Abstract
Background and Purpose
Serine proteases have been re suggested as important mediators of visceral pain. We investigated their effect by using newly developed serine protease inhibitors with a well‐characterized inhibitory profile in a rat model of post‐inflammatory irritable bowel syndrome (IBS).
Experimental Approach
Colitis was induced in rats receiving intrarectal trinitrobenzenesulphonic acid; controls received 0.9% NaCl. Colonoscopies were performed on day 3, to confirm colitis, and later until mucosal healing. Visceral hypersensitivity was quantified by visceromotor responses (VMRs) to colorectal distension, 30 min after i.p. injection of the serine protease inhibitors nafamostat, UAMC‐00050 or UAMC‐01162. Serine proteases, protease‐activated receptors (PARs) and TRP channels were quantified by qPCR and immunohistochemistry. Proteolytic activity was characterized using fluorogenic substrates.
Key Results
VMR was significantly elevated in post‐colitis rats. Nafamostat normalized VMRs at the lowest dose tested. UAMC‐00050 and UAMC‐01162 significantly decreased VMR dose‐dependently. Expression of mRNA for tryptase‐αβ‐1and PAR4, and tryptase immunoreactivity was significantly increased in the colon of post‐colitis animals. Trypsin‐like activity was also significantly increased in the colon but not in the faeces. PAR2 and TRPA1 immunoreactivity co‐localized with CGRP‐positive nerve fibres in control and post‐colitis animals.
Conclusions and Implications
Increased expression of serine proteases and activity together with increased expression of downstream molecules at the colonic and DRG level and in CGRP‐positive sensory nerve fibres imply a role for serine proteases in post‐inflammatory visceral hypersensitivity. Our results support further investigation of serine protease inhibitors as an interesting treatment strategy for IBS‐related visceral pain.
Abbreviations
- AP
activating peptide
- DRGs
dorsal root ganglia
- IBS
irritable bowel syndrome
- IBS‐C
irritable bowel syndrome constipation
- IBS‐D
irritable bowel syndrome diarrhoea
- KLK
kallikrein
- MPO
myeloperoxidase
- PRSS
protease serine, trypsin precursor
- S‐N‐K
Student–Newman–Keuls
- TNBS
trinitrobenzenesulphonic acid
- VMR
visceromotor response
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder, characterized by chronic abdominal pain and altered defecation patterns, in the absence of any organic cause (Chey et al., 2015). Visceral hypersensitivity is a major factor underlying abdominal pain in IBS patients (Barbara et al., 2011) and an important contributor to gastrointestinal symptom generation in IBS patients, thus making it a relevant treatment target (Simren et al., 2017). Remarkably, only 24% of the IBS patients report complete relief of abdominal pain after treatment, which is usually only a symptomatic treatment of the most explicit motility‐related symptom (Hungin et al., 2003). Therefore, further research towards new treatments for abdominal pain in IBS patients is of utmost importance.
Gastrointestinal inflammation is considered as an important trigger for the onset of visceral hypersensitivity (De Schepper et al., 2008). Upon activation of inflammatory cells, such as mast cells and T‐cells/B‐cells, at the peripheral level, excessive amounts of mediators (histamine, 5‐HT, cytokines, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=759, bradykinin and ATP) are released, thereby sensitizing peripheral afferent neurons contributing to visceral hypersensitivity. Considerable research efforts has been devoted to the role of various inflammatory mediators in visceral hypersensitivity, but the role of proteases has only begun to be understood (Ceuleers et al., 2016; Vergnolle, 2016).
The class of serine proteases attracts particular attention in visceral nociception research because serine protease expression and/or activity is frequently elevated in colonic and faecal samples of IBS patients (Barbara et al., 2004; Cenac et al., 2007; Roka et al., 2007; Gecse et al., 2008; Annahazi et al., 2009; Buhner et al., 2009; Tooth et al., 2014). Despite suggestions of serine protease inhibitors as a possible treatment option for visceral pain, only a few preclinical animal studies actually investigated the effect of serine protease inhibitors on visceral sensitivity (Ceuleers et al., 2016; Vergnolle, 2016). A clear role for http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=59 (PARs) in visceral hypersensitivity is shown in preclinical in vitro and in vivo studies, but only a few animal studies have evaluated serine protease inhibitors, thereby focusing on proteases rather than PARs. The supernatant from colonic biopsies from IBS patients loses its capacity to induce visceral hypersensitivity in mice if the supernatant is pretreated in vitro with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4262, a broad‐spectrum serine protease inhibitor (Cenac et al., 2007; Wang et al., 2015). However, proof of an in vivo effect remains scarce. To our knowledge, the only evidence that in vivo serine protease inhibition reduces visceral pain was provided by Zhao et al. (2011), showing that intragastric pretreatment with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6432, structurally related to nafamostat, reduced pain in a rat model of stress‐induced visceral hypersensitivity. This highlights the importance of more extensive research on serine protease inhibitors as a possible treatment option for visceral pain.
Therefore, we aimed at investigating the in vivo symptomatic effect of two newly developed serine protease inhibitors (UAMC‐00050 and UAMC‐01162; patent WO2007045496) (Joossens et al., 2007) in a rat model of post‐inflammatory IBS. We compared the effects of UAMC‐00050 and UAMC‐01162 with those of nafamostat and attempted to unravel the type of serine proteases contributing to visceral hypersensitivity to define the optimal inhibition profile for serine protease inhibitors targeting abdominal pain in IBS patients.
Methods
Animals
All animal care and experimental procedures were approved by the Ethical Committee for Animal Experiments of the University of Antwerp (EC nr. 2014‐41) and performed in accordance with Directive 2010/63/EU of the European Parliament and the Council on the protection of animals used for scientific purposes. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Male Sprague–Dawley rats (200–225 g; Charles River, Calco, Italy) were housed at constant room temperature (22 ± 2°C) and humidity (60%) with two rats per cage. Rats had unlimited access to water and food and were kept on a 12:12 h day–night cycle.
Induction of TNBS colitis
2,4,6‐Trinitrobenzenesulphonic acid (TNBS) colitis was induced at day 0 by a TNBS enema containing 4 mg TNBS (Sigma‐Aldrich Inc, St Louis, MO, USA) in 50% ethanol (Acros Organics, Geel, Belgium) as previously described (Deiteren et al., 2015). This post‐TNBS rat model for IBS is validated and routinely used in our lab and described in review papers as a relevant animal model to study visceral pain (Deiteren et al., 2015; Greenwood‐Van Meerveld et al., 2015). After an overnight fast and under ketamine [35 mg·kg−1, i.p.; Ketalar® Pfizer (Puurs, Belgium)] and xylazine [5 mg·kg−1, i.p.; Rompun® Bayer (Leverkusen, Germany)] anaesthesia, 0.25 mL of the TNBS solution was administered intrarectally using a flexible catheter (18G, length 4.5 cm). Control animals received 0.25 mL 0.9% NaCl (Braun®, Diegem, Belgium) intrarectally, under ketamine (35 mg·kg−1, i.p.) and xylazine (5 mg·kg−1, i.p.) anaesthesia. The animals were kept in tail‐up position during 1 min and were then allowed to recover in a Trendelenburg position in a temperature‐controlled cage (28°C) up until 1 h until they regained consciousness. No post‐operative analgesia was administered as approved by the Ethical Committee for Animal Experiments of the University of Antwerp (EC nr. 2014‐41). Subsequently, animals were brought back to their cages with free access to food and water.
Experimental design
The experimental course is shown in Figure 1. A mild colitis was induced on day 0 by a TNBS enema. Controls received an intrarectal administration with 0.9% NaCl. Colitis was verified by colonoscopy on day 3. From day 10 onwards, colonoscopy was performed every 4 days to follow the healing of the colonic mucosa. Functional experiments [visceromotor response (VMR)] were performed 3 days after complete resolution of colitis. The compounds (0.1–10 mg·kg−1 nafamostat mesilate, 0.01–1 mg·kg−1 UAMC‐00050 and 1–2.5 mg·kg−1 UAMC‐01162) or vehicle (sterile water for nafamostat, 5% DMSO for UAMC‐00050 and UAMC‐01162) were injected i.p. 30 min before the start of the VMR experiment (n = 7–10 per group). Group sizes are unequal due to experimental loss (electrode failure, anaesthesia and remaining colonic inflammation). The active compounds are described in the Materials section. Colonic compliance was evaluated before animals were killed (exsanguination under 45 mg·kg−1, i.p. pentobarbital anaesthesia) to assess colonic inflammatory parameters [colonoscopy, macroscopy, microscopy and myeloperoxidase (MPO) activity]. Colonic samples were taken for immunohistochemistry, qPCR and proteolytic activity experiments. Dorsal root ganglion (DRG) samples were obtained from another group of control and post‐colitis IBS rats (n = 12 per group). All animals were randomized for treatment, and data analysis was carried out by a person blinded to this study.
Figure 1.
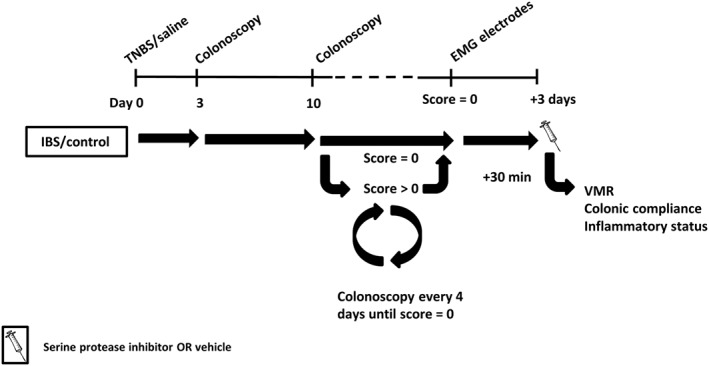
Overview of the experimental design. On day 0, rats received an intrarectal treatment with TNBS (colitis) or saline (control). The severity of colitis and the mucosal healing were monitored in vivo using colonoscopy: on day 3 to confirm the presence of colitis and from day 10 onwards every 4 days until complete mucosal healing. Hereafter, EMG electrodes were implanted, and 3 days later, all experiments were conducted after a single i.p. injection with vehicle/serine protease inhibitor.
