Abstract
Peripheral arterial disease (PAD) is associated with augmented blood pressure (BP) and impaired coronary blood flow responses to exercise, which may increase cardiovascular risk. We investigated the effects of leg revascularization on the BP and coronary blood flow responses to exercise in PAD. Seventeen PAD patients (11 men, 66 ± 2 yr) performed single-leg plantar flexion exercise 24 h before and 1 mo following leg revascularization. BP and heart rate (HR) were measured continuously, and rate pressure product (systolic BP × HR) was calculated as an index of myocardial oxygen demand. Coronary blood velocity was obtained by transthoracic Doppler echocardiography in 8/17 subjects. The mean BP response to plantar flexion exercise was attenuated by leg revascularization (pre-revascularization: 15 ± 4 vs. post-revascularization: 7 ± 3 mmHg, P = 0.025). The HR response to plantar flexion was also attenuated following leg revascularization (pre-revascularization: 9 ± 1 vs. post-revascularization: 6 ± 1 beats/min, P = 0.006). The change in coronary blood velocity with exercise was greater at the post-revascularization visit: 4 ± 1 vs. pre-revascularization: −1 ± 2 cm/s (P = 0.038), even though the change in rate pressure product was not greater following revascularization in these subjects (pre-revascularization: 2,796 ± 871 vs. post-revascularization: 1,766 ± 378 mmHg·beats/min, P = 0.082). These data suggest that leg revascularization alters reflex control of BP, HR, and coronary blood flow in response to exercise in patients with PAD.
NEW & NOTEWORTHY We found that peripheral revascularization procedures lowered exercise blood pressure and improved coronary blood flow in patients with peripheral arterial disease.
Keywords: coronary circulation, endovascular therapy, heart rate, sympathetic nervous system, vascular surgery
INTRODUCTION
Peripheral arterial disease (PAD) is a manifestation of atherosclerotic disease that involves the descending aorta and arteries in the lower limbs. It is estimated that 8 million people in the United States have PAD, although many are asymptomatic and undiagnosed (12). PAD is associated with substantial systemic atherosclerotic burden, and patients with PAD are at an increased risk for cardiovascular morbidity and mortality, as compared with patients with coronary or carotid artery disease (24). The standard of care for PAD includes medical management, exercise therapy, and endovascular or surgical interventions. Endovascular and surgical interventions are used to achieve symptomatic relief in PAD, and the medical management of associated risk factors decreases cardiovascular risk (24). Many studies have investigated the effects of pharmacological, endovascular, and surgical interventions at improving symptoms and cardiovascular risk in PAD (26). Lower limb revascularization procedures (angioplasty, stenting, and bypass grafts) in PAD decrease the risk of cardiovascular events, amputation-free survival, and quality of life (11, 17). Although some studies suggest that global endothelial function improves in PAD patients following revascularization (4, 15, 29), it is unknown whether hemodynamic or coronary responses to exercise are improved.
PAD patients have an augmented blood pressure (BP) response to leg exercise, which may be predictive of cardiovascular and all-cause mortality in PAD (6). The exaggerated BP response to exercise (exaggerated exercise pressor reflex) in PAD appears to be triggered by leg ischemia since the exaggerated exercise pressor reflex in PAD is not observed with upper body exercise (2, 28).
We recently observed that PAD patients without overt coronary artery disease have attenuated coronary hyperemic responses to exercise (28), which may contribute to cardiovascular risk. It is unclear whether peripheral vascular interventions in PAD would alter the BP or coronary vascular responses to leg exercise. Therefore, in this study, we investigated BP and coronary blood flow responses to leg exercise before and 1 mo following leg revascularization. We hypothesized 1) that leg revascularization would reduce the BP response to plantar flexion exercise in PAD and 2) that leg revascularization would not alter the coronary blood flow response to this exercise. We did not expect leg revascularization to alter coronary responses to exercise since the coronary arteries were not treated directly and the anticipated reduction in exercise BP would lead to less metabolic demand for coronary flow.
METHODS
Subjects and design.
In these laboratory studies, we used a repeated-measure, within-subject design. We studied 17 patients with PAD (66 ± 2 yr, 11 men) before and 1 mo following a leg revascularization procedure. PAD patients were recruited from vascular clinic lists at the Penn State Hershey Medical Center and from our database of subjects who had participated in prior studies. The sample size was determined after the first three subjects had completed the study. We determined that if the true difference in the coronary blood velocity (CBV) response to exercise from pre- to post-revascularization was 4 cm/s with a standard deviation of 2 cm/s, then we would need to enroll seven subjects for this variable to have 90% power and α = 0.01. Since CBV cannot be obtained in all subjects, we enrolled 17 subjects total, and CBV measurements were obtained in 9 subjects.
