Abstract
Background and Purpose
The sonic hedgehog pathway (Shh) plays a central role in maintaining stem cell function and behaviour in various processes related to self‐renewal and tissue regeneration. However, the therapeutic effect of Shh on mouse embryonic stem cells (mESCs) has not yet been clearly elucidated. Thus, we investigated the effect of Shh on the regulation of mESC behaviour as well as the effect of Shh‐pretreated mESCs in skin wound healing.
Experimental Approach
The underlying mechanisms of Shh signalling pathway in growth and motility of mESCs were investigated using Western blot analysis, a cell proliferation assay and cell migration assay. In addition, the effect of Shh‐pretreated mESCs in skin wound healing was determined using a mouse excisional wound splinting model.
Key Results
Shh disrupted the adherens junction through proteolysis by activating MMPs. In addition, the release of β‐catenin from adherens junctions mediated by Shh led to cell cycle‐dependent mESC proliferation. Shh‐mediated Gli1 expression led to integrin β1 up‐regulation, followed by FAK and Src phosphorylation. Furthermore, among the Rho‐GTPases, Rac1 and Cdc42 were activated in a Shh‐dependent manner while F‐actin expression was suppressed by Rac1 and Cdc42 siRNA transfection. Consistent with the in vitro results, the skin wound healing assay revealed that Shh‐treated mESCs increased angiogenesis and skin wound repair compared to that in Shh‐treated mESCs transfected with integrin β1 siRNA in vivo.
Conclusions and Implications
Our results imply that Shh induces adherens junction disruption and integrin β1‐dependent F‐actin formation by a mechanism involving FAK/Src and Rac1/Cdc42 signalling pathways in mESCs.
Abbreviations
- ECM
extracellular matrix
- F‐actin
filamentous actin
- FAK
focal adhesion kinase
- Hh
hedgehog
- mESCs
mouse embryonic stem cells
- Ptch1
patched
- Shh
Sonic hedgehog
- Smo
smoothened
- Src
steroid receptor coactivator
Introduction
The hedgehog (Hh) signalling pathway contributes to embryonic development, cell‐fate specification of stem cells, organogenesis and tissue repair/regeneration. Sonic hedgehog (Shh), which is one of the Hh family members, has been studied due to its potential as a target in therapeutic medicine (Rimkus et al., 2016). The Shh canonical pathway, by interacting with the patched (Ptch1) protein family, modulates Gli‐dependent transcription targets, such as Gli1 and Gli2, which suggests the possibility of autocrine Shh signalling in cells, affecting them in a paracrine manner (Delloye‐Bourgeois et al., 2013; Li et al., 2014). In contrast, Gli3 acts as a repressor to inhibit their target gene expression. In the absence of Shh, however, the Hh receptor Ptch1 inhibits the action of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=239. Shh activation leads to the migration and proliferation of various stem cells (Wu et al., 2010; Plaisant et al., 2011; Reinchisi et al., 2013; Yan et al., 2013). For these reasons, blockage of Shh signalling by therapeutic agents such as cyclopamine, which decreases cell proliferation, survival and self‐renewal, has been extended into clinical usage (Bar et al., 2007; Clement et al., 2007; Justilien and Fields, 2015).
Cadherins and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=760, two major adhesion molecule families, notably appear to be dependent on Shh signalling with regard to their involvement in cell adhesion and self‐renewal. The cadherin‐based adherens junction is involved in the cytoskeletal network, which interacts with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5371 and α‐catenin in order to form stable adherens junctions (Drees et al., 2005). Proteolytic disruption of E‐cadherin‐based adherens junctions induces β‐catenin‐induced transcriptional events that control the cellular response (Klinke 2nd et al., 2015). Integrins, transmembrane receptors composed of two subunits (α and β), are well‐organized proteins involved in wound healing that collaborate with cell surface receptors and molecules. Integrin‐mediated cell‐matrix interactions, which lead to cell adhesion, polarity and migration, are essential for re‐epithelialization and wound healing (Kenny and Connelly, 2015). Importantly, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2455 is required for angiogenesis, as mutant embryos die before vascular development (Carlson et al., 2008). Several studies have shown that the http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=897 http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5250/Cdc42 crosstalk with integrin‐dependent focal adhesions is involved in a number of cell functions in vitro, including motility, proliferation and spreading (Price et al., 1998; Wang et al., 2015; Huang et al., 2016). Therefore, further studies are required to determine the involvement of integrins in skin wound repairs in vivo, such as extracellular matrix (ECM) remodelling or autocrine/paracrine signalling.
Stem cells, by regulating the cellular micro‐environment, have an efficient endogenous repair mechanism (Lane et al., 2014). Moreover, stem cell‐based therapies have been widely reported to enhance tissue repair and regeneration through cellular engraftment and paracrine activity (Forbes and Rosenthal, 2014). Pluripotent stem cells, including ESCs, can be induced to repair tissues. Therefore, they have attracted attention as an ideal cell source for use in regenerative medicine (Thomson et al., 1998; Ko et al., 2014). Skin wound healing is a dynamic process that involves the orchestration of cellular and molecular mechanisms for increasing re‐epithelialization, granulation tissue formation and blood vessel density (Takeo et al., 2015). Various types of cells modulate growth and motility in the wound site during the healing process (Guo and DiPietro, 2010; Pastar et al., 2014). Although the undifferentiated transplantation of ESCs has limited clinical use due to its possible ability to cause cancer, many previous investigators demonstrated the therapeutic effects of ESCs in regenerative medicine (Hodgson et al., 2004; Laflamme et al., 2007; Lee et al., 2011). Considerable evidence has demonstrated that the topical application of undifferentiated ESCs can enhance the formation of granulation tissue, re‐epithelialization and angiogenesis during skin wound healing in rodent models (Lee et al., 2011; Kim et al., 2015). In addition, the differentiation of ESCs from keratinocytes has been used to reconstitute the epidermis in regenerative medicine (Guenou et al., 2009). In this regard, in vivo studies are needed to elucidate the interaction between stem cells and related signalling molecules in wound sites. Based on our investigation, we have proposed a complementary mechanism whereby Shh can promote tissue regeneration and regenerative medicine in mouse ESCs, which may be beneficial for wound healing. Furthermore, our study on the regulation of ESC's behaviour will provide new insight into understanding the physiology of pluripotent stem cells, such as ESCs and induced pluripotent stem cells, and clinical applications of pluripotent stem cell‐derived somatic cells in regenerative medicine.