Visceromotor response
The VMR is the nociceptive reflex in which abdominal muscles contract in response to a colorectal balloon distension (Ness and Gebhart, 1988). This is a validated and objective method to quantify visceral sensitivity in rats (Vermeulen et al., 2013; Deiteren et al., 2014; Deiteren et al., 2015). Under ketamine (35 mg·kg−1, i.p.) and xylazine (5 mg·kg−1, i.p.) anaesthesia, non‐isolated ends of a pair of EMG electrodes were implanted into the external abdominal muscle, and the other electrode ends were s.c. tunnelled to the neck, exteriorized and accessibly immobilized in between the shoulder blades. Rats were allowed to recover in a temperature‐controlled cage (28°C) up until 1 h until they regained consciousness. No post‐operative analgesia was administered as approved by the Ethical Committee for Animal Experiments of the University of Antwerp (EC nr. 2014‐41). Subsequently, animals were brought back to their cages with free access to food and water. Three days later, the VMR was quantified in conscious rats by introducing into the colorectum a lubricated balloon (length 5 cm), which was connected to a barostat (Distender Series II Barostat; G&J Electronics, Toronto, ON, Canada). The EMG signal was recorded by a data acquisition during a phasic distension protocol (10–20–30–40–60 mmHg, 20 s, 4 min interval) generated by the barostat. The EMG signal was registered, amplified (Neurolog; Digitimer Ltd, Welwyn Garden City, UK), digitalized (CED 1401; Cambridge Electronic Design, Cambridge, UK) and analysed using Spike2 V.5.16 (Cambridge Electronic Design). The VMR was quantified by calculating the AUC of the EMG signal during colorectal distension (20 s) corrected for the EMG signal before the distension (20 s).
Colonic compliance
Colonic compliance is the resistance of the colon against deformation and calculated as the ratio of change in volume over change in pressure. The effect of the serine protease inhibitors was studied on colonic compliance to assess possible changes in the viscoelastic properties of the colon. Under pentobarbital anaesthesia (45 mg·kg−1, i.p. Nembutal®), a lubricated balloon was introduced into the colorectum of the rat and filled with increasing volumes of water (0–0.5–1.0–1.5–2.0 mL, 80 s interval). The corresponding pressure in the colon of the rat was measured, and the resulting pressure–volume curves display colonic compliance.
Inflammatory parameters
Colonoscopy
Colonoscopy was performed with a paediatric endoscope (Olympus GIF‐N30; Olympus Europe GmbH, Hamburg, Germany). Under ketamine/xylazine anaesthesia (35/5 mg·kg−1, i.p.), the lubricated tip of the endoscope was introduced into the colon and advanced under endoscopic view until the hepatic flexure was reached (±10 cm proximal to the anus). During withdrawal of the endoscope, intestinal inflammation was evaluated using a standardized scoring system (total score 0–19) (Vermeulen et al., 2011).
Post‐mortem inflammatory markers
At the end of the experimental period, the rat was killed, and the colon was isolated, rinsed with Krebs solution, opened longitudinally and macroscopically evaluated using a validated scoring system (total score 0–10) (Vermeulen et al., 2011). For microscopic evaluation, a colonic segment of approximately 1 cm2 was fixed in 4% formaldehyde, embedded in paraffin and stained with haematoxylin and eosin. A microscopic score of 0–10 was given using a previously published scoring system (Vermeulen et al., 2011). Finally, colonic MPO activity was measured as previously published (Vermeulen et al., 2011). The MPO activity is defined as the quantity needed to transform 1 μmol H2O2 to H2O within 1 min at 25°C and is expressed as U·g−1 tissue (Pulli et al., 2013).
Quantitative RT‐PCR
Rats were killed, and distal colonic segments of approximately 50 mg were collected. DRGs T13‐L2 (splanchnic colonic afferents) and L6‐S1 (pelvic colonic afferents) were harvested bilaterally. Briefly, the spinal column was dissected and cut down the midline, and after removal of the spinal cord and meninges, DRGs of interest were extracted as previously described (Sleigh et al., 2016). Samples were snap‐frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted from colon or DRG (Isolate II RNA Mini Kit; Bioline, London, UK), and RNA was converted to cDNA by reverse transcription (SensiFAST cDNA Synthesis Kit; Bioline, London, UK). A TaqMan gene expression assay (Thermo Fisher, Waltham, MA, USA, list of primers in Table A1) was executed on an ABIPrism 7300 sequent detector system (Applied Biosystems, Foster City, CA, USA) in a 25 μL reaction volume containing 2 μL cDNA, 12.5 μL TaqMan Universal PCR Master Mix (Thermo Fisher, Waltham, MA, USA), 1.25 μL TaqMan assay probe and 9.25 μL RNase‐free H2O. Gene expression assays were executed following the MIQE guidelines (Bustin et al., 2009). GAPDH and β‐actin were reference genes. The parameters for PCR amplification were 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min (Bustin et al., 2009). The outcome values were analysed using qBASEPLUS software (Biogazelle N.V., Zwijnaarde, Belgium).
Immunohistochemistry for mast cell tryptase
Colonic samples were fixed (4% formaldehyde; Merck, Darmstadt, Germany), embedded in paraffin and cut into 5 μm sections. Sections were pretreated with trypsin (37°C, 10 min; Sigma‐Aldrich) and citrate buffer pH 6 (microwave, 10 min). Slides were incubated overnight in a moist chamber with mouse mast cell http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2424 monoclonal antibody (1:10 000; clone AA1; Abcam, Cambridge, UK). After washing with Tris‐buffered saline, slides were incubated (30 min) with biotinylated goat anti‐mouse IgG antibody (1:200; Vector Laboratories, Burlingame, CA, USA) and rat serum (1:20; Vector Laboratories) and incubated (60 min) with a Vectastain® avidin–biotin complex (Vector Laboratories). Slides were washed, developed in an aminoethylcarbozole solution with hydrogen peroxide (Sigma‐Aldrich) (10 min), counterstained with haematoxylin (2 min) and covered. Colon sections were screened (100× magnification), and the total number of tryptase‐positive cells in the mucosa was quantified using ImageJ 1.51 J8 (National Institutes of Health, Bethesda, MD, USA) and expressed per mm2.
Immunohistochemistry for PAR2, PAR4 and TRPA1 channels
Distal colon samples were fixed in 4% paraformaldehyde and further processed for cryoembedding. Cryosections (12 μm) were thaw‐mounted on poly‐l‐lysine‐coated slides and dried at room temperature for 2 h. Subsequently, these sections were permeabilized and blocked in 0.01 M PBS containing 0.3% Triton X‐100 and 10% normal horse serum for 1 h at room temperature, after which they were incubated with primary antibodies for 16 h at 4°C. All antibodies were diluted in 0.01 M PBS containing 0.3% Triton X‐100 and 1% BSA. The following primary antibodies were used: rabbit anti‐http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=350 (1/200; Alomone Labs, Jerusalem, Israel), rabbit anti‐http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=348 (1/100; Santa Cruz Biotechnology, Heidelberg, Germany), rabbit anti‐http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=485 (1/1000; Abcam) and goat anti‐http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=695 (1/3000; Abcam). After several washing steps with 0.01 M PBS, visualization was carried out with CY3‐conjugated donkey anti‐rabbit immunoglobulins (1/1000; Jackson ImmunoResearch, Ely, UK) or CY5‐conjugated donkey anti‐goat immunoglobulins (1/1000; Jackson ImmunoResearch). Specificity was confirmed using negative controls and isotype controls. High‐resolution images were obtained on a Leica, Wetzlar, Germany TCS SP8 confocal laser scanning microscope, and images were processed using the ImageJ software.
Proteolytic activities
To assess the activity of serine proteases in post‐inflammatory visceral hypersensitivity, trypsin‐like, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2342&familyId=751&familyType=ENZYME, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2358&familyId=751&familyType=ENZYME, pancreas elastase and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2865 activities were determined in colonic and faecal samples. Distal colon samples of approximately 50 mg were taken after death, rinsed with Krebs solution, snap‐frozen within 5 min and stored at −80°C until further processing. The first step was to crush the colonic samples on dry ice using liquid nitrogen, to avoid loss of activity due to temperature increase. Afterwards, the samples were dissolved in lysis buffer (composition depending on assessed activity) for 15 min and centrifuged (4°C, 5 min, 12 000× g). The supernatant was collected and used immediately to measure enzymic activity. Lysis buffer for trypsin‐like activity contained 1% octylglucoside (Roth, Wiesbaden, Germany) and 0.05% heparin (Sigma Life Science, Munich, Germany) in 50 mM Tris–HCl pH 7.4. The buffers for the other enzyme activities were the same (1% octylglucoside in 50 mM Tris–HCl pH 7.4) except for the addition of 0.1% heparin in the buffer for chymotrypsin‐like activity.
Boc‐Gln‐Ala‐Arg‐AMC (75 μM) and n‐Tosyl‐Gly‐Pro‐Arg‐AMC (100 μM) (both Bachem, Bubendorf, Switzerland) were used to assess the trypsin‐like activity. Colonic supernatant was placed in a cold microtiter plate, and preheated (37°C) substrate solutions in 50 mM Tris–HCl pH 8.0 were added to the samples to start the incubation. Fluorescence was measured kinetically for 20 min at 37°C on Tecan, Männedorf, Switzerland Infinite F200 Pro. The activities (U·L−1) were transformed to specific activities (U·g−1) using the protein concentration in the samples as determined by Bradford analysis. Chymotrypsin‐like, neutrophil elastase, pancreas elastase and KLK activities were measured using Suc‐Ala‐Ala‐Pro‐Phe‐AMC (0.45 mM), Suc‐Ala‐Ala‐Pro‐Val‐AMC (0.45 mM), Suc‐Ala‐Ala‐Ala‐AMC (0.45 mM) and H‐Pro‐Phe‐Arg‐AMC (0.45 mM) (all Bachem) in 50 mM Tris–HCl pH 8.0 respectively. The protocol for these activity experiments was identical to the one for trypsin‐like activity except for a 5 min pre‐incubation of the samples.