The patients in this study underwent various leg revascularization procedures. Most of these procedures were unilateral. Two subjects had bilateral stents placed. One out of 17 of the PAD patients underwent vascular bypass surgery during the study; this patient had a right femoral to popliteal artery bypass graft. Sixteen of the PAD patients underwent angioplasty and 9/16 of these patients also had a stent placed. The stents were placed in the common iliac (8/16), superficial femoral (8/16), popliteal (4/16), and tibial (2/16) arteries. Three out of the 16 patients who had a stent placed underwent concurrent common femoral endarterectomy. Patients with other vascular diseases or diabetes were not excluded from the study; 5 subjects had coronary artery disease, 14 were hypertensive, and 4 were diabetic.
At the study visits, subjects were on several medications and supplements, including statins (n = 13), fenofibrate (n = 1), aspirin (n = 13), clopidogrel (n = 12), cilostazol (n = 1), ACE inhibitors (n = 10), amlodipine (n = 5), selective β1-blockers (n = 8), diuretics (n = 4), α1-blocker (n = 1), proton pump inhibitors (n = 4), antidiabetic medications (n = 5), antidepressants (n = 3), levothyroxine (n = 2), bone health medications (n = 2), albuterol (n = 1), multivitamins (n = 6), vitamin D (n = 2), iron (n = 2), fish oil (n = 1), coenzyme Q10 (n = 1), and St. John’s wort (n = 1). Subjects were on the same medications and same dosages for both study visits with the exception that five subjects had clopidogrel added to their medications following their peripheral intervention; at the post-revascularization visit, all 17 subjects were taking clopidogrel.
Experimental protocol.
Each subject completed two identical laboratory visits: the pre-revascularization visit was ~24 h before their procedure, and the post-revascularization visit was approximately 1 mo after their procedure. All study protocols were approved in advance by the Institutional Review Board of Penn State Hershey and conformed to the Declaration of Helsinki. All subjects provided written informed consent. The study protocols were performed in the supine position in a thermoneutral laboratory (20–21°C). At each visit, ankle-brachial indices (ABIs) were measured. Subjects were then instrumented with a three-lead EKG (Cardiocap/5, GE Healthcare), a finger BP cuff (Finometer, FMS), and pneumotrace to monitor respiratory activity; these variables were continuously collected at 200 Hz by PowerLab (ADInstruments) and analyzed offline. Resting BPs were obtained in triplicate by automated oscillometry of the right brachial artery (Phillips Sure Signs VS3) after 15 min of quiet rest, and these values were used to verify the Finometer values, as previously described (23).
In nine subjects, peak diastolic CBV in the left anterior descending artery (LAD) was measured by transthoracic Doppler echocardiography. Images were obtained from the adjusted apical four-chamber view using a 7S probe (all images acquired by Z. Gao). The specific procedures for measuring CBV in LAD have been described, and the reproducibility within subjects has been verified (8–10, 20). Although we cannot measure absolute coronary blood flow with technique, changes in LAD CBV are indicative of changes in LAD flow since LAD diameter is not significantly altered by physiological stressors (7).
Subjects exercised with their leg undergoing the revascularization procedure. If a leg revascularization procedure was to be performed on both legs, subjects exercised with their most symptomatic leg. Subjects performed supine single-leg dynamic plantar flexion exercise by contracting the calf muscles of a single leg 30 times per minute. Subjects were instrumented with a boot on their foot, which was attached to a pulley system in which weight could be added. The amount of weight attached to the pulley device was increased every minute. The resistance started at 0.5 kg and increased by 0.5 kg every minute until subjects finished the 3.0-kg workload or fatigue, significant pain, or inability to maintain the cadence occurred. Ratings of perceived exertion (6 = no exertion, 20 = maximal exertion) and pain (0 = no pain, 10 = worst pain possible) were obtained each minute using the Borg scales (3).
Data collection and statistical analysis.