Methods
Mouse ESC culture
Mouse ESCs were cultured with DMEM (Thermo Fisher Scientific) supplemented with 3.7 g·L−1 sodium bicarbonate, 1% penicillin and streptomycin, 1.7 mM L‐glutamine, 0.1 mM β–mercaptoethanol, 5 ng·mL−1 mouse LIF and 15% FBS. Cells were grown on gelatinized 60 mm diameter culture dishes in an incubator maintained at 37°C with 5% CO2. When cells were approximately 70–80% confluent, the medium was replaced with serum‐free DMEM (5% serum replacement instead of 15% FBS) for 24 h before the experiments.
Immunofluorescence staining
Cells were washed with cold PBS and fixed with 4% paraformaldehyde for 10 min. They were then incubated with 0.1% Triton X‐100 for permeabilization. The cells were washed three times with PBS and then incubated with 5% normal goat serum for 30 min to decrease nonspecific antibody binding. The cells were treated with 1:100 dilution of primary antibody against target proteins. Next, cells were incubated with either a 1:100 dilution of FITC‐conjugated anti‐rabbit and anti‐mouse IgG antibody or Alexa Fluor488/555‐conjugated secondary antibody (Thermo Fisher Scientific). Fluorescent images were visualized with a FluoView 300 fluorescence microscope (Olympus, Tokyo, Japan). BrdU was normalized to PI for the entire images including wound area, and the data were analysed by using MetaMorph software (Universal Imaging, West Chester, PA, USA).
Western blot analysis
Cells were washed twice with cold‐PBS followed by incubation on ice and lysing with lysis buffer [20 mM Tris (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1% Triton X‐100, 1 mg·mL−1 aprotinin, 1 mM PMSF and 0.5 mM sodium orthovanadate] and separated with one set of 3 s pulses on ice using a Branson Sonifier 250 with output power set to 3. The lysates were centrifuged at 21 055× g for 40 min at 4°C, and protein concentration was determined by using a BCA protein assay kit (Thermo Fisher Scientific). Sample protein separations were resolved by SDS‐PAGE and transferred to PVDF membranes. The membranes were incubated with primary antibody (1:800 or 1:1000 dilutions) overnight at 4°C in an incubator after being blocked with TBST [10 mM Tris–HCl (pH 7.6), 150 mM NaCl and 0.01% Tween‐20] containing 5% skimmed milk for 1 h. Next day, the membranes were washed with TBST and then incubated with HRP‐conjugated secondary antibodies. Specific bands were detected by ChemiDoc™ XRS+ System (Bio‐Rad, Hercules, CA, USA). The positive pixel area of specific bands was quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA), and the bands were normalized to β‐actin, pan‐cadherin or lamin A/C.
Trichloroacetic acid (TCA) precipitation
The 10% TCA was added to 1 mL of mESC culture media. The mixture was centrifuged at 18 341× g for 5 min, and the supernatant was removed. The pellet was washed with 200 μL cold acetone and then dried by using heat block at 95°C for 5 min. Sample buffer was added to the pellet, and the mixture was boiled in heat block for 10 min. Samples were loaded onto polyacrylamide gels for SDS‐PAGE.
Compliance with requirements for studies using animals
All animal care and procedures were performed following the National Institutes of Health Guidelines for the Humane Treatment of Animals of Seoul National University and were approved by the Institutional Animal Care and Use Committees at Seoul National University (SNU‐170206‐1). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). A total of 48 animals was used in the experiments. Eight‐week‐old male ICR mice weighing 22–25 g were purchased from Han Lim Experimental Animal (Suwon, Korea). The ICR mouse, which appears to mimic natural, generically heterogeneous populations, has been widely utilized in many fields such as basic research since it is a general‐purpose stock, easy to handle and is fairly docile. Animals were housed in individual cages under standard environmental conditions (24 ± 2°C, 12/12 h light/dark cycle with lights on at 07:00, ad libitum access to food and purified water). Mice were provided with beta chip bedding, which was substituted with new bedding twice a week. Mice were stabilized in a new environment for 4 days to enhance both animal welfare and experimental results. Mice were anaesthetized with 3% isoflurane in a mixture of N2O/O2 gas and placed on a heating table to maintain a constant body temperature (37°C). Anesthesia depth was monitored by reflexes. Mouse's mouth was gently open and the degree of muscle relaxation was assessed. Jaw was relaxed and easy to open. Withdrawal reflex of tow had also checked. Two 6 mm excisional wounds were created on the back of each mouse surgically by a 6 mm diameter sterile biopsy punch (Kai Medical, Seki, Japan). A silicone splinting ring was placed around the wound with several stitches, and then the wounds were dressed with sterile transparent dressing Tegaderm (3 M, St. Paul, MN, USA). After the surgical procedures, meloxicam was used to reduce the pain (1 mg·kg−1 of meloxicam was injected i.p.). The health status of animals was checked daily before the surgical procedures and three times a day after the surgical procedures. The allocation of mice into each experimental group was randomized, meaning that all the surgical procedures and injection of mESCs or drug were performed blindly. The skin tissues, once removed, were mounted on slides, and several sections were randomly selected from each animal. We were carefully concerned with the ‘3R principle’ (reduction, replacement and refinement) in the context of animal testing ethics.
Mouse excisional wound splinting model
Methods for the mouse skin excision wound splinting model and stem cell transplantation were performed as described previously (Wang et al., 2013). To examine the functional effects of mESCs pretreated with Shh, mice were randomly divided into four groups (n = 6 each group): vehicle, Shh, mESCs and Shh‐pretreated mESCs. To examine the role of integrin in the migration of mESCs towards the wound site, mice were divided into another four groups (n = 6 each group): integrin β1 siRNA + mESCs; integrin β1 siRNA + Shh‐pretreated mESCs; non‐targeting (NT) siRNA + mESCs; and NT siRNA + Shh‐pretreated mESCs. The mESCs were pretreated with bromo‐2′‐deoxyuridine (BrdU, 2 μM) for 24 h prior to injection. In the cell treatment groups, 1 × 106 mESCs in 100 μL saline were injected into the dermis at four sites around the wound. Wounds were photographed at different times (days 0, 3, 6 and 9) with a digital camera system (Canon, Tokyo, Japan) at the same distance from the wound, and % of wound size that healed was calculated. At day 9, all mice were killed by CO2, and wound tissue samples were harvested. The samples were fixed by 4% paraformaldehyde in PBS at room temperature (RT) for 4 h. The tissues were incubated in 30% sucrose in PBS at RT for 2 h and then incubated in 40% sucrose at 4°C overnight. The fixed tissues were embedded in O.C.T. compound (Sakura Finetek, Torrance, CA, USA) and were then frozen and stored immediately at −70°C. The tissues were cut to 6 μm thick sections using a cryostat (Leica Biosystems, Nussloch, Germany) and assembled on SuperFrost Plus slides (Thermo Fisher Scientific, Waltham) for haematoxylin and eosin (H&E) staining and immunohistochemical analysis.