Experiments to determine proteolytic activities in the faecal samples were performed in the same way except for the lysis buffer, which did not contain heparin or octylglucoside, and lysis time, which was 10 min instead of 15 min.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). All data are presented as mean ± SEM. The statistical analysis was performed using SPSS Statistics (version 24.0; IBM, Armonk, NY, USA). Results (VMR and compliance) were analysed using a generalized estimating equations test with least significant difference post hoc test. Inflammatory parameters and immunohistochemistry results were analysed using a two‐way ANOVA with Student–Newman–Keuls (S‐N‐K) post hoc test, as appropriate. Post hoc tests were only carried out if F achieved P < 0.05 and there was no significant variance in homogeneity. qPCR results were analysed using unpaired Student's t‐test or two‐way ANOVA with S‐N‐K post hoc test. Proteolytic activities were analysed using a Mann–Whitney U‐test. A P‐value <0.05 was considered statistically significant. The graphs were made using GraphPad Prism 6.0.
Materials
Active compounds
Nafamostat mesilate (Selleckchem®, also named FUT‐175) is a broad‐spectrum serine protease inhibitor, commercially available in Japan for the treatment of acute pancreatitis and disseminated intravascular coagulation (Isozaki et al., 2006). UAMC‐00050 and UAMC‐01162 are serine protease inhibitors with a well‐defined multi‐target inhibition profile, developed by the Antwerp Drug Discovery Network (patent WO2007045496) (Joossens et al., 2007). These compounds slightly differ in both structural properties as well as in inhibitory potency; UAMC‐00050 (bis(acetamidophenyl) guanidinophenylethylphosphonate) is a more potent inhibitor than UAMC‐01162 with a highly similar structure, namely, diphenyl guanidinophenylethylphosphonate. The inhibition profiles (displayed as IC50 values) of the three serine protease inhibitors used in the present study are provided in Table A2 and have been described in detail previously (van Soom et al., 2015).
Other chemicals and reagents
Heparin, hydrogen peroxide, TNBS and trypsin were purchased from Sigma‐Aldrich (Steinheim, Germany). Ketamine (Ketalar) was purchased from Pfizer. Xylazine (Rompun) was purchased from Bayer. Sodium chloride 0.9% was purchased from Braun. Pentobarbital 60 mg·mL−1 (Nembutal) was purchased from Ceva (Brussels, Belgium). The Krebs–Ringer solution used had the following composition: 118.3 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 2 mM NaHCO3, 0.026 mM CaEDTA and 11.1 mM glucose. Mouse anti‐mast cell tryptase monoclonal antibody, rabbit anti‐TRPA1 and goat anti‐CGRP were purchased from Abcam. Goat anti‐mouse IgG antibody, rat serum and Vectastain® avidin–biotin complex were obtained from Vector Laboratories. Rabbit anti‐PAR4 was purchased from Alomone Labs. Rabbit anti‐PAR2 was obtained from Santa Cruz Biotechnology. CY3‐conjugated donkey anti‐rabbit immunoglobulins and CY5‐conjugated donkey anti‐goat immunoglobulins were obtained from Jackson ImmunoResearch. Octylglucoside was purchased from Roth. Boc‐Gln‐Ala‐Arg‐AMC, n‐Tosyl‐Gly‐Pro‐Arg‐AMC, Suc‐Ala‐Ala‐Pro‐Phe‐AMC, Suc‐Ala‐Ala‐Pro‐Val‐AMC, Suc‐Ala‐Ala‐Ala‐AMC and H‐Pro‐Phe‐Arg‐AMC were obtained from Bachem.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c).
Results
Nafamostat mesilate decreases visceral hypersensitivity in a post‐inflammatory setting
Rats treated with TNBS developed colitis on day 3, as demonstrated by the significantly increased colonoscopic inflammatory scores compared with controls (Table 1A). At the day of the VMR, the post‐inflammatory status of the animals was confirmed by colonoscopy, macroscopy, microscopy and colonic MPO activity.
Table 1.
Inflammatory parameters of nafamostat mesilate, UAMC‐00050 and UAMC‐01162 in control and post‐colitis rats
| A. Nafamostat mesilate | |||||||
|---|---|---|---|---|---|---|---|
| Group | Drug | n | Colonoscopy (0–19) | Macroscopy | Microscopy | MPO activity | |
| Day 3 | Day VMR | ||||||
| Control | Vehicle | 8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.2 | 0.9 ± 0.3 |
| 0.1 mg·kg−1 | 8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.6 ± 0.4 | 0.8 ± 0.3 | |
| Post‐colitis | Vehicle | 8 | 5.8 ± 0.8* | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.8 ± 0.3 | 1.1 ± 0.3 |
| 0.01 mg·kg−1 | 8 | 6.6 ± 0.7* | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 0.5 | 1.5 ± 0.5 | |
| 0.1 mg·kg−1 | 8 | 7.0 ± 0.5* | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.2 | 1.7 ± 0.4 | |
| 1 mg·kg−1 | 8 | 5.0 ± 0.4* | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.6 ± 0.4 | 1.4 ± 0.5 | |
| 10 mg·kg−1 | 7 | 6.0 ± 0.7* | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.7 ± 0.7 | 2.1 ± 0.5 | |
| B. UAMC‐00050 | |||||||
|---|---|---|---|---|---|---|---|
| Group | Drug | n | Colonoscopy (0–19) | Macroscopy | Microscopy | MPO activity | |
| Day 3 | Day VMR | ||||||
| Control | Vehicle | 7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.2 | 0.9 ± 0.2 |
| 1 mg·kg−1 | 6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.5 ± 0.1 | |
| Post‐colitis | Vehicle | 8 | 5.4 ± 0.7* | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.5 ± 0.2 | 1.6 ± 0.5 |
| 0.01 mg·kg−1 | 7 | 7.0 ± 0.9* | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.2 | 1.2 ± 0.5 | |
| 0.1 mg·kg−1 | 6 | 5.3 ± 0.9* | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.2 | 1.8 ± 0.9 | |
| 1 mg·kg−1 | 9 | 4.3 ± 0.6* | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 1.2 ± 0.6 | |
| C. UAMC‐01162 | |||||||
|---|---|---|---|---|---|---|---|
| Group | Drug | n | Colonoscopy (0–19) | Macroscopy | Microscopy | MPO activity | |
| Day 3 | Day VMR | ||||||
| Control | Vehicle | 8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.9 ± 0.3 |
| 2.5 mg·kg−1 | 8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.6 ± 0.2 | |
| Post‐colitis | Vehicle | 8 | 6.5 ± 0.9* | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.6 ± 0.2 | 0.9 ± 0.4 |
| 1 mg·kg−1 | 8 | 6.4 ± 0.8* | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.2 | 1.3 ± 0.2 | |
| 2.5 mg·kg−1 | 8 | 7.9 ± 0.9* | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.1 | 1.0 ± 0.2 | |
Data are presented as mean ± SEM. Two‐way ANOVA followed by S‐N‐K post hoc test. No signficant interaction, no signficant effect of the factor ‘drug’. Signficant effect of the factor ‘group’ for colonoscopy at day 3; *P < 0.05.
Compared with control rats, vehicle‐treated post‐colitis rats showed significantly increased VMRs for distension pressures (20–60 mmHg), indicating post‐inflammatory visceral hypersensitivity (Figure 2A). A single i.p. administration of nafamostat (0.01–10 mg·kg−1) 30 min before the VMR experiment attenuated visceral sensitivity. After a dose of 0.01 mg·kg−1, VMRs were significantly decreased at 20–60 mmHg (Figure 2A), whereas a dose of 0.1 and 1 mg·kg−1 significantly lowered VMRs at 30–40–60 mmHg (Figure 2B), and a dose of 1 mg·kg−1 only affected VMRs at 20 and 30 mmHg (Figure 2C). In the highest dose (10 mg·kg−1), no significant effect of nafamostat was observed in post‐colitis rats (Figure 2D). In control animals, the dose of 0.1 mg·kg−1 had no effect on visceral sensitivity (Figure 2E). Nafamostat (0.1 mg·kg−1) had no effect on colonic compliance (Figure 2F). Moreover, nafamostat treatment did not affect the inflammatory parameters compared with vehicle‐treated controls (Table 1A).
Figure 2.
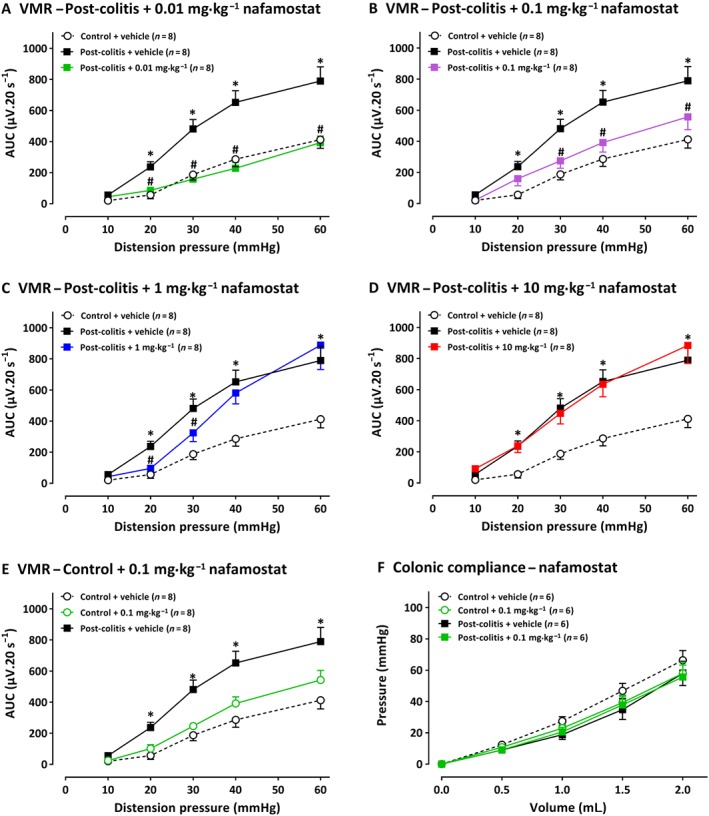
The effect of nafamostat mesylate (0.01–10 mg·kg−1) and its vehicle (water for injection) on VMRs (n = 8 per group and n = 7 per group for 10 mg·kg−1) and colonic compliance (n = 6 per group) in post‐colitis and control rats. The statistical analysis was performed on the complete dataset, but separate graphs were made for each dose for greater clarity. Thus, in rats post‐colitis, the effects of 0.01 mg·kg−1 nafamostat are shown in A; 0.1 mg·kg−1 in B, 1.0 mg·kg−1 in C and 10 mg·kg−1 in D. In E, 0.1 mg·kg−1 of nafamostat was given to control rats, i.e. without colitis and did not change the VMR responses. In F, 0.1 mg·kg−1 nafamostat did not change colonic compliance in either control or post‐colitis rats. Data are presented as mean ± SEM. *P < 0.05; significantly different from control + vehicle; # P < 0.05; significantly different from post‐colitis + vehicle; generalized estimating equations + least significant difference post hoc test.