All variables were measured continuously, except for CBV, and analyzed offline. Since CBV was acquired during the last 20 s of each minute, an average of the last 20 s of each minute was reported for continuous variables. The baseline value is an average of the last 20 s of each of the 3 min before exercise. For exercise data, an average of the last 20 s of each minute/workload is presented. Rate pressure product [RPP, the product of systolic BP and heart rate (HR)] was used an index of myocardial O2 demand. Each subjects’ maximum workload from the pre-revascularization visit was time matched to the workload at their post-revascularization visit and used for statistical comparisons (since most subjects exercised longer at the post-revascularization visit). Statistical analyses were performed using SPSS 24 (IBM). Two-factor, repeated-measures ANOVA were conducted on the raw physiological variables to analyze responses to plantar flexion exercise over five time points (baseline, exercise at 0.5 kg, 1.0 kg, 1.5 kg, and maximum workload) and two treatments (pre-revascularization and post-revascularization, Fig. 1). Statistical comparisons were made at the same absolute workload up to the 1.5-kg workload (3 min of exercise), which is the last workload that 16/17 subjects completed. Data are also shown at each subjects’ last workload at the pre-revascularization visit; data from the post-revascularization visit is time matched to each subject’s initial stopping point. For significant interactions, post hoc paired Student’s t-tests with modified Holm adjustments were performed. Changes (Δ) from baseline to plantar flexion exercise at each subjects’ maximum workload were compared between groups using paired Student’s t-tests. Wilcoxon signed-rank tests were used to compare nonparametric and anthropometric data between visits. All data are shown as means ± SE unless otherwise stated. Significance was set at P < 0.05 for all tests.
Fig. 1.
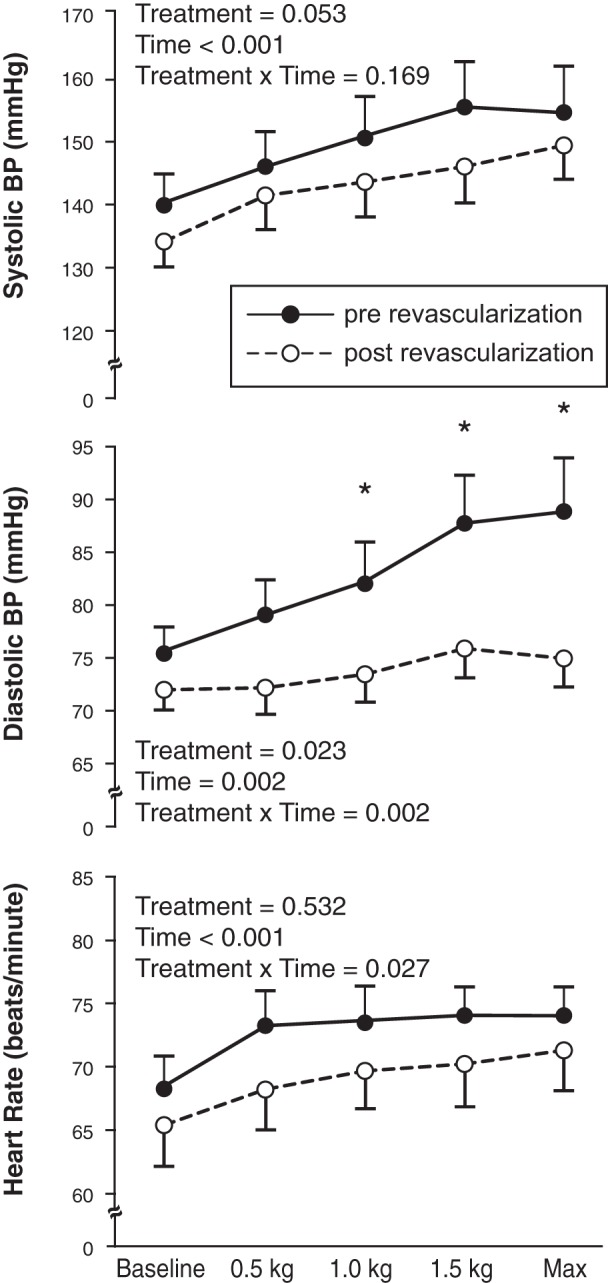
Systolic blood pressure (BP), diastolic BP, and heart rate responses to plantar flexion exercise over time (baseline, 0.5 kg, 1.0 kg, 1.5 kg, maximum workload) and by treatment (pre-revascularization and post-revascularization) (n = 17). *P < 0.05 between treatments.
RESULTS
Data on demographic, resting hemodynamics, and exercise tolerance are shown in Table 1. There were no changes in weight, body mass index, or resting BP and HR data between study visits. ABI increased from the pre- to post- intervention visit in the leg that was revascularized. ABI in the opposite leg did not change between visits. Fifteen out of 17 subjects had a history of smoking; three were current smokers, and total pack years ranged from 0 to 105 in our subjects. All subjects had symptomatic PAD with claudication, but not rest pain (i.e., Fontaine stage II). On average, subjects were able to complete exercise 2 min longer and reach a higher maximal workload (1.0 kg greater) at the post-revascularization visit compared with the pre-revascularization visit (Table 1). Subjects also had less pain and lower perceived exertion at their maximal workload or end of exercise at the post-revascularization visit compared with the pre-revascularization visit.
Table 1.