Affinity precipitation of cellular GTP‐Rac1 and ‐Cdc42
Activation of Rac1 and Cdc42 was determined by using affinity precipitation assay kits (EMD Millipore, Billerica, MA, USA) according to the manufacturer's instructions. Briefly, cells were lysed with Mg2+ lysis/wash buffer. Next, the cell lysates were incubated for 1 h with agarose‐conjugated Rac1/Cdc42‐binding domain (GST‐PAK‐PBD). After the incubation, the agarose beads were boiled in sample buffer to release active Rac1 and Cdc42 and were analysed via Western blotting using anti‐Rac1 and anti‐Cdc42 antibodies respectively.
Co‐immunoprecipitation (co‐IP)
Cells were washed twice with cold‐PBS and lysed, with co‐IP buffer containing protease inhibitor, by a Sonicator (Branson Sonifier at a power output of 3 and duty cycle of 30%) and centrifuged at 14 000× g for 30 min at 4°C. Cell lysates (200–400 μg) were incubated with primary antibody against E‐cadherin for 4 h at 4°C on a gentle shaker. Next, the cell lysates were incubated with the protein A/G PLUS‐agarose IP reagent (Pierce, Rockford, IL, USA) overnight. Next day, the cell lysates were washed with co‐IP buffer, three times. For SDS‐PAGE analysis, sample buffer was added to the cell lysates, and then samples were boiled at 95°C for 5 min. Samples were analysed by Western blotting using anti‐E‐cadherin and anti‐β‐catenin antibodies respectively.
Subcellular fractionation
Prior to harvesting, cells were washed twice with cold‐PBS. Cells were resuspended in buffer A [137 mM NaCl, 8.1 mM Na2HPO4, 2.7 mM KCl, 1.5 mM KH2PO4, 2.5 mM EDTA, 1 mM DTT, 0.1 mM PMSF, and 10 μg·mL−1 leupeptin (pH 7.5)]. The lysates were centrifuged at 1000× g for 20 min at 4°C. The pellet, as a nuclear fraction, was resuspended in buffer A containing 1% (v.v−1) Triton X‐100.
Small interfering RNA (siRNA) transfection
Cells at approximately 70–80% confluence were transfected with a SMARTpool of siRNAs specific for Gli1, E‐cadherin, β‐catenin, integrin β1, integrin β4, integrin β6, Rac1, Cdc42, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2133 (20 nM; Dhamacon, Lafayette, CO, USA) or NT siRNA as a negative control (Thermo Fisher Scientific, Waltham) for 24 h, according to the manufacturer's instructions. The efficacies of the siRNAs were confirmed by Western blotting (Supporting Information Figure S9).
Cell cycle analysis
Cells were washed twice with cold‐PBS and treated with 0.05% trypsin/EDTA for cell dissociation. Cells were fixed with 70% cold ethanol for 40 min in an incubator at 4°C. For DNA staining, fixed cells were centrifuged 5 min at 211× g and incubated with 250 μg·mL−1 PI and 100 μg·mL−1 RNase for 30 min at 37°C. Samples were analysed by CXP software (Beckman Coulter, Fullerton, CA, USA), and cell cycle histograms represent the proliferation indices after analysis of the PI‐stained cells by FACS (Beckman Coulter).
Cell proliferation assay
The mESCs were seeded at equal densities (5 × 102 cells per well) in 96‐well plates. After the incubation period, 10 μL of EZ‐Cytox reagent (DAEIL Lab, Seoul, Korea) was added, and the cells were incubated in darkness for 2 h at 37°C with 5% CO2. Cell proliferation was determined by measuring absorbance at 450 nm using a multiplate reader (Bio‐Rad, Hercules, CA, USA).
Cell migration assays
Wound healing migration assay
Cells were seeded at 1 × 103 cells per well in 35 mm diameter plates (IBIDI, Martinsried, Germany). When cells had achieved 70–80% confluence, after serum‐free treatment for 24 h, the silicone inserts were carefully removed with sterile forceps to create a wound. Migrated cells were visualized by Olympus IX81‐ZDC zero‐drift microscope and a Cascade 512B camera (Roper Scientific, Tucson, AZ, USA).
Oris™ migration assay
Cells were seeded at 3 × 102 cells. 100 μL−1 in an Oris well (Platypus Technologies, Madison, WI, USA; cat# CMA 1.101). Inserts were carefully removed when cells had reached 70–80% confluence and treated with Shh or siRNAs. Migrated cells were stained with 5 μM calcein AM and quantified by measurement of fluorescence signals using a microplate reader (PerkinElmer, Waltham, MA, USA) at excitation and emission wavelengths of 485 and 515 nm respectively.
Live cell migration assay
Cells were seeded at 5 × 102 cells on gelatinized 35 mm confocal dishes and incubated in an incubator maintained at 37°C with 5% CO2. Cells were placed in temperature/CO2 control chambers (Tokai, Tokyo, Japan) installed with an Olympus IX81‐ZDC zero‐drift microscope. A series of images were acquired for 0–24 h at 5 min intervals by using a Cascade 512B camera (Roper Scientific, Tucson, AZ, USA) operated by the multidimensional acquisition package of MetaMorph v. 7.01 software (Molecular Devices, Sunnyvale, CA, USA).
Reverse transcription and quantitative real‐time PCR
Total RNA was extracted with an RNeasy Extraction Kit (Takara Bio, Shiga, Japan), and a reverse transcription was performed with 1 μg of RNA using a Maxime RT premix kit (iNtRON Biotechnology, Sungnam, Korea). Next, cDNA was amplified by specific primers using a PCR kit (iNtRON Biotechnology, Sungnam, Korea). Quantification of mRNA was then performed in a Rotor‐Gene 6500 real‐time thermal cycling system (Corbett Research, Sydney, Australia) using the Quantinova SYBR Green PCR Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer's instructions. Sequences of primers used for qPCR are described in Supporting Information Table S1.