UAMC‐00050 decreases visceral hypersensitivity in a post‐inflammatory setting
On day 3, all TNBS rats displayed significantly higher colonoscopic inflammatory scores compared with controls, indicating the presence of a mild colitis (Table 1B). At the day of the VMR, the post‐inflammatory status of all animals was confirmed by colonoscopy and post‐mortem inflammatory markers.
Rats in the post‐colitis group displayed significantly higher VMRs compared with controls, confirming visceral hypersensitivity (Figure 3A). A single i.p. administration with UAMC‐00050 decreased VMRs in a dose‐dependent manner in post‐colitis animals. Thus, 0.01 mg·kg−1 had no effect (Figure 3A), whereas 0.1 mg·kg−1 UAMC‐00050 significantly decreased VMRs at 30–40–60 mmHg (Figure 3B), and 1 mg·kg−1 UAMC‐00050 completely restored sensitivity to normal values (Figure 3C). The most effective dose of 1 mg·kg−1 UAMC‐00050 had no effect on VMRs in control animals (Figure 3D) and had no effect on colonic compliance (Figure 3E). UAMC‐00050 (0.01–1 mg·kg−1) had no effect on the inflammatory markers (Table 1B).
Figure 3.
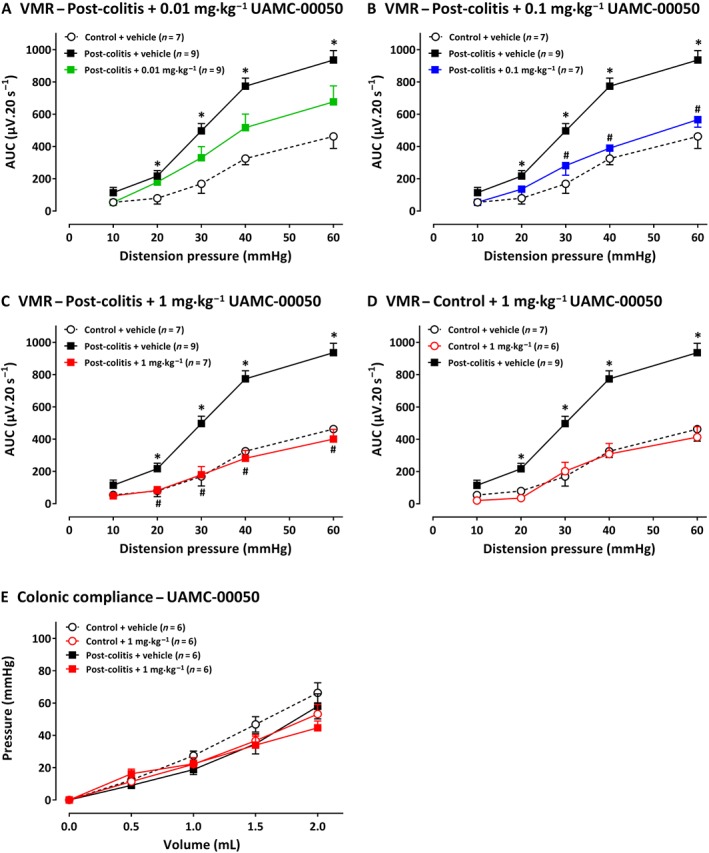
The effect of UAMC‐00050 (0.01–1 mg·kg−1) and its vehicle (5% DMSO) on VMRs. In A, results presented are from post‐colitis rats + 0.01 mg·kg−1 (n = 9); in B, post‐colitis + 0.1 mg·kg−1 (n= 7); in C, post‐colitis + 1 mg·kg−1, (n = 7); in D, results are from control rats treated with 1 mg·kg−1, UAMC‐00050 (n = 6). In E, the lack of effects of 1 mg·kg−1 UAMC‐00050 on colonic compliance are shown, for control rats and post colitis rats (n = 6). The statistical analysis was performed on the complete dataset, but separate graphs were made for each dose for greater clarity. Data are presented as mean ± SEM. *P < 0.05; significantly different from control + vehicle. # P < 0.05, significantly different from post‐colitis + vehicle; generalized estimating equations + least significant difference post hoc test.
UAMC‐01162 decreases visceral hypersensitivity in a post‐inflammatory setting
A mild colitis was present in all TNBS animals, as confirmed by the significant higher colonoscopic inflammatory score at day 3 compared with controls (Table 1C). At the end of the experiments, the post‐inflammatory status of all animals was confirmed. Vehicle‐treated post‐colitis rats displayed significantly higher VMRs compared with controls, demonstrating visceral hypersensitivity (Figure 4A). A single i.p. injection of 1 mg·kg−1 UAMC‐01162 in post‐colitis animals had no effect on VMR (Figure 4A), whereas 2.5 mg·kg−1 UAMC‐01162 completely reversed visceral hypersensitivity (Figure 4B). In control animals, no changes in visceral sensitivity were observed after a single i.p. injection with 2.5 mg·kg−1 UAMC‐01162 (Figure 4C). UAMC‐01162 did not alter colonic compliance (Figure 4D) and had no effect on colonic inflammatory markers (Table 1C).
Figure 4.
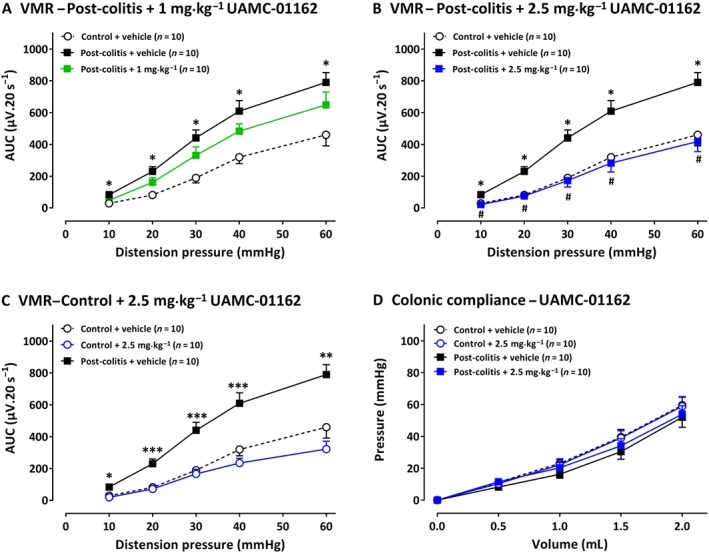
The effect of UAMC‐01162 (1–2.5 mg·kg−1) and its vehicle (5% DMSO) on VMRs and colonic compliance in post‐colitis and control rats (n = 10 per group). In A, results presented are from post‐colitis rats + 1 mg·kg−1; in B, post‐colitis + 2.5 mg·kg−1. In C, the results are from control rats treated with 2.5 mg·kg−1 UAMC‐01162. In D, the results show the lack of effects of 2.5 mg·kg−1 UAMC‐01162 on colonic compliance are shown, for control rats and post‐colitis rats. The statistical analysis was performed on the complete dataset, but separate graphs were made for each dose for greater clarity. Data are presented as mean ± SEM. *P < 0.05; significantly different from control + vehicle. # P < 0.05; significantly different from post‐colitis + vehicle; generalized estimating equations + least significant difference post hoc test.
mRNA quantification of serine proteases, PARs and TRP cation channels
The selection of serine proteases for qPCR analysis was based on the inhibition profiles of the respective inhibitors. The relative mRNA expression of Tpsab1 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2424&familyId=751&familyType=ENZYME) was increased in the colon of post‐colitis rats, while the expression of Plau (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2393), St14 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2418) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2378 was comparable between control and post‐colitis groups. http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2372, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2374 and Ctsg (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3572) could not be detected (Table 2). Nafamostat, UAMC‐00050 or UAMC‐01162 did not affect serine protease expression at the mRNA level (Table 2).
Table 2.
Relative mRNA expression of serine proteases in colon samples
| A. Nafamostat mesylate (NFM) | ||||
|---|---|---|---|---|
| Gene | Control | Post‐colitis | ||
| Vehicle | NFM 0.1 mg·kg−1 | Vehicle | NFM 0.1 mg·kg−1 | |
| Tryptase αβ1 | 1.30 ± 0.37 | 1.32 ± 0.45 | 4.65 ± 1.07* | 4.26 ± 1.41* |
| Matriptase | 1.07 ± 0.15 | 1.25 ± 0.17 | 1.00 ± 0.16 | 1.05 ± 0.15 |
| Cathepsin G | <LOD | <LOD | <LOD | <LOD |
| uPA | 1.12 ± 0.21 | 1.77 ± 0.33 | 1.43 ± 0.31 | 1.07 ± 0.23 |
| KLK2 | <LOD | <LOD | <LOD | <LOD |
| KLK4 | <LOD | <LOD | <LOD | <LOD |
| KLK8 | 1.21 ± 0.24 | 2.02 ± 0.57 | 1.18 ± 0.18 | 1.67 ± 0.63 |
| n = 8 | n = 8 | n = 8 | n = 8 | |
| B. UAMC‐00050 | ||||
|---|---|---|---|---|
| Gene | Control | Post‐colitis | ||
| Vehicle | UAMC‐00050 | Vehicle | UAMC‐00050 | |
| Tryptase αβ1 | 1.30 ± 0.37 | 3.14 ± 1.19 | 4.65 ± 1.07* | 2.63 ± 0.44 |
| Matriptase | 1.07 ± 0.15 | 0.88 ± 0.09 | 1.00 ± 0.16 | 1.00 ± 0.13 |
| Cathepsin G | <LOD | <LOD | <LOD | <LOD |
| uPA | 1.12 ± 0.21 | 1.43 ± 0.31 | 1.43 ± 0.31 | 1.23 ± 0.19 |
| KLK2 | <LOD | <LOD | <LOD | <LOD |
| KLK4 | <LOD | <LOD | <LOD | <LOD |
| KLK8 | 1.21 ± 0.24 | 1.50 ± 0.37 | 1.18 ± 0.18 | 1.28 ± 0.21 |
| n = 8 | n = 6 | n = 8 | n = 7 | |
| C. UAMC‐01162 | ||||
|---|---|---|---|---|
| Gene | Control | Post‐colitis | ||
| Vehicle | UAMC‐01162 | Vehicle | UAMC‐01162 | |
| Tryptase αβ1 | 1.29 ± 0.39 | 1.11 ± 0.37 | 2.27 ± 1.00 | 0.75 ± 0.22 |
| Matriptase | 1.05 ± 0.10 | 0.65 ± 0.11* | 0.71 ± 0.11* | 0.64 ± 0.07* |
| Cathepsin G | <LOD | <LOD | <LOD | <LOD |
| uPA | 1.09 ± 0.16 | 1.09 ± 0.20 | 1.08 ± 0.16 | 1.07 ± 0.12 |
| KLK2 | <LOD | <LOD | <LOD | <LOD |
| KLK4 | <LOD | <LOD | <LOD | <LOD |
| KLK8 | 1.19 ± 0.21 | 1.09 ± 0.12 | 0.91 ± 0.20 | 0.88 ± 0.16 |
| n = 10 | n = 10 | n = 10 | n = 10 | |
Data are expressed as relative mRNA expression and presented as mean ± SEM for n = 8 per group (NFM), n = 8 per group (control/post‐colitis + vehicle), n = 6 per group (control + UAMC‐00050), n = 7 per group (post‐colitis + UAMC‐00050) and n = 10 per group (UAMC‐01162). Two‐way ANOVA followed by one‐way ANOVA + least significant difference post hoc test if applicable. NFM, nafamostat mesylate; LOD, limit of detection.