Demographic, resting hemodynamic, and exercise tolerance data
| PAD Patients (n = 17) | Pre-Revascularization | Post-Revascularization | P Values |
|---|---|---|---|
| Male/Female | 11/6 | ||
| Age, yr | 66 ± 2 | ||
| Height, m | 1.68 ± 0.03 | ||
| Weight, kg | 84 ± 5 | 84 ± 5 | 0.514 |
| BMI, kg/m2 | 29.5 ± 1.3 | 29.3 ± 1.2 | 0.511 |
| ABI of worse leg | 0.61 ± 0.03 | 0.84 ± 0.04 | <0.001 |
| ABI of other leg | 0.81 ± 0.05 | 0.82 ± 0.05 | 0.421 |
| Smoking | |||
| Pack years | 33 ± 8 | ||
| Smoking history/current smokers | 15/3 | ||
| Resting hemodynamics | |||
| Systolic BP, mmHg | 140 ± 5 | 134 ± 4 | 0.186 |
| Diastolic BP, mmHg | 75 ± 3 | 72 ± 2 | 0.226 |
| Heart rate, beats/min | 68 ± 3 | 65 ± 3 | 0.265 |
| Exercise time, min | 4 (2–6) | 6 (4–6) | 0.004 |
| Maximal workload, kg | 2.0 (1.0–3.0) | 3.0 (2.0–3.0) | 0.004 |
| Pain at end (0–10) | 5 (0–8) | 0 (0–6) | 0.375 |
| RPE at end (6–20) | 14 (8–17) | 12 (7–17) | 0.875 |
Data are shown as means ± SE except for nonparametric data, which are shown as median (max–min). ABI, ankle-brachial index; BMI, body-mass index; BP, blood pressure; PAD, peripheral artery disease; RPE, rating of perceived exertion.
Effect of revascularization on the exercise pressor reflex.
The HR and BP responses to exercise are displayed in Fig. 1. Significant interactions were observed for diastolic BP and HR. Although the change in systolic BP from baseline to maximum plantar flexion exercise tended to be lower following leg revascularization, the difference was not statistically significant (pre-revascularization: 21 ± 5 vs. post-revascularization: 15 ± 3 mmHg; P = 0.058). Diastolic BP increased less in response to plantar flexion post-revascularization: 14 ± 4 vs. pre-revascularization: 4 ± 2 mmHg (P = 0.014). The mean BP response to plantar flexion exercise was attenuated by leg revascularization (pre-revascularization: 15 ± 4 vs. post-revascularization: 7 ± 3 mmHg; P = 0.025, Fig. 2). The HR response to plantar flexion was also attenuated following leg revascularization (pre-revascularization: 9 ± 1 vs. post-revascularization: 6 ± 1 beats/min; P = 0.006, Fig. 2).
Fig. 2.
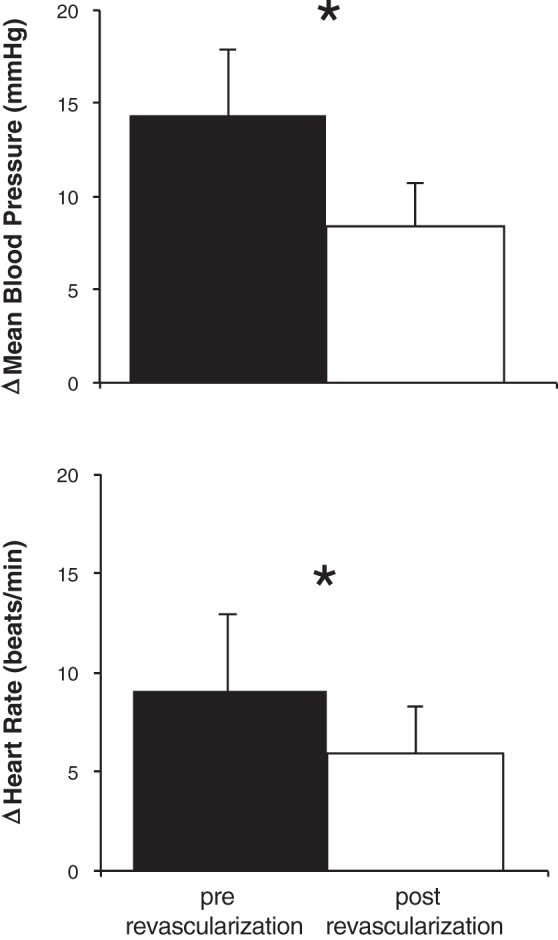
Mean blood pressure and heart rate responses (changes from baseline to maximum exercise) to single-leg plantar flexion exercise pre-revascularization (solid bars) and 1 mo post-revascularization (open bars) (n = 17). *P < 0.05 between treatments.