In situ proximity ligation assay (PLA)
Duolink™ in situ PLA was performed according to the manufacturer's instructions (Olink Bioscience, Uppsala, Sweden). After being blocked with blocking solution for 30 min at 37°C, primary antibodies against rabbit anti‐E‐cadherin and mouse anti‐β‐catenin were diluted in TBST containing 1% BSA and then incubated overnight at 4°C as standard conditions. The Duolink secondary antibodies with PLA probe against the particular primary antibodies were applied for 1 h at 37°C. The DNA oligonucleotides and the ligation enzyme were added and incubated for 1 h at 37°C. For amplification, polymerase in amplification buffer were added and then incubated for 100 min at 37°C. Fluorescent images (fluorescently labelled oligonucleotides; green dot, PI staining; red dot) were visualized with a FluoView 300 fluorescence microscope (Olympus, Tokyo, Japan).
Data analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). All data are represented as mean ± SEM. Statistical analysis was performed with GraphPad Prism Version 5.0 (GraphPad Inc., San Diego, CA, USA). Statistical analysis was performed using the ANOVA, and Bonferroni–Dunn test allows for multiple comparisons in some cases. A P value of <0.05 was accepted as statistical significance.
Materials
Mouse embryonic stem cells (mESCs; ES E14TG2a) were obtained from the American Type Culture Collection (Manassas, VA, USA). FBS and PBS were purchased from GE Healthcare Life Sciences (South Logan, Utah, USA). Sonic hedgehog (Shh), cyclopamine, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5535, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9404, fluorescein isothiocyanate (FITC)‐anti‐rabbit antibody and 5‐bromo‐2′‐deoxyuridine (BrdU) were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Propidium iodide (PI) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Leukaemia inhibitory factor (LIF; cat#, sc‐4378), Gant 61, MMP inhibitor, primary antibodies against Gli1 (cat#, sc‐20687), Gli2 (cat#, sc‐28674), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1629 (cat#, sc‐13594), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1633 (cat#, sc‐21733), β‐catenin (cat#, sc‐7963), E‐cadherin (cat#, sc‐7870), integrin β1 (cat#, scβ8978), p‐FAK (S722) (cat#, sc‐16662‐R), p‐FAK (Tyr 397) (cat#, sc‐11765‐R), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2180 (cat#, sc‐932), Paxillin (cat#, sc‐5574), Rac1 (cat#, sc‐217), Cdc42 (cat#, sc‐8401), β‐actin (cat#, sc‐47778), pan‐cadherin (cat#, sc‐59876), Lamin A/C (cat#, sc‐20681) and secondary antibodies against HRP‐conjugated goat anti‐rabbit IgG, goat anti‐mouse IgG and rabbit anti‐goat IgG were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Primary antibodies against filamentous actin (F‐actin) (cat#, ab205) was purchased from Abcam (Cambridge, MA, USA) and phospho (p)‐β‐catenin (T41/S45) (cat#, 9565S) was purchased from Cell Signaling Technology (Danvers, MA, USA). All other reagents were of the highest commercial purity.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data form the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c).
Results
Regulation of Shh activation affects MMP on adherens junction disruption
When extracellular Hh is present, the Hh signalling is triggered by the Hh binding to the transmembrane receptor Ptch1, which normally inhibits Smo, and activates the transcriptional activity of Gli factors. Therefore, we investigated which Gli isoform is involved in mESCs. The mRNA expression levels of Gli1 and Gli2, which are the activator transcription factors of Hh signalling, were increased. Meanwhile, the mRNA expression level of Gli3, which is a transcriptional repressor, was not significantly changed (Figure 1A). Gli1 and Gli2 were increased in a time‐dependent manner until 12 h of Shh treatment, while the Gli2 level was lower at 24 h when compared to the level at 12 h (Figure 1B). Shh‐dependent mESCs reacted positively in immunofluorescence staining with antibodies against the Gli1. Additionally, Shh‐induced Gli1 activation was blocked by the Shh inhibitor cyclopamine (Figure 1C). To determine the role of Shh‐induced Gli in the maintenance of undifferentiation status in mESCs, we investigated the lineage‐specific differentiation marker genes and the pluripotent marker genes by using real‐time PCR. Shh treatment or Gant 61‐pretreated Shh did not affect mRNA expression levels of the self‐renewal and differentiation marker genes (Supporting Information Figure S1), which means that Shh‐induced Gli1 does not affect differentiation.
Figure 1.
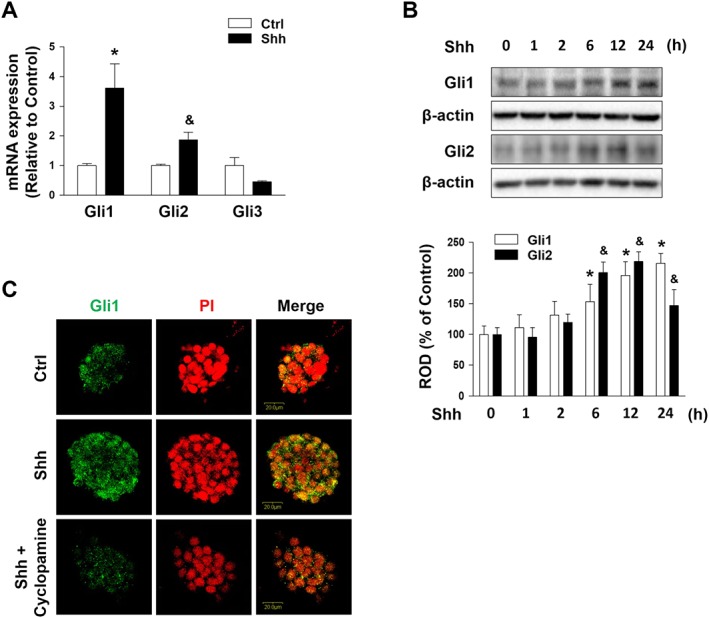
Effect of Shh on Gli family. (A) mESCs were treated with Shh for 24 h, and then Gli1, Gli2, Gli3 and β‐actin mRNA were amplified by PCR and quantified by real‐time PCR. Each mRNA expression level was normalized to the β‐actin mRNA expression. The data are expressed as mean ± SEM for experiments performed five times. *P < 0.05 versus control of Gli1; & P < 0.05 versus control of Gli2. (B) mESCs were treated with Shh (500 ng·mL−1) for various time (0–24 h), and the expressions of Gli1 and Gli2 were detected by Western blotting. β‐Actin was used as the loading control. The data are expressed as mean ± SEM for experiments performed five times. *P < 0.05 versus 0 h of Gli1; & P < 0.05 versus 0 h of Gli2. (C) mESCs were pretreated with cyclopamine (Smo inhibitor; 10−5 M) for 30 min prior to Shh treatment for 24 h. The expression of Gli1 was detected by immunofluorescence staining and visualized via confocal microscopy. Representative images from five independent experiments are shown. Scale bar = 20 μm. Ctrl, control; ROD, relative optical density.