A: For tryptase αβ1, *P<0.05, significant effect of the factor ‘group’; no significant effect of the factor ‘drug’; no significant interaction.
B: *P < 0.05 significantly different from control + vehicle. No significant effect of the factor ‘group’. No significant effect of the factor ‘drug’. Significant interaction for tryptase αβ1.
C: *P < 0.05 significantly different from control + vehicle. No significant effect of the factor ‘group’. No significant effect of the factor ‘drug’. Significant interaction for matriptase.
Recently, the serine protease trypsin was shown to be involved in visceral hypersensitivity (Rolland‐Fourcade et al., 2017). We therefore also investigated the colonic mRNA expression levels of the three trypsin isoforms: http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2397 (trypsin‐1 precursor), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2398 (trypsin‐2 precursor) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2399 (trypsin‐3 precursor). The mRNA expression level of PRSS1 was comparable in control and post‐colitis rats (1.26 ± 0.28 vs. 1.43 ± 0.40; n = 12; ns), while PRSS3 was significantly up‐regulated in the post‐colitis group (1.09 ± 0.31 vs. 3.84 ± 1.20; n = 12; P = 0.03). PRSS2 was below the limit of detection (data not shown).
We also assessed colonic mRNA expression of receptors involved in the serine protease signalling cascade, that is, PARs and the TRP cation channels (Cenac, 2013). mRNA expression of PAR4 was significantly up‐regulated in colonic samples of post‐colitis animals, while that for http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=510 tended to be increased, but this up‐regulation did not reach significance (P > 0.05). No significant differences were detected in the mRNAs for PAR2, TRPA1 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=507 in the colon of control versus post‐colitis animals (Table 3).
Table 3.
Relative mRNA expression of PARs and TRP channels in samples from colons, DRG T13‐L2 and DRG L6‐S1
| Gene | Colon | DRG T13‐L2 | DRG L6‐S1 | |||
|---|---|---|---|---|---|---|
| Control | Post‐colitis | Control | Post‐colitis | Control | Post‐colitis | |
| PAR2 | 1.05 ± 0.11 | 0.90 ± 0.14 | 1.07 ± 0.11 | 1.31 ± 0.08 | 1.07 ± 0.13 | 0.75 ± 0.04a |
| PAR4 | 0.94 ± 0.15 | 1.87 ± 0.30a | 1.03 ± 0.08 | 1.60 ± 0.17a | 1.05 ± 0.12 | 1.21 ± 0.25 |
| TRPA1 | 1.09 ± 0.19 | 1.27 ± 0.28 | 1.04 ± 0.08 | 1.40 ± 0.12a | 1.10 ± 0.15 | 0.81 ± 0.09 |
| TRPV1 | 1.03 ± 0.10 | 0.81 ± 0.15 | 1.04 ± 0.08 | 1.19 ± 0.07 | 1.07 ± 0.12 | 1.49 ± 0.20 |
| TRPV4 | 1.04 ± 0.11 | 1.73 ± 0.36b | 1.10 ± 0.18 | 1.48 ± 0.17 | 1.05 ± 0.11 | 0.88 ± 0.10 |
| n = 8 | n = 8 | n = 12 | n = 12 | n = 12 | n = 12 | |
Data are expressed as relative mRNA expression and presented as mean ± SEM for n = 8 per group (colon) and n = 12 per group (DRG). Independent samples t‐test.
P < 0.05; significant effect of the factor group;
P > 0.05, not significant.
qPCR experiments on DRGs were executed at the level of colonic splanchnic afferent nerves (T13‐L2) and colonic pelvic afferent nerves (L6‐S1). qPCR on DRG level T13‐L2 revealed a clear trend towards up‐regulation of all PARs and TRPs in the post‐colitis group, showing significant increases for PAR4 and TRPA1. At DRG level L6‐S1, a trend towards a lower mRNA expression of PARs and TRPs was seen in the post‐colitis animals, showing significant results only for PAR2 (Table 3, *).
Immunohistochemistry for mast cell tryptase
The total number of mast cell tryptase‐positive cells per mm2, quantified by immunohistochemistry in colonic mucosa, was significantly increased in post‐colitis rats compared with control animals (Figure 5). Similar to the mRNA results, treatment with serine protease inhibitors did not affect mast cell tryptase expression at the protein level (Figure 5).
Figure 5.
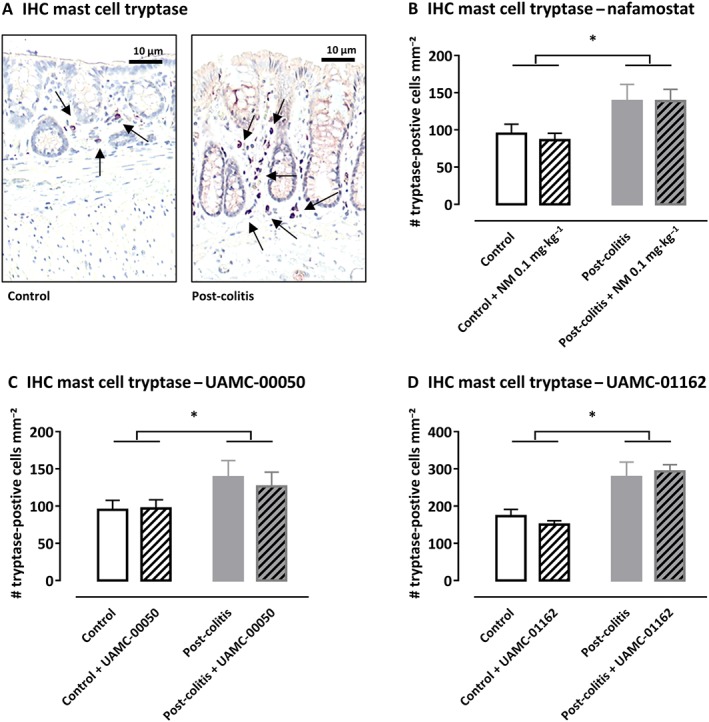
Immunohistochemistry with mast cell tryptase antibody in rat colon. (A) Representative images of the colonic mucosa of a control and a post‐colitis animal with mast cell tryptase (arrow). (B–D) The number of tryptase‐positive mast cells mm−2 in the colonic mucosa of control and post‐colitis rats with or without treatment with a serine protease inhibitor. Data shown are means +SEM; n = 8 per group. *P < 0.05; significant effect of the factor ‘group’; no significant effect of the factor ‘drug’; no significant interaction between the factors ‘group’ and ‘drug’; two‐way ANOVA.
Immunohistochemistry for PAR2, PAR4 and TRPA1
To investigate the potential involvement of the protease‐PAR‐TRP axis, as reviewed by Balemans et al. (2017), we determined the expression of PAR2, PAR4 and TRPA1 in nerve fibres in the lamina propria of the distal colon. CGRP‐immunoreactive sensory nerve fibres showed a clear co‐expression of PAR2 (Figure 6A–C), whereas PAR4 appeared to be absent in these fibres (Figure 6D–F). TRPA1, which is a downstream target of PAR2 signalling, was also co‐expressed with CGRP in lamina propria nerve fibres (Figure 6G–I).
Figure 6.
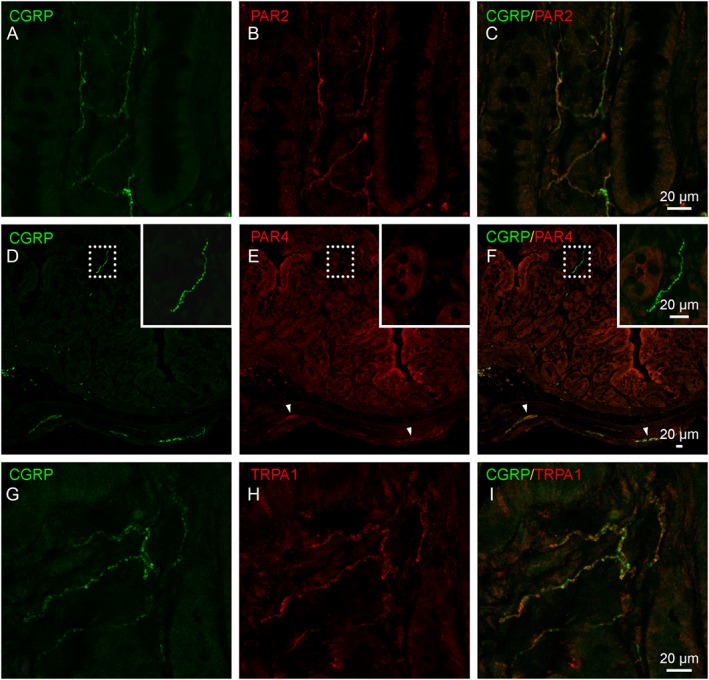
Immunohistochemical localization of PAR2, PAR4 and TRPA1 channels in sensory nerve fibres of the distal colon. (A–C) Representative images showing co‐localization of PAR2 (red) in CGRP‐immunopositive nerve fibres (green). (D–F) Representative images showing the presence of PAR4 immunoreactivity (red) in the colonic epithelium and in enteric nerve plexuses (arrowheads) but not in the CGRP‐immunoreactive nerve fibre population (inset). (G–I) Representative images showing co‐localization of TRPA1 protein (red) in CGRP‐immunopositive nerve fibres (green).