Effect of revascularization on coronary blood flow.
CBV was measured before and after revascularization in 9/17 subjects. One subject was excluded from analysis because his CBV measurements were more than two SDs above the mean. CBV responses to plantar flexion were greater following leg revascularization in 6/8 subjects (Fig. 3). At the pre-revascularization visit, subjects had no increase in CBV in response to plantar flexion exercise on average (baseline: 22.6 ± 3.14 vs. max: 22.2 ± 4.5 cm/s; P = 0.282). Yet, at the post-revascularization visit CBV increased with exercise (baseline: 20.0 ± 2.6 cm/s vs. max: 24.1 ± 2.9 cm/s; P = 0.013). The change in CBV with exercise was significantly greater at the post-revascularization visit: 4.1 ± 1.6 cm/s vs. pre-revascularization: −1.20 ± 2.09 cm/s (P = 0.038, Fig. 3). The change in RPP with exercise was not significantly altered by revascularization (pre-revascularization: 2,796 ± 871 vs. post-revascularization: 1,766 ± 378 mmHg·beats/min; P = 0.082, Fig. 3). However, the RPP response to plantar flexion was attenuated following revascularization in 5/8 subjects.
Fig. 3.
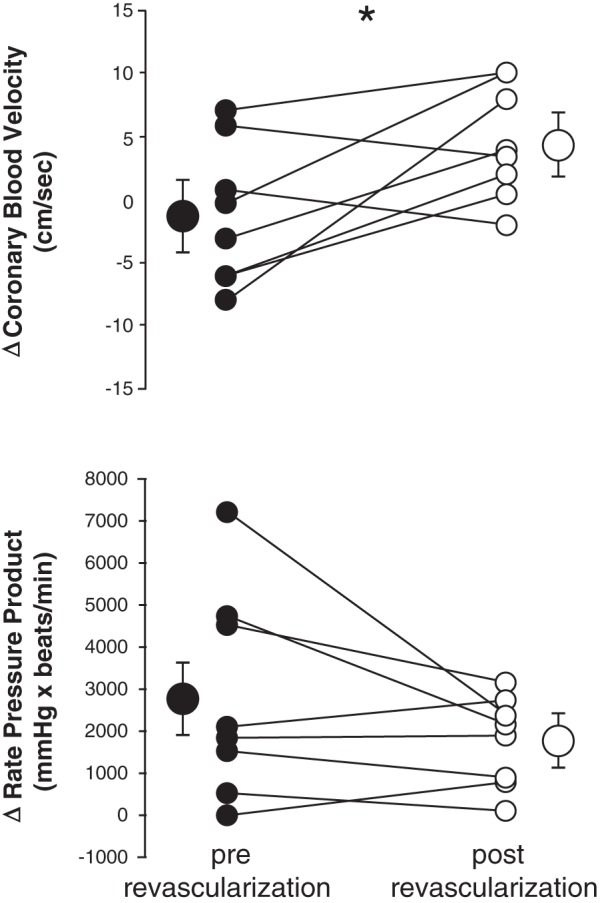
Coronary blood velocity and rate pressure product responses to single-leg plantar flexion exercise pre-revascularization (●) and 1 mo post-revascularization (○). Each circle represents the change from baseline to maximum exercise in one subject (n = 8). Values expressed as means ± SE are also shown on the outside of the individual data as larger circles with brackets. *P < 0.05 between treatments.
DISCUSSION
Summary and main findings.
The purpose of this study was to investigate the effects of leg revascularization on the cardiovascular response to exercise in PAD. This study produced two novel findings. First, the BP and HR responses to exercise in PAD were attenuated following leg revascularization. Second, coronary exercise hyperemia was improved 1 mo after leg revascularization when exercising at the same workload with a similar myocardial oxygen demand. Together, these data suggest that leg revascularization improves reflex control of blood flow in PAD.
Effect of revascularization on the exercise pressor reflex.
The BP response to plantar flexion exercise is augmented in PAD patients compared with healthy subjects (18, 21, 28). This heightened exercise pressor reflex in PAD may be caused by increased sympathetic nervous system activation to exercise and sensitized skeletal muscle afferents (19). Reducing oxidative stress with vitamin C or prostanoids with the cyclooxygenase inhibitor ketorolac attenuates the BP response to exercise in PAD (21, 22). The current study adds to this literature by showing that restoring large artery blood flow, via leg revascularization, decreases the heightened BP and HR responses to exercise in PAD. In addition, the patients were able to perform plantar flexion longer (~2 min on average) following revascularization. These data suggest that ischemia in the leg contributes to the heightened BP and HR responses to exercise in PAD. Following revascularization, ischemia in the limb is improved (increased ABI), which may decrease the muscle mechanoreflex and/or metaboreflex responses to exercise. Further studies are needed to explore how revascularization affects these mechanisms.