MMPs are essential enzymes degrading proteins of the basement membrane that are active in various cell behaviours such as cell proliferation and migration. To investigate the adherens junction disruption of mESCs pretreated with Shh, we distinguished the specific isotype of MMP. As shown in Figure 2A, there were increases in mRNA expression levels of MMP2 and MMP9 among the various kinds of MMP mRNA amplicons. Gli1 inhibition by Gli1 siRNA inhibited the Shh‐induced increase in MMP2/9 mRNA expression. Shh stimulated the levels of secreted and total MMP2/9 in a time‐dependent manner (Figure 2B). Blockade of the Shh signalling by cyclopamine prevented the increases in secreted and total MMP2/9. Furthermore, Shh‐pretreated Gli1 siRNA transfection blocked the increase in secreted and total MMP2/9 (Figure 2C). However, Gli2 silencing did not significantly change the expression levels of MMP2/9 compared to NT siRNA‐transfected mESCs with Shh (Supporting Information Figure S2). Therefore, our data showed that Shh‐induced increases in MMP2/9 are mainly regulated by Gli1 activation, and not Gli2. To examine the relationship between MMP2/9 and the effects of Shh on the adherens junction in mESCs, co‐IP was performed. The extract from mESCs co‐IP by rabbit anti‐E‐cadherin antibody was subjected to immunoblotting with mouse anti‐β‐catenin antibody. The results showed that the interaction between E‐cadherin and β‐catenin was detached by Shh (Figure 2D). The mESCs were fixed and subjected to Duolink® fluorescence PLA by using rabbit anti‐E‐cadherin and mouse anti‐β‐catenin antibodies. The interaction between E‐cadherin and β‐catenin is shown as green dots. When the Gli1 inhibitor Gant 61 or MMP inhibitor was combined with Shh treatment the decrease in the E‐cadherin/β‐catenin interaction was reversed (Figure 2E). Canonical http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3672 signalling results in β‐catenin stabilization and nuclear translocation for the activation of target genes. In addition, the Wnt/β‐catenin pathway is involved in EMT. Therefore, we investigated the effect of Shh‐induced Gli1/2 on Wnt signalling and Snail, a representative EMT inducer. Our results showed that Shh treatment induced Wnt expression and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2030 phosphorylation. We also confirmed that Gli1 siRNA inhibited the increased expression of Wnt and phosphorylation of GSK‐3β (Supporting Information Figure S4A). Our data also showed that Shh treatment induced Snail expression was reversed by Gant 61 pretreatment (Supporting Information Figure S3A). Subsequently, we analysed the effect of Snail silencing on the expressions of MMP2, MMP9, E‐cadherin, β‐catenin and integrin β1. We found that Shh‐induced down‐regulation of E‐cadherin was blocked by Snail silencing, but Shh‐induced expressions of MMPs, β‐catenin and integrin β1 were not reversed by Snail silencing (Supporting Information Figure S3B). These findings indicate that Shh‐induced Gli1 stimulates Wnt signals and Shh‐induced Snail expression stimulates β‐catenin‐mediated suppression of E‐cadherin in mESCs.
Figure 2.
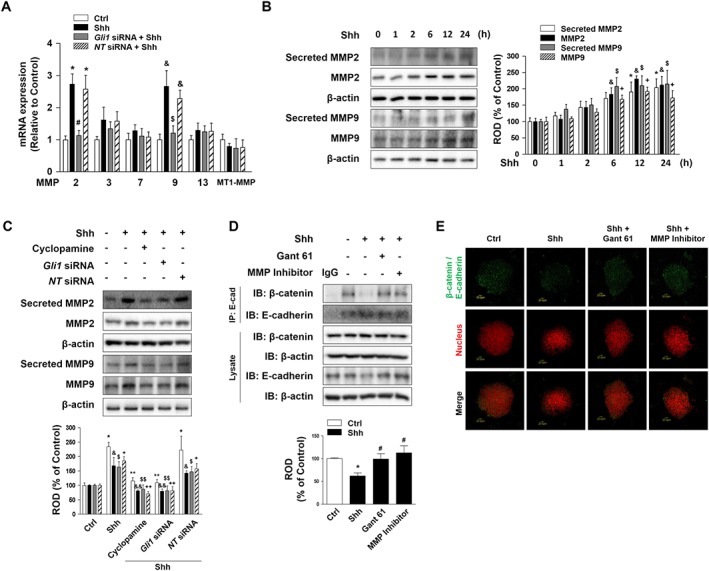
Effect of different treatments on the action of Shh on the adherens junction. (A) mESCs were transfected with Gli1 siRNA or NT siRNA (20 nM) for 24 h prior to Shh treatment for 24 h. The levels of MMP isotypes and MT1‐MMP mRNA expressions were measured by real‐time PCR. The data are expressed as mean ± SEM for five experiments. *P < 0.05 versus control of MMP2; # P < 0.05 versus Shh + NT siRNA of MMP2; & P < 0.05 versus control of MMP9; $ P < 0.05 versus Shh + NT siRNA of MMP9. (B) mESCs were treated with Shh for various time (0–24 h), and the secretion of MMP2 and MMP9 into the medium and the expression of MMP2 and MMP9 were detected by Western blotting. β‐Actin was used as the loading control to normalize the levels of total MMP2/9. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus 0 h of secreted MMP2; & P < 0.05 versus 0 h of MMP2; $ P < 0.05 versus 0 h of secreted MMP9; + P < 0.05 versus 0 h of MMP9. (C) mESCs were pretreated with cyclopamine for 30 min and transfected with Gli1 siRNA or NT siRNA for 24 h prior to Shh treatment for 24 h. The secretion of MMP2 and MMP9 into the medium and the expression of MMP2 and MMP9 were detected by Western blotting. β‐Actin was used as the loading control to normalize the levels of total MMP2/9. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control of secreted MMP2; **P < 0.05 versus Shh of secreted MMP2; & P < 0.05 versus control of MMP2; && P < 0.05 versus Shh of MMP2; $ P < 0.05 versus control of secreted MMP9; $$ P < 0.05 versus Shh of secreted MMP9; + P < 0.05 versus control of MMP9; ++ P < 0.05 versus Shh of MMP9. (D) mESCs were pretreated with Gant 61 (Gli inhibitor; 10 μM) or MMP inhibitor (10−7 M) prior to Shh treatment for 24 h. Complexes were immunoprecipitated from cell lysate with an anti‐E‐cadherin antibody and blotted with antibodies against E‐cadherin and β‐catenin. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh. (E) mESCs were pretreated with Gant 61 or MMP inhibitor prior to Shh treatment for 24 h. In situ PLA assay result demonstrates the interaction between β‐catenin and E‐cadherin in mESCs (Green dot, β‐catenin/E‐cadherin; Red, nuclear). Scale bar = 20 μm. Ctrl, control; NT, non‐targeting; ROD, relative optical density.
