels (BK) via estrogen receptor β. J Mol Neurosci 56: 799–807, 2015. doi: 10.1007/s12031-015-0502-0. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Hudetz AG, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats: evidence for their protection against cerebral vasospasm. Circ Res 82: 729–737, 1998. doi: 10.1161/01.RES.82.6.729. [DOI] [PubMed] [Google Scholar]
- 50.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 51.Love MP, Haynes WG, Gray GA, Webb DJ, McMurray JJ. Vasodilator effects of endothelin-converting enzyme inhibition and endothelin ETA receptor blockade in chronic heart failure patients treated with ACE inhibitors. Circulation 94: 2131–2137, 1996. doi: 10.1161/01.CIR.94.9.2131. [DOI] [PubMed] [Google Scholar]
- 52.Love MP, Haynes WG, Webb DJ, McMurray JJ. Venous endothelin receptor function in patients with chronic heart failure. Clin Sci (Lond) 98: 65–70, 2000. doi: 10.1042/cs0980065. [DOI] [PubMed] [Google Scholar]
- 53.Marshall KD, Muller BN, Krenz M, Hanft LM, McDonald KS, Dellsperger KC, Emter CA. Heart failure with preserved ejection fraction: chronic low-intensity interval exercise training preserves myocardial O2 balance and diastolic function. J Appl Physiol (1985) 114: 131–147, 2013. doi: 10.1152/japplphysiol.01059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonald KS, Emter CA. Exploring new concepts in the management of heart failure with preserved ejection fraction: is exercise the key for improving treatment? J Appl Physiol (1985) 119: 724–725, 2015. doi: 10.1152/japplphysiol.00570.2015. [DOI] [PubMed] [Google Scholar]
- 55.Merkus D, Houweling B, van den Meiracker AH, Boomsma F, Duncker DJ. Contribution of endothelin to coronary vasomotor tone is abolished after myocardial infarction. Am J Physiol Heart Circ Physiol 288: H871–H880, 2005. doi: 10.1152/ajpheart.00429.2004. [DOI] [PubMed] [Google Scholar]
- 56.Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol 288: H1233–H1241, 2005. doi: 10.1152/ajpheart.00732.2004. [DOI] [PubMed] [Google Scholar]
- 57.Mokelke EA, Hu Q, Song M, Toro L, Reddy HK, Sturek M. Altered functional coupling of coronary K+ channels in diabetic dyslipidemic pigs is prevented by exercise. J Appl Physiol (1985) 95: 1179–1193, 2003. doi: 10.1152/japplphysiol.00972.2002. [DOI] [PubMed] [Google Scholar]
- 58.Muller JM, Myers PR, Laughlin MH. Exercise training alters myogenic responses in porcine coronary resistance arteries. J Appl Physiol (1985) 75: 2677–2682, 1993. doi: 10.1152/jappl.1993.75.6.2677. [DOI] [PubMed] [Google Scholar]
- 59.Nieves-Cintrón M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem 282: 3231–3240, 2007. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 60.Nourian Z, Li M, Leo MD, Jaggar JH, Braun AP, Hill MA. Large conductance Ca2+-activated K+ channel (BKCa) α-subunit splice variants in resistance arteries from rat cerebral and skeletal muscle vasculature. PLoS One 9: e98863, 2014. doi: 10.1371/journal.pone.0098863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL; HF-ACTION Investigators . Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1439–1450, 2009. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olver TD, Hiemstra JA, Edwards JC, Ferguson BS, Laughlin MH, Emter CA. The protective role of sex hormones in females and exercise prehabilitation in males on sternotomy-induced cranial hypoperfusion in aortic banded mini-swine. J Appl Physiol (1985) 122: 423–429, 2017. doi: 10.1152/japplphysiol.00817.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olver TD, Hiemstra JA, Edwards JC, Schachtman TR, Heesch CM, Fadel PJ, Laughlin MH, Emter CA. Loss of female sex hormones exacerbates cerebrovascular and cognitive dysfunction in aortic banded miniswine through a neuropeptide Y-Ca2+-activated potassium channel-nitric oxide mediated mechanism. J Am Heart Assoc 6: e007409, 2017. doi: 10.1161/JAHA.117.007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olver TD, Klakotskaia D, Ferguson BS, Hiemstra JA, Schachtman TR, Laughlin MH, Emter CA. Carotid artery vascular mechanics serve as biomarkers of cognitive dysfunction in aortic-banded miniature swine that can be treated with an exercise intervention. J Am Heart Assoc 5: e003248, 2016. doi: 10.1161/JAHA.116.003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 8: 33–40, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piepoli MF, Conraads V, Corrà U, Dickstein K, Francis DP, Jaarsma T, McMurray J, Pieske B, Piotrowicz E, Schmid JP, Anker SD, Solal AC, Filippatos GS, Hoes AW, Gielen S, Giannuzzi P, Ponikowski PP. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 13: 347–357, 2011. doi: 10.1093/eurjhf/hfr017. [DOI] [PubMed] [Google Scholar]