Effect of leg revascularization on coronary exercise hyperemia.
In our previous study, we showed that the CBV response to leg exercise is attenuated in PAD (28). We speculated that the impaired coronary exercise hyperemia in PAD is attributed to increased α-adrenergic vasoconstriction in the coronaries or global endothelial dysfunction. In the current study, we hypothesized that leg revascularization would not alter the CBV response to leg exercise in PAD because the coronary vasculature was not treated as part of the subjects’ clinical interventions during the timeframe of the study. Contrary to our hypothesis, we observed a significant change in CBV responses to plantar flexion exercise in 6/8 PAD patients following leg revascularization. At the pre-revascularization visit, 4/8 subjects had a paradoxical decrease in CBV in response to exercise (i.e., their hearts were getting less blood flow and oxygen during exercise compared with at rest). In all four of these subjects, their CBV response increased in response to exercise at their post-revascularization visit. Despite this beneficial effect of peripheral revascularization, the CBV response in PAD post-revascularization is still attenuated compared with healthy subjects’ CBV response using a similar exercise protocol (28).
There are a few possible mechanisms that may underlie this change in coronary blood physiology occurring 1 mo following leg revascularization. First, revascularization may have decreased sympathetic nervous system activity. Although we did not directly measure sympathetic nerve activity in this study, less sympathetic tone during exercise may have decreased BP and α-adrenergic coronary vasoconstriction. Second, there may have been alterations in the timing and amplitude of the arterial pulse-wave reflection following revascularization, which could alter CBV. Peripheral arterial obstruction causes premature arterial pulse wave reflection in PAD and augments afterload (aortic blood pressure), reducing coronary perfusion (30). Removing the arterial obstruction may delay pulse wave reflection and improve coronary perfusion. This hypothesis is supported by a decrease in augmentation index in PAD patients 3 mo following a peripheral stent placement (16). Therefore, revascularization could delay the arrival of the reflected pulse wave and normalize CBV responses in PAD. Third, there is evidence of improved endothelium-dependent vasodilation in the arm of PAD patients following leg revascularization (4, 15, 29). Furthermore, endothelium-dependent vasodilation in the arm is correlated to coronary vasodilation in PAD (27). Therefore, if flow-mediated dilation is improved in the arm following leg revascularization as the literature suggests (4, 15, 29), it is likely that endothelium-dependent vasodilation in the coronary arteries is improved as well, although this has not been investigated directly. These previous studies suggest that leg revascularization may improve endothelial function in PAD in multiple vascular beds, not just the leg, although the mechanism of physiological improvement remains unclear. Finally, it is also possible that post-revascularization, the PAD patients exercised more in their daily lives, which could have profound physiological effects (13). We do not have data on how much our study subjects walked pre- or post-revascularization and the literature does not support that PAD patients walk significantly more following leg revascularization, even if their pain decreases (1, 25).
Limitations.
The main limitations of this study are typical of human physiology experiments in clinical populations. Subjects underwent different types of interventions including femoral to popliteal bypass (n = 1), angioplasty with stent placed (n = 9), and angioplasty without stent placed (n = 7). While our study was not powered to investigate whether one type of peripheral vascular intervention affected the cardiovascular response to exercise more, there does not appear to be a difference between the treatment groups. Some subjects experienced pain during the exercise protocol, which may have augmented their BP reactivity. The subjects were on several medications—such as β1-blockers, α1-blockers, amlodipine, and ACE inhibitors—during the study visits, and this may have affected their BP responses. All subjects were also on platelet inhibitors such as aspirin and clopidogrel, and one subject was taking the vasodilator cilastazol; these medications may increase blood flow. All patients took their usual medications during both study visits, and we did not have patients withhold certain medications for safety and ethical reasons. Yet, the effect of medications on physiological responses is probably minimized, since patients were taking the same medications at both study visits, and the study is a within-subjects design (all patients were their own control). However, clopidogrel was added to six subjects’ medication lists following revascularization, since it is prescribed specifically following stent implantation, and this drug can improve endothelial function (5, 14). Our study was not randomized, and we did not have a control group or control treatment such as sham surgery or medical management. In addition, the PAD patients in this study had comorbid conditions, including coronary artery disease and diabetes. Since some PAD patients also had coronary artery disease, this may have affected their CBV responses, but every subject served as his or her own control, and no procedure was performed on their coronary arteries between visits. Finally, we studied cardiovascular responses to exercise in PAD patients 1 mo following their procedure, and we do not know whether the changes that we observed occurred earlier than 1 mo or remained long-term.