Shh‐induced release of β‐catenin from adherens junction regulates cell cycle
Degradation of E‐cadherin is regulated through lysosomal proteolysis. The inhibition of protein degradation by chloroquine with Shh treatment prevented the Shh‐induced decrease in E‐cadherin expression (Figure 3A). In addition, we observed that E‐cadherin distribution was affected when mESCs were treated with Shh, and this effect was reversed by cotreatment with chloroquine, as seen through immunofluorescence staining (Figure 3B). Cells were transfected with E‐cadherin siRNA for 24 h, and Western blotting was performed to detect the β‐catenin activation. Increased levels of p‐β‐catenin (T41, S45) and active‐β‐catenin were observed after E‐cadherin siRNA transfection (Figure 3C). To assess the migration of β‐catenin to the nucleus induced by Shh, we undertook nuclear fractionation after Shh treatment for 24 h. β‐Catenin was in high abundance in the nucleus after Shh treatment when compared to control cells. In addition, pretreatment with an MMP inhibitor blocked β‐catenin nuclear translocation (Figure 3D). The mESCs were incubated with or without Shh for 24 h and analysed by confocal microscopy for nuclear translocation by using specific rabbit E‐cadherin or mouse β‐catenin antibodies. A high portion of Shh‐treated cells displayed cytosolic localization and nuclear translocation of β‐catenin (Figure 3E). We further analysed the effect of Gant 61 or an MMP inhibitor on Shh‐phosphorylated β‐catenin at T41/S45. We showed that Shh‐induced β‐catenin phosphorylation is inhibited by the pretreatment with Gant 61 or an MMP inhibitor (Supporting Information Figures S5A and S6A). Consequently, pretreatment with the MMP inhibitor prevented the Shh‐induced reduction in E‐cadherin expression (Supporting Information Figure S5B). These findings indicate that Gli1‐induced MMP2 and MMP9 stimulate proteolysis of E‐cadherin, which leads to the nuclear translocation of β‐catenin. To determine the influence of the translocated β‐catenin in the nucleus, we examined whether Shh affected proliferation of the treated cells. Shh treatment increased the proliferation of mESCs in a time‐dependent manner, as shown in Figure 3F. In addition, flow cytometric analysis of PI‐stained cells and a cell proliferation assay were used to measure proliferation. Shh‐treated mESCs showed increased proliferation compared to control cells, whereas proliferation was blocked by β‐catenin siRNA in Shh‐treated cells (Figure 3G, H), showing that β‐catenin regulates the cell cycle.
Figure 3.
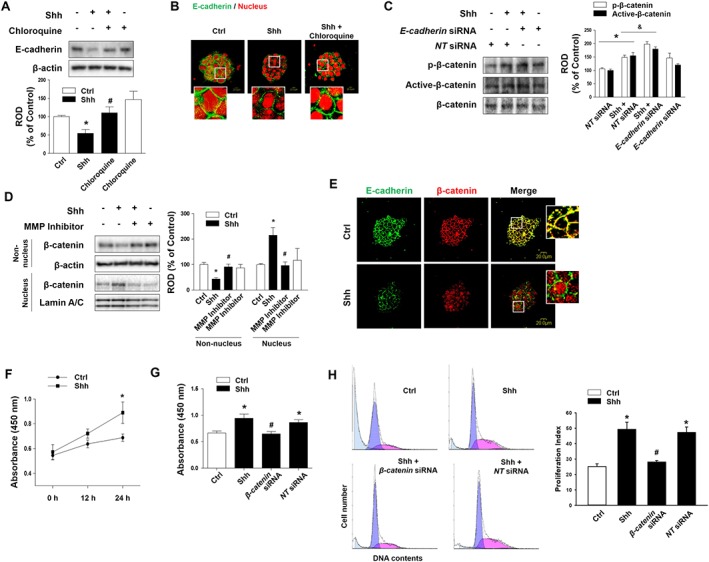
Effect of Shh on mESC proliferation. (A) mESCs were pretreated with chloroquine (lysosomal protease inhibitor; 10 μM) for 30 min prior to Shh treatment for 24 h. E‐cadherin protein was detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh. (B) mESCs were pretreated with chloroquine for 30 min prior to Shh treatment for 24 h. The expression of E‐cadherin was detected by immunofluorescence staining and visualized by confocal microscopy. Representative images from five independent experiments are shown. Scale bar = 20 μm. (C) mESCs were transfected with E‐cadherin siRNA (20 nM) or NT siRNA for 24 h prior to Shh treatment, and p‐β‐catenin and active‐β‐catenin were detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. * P < 0.05 versus NT siRNA; & P < 0.05 versus Shh + NT siRNA. (D) mESCs were pretreated with MMP inhibitor prior to Shh treatment, and the expressions of β‐catenin in the nucleus and non‐nucleus fraction were detected by Western blotting. β‐Actin was used as the cytosol marker, and Lamin A/C was used as the nuclear marker. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh. (E) mESCs were treated with Shh for 24 h. The expressions of E‐cadherin and counter‐labelled with β‐catenin were detected by immunofluorescence staining and visualized by confocal microscopy. Representative images from five independent experiments are shown. Scale bar = 20 μm. (F) mESCs were treated with Shh for 0–24 h and then incubated with 10 μL EZ‐Cytox reagent for 2 h. Cell proliferation was determined by measuring absorbance at 450 nm by using a multiplate reader. The data are reported as mean ± SEM for six independent experiments. *P < 0.05 versus control. (G) mESCs were transfected with β‐catenin siRNA or NT siRNA prior to Shh treatment for 24 h and then incubated with 10 μL EZ‐Cytox reagent for 2 h. Cell proliferation was determined by measuring absorbance at 450 nm using a multiplate reader. The data are reported as mean ± SEM for six independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh + NT siRNA. (H) mESCs were transfected with β‐catenin siRNA or NT siRNA prior to Shh treatment for 24 h. The cells were washed with PBS and subjected to PI staining for cell cycle analysis using flow cytometry. Numbers of cells in each section were configured manually to identify the percentage of cells in S phase. Proliferation index = (S + G2/M)/(G0/G1 + S + G2/M). The data are reported as the mean ± SEM for five independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh + NT siRNA. Ctrl, control; E‐cad, E‐cadherin; NT, non‐targeting; ROD, relative optical density.