Proteolytic activities
In order to assess the proteolytic activities in control versus post‐colitis rats, trypsin‐like, chymotrypsin‐like, neutrophil elastase, pancreas elastase and KLK activities were determined in colon and faecal samples. Trypsin‐like activity was found to be significantly up‐regulated in the colon of post‐colitis rats compared with controls using both Boc‐Gln‐Ala‐Arg‐AMC (Figure 7A) and n‐Tosyl‐Gly‐Pro‐Arg‐AMC (Figure 7B) as a substrate. Neutrophil elastase, pancreas elastase, KLK and chymotrypsin‐like activities were not significantly different in colonic samples from post‐colitis versus control animals (Figure 7C–F). In faecal samples, no significant differences could be detected between control and post‐colitis rats (Figure A1 A–F).
Figure 7.
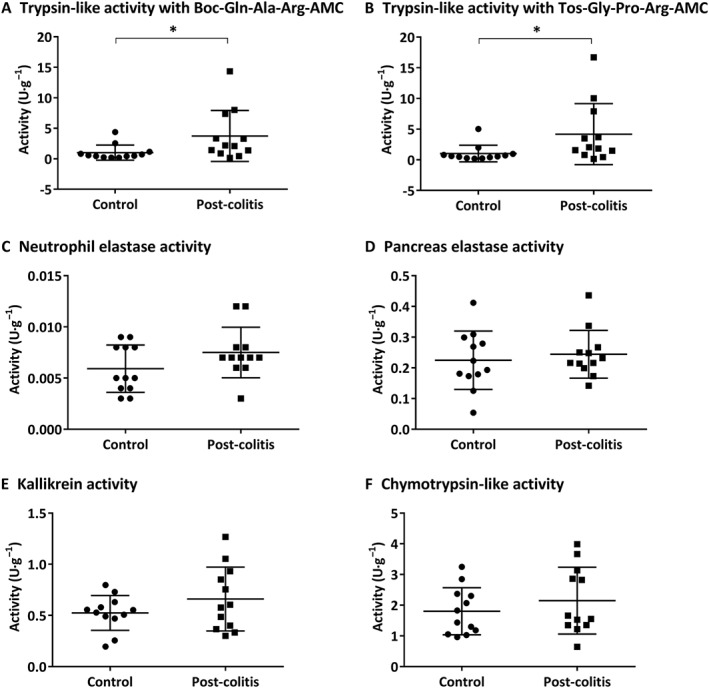
Proteolytic activities determined in colon samples. Trypsin‐like activity was measured using Boc‐Gln‐Ala‐Arg‐AMC (in A) and n‐Tos‐Gly‐Pro‐Arg‐AMC (in B). To determine the neutrophil elastase (in C), pancreas elastase (in D), KLK (in E) and chymotrypsin‐like activity (in F), Suc‐Ala‐Ala‐Pro‐Val‐AMC, Suc‐Ala‐Ala‐Ala‐AMC, H‐Pro‐Phe‐Arg‐AMC and Suc‐Ala‐Ala‐Pro‐Phe‐AMC were used respectively. Data are presented as mean ± SEM (n = 12). *P < 0.05; Mann–Whitney U‐test.
Discussion
The aim of this study was to elucidate the effects of serine protease inhibitors on post‐inflammatory visceral hypersensitivity and their targets of action. Our results suggest that treatment with serine protease inhibitors reverses the visceral pain response to colonic distension in rats with post‐inflammatory visceral hypersensitivity. Nafamostat significantly decreased visceral hypersensitivity, with a complete reversal in the lowest dose tested. Furthermore, both UAMC‐00050 and UAMC‐01162, two newly developed serine protease inhibitors [patent WO2007045496 (A1)] (Joossens et al., 2007), significantly lowered visceral hypersensitivity dose‐dependently, completely restoring visceral sensitivity in the highest dose used. The serine protease inhibitors had no effect in healthy controls and did not affect colonic compliance, excluding an effect on viscoelastic properties of the colon.
Next, we tried to further identify the specific serine proteases involved and their possible mode of action. Firstly, in order to define the serine proteases involved in a state of post‐inflammatory visceral hypersensitivity, mRNA gene expression assays were performed. Colon tissue was tested for the expression of a panel of serine proteases, defined by those with an IC50 < 10−6 M for the serine protease inhibitors tested in this study. Our data revealed a fourfold increased mRNA expression of the serine protease tryptase as well as a significant up‐regulation of PRSS3 at the colonic level in post‐colitis rats, while all other serine proteases tested in our panel were not significantly altered or below the detection limit in colon tissue. We confirmed this result at the protein level, by demonstrating a significantly increased number of mast cell tryptase‐positive cells in the colonic mucosa of post‐colitis rats compared with controls. Our results, therefore, point towards an important role for the serine protease tryptase in post‐inflammatory visceral hypersensitivity. Moreover, we could not find evidence for the involvement of KLK2, KLK4, KLK8, cathepsin G and urokinase plasminogen activator at the colonic level.
These serine protease profile determinations could be of great importance towards the development of a new biomarker for IBS (Barbara, 2015). Previous studies showed an increased faecal protease activity in IBS‐D (serine proteases) and IBS‐C (cysteine proteases) patients compared with healthy controls (Roka et al., 2007; Gecse et al., 2008; Annahazi et al., 2013), highlighting the potential of proteases as a diagnostic tool.
Interestingly, the three serine protease inhibitors did not affect expression of mRNA and protein, suggesting a direct effect on the activity of the enzymes. It is of great interest to determine the serine protease activity as well as the effect of the serine protease inhibitors, but the currently available tools to study protease activity, such as the azocasein assay, are very non‐specific (Edgington‐Mitchell, 2016). The fluorogenic substrates used here allowed the proteolytic activities to be to better characterized. Trypsin‐like activity was found to be significantly increased in the colon of post‐colitis rats compared with controls with both substrates, while no significant differences could be detected in faecal samples. However, more specific probes and/or inhibitors to directly assess the activity of individual proteases are essential for a more precise interpretation of the results. Edgington‐Mitchell et al. (2017) recently demonstrated the efficacy of two fluorescent activity‐based probes detecting serine proteases in vitro, but their use was less valuable in two in vivo animal models for acute inflammation (pancreatitis and colitis). Optimization of these probes for in vivo use is of great importance when studying the role of serine proteases in several pathologies.
Remarkably, nafamostat showed its greatest potential at the lowest dose used pointing to a loss of specificity of the compound on visceral hypersensitivity: nafamostat 0.01 mg·kg−1 was more effective than 0.1 and 1 mg·kg−1, and no effect was observed in a dose of 10 mg·kg−1. Amongst all of the examined proteases, the affinity of nafamostat is the highest for human tryptase (9.53 × 10−11 M). Thus, when used in a relatively low concentration, nafamostat is an extremely potent and selective inhibitor of human tryptase (Mori et al., 2003) suggesting that by using higher concentrations, the selectivity for tryptase might be lost, altering the protease profile in a more widespread way.
Besides investigating the type of serine proteases involved in visceral hypersensitivity, we also studied the source of the proteases and site of action of the serine protease inhibitors. Sources of proteases in the gastrointestinal tract are diverse and include pancreas, the microbiome, epithelial cells, neutrophils, macrophages and mast cells (Vergnolle, 2016). Our results provide evidence for the presence of tryptase in mast cells. Recently, the group of Vergnolle demonstrated the release of trypsin‐3 by the intestinal epithelium and showed its involvement in visceral hypersensitivity (Rolland‐Fourcade et al., 2017). This highlights the need for further study of the source of proteases involved in visceral pain.
We hypothesize that the compounds used in our study directly inhibit serine proteases early in the signalling cascade, thereby preventing the proteases from activating PARs which, in turn, cannot sensitize TRP channels, thereby preventing visceral hypersensitivity. A recent review on TRP channels reports that pro‐inflammatory mediators, including 5‐HT, histamine, bradykinins and proteases can activate various GPCRs such as PAR receptors, thereby triggering TRP channel sensitization in visceral hypersensitivity (Balemans et al., 2017). To investigate this hypothesis further in our model, we assessed the mRNA expression of PARs and TRP channels, which play a role in visceral hypersensitivity and serine protease downstream signalling pathways, at the colonic level and at DRGs. qPCR analysis revealed a significant up‐regulation of PAR4 in IBS rats at the colonic level and DRG T13‐L2. A significant up‐regulation of TRPA1 channels was found in DRG T13‐L2, while TRPV4 channels showed a tendency to increase in the colon of IBS rats. Interestingly, no significant upregulations of PARs and/or TRP channels were found in DRGs L6‐S1, highlighting the importance of thoracolumbar spinal cord (T13‐L2) in the processing of visceral pain signals (Traub, 2000; Christianson et al., 2006). These mRNA data support the involvement of both PARs and TRP channels in the downstream pathways of serine proteases involved in post‐inflammatory visceral hypersensitivity.
In order to reinforce our hypothesis on the involvement of PAR and TRP receptors in visceral hypersensitivity, immunohistochemical experiments were performed after the qPCR experiments. Increased expression of mast cell tryptase was observed both at the mRNA level as well as immunohistochemically in the colon of post‐colitis rats compared with control animals. Tryptase is a PAR2 ligand, and the involvement of both tryptase and the PAR2 receptor in visceral hypersensitivity has already been reported (Vergnolle et al., 2001; Cenac et al., 2007). We now demonstrated the presence of PAR2‐immunopositive nerve fibres in the colon of post‐colitis rats, for which double labelling with CGRP confirmed their sensory origin. We thus provide evidence at the protein level of the presence of both tryptase and PAR2 in the colon of post‐colitis animals. Furthermore, we could also demonstrate the presence of TRPA1 channels in CGRP‐positive nerve fibres in the lamina propria of the colon. Previously, Cattaruzza et al. (2010) already demonstrated that TRPA1 channels mediate PAR2‐induced visceral hypersensitivity. Thus, our results fit in the general hypothesis described in the review by Balemans et al. (2017).