Conclusions.
We found that leg revascularization decreased the BP and HR responses to exercise and increased coronary exercise hyperemia in patients with PAD. These findings suggest that leg revascularization may have broad systemic effects in patients with peripheral arterial disease that include improvements in reflex control of blood pressure and flow.
GRANTS
This project was supported by NIH Grant P01 HL-134609 (to L. I. Sinoway) and American Heart Association Grant 15PRE24470033 (to A. J. Miller). The project described was supported, in part, by the National Center for Advancing Translational Sciences, NIH, through Grant UL1 TR-000127 and UL1 TR-002014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.M., J.C.L., U.A.L., L.I.S., and M.D.M. conceived and designed research; A.J.M., J.C.L., D.J.-K.K., and M.D.M. performed experiments; A.J.M., J.C.L., and M.D.M. analyzed data; A.J.M., J.C.L., D.J.-K.K., U.A.L., F.A., J.F.R., L.I.S., and M.D.M. interpreted results of experiments; A.J.M., J.C.L., and M.D.M. prepared figures; A.J.M., J.C.L., D.J.-K.K., and M.D.M. drafted manuscript; A.J.M., J.C.L., D.J.-K.K., U.A.L., F.A., J.F.R., L.I.S., and M.D.M. edited and revised manuscript; A.J.M., J.C.L., D.J.-K.K., U.A.L., F.A., J.F.R., L.I.S., and M.D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Cheryl Blaha and Aimee Cauffman for their nursing assistance and Jen Stoner and Kris Gray for administrative support.
REFERENCES
- 1.Afaq A, Patel JH, Gardner AW, Hennebry TA. Predictors of change in walking distance in patients with peripheral arterial disease undergoing endovascular intervention. Clin Cardiol 32: E7–E11, 2009. doi: 10.1002/clc.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakke EF, Hisdal J, Kroese AJ, Jørgensen JJ, Stranden E. Blood pressure response to isometric exercise in patients with peripheral atherosclerotic disease. Clin Physiol Funct Imaging 27: 109–115, 2007. doi: 10.1111/j.1475-097X.2007.00720.x. [DOI] [PubMed] [Google Scholar]
- 3.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics, 1998. [Google Scholar]
- 4.Budzynski J, Ciecierski C, Suppan K. The effect of lower limb revascularization on the global endothelial function and cardiovascular risk. J Exp Clin Cardiol 1–9, 2013. [Google Scholar]
- 5.Dahmus JD, Bruning RS, Kenney WL, Alexander LM. Oral clopidogrel improves cutaneous microvascular function through EDHF-dependent mechanisms in middle-aged humans. Am J Physiol Regul Integr Comp Physiol 305: R452–R458, 2013. doi: 10.1152/ajpregu.00366.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Liefde II, Hoeks SE, van Gestel YRBM, Bax JJ, Klein J, van Domburg RT, Poldermans D. Usefulness of hypertensive blood pressure response during a single-stage exercise test to predict long-term outcome in patients with peripheral arterial disease. Am J Cardiol 102: 921–926, 2008. doi: 10.1016/j.amjcard.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Dean J, Cruz SD, Mehta PK, Merz CN. Coronary microvascular dysfunction: sex-specific risk, diagnosis, and therapy. Nat Rev Cardiol 12: 406–414, 2015. doi: 10.1038/nrcardio.2015.72. [DOI] [PubMed] [Google Scholar]
- 8.Gao Z, Novick M, Muller MD, Williams RJ, Spilk S, Leuenberger UA, Sinoway LI. Exercise and diet-induced weight loss attenuates oxidative stress related-coronary vasoconstriction in obese adolescents. Eur J Appl Physiol 113: 519–528, 2013. doi: 10.1007/s00421-012-2459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Z, Spilk S, Momen A, Muller MD, Leuenberger UA, Sinoway LI. Vitamin C prevents hyperoxia-mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol 112: 483–492, 2012. doi: 10.1007/s00421-011-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Z, Wilson TE, Drew RC, Ettinger J, Monahan KD. Altered coronary vascular control during cold stress in healthy older adults. Am J Physiol Heart Circ Physiol 302: H312–H318, 2012. doi: 10.1152/ajpheart.00297.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giugliano G, Di Serafino L, Perrino C, Schiano V, Laurenzano E, Sannino A, Brevetti L, Petretta MP, Trimarco B, Esposito G. Effects of successful percutaneous lower extremity revascularization on cardiovascular outcome in patients with peripheral arterial disease. Int J Cardiol 167: 2566–2571, 2013. doi: 10.1093/eurheartj/ehr457. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129: e28–e292, 2014. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 123: 87–97, 2011. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heitzer T, Rudolph V, Schwedhelm E, Karstens M, Sydow K, Ortak M, Tschentscher P, Meinertz T, Böger R, Baldus S. Clopidogrel improves systemic endothelial nitric oxide bioavailability in patients with coronary artery disease: evidence for antioxidant and antiinflammatory effects. Arterioscler Thromb Vasc Biol 26: 1648–1652, 2006. doi: 10.1161/01.ATV.0000225288.74170.dc. [DOI] [PubMed] [Google Scholar]