Shh‐induced integrin β1 assists mESC migration through the cytoskeleton
To determine whether Gli1 affects integrin expression, we screened the expression pattern of the integrin subunits (β1, β3, β4, β5, β6 and β8) in mESCs when Gli1 siRNA was transfected. Shh increased the mRNA expression levels of integrins β1, β4 and β6 but failed to regulate other integrin subunits, including integrins β3, β5 and β8 mRNA (Figure 4A). Moreover, to confirm the role of integrin β1 on mESC migration, mESCs were transfected with integrin β1, integrin β4, integrin β6 or NT siRNA, and the migration of Shh‐treated cells was quantified by an Oris migration assay. The results showed that transfection with integrin β1 siRNA decreased the Shh‐induced cell migration but integrin β4 and β6 siRNA treatment had no effect on the migration of cells induced by Shh (Figure 4B). The Shh‐induced increase in integrin β1 expression was inhibited by pretreatment with cyclopamine and Gant 61, a Smo inhibitor and a Gli inhibitor respectively (Figure 4C). These results suggest that integrin β1 is the most important integrin isotype in Shh‐induced mESC migration, and downstream signalling pathways of Shh might be involved in regulating integrin β1 expression. Furthermore, we investigated the relationships between integrin β1 and β‐catenin in the Shh‐treated cells. Our data showed that Shh‐induced β‐catenin nuclear translocation was not blocked by integrin β1 siRNA transfection, and Shh‐induced integrin β1 expression was not inhibited by β‐catenin siRNA transfection (Supporting Information Figure S7). Thus, these findings suggest independent relationships between Shh‐induced integrin β1 expression and β‐catenin nuclear translocation.
Figure 4.
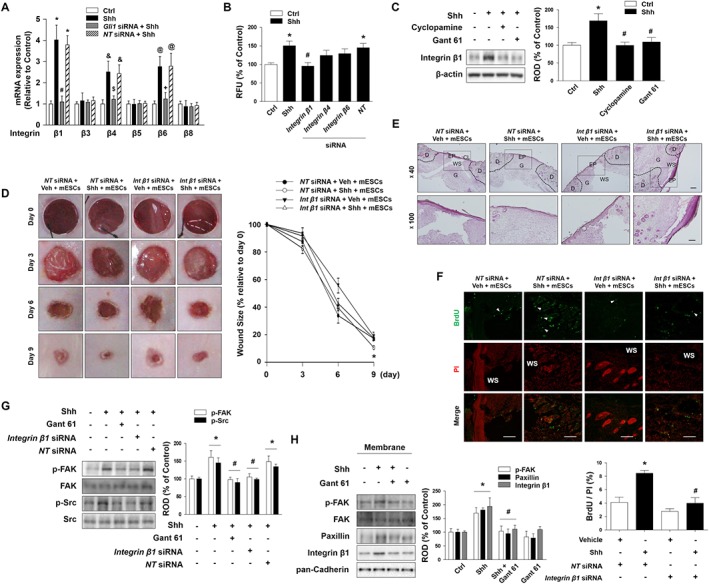
The role of integrin β1 on mESC migration. (A) mESCs were transfected with Gli1 siRNA (20 nM) or NT siRNA for 24 h prior to Shh treatment for 24 h. The levels of integrins β1, β3, β4, β5, β6 and β8 mRNA expression were measured by real‐time PCR. The data are expressed as mean ± SEM for five experiments. *P < 0.05 versus control of integrin β1; # P < 0.05 versus Shh + NT siRNA of integrin β1; & P < 0.05 versus control of integrin β4; $ P < 0.05 versus Shh + NT siRNA of integrin β4; @ P < 0.05 versus control of integrin β6; + P < 0.05 versus Shh + NT siRNA of integrin β6. (B) mESCs were transfected with integrins β1, β4, β6 or NT siRNA for 24 h prior to Shh treatment for 24 h. Migration of Shh‐treated cells was quantified using the Oris™ migration assay kit. The data are reported as the mean ± SEM for five independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh + NT siRNA. (C) mESCs were pretreated with cyclopamine or Gant 61 prior to Shh treatment for 24 h, and integrin β1 was detected by Western blotting. The data are expressed as mean ± SEM for six independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh. (D) Mice were randomly divided into four groups (n = 6 each group): NT siRNA + Vehicle + mESCs, NT siRNA + Shh + mESCs, integrin β1 siRNA + Vehicle + mESCs, or integrin β1 siRNA + Shh + mESCs. Wounds (6 mm diameter) were created by using a biopsy punch. Representative gross images of wounds at days 0, 3, 6 and 9 are shown. (left panel) Wound sizes compared to original wound size at day 0 were quantified. (right panel) The values are reported as the mean ± SEM. n = 6. *P < 0.05 versus NT siRNA + Vehicle + mESC group. (E) Wound tissues were stained with H&E at day 9 after wounding. Scale bars = 200 μm or 100 μm, magnification; ×40 or ×100. (F) mESCs were pretreated with BrdU (2 μM) for 24 h prior to injection. Wound tissues were immunostained with BrdU (green) and PI (red) and then visualized by confocal microscopy (upper panel). Scale bar = 100 μm. The percentage of BrdU‐positive cells in total cells was analysed by using Metamorph software (lower panel). The data are expressed as mean ± SEM for six independent experiments. *P < 0.05 versus Veh + NT siRNA; # P < 0.05 versus Shh + NT siRNA. (G) mESCs were pretreated with Gant 61, integrin β1 siRNA or NT siRNA prior to Shh treatment for 1 h, and p‐FAK and p‐Src were detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh + NT siRNA. (H) mESCs were pretreated with Gant 61 prior to Shh treatment and the expressions of p‐FAK, paxillin and integrin β1 in the membrane fraction were detected by western blotting. Pan‐cadherin was used as the membrane marker. The data are expressed as mean ± SEM for six independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh. CL, cornified layer; Ctrl, control; D, dermis; EP, epidermis; G, granulation tissue; NT, non‐targeting; RFU, relative fluorescence units; ROD, relative optical density; WS, wound site.