Interestingly, a down‐regulation of PAR4 was shown in colonic biopsies of IBS‐D, IBS‐C and post‐infectious IBS patients (Han et al., 2012; Zhao et al., 2012). This discrepancy may be explained by a study of Annahazi et al. (2012). In a low‐grade TNBS colitis mouse model, PAR4 antagonism increases colorectal hyperalgesia. Their proposed mechanism is an endogenous activation of PAR4 (possibly by cathepsin G), inducing a feedback antinociceptive effect. Moreover, PAR4 activation has been shown to result in antinociceptive effects as demonstrated by the inhibition of the excitability of mouse and rat colonic DRGs after the application of a PAR4‐activating peptide (AP) (Asfaha et al., 2007; Karanjia et al., 2009), a decrease in carrageenan‐induced inflammatory visceral hypersensitivity after an injection with a PAR4‐AP (Asfaha et al., 2007) and a reduced visceral hypersensitivity in mice after an intracolonic administration with the PAR4‐agonist AYPGKF‐NH2 (Auge et al., 2009). We therefore hypothesize that the increased mRNA expression of PAR4 in the colon and DRG samples of post‐colitis rats might be a consequence of post‐inflammatory visceral hypersensitivity, rather than a cause. We were also able to localize PAR‐4 in epithelial cells, as well as in myenteric and submucosal neurons.
The trend towards increased expression of TRPV4 channels in colon of post‐inflammatory IBS rats is in line with the increased expression of these channels in human colon biopsies in IBD patients (Fichna et al., 2012). However, to our knowledge, no such data are available for IBS patients. Also, the up‐regulation of TRPA1 channels in DRGs of rats with visceral hypersensitivity following TNBS‐induced colitis is in line with the findings from Yang et al. (2008). Also in a water‐avoidance stress‐induced rat model for IBS, a significant up‐regulation of TRPA1 channels was seen in DRGs (Yu et al., 2010).
In summary, we have investigated the effect of three different serine protease inhibitors in a rat model of post‐inflammatory visceral hypersensitivity. After a single administration of either of these compounds, a decrease in visceral pain was shown in post‐colitis rats, but no effect was observed in healthy controls. Our work differs from previous studies in three key aspects. Firstly, we opted for a direct in vivo treatment strategy, with a single i.p. injection of the animals, while preceding studies employed an in vitro treatment strategy (Cenac et al., 2007; Wang et al., 2015). Secondly, we are the first to demonstrate a positive outcome after a symptomatic treatment instead of a preventive treatment in earlier studies (Cenac et al., 2007; Zhao et al., 2011; Wang et al., 2015). Thirdly, the animal models used are different. Previous studies employed intracolonic administration in mice of supernatants from IBS patients (Cenac et al., 2007; Wang et al., 2015) or acute stress in rats (Zhao et al., 2011) to induce visceral hypersensitivity, whereas we used an inflammation‐triggered, post‐colitis, model based on chemically induced colitis in rats. We provide further evidence for the role for the serine proteases tryptase and trypsin‐3 in this post‐inflammatory rat model for IBS, shown by the elevated mRNA expression and an increased number of mast cell tryptase‐positive cells. Moreover, the increased tryptase expression at the colonic level of post‐colitis rats, increased trypsin‐like serine protease activity in the colon of post‐colitis rats, the localization of PAR2 and TRPA1 in the sensory nerve fibres in the colon together with the increased TRPA1 mRNA expression at the DRG level in post‐colitis rats suggest their possible involvement in post‐inflammatory visceral hypersensitivity.
Our results indicate that serine protease inhibition represents an interesting new treatment strategy for abdominal pain in IBS patients. Regarding the search for new biomarkers in IBS patients, our study points towards serine proteases and more specifically tryptase as a possible candidate.
Author contributions
All authors contributed to the conception and design of the study, interpreted the data, revised the manuscript critically for important intellectual content and approved the final version. In addition, H.C. collected the data, and H.C., J.G.D.M. and B.Y.D.W. drafted the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
A special thanks to our laboratory technicians, P. Aerts, I. Goolaerts, A. Jürgens, M. Vinckx and L. Vits, for their assistance in the qPCR experiments and R. Van Den Bossche for the immunohistochemistry.
This study was funded by the University Research Fund Doctoral Projects (BOF‐DOCPRO) no. DOCPRO4 2014/ID 2964, the Research Foundation Flanders (FWO) no. G034113N and the Research Foundation Flanders‐Strategic Basic Research (FWO‐SBO) no. S001017N.
Appendix A.
Table A1.
TaqMan primers used for qPCR analysis of colonic and DRG samples
| Protein | Gene ID |
|---|---|
| Tryptase αβ1 | Rn00570928_m1 |
| Matriptase | Rn00586242_m1 |
| Cathepsin G | Rn01489144_g1 |
| Urokinase plasminogen activator | Rn00565261_m1 |
| KLK2 | Rn00820615_m1 |
| KLK4 | Rn01498534_g1 |
| KLK8 | Rn01476995_m1 |
| PAR2 | Rn00588089_m1 |
| PAR4 | Rn00587480_m1 |
| TRPA1 | Rn01473803_m1 |
| TRPV1 | Rn00583117_m1 |
| TRPV4 | Rn00576745_m1 |
| GAPDH | Rn01775763_g1 |
| β‐actin | Rn00667869_m1 |
Table A2.
Inhibitory profiles for the serine protease inhibitors UAMC‐00050, UAMC‐01162 and nafamostat mesilate
| Proteases | UAMC‐00050 | UAMC‐01162 | Nafamostat mesilate |
|---|---|---|---|
| IC50 (μM) | IC50 (μM) | Est. IC50 (μM) | |
| uPA | 0.0042 | 0.0031 | <0.001 |
| tPA | 7 | 23 | >2.5 |
| Plasmin | 0.9 | 13 | ±0.05 |
| Thrombin | 0.39 | 17 | ±0.5 |
| FXa | 100 | ±250 | ±2 |
| FXIIa | 1.61 | >2.5 | ±0.1 |
| Matriptase | 0.0025 | 0.083 | <0.001 |
| Tryptase | 0.028 | 0.093 | <0.001 |
| Cathepsin G | 0.12 | 0.33 | ±0.25 |
| HNE | >2.5 | >2.5 | >2.5 |
| Plasma KLK | >2.5 | >2.5 | ±0.005 |
| KLK1 | >2.5 | >2.5 | ±1 |
| KLK2 | 0.11 | 3 | ±0.1 |
| KLK4 | 0.0017 | 0.009 | ±0.005 |
| KLK8 | 0.0016 | 0.028 | ±0.015 |
| AChE | >20 | >10 | >2.5 |
Data are presented as mean. Est., estimated; FXa, factor Xa; FXIIa, factor XIIa; HNE, human neutrophil elastase; tPA, tissue plasminogen activator; uPA, urokinase plasminogen activator.
Figure A1.
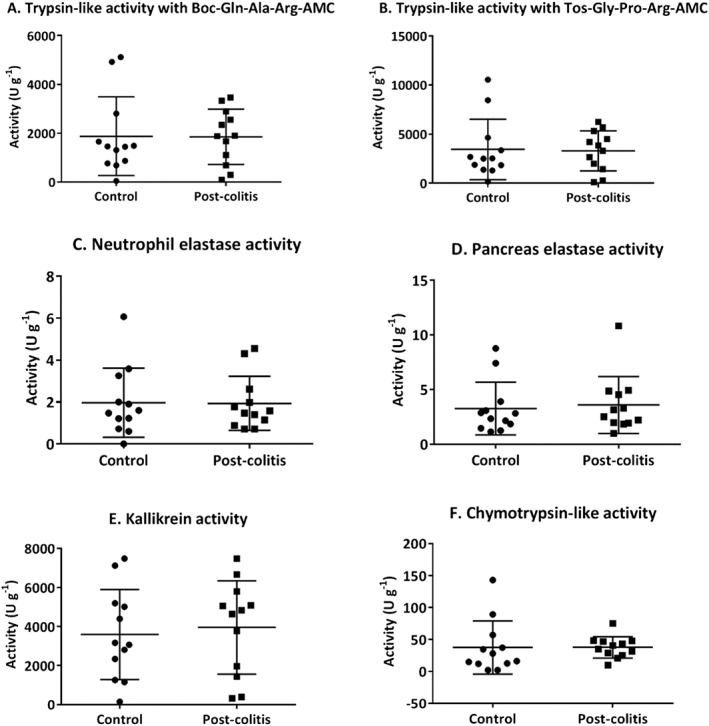
Proteolytic activities determined in faecal samples. Trypsin‐like activity was measured using Boc‐Gln‐Ala‐Arg‐AMC (in A) and n‐Tos‐Gly‐Pro‐Arg‐AMC (in B). To determine the neutrophil elastase (in C), pancreas elastase (in D), KLK (in E) and chymotrypsin‐like activity (in F), Suc‐Ala‐Ala‐Pro‐Val‐AMC, Suc‐Ala‐Ala‐Ala‐AMC, H‐Pro‐Phe‐Arg‐AMC and Suc‐Ala‐Ala‐Pro‐Phe‐AMC were used respectively. Data are presented as mean ± SEM. Mann–Whitney U‐test; n = 12.