- 15.Husmann M, Dörffler-Melly J, Kalka C, Diehm N, Baumgartner I, Silvestro A. Successful lower extremity angioplasty improves brachial artery flow-mediated dilation in patients with peripheral arterial disease. J Vasc Surg 48: 1211–1216, 2008. doi: 10.1016/j.jvs.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Jacomella V, Shenoy A, Mosimann K, Kohler MK, Amann-Vesti B, Husmann M. The impact of endovascular lower-limb revascularisation on the aortic augmentation index in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg 45: 497–501, 2013. doi: 10.1016/j.ejvs.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Kalbaugh CA, Taylor SM, Blackhurst DW, Dellinger MB, Trent EA, Youkey JR. One-year prospective quality-of-life outcomes in patients treated with angioplasty for symptomatic peripheral arterial disease. J Vasc Surg 44: 296–302, 2006. doi: 10.1016/j.jvs.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 18.Lorentsen E. Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation 46: 257–263, 1972. doi: 10.1161/01.CIR.46.2.257. [DOI] [PubMed] [Google Scholar]
- 19.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, Boehmer JP, Sinoway LI, Leuenberger UA. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol 296: H854–H861, 2009. doi: 10.1152/ajpheart.01075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012. doi: 10.1113/jphysiol.2012.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller MD, Drew RC, Ross AJ, Blaha CA, Cauffman AE, Kaufman MP, Sinoway LI. Inhibition of cyclooxygenase attenuates the blood pressure response to plantar flexion exercise in peripheral arterial disease. Am J Physiol Heart Circ Physiol 309: H523–H528, 2015. doi: 10.1152/ajpheart.00267.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller MD, Gao Z, McQuillan PM, Leuenberger UA, Sinoway LI. Coronary responses to cold air inhalation following afferent and efferent blockade. Am J Physiol Heart Circ Physiol 307: H228–H235, 2014. doi: 10.1152/ajpheart.00174.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45 Suppl S: S5–S67, 2007. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 25.Otsuka S, Morisawa T, Yuguchi S, Hojo Y, Matsuo T, Nakajima M, Ishida A, Takahashi T. Clinical importance of change in physical activity after endovascular treatment combined with exercise training in patients with peripheral arterial disease. Heart Vessels 32: 143–148, 2017. doi: 10.1007/s00380-016-0856-4. [DOI] [PubMed] [Google Scholar]
- 26.Owens CD, Conte MS. Medical management of peripheral arterial disease: bridging the “gap”? Circulation 126: 1319–1321, 2012. doi: 10.1161/CIRCULATIONAHA.112.129692. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrino T, Storto G, Filardi PP, Sorrentino AR, Silvestro A, Petretta M, Brevetti G, Chiariello M, Salvatore M, Cuocolo A. Relationship between brachial artery flow-mediated dilation and coronary flow reserve in patients with peripheral artery disease. J Nucl Med 46: 1997–2002, 2005. [PubMed] [Google Scholar]
- 28.Ross AJ, Gao Z, Luck JC, Blaha CA, Cauffman AE, Aziz F, Radtka JF III, Proctor DN, Leuenberger UA, Sinoway LI, Muller MD. Coronary exercise hyperemia is impaired in patients with peripheral arterial disease. Ann Vasc Surg 38: 260–267, 2017. doi: 10.1016/j.avsg.2016.05.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unal O, Karatepe O, Ugurlucan M, Koc B, Filizcan U, Aksoy M. Effects of lower extremity revascularization on the endothelial functions measured with noninvasive brachial artery flow-mediated dilatation. Ann Vasc Surg 25: 969–974, 2011. doi: 10.1016/j.avsg.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Zagura M, Kals J, Kilk K, Serg M, Kampus P, Eha J, Soomets U, Zilmer M. Metabolomic signature of arterial stiffness in male patients with peripheral arterial disease. Hypertens Res 38: 840–846, 2015. doi: 10.1038/hr.2015.71. [DOI] [PubMed] [Google Scholar]