- 67.Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res 87: E53–E60, 2000. doi: 10.1161/01.RES.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 68.Rusch NJ. BK channels in cardiovascular disease: a complex story of channel dysregulation. Am J Physiol Heart Circ Physiol 297: H1580–H1582, 2009. doi: 10.1152/ajpheart.00852.2009. [DOI] [PubMed] [Google Scholar]
- 69.Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, Krippeit-Drews P, Feil R, Hofmann F, Knaus HG, Kenyon C, Shipston MJ, Storm JF, Neuhuber W, Korth M, Schubert R, Gollasch M, Ruth P. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation 112: 60–68, 2005. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- 70.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol 26: 562–568, 2011. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 71.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci 22: 505–512, 2001. doi: 10.1016/S0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 72.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 134: 73–90, 2016. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sorop O, Olver TD, van de Wouw J, Heinonen I, van Duin RW, Duncker DJ, Merkus D. The microcirculation: a key player in obesity-associated cardiovascular disease. Cardiovasc Res 113: 1035–1045, 2017. doi: 10.1093/cvr/cvx093. [DOI] [PubMed] [Google Scholar]
- 74.Srere PA. [1] Citrate synthase: [EC 4.1.3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. Methods Enzymol 13: 3–11, 1969. doi: 10.1016/0076-6879(69)13005-0. [DOI] [Google Scholar]
- 75.Tadano K, Hosokawa A, Fukuroda T, Nishikibe M. The functional shift of endothelin receptor subtypes in dogs with heart failure produced by rapid ventricular pacing. J Cardiovasc Pharmacol 44, Suppl 1: S350–S353, 2004. doi: 10.1097/01.fjc.0000166291.72324.eb. [DOI] [PubMed] [Google Scholar]
- 76.Thorin E, Lucas M, Cernacek P, Dupuis J. Role of ETA receptors in the regulation of vascular reactivity in rats with congestive heart failure. Am J Physiol Heart Circ Physiol 279: H844–H851, 2000. doi: 10.1152/ajpheart.2000.279.2.H844. [DOI] [PubMed] [Google Scholar]
- 77.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science 285: 1929–1931, 1999. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 78.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res 98: 868–878, 2006. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 79.White RE, Han G, Maunz M, Dimitropoulou C, El-Mowafy AM, Barlow RS, Catravas JD, Snead C, Carrier GO, Zhu S, Yu X. Endothelium-independent effect of estrogen on Ca2+-activated K+ channels in human coronary artery smooth muscle cells. Cardiovasc Res 53: 650–661, 2002. doi: 10.1016/S0008-6363(01)00428-X. [DOI] [PubMed] [Google Scholar]
- 80.Williams JL, Hathaway CA, Kloster KL, Layne BH. Low power, type II errors, and other statistical problems in recent cardiovascular research. Am J Physiol Heart Circ Physiol 273: H487–H493, 1997. doi: 10.1152/ajpheart.1997.273.1.H487. [DOI] [PubMed] [Google Scholar]
- 81.Yang Y, Jones AW, Thomas TR, Rubin LJ. Influence of sex, high-fat diet, and exercise training on potassium currents of swine coronary smooth muscle. Am J Physiol Heart Circ Physiol 293: H1553–H1563, 2007. doi: 10.1152/ajpheart.00151.2007. [DOI] [PubMed] [Google Scholar]
- 82.Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L. Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol 306: H628–H640, 2014. doi: 10.1152/ajpheart.00859.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]