Ceuleers, H. , Hanning, N. , Heirbaut, J. , Van Remoortel, S. , Joossens, J. , Van Der Veken, P. , Francque, S. M. , De bruyn, M. , Lambeir, A.‐M. , De Man, J. G. , Timmermans, J.‐P. , Augustyns, K. , De Meester, I. , and De Winter, B. Y. (2018) Newly developed serine protease inhibitors decrease visceral hypersensitivity in a post‐inflammatory rat model for irritable bowel syndrome. British Journal of Pharmacology, 175: 3516–3533. 10.1111/bph.14396.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annahazi A, Dabek M, Gecse K, Salvador‐Cartier C, Polizzi A, Rosztoczy A et al (2012). Proteinase‐activated receptor‐4 evoked colorectal analgesia in mice: an endogenously activated feed‐back loop in visceral inflammatory pain. Neurogastroenterol Motil 24: 76–85, e13. [DOI] [PubMed] [Google Scholar]
- Annahazi A, Ferrier L, Bezirard V, Leveque M, Eutamene H, Ait‐Belgnaoui A et al (2013). Luminal cysteine‐proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation‐predominant IBS. Am J Gastroenterol 108: 1322–1331. [DOI] [PubMed] [Google Scholar]
- Annahazi A, Gecse K, Dabek M, Ait‐Belgnaoui A, Rosztoczy A, Roka R et al (2009). Fecal proteases from diarrheic‐IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain 144: 209–217. [DOI] [PubMed] [Google Scholar]
- Asfaha S, Cenac N, Houle S, Altier C, Papez MD, Nguyen C et al (2007). Protease‐activated receptor‐4: a novel mechanism of inflammatory pain modulation. Br J Pharmacol 150: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auge C, Balz‐Hara D, Steinhoff M, Vergnolle N, Cenac N (2009). Protease‐activated receptor‐4 (PAR 4): a role as inhibitor of visceral pain and hypersensitivity. Neurogastroenterol Motil 21: 1189–e1107. [DOI] [PubMed] [Google Scholar]
- Balemans D, Boeckxstaens GE, Talavera K, Wouters MM (2017). Transient receptor potential ion channel function in sensory transduction and cellular signaling cascades underlying visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 312: G635–G648. [DOI] [PubMed] [Google Scholar]
- Barbara G (2015). IBS: biomarkers for IBS: ready for prime time? Nat Rev Gastroenterol Hepatol 12: 9–10. [DOI] [PubMed] [Google Scholar]
- Barbara G, Cremon C, De Giorgio R, Dothel G, Zecchi L, Bellacosa L et al (2011). Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep 13: 308–315. [DOI] [PubMed] [Google Scholar]
- Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D et al (2004). Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126: 693–702. [DOI] [PubMed] [Google Scholar]
- Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V et al (2009). Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137: 1425–1434. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M et al (2009). The MIQE guidelines: minimum information for publication of quantitative real‐time PCR experiments. Clin Chem 55: 611–622. [DOI] [PubMed] [Google Scholar]
- Cattaruzza F, Spreadbury I, Miranda‐Morales M, Grady EF, Vanner S, Bunnett NW (2010). Transient receptor potential ankyrin‐1 has a major role in mediating visceral pain in mice. Am J Physiol Gastrointest Liver Physiol 298: G81–G91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N (2013). Protease‐activated receptors as therapeutic targets in visceral pain. Curr Neuropharmacol 11: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade‐Gordon P et al (2007). Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest 117: 636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuleers H, Van Spaendonk H, Hanning N, Heirbaut J, Lambeir AM, Joossens J et al (2016). Visceral hypersensitivity in inflammatory bowel diseases and irritable bowel syndrome: the role of proteases. World J Gastroenterol 22: 10275–10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey WD, Kurlander J, Eswaran S (2015). Irritable bowel syndrome: a clinical review. JAMA 313: 949–958. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Traub RJ, Davis BM (2006). Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol 494: 246–259. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA et al (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Brit J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper HU, De Man JG, Moreels TG, Pelckmans PA, De Winter BY (2008). Review article: gastrointestinal sensory and motor disturbances in inflammatory bowel disease – clinical relevance and pathophysiological mechanisms. Aliment Pharmacol Ther 27: 621–637. [DOI] [PubMed] [Google Scholar]
- Deiteren A, De Man JG, Ruyssers NE, Moreels TG, Pelckmans PA, De Winter BY (2014). Histamine H4 and H1 receptors contribute to postinflammatory visceral hypersensitivity. Gut 63: 1873–1882. [DOI] [PubMed] [Google Scholar]
- Deiteren A, van der Linden L, de Wit A, Ceuleers H, Buckinx R, Timmermans JP et al (2015). P2X3 receptors mediate visceral hypersensitivity during acute chemically‐induced colitis and in the post‐inflammatory phase via different mechanisms of sensitization. PLoS One 10: e0123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington‐Mitchell LE (2016). Pathophysiological roles of proteases in gastrointestinal disease. Am J Physiol Gastrointest Liver Physiol 310: G234–G239 [DOI] [PubMed] [Google Scholar]
- Edgington‐Mitchell LE, Barlow N, Aurelio L, Samha A, Szabo M, Graham B et al (2017). Fluorescent diphenylphosphonate‐based probes for detection of serine protease activity during inflammation. Bioorg Med Chem Lett 27: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichna J, Mokrowiecka A, Cygankiewicz AI, Zakrzewski PK, Malecka‐Panas E, Janecka A et al (2012). Transient receptor potential vanilloid 4 blockade protects against experimental colitis in mice: a new strategy for inflammatory bowel diseases treatment? Neurogastroenterol Motil 24: e557–e560. [DOI] [PubMed] [Google Scholar]
- Gecse K, Roka R, Ferrier L, Leveque M, Eutamene H, Cartier C et al (2008). Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57: 591–599. [DOI] [PubMed] [Google Scholar]
- Greenwood‐Van Meerveld B, Prusator DK, Johnson AC (2015). Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol 308: G885–G903. [DOI] [PubMed] [Google Scholar]
- Han W, Wang Z, Lu X, Guo C (2012). Protease activated receptor 4 status of mast cells in post infectious irritable bowel syndrome. Neurogastroenterol Motil 24 (113–119): e182. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 46: D1091–d1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungin AP, Whorwell PJ, Tack J, Mearin F (2003). The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther 17: 643–650. [DOI] [PubMed] [Google Scholar]
- Isozaki Y, Yoshida N, Kuroda M, Handa O, Takagi T, Kokura S et al (2006). Anti‐tryptase treatment using nafamostat mesilate has a therapeutic effect on experimental colitis. Scand J Gastroenterol 41: 944–953. [DOI] [PubMed] [Google Scholar]
- Joossens J, Ali OM, El‐Sayed I, Surpateanu G, Van der Veken P, Lambeir AM et al (2007). Small, potent, and selective diaryl phosphonate inhibitors for urokinase‐type plasminogen activator with in vivo antimetastatic properties. J Med Chem 50: 6638–6646. [DOI] [PubMed] [Google Scholar]
- Karanjia R, Spreadbury I, Bautista‐Cruz F, Tsang ME, Vanner S (2009). Activation of protease‐activated receptor‐4 inhibits the intrinsic excitability of colonic dorsal root ganglia neurons. Neurogastroenterol Motil 21: 1218–1221. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Itoh Y, Shinohata R, Sendo T, Oishi R, Nishibori M (2003). Nafamostat mesilate is an extremely potent inhibitor of human tryptase. J Pharmacol Sci 92: 420–423. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF (1988). Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res 450: 153–169. [DOI] [PubMed] [Google Scholar]
- Pulli B, Ali M, Forghani R, Schob S, Hsieh KL, Wojtkiewicz G et al (2013). Measuring myeloperoxidase activity in biological samples. PLoS One 8: e67976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roka R, Rosztoczy A, Leveque M, Izbeki F, Nagy F, Molnar T et al (2007). A pilot study of fecal serine‐protease activity: a pathophysiologic factor in diarrhea‐predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 5: 550–555. [DOI] [PubMed] [Google Scholar]
- Rolland‐Fourcade C, Denadai‐Souza A, Cirillo C, Lopez C, Jaramillo JO, Desormeaux C et al (2017). Epithelial expression and function of trypsin‐3 in irritable bowel syndrome. Gut 66: 1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simren M, Tornblom H, Palsson OS, Whitehead WE (2017). Management of the multiple symptoms of irritable bowel syndrome. Lancet Gastroenterol Hepatol 2: 112–122. [DOI] [PubMed] [Google Scholar]
- Sleigh JN, Weir GA, Schiavo G (2016). A simple, step‐by‐step dissection protocol for the rapid isolation of mouse dorsal root ganglia. BMC Res Notes 9: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooth D, Garsed K, Singh G, Marciani L, Lam C, Fordham I et al (2014). Characterisation of faecal protease activity in irritable bowel syndrome with diarrhoea: origin and effect of gut transit. Gut 63: 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ (2000). Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. Neuroreport 11: 2113–2116. [DOI] [PubMed] [Google Scholar]
- van Soom J, Cuzzucoli Crucitti G, Gladysz R, van der Veken P, Di Santo R, Stuyver I et al (2015). The first potent diphenyl phosphonate KLK4 inhibitors with unexpected binding kinetics. Med Chem Commun 6: 1954–1958. [Google Scholar]
- Vergnolle N (2016). Protease inhibition as new therapeutic strategy for GI diseases. Gut 65: 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF et al (2001). Proteinase‐activated receptor‐2 and hyperalgesia: a novel pain pathway. Nat Med 7: 821–826. [DOI] [PubMed] [Google Scholar]
- Vermeulen W, De Man JG, De Schepper HU, Bult H, Moreels TG, Pelckmans PA et al (2013). Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur J Pharmacol 698: 404–412. [DOI] [PubMed] [Google Scholar]
- Vermeulen W, De Man JG, Nullens S, Pelckmans PA, De Winter BY, Moreels TG (2011). The use of colonoscopy to follow the inflammatory time course of TNBS colitis in rats. Acta Gastroenterol Belg 74: 304–311. [PubMed] [Google Scholar]
- Wang P, Chen FX, Du C, Li CQ, Yu YB, Zuo XL et al (2015). Increased production of BDNF in colonic epithelial cells induced by fecal supernatants from diarrheic IBS patients. Sci Rep 5: 10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L (2008). Transient receptor potential ankyrin‐1 participates in visceral hyperalgesia following experimental colitis. Neurosci Lett 440: 237–241. [DOI] [PubMed] [Google Scholar]
- Yu YB, Yang J, Zuo XL, Gao LJ, Wang P, Li YQ (2010). Transient receptor potential vanilloid‐1 (TRPV1) and ankyrin‐1 (TRPA1) participate in visceral hyperalgesia in chronic water avoidance stress rat model. Neurochem Res 35: 797–803. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wang J, Dong L, Shi H, Wang Z, Ding H et al (2011). A protease inhibitor against acute stress‐induced visceral hypersensitivity and paracellular permeability in rats. Eur J Pharmacol 654: 289–294. [DOI] [PubMed] [Google Scholar]
- Zhao JH, Dong L, Shi HT, Wang ZY, Shi HY, Ding H (2012). The expression of protease‐activated receptor 2 and 4 in the colon of irritable bowel syndrome patients. Dig Dis Sci 57: 58–64. [DOI] [PubMed] [Google Scholar]