To confirm the effect of integrin β1 on Shh‐induced mESC migration in vivo, we transfected integrin β1 siRNA into mESCs before injecting it into the wounds. The wound sizes of mice injected with Shh‐pretreated mESCs were decreased significantly 9 days after wounding, whereas integrin β1 siRNA transfection abolished the effect observed in mESCs pretreated with Shh. This means that integrin β1 expression is important in enhancing the skin wound healing effect of Shh‐stimulated mESCs (Figure 4D). Histological analysis using H&E staining showed wound healing was almost complete in the Shh‐treated mESC group at day 9. In addition, the wound sites were covered with granulation tissues in the Shh‐treated mESC group (Figure 4E). To verify mESC migration towards the wound sites during wound healing, mESCs were pretreated with BrdU (2 μM) for 24 h before injection. Our results showed that transplantation of mESCs with Shh pretreatment increased BrdU‐positive cells at the wounds compared to NT siRNA‐transfected mESC transplantation group (Figure 4F). Integrin/FAK/http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2206 signalling can regulate cellular processes such as cell adhesion, spreading and migration. Shh increased the phosphorylations of FAK and Src in a time‐dependent manner (data not shown), but such phosphorylations were blocked by Gant 61 or integrin β1 siRNA (Figure 4G). Furthermore, we analysed the role of Gli1 in Shh‐induced focal adhesion formation. As shown in Figure 4H, Shh‐induced p‐FAK, FAK, paxillin and integrin β1 expressions in the membrane fraction are reversed by Gant 61 pretreatment. These data suggest that Shh‐induced Gli1 stimulates focal adhesion formation.
Shh induces F‐actin formation through Rho GTPase
Next, we examined whether Rho GTPase activation is responsible for Shh‐induced mESC migration. To verify the effect of Shh on the GTP‐binding form of Rac1 and Cdc42, mESCs were treated with cyclopamine or the Src‐family kinase inhibitor PP2. The GTP‐binding forms of Rac1 and Cdc42 were significantly increased in mESCs during Shh treatment, which suggests that GTP‐binding forms were involved in increasing mESC motility. The Shh‐induced increase in Rac1‐ and Cdc42‐GTP were reversed by treating Shh with cyclopamine or PP2 to prevent Rac1/Cdc42‐GTP binding (Figure 5A). Furthermore, to verify the role of Rho GTPase in mESC migration, the cells were transfected with Rac1, Cdc42 or PAK1 siRNAs. The IBIDI migration assay showed that Shh‐induced mESC migration was prevented by the knockdown of Rac1, Cdc42 or PAK1 (Figure 5B). Additionally, their migration levels were quantified by the Oris cell migration assay (Figure 5C). In detail, the cells were transfected with Rac1, Cdc42 or PAK1 siRNAs before Shh treatment for 24 h. Live cell imaging was used to visualize cell migration, and the Shh‐treated mESCs showed a significant increase in total migration distance (Figure 5D). We confirmed the localization of F‐actin in the mESCs through immunofluorescence staining (Figure 5E).
Figure 5.
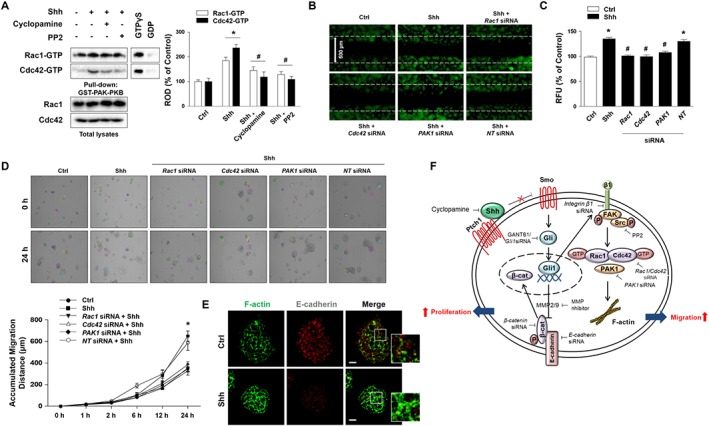
Effect of Shh on mESC migration through F‐actin formation. (A) mESCs were pretreated with cyclopamine or PP2 (Src inhibitor; 10 μM) prior to Shh treatment and analysed with affinity precipitation. GTPγS was used as the positive control, and GDP was used as the negative control. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh. (B) mESCs were cultured in IBIDI dishes and were transfected with Rac1, Cdc42, PAK1 or NT siRNA for 24 h prior to Shh for 24 h. Wound width = 500 μm. (C) mESCs were transfected with Rac1, Cdc42, PAK1 or NT siRNA for 24 h prior to Shh treatment. Migration of Shh‐treated cells was quantified by using the Oris™ migration assay kit. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; # P < 0.05 versus Shh + NT siRNA. (D) mESCs were transfected with Rac1, Cdc42, PAK1 or NT siRNA for 24 h prior to Shh for 0–24 h and microscopic images of live cells are shown. A series of images were acquired for 0–24 h at 5 min intervals. Coloured line and number in images represent the tracked cells for measurement of migration distance. (upper panel) The accumulated migration distance was analysed by Meta Morph software. (lower panel) Error bars indicate a mean ± SEM for five independent experiments. *P < 0.05 versus control. (E) mESCs were treated with Shh for 24 h. The expressions of F‐actin (green) and E‐cadherin (red) were detected by immunofluorescence staining and visualized by confocal microscopy. Representative images from five independent experiments are shown. Scale bar = 20 μm. (F) The hypothesized model for the signal pathway involved in the effect of Shh on mESC proliferation and migration through adherens junction disruption and integrin β1‐dependent F‐actin formation. Ctrl, control; NT, non‐targeting; Ptch1, patched; ROD, relative optical density; Shh, sonic hedgehog; Smo, smoothened.
Discussion
In this study, we reported that Shh‐induced MMP2/9 activation decreased E‐cadherin expression through adherens junction disruption, and Shh‐induced Gli1 expression increased F‐actin formation through integrin β1‐dependent activation of Rho GTPase. This stimulated the proliferation and migration of mESCs. In our previous report, we confirmed that mESCs pretreated with Shh enhanced re‐epithelialization and were highly efficient in wound repair compared to mESCs pretreated with vehicle in a mouse wound healing model (Suh and Han, 2015). This motivated us to investigate the exact mechanism involved and how Shh acts as a critical regulator of cell‐fate determination during wound healing. Through mRNA expression and Oris migration assay analyses, we observed that of the integrin family, integrin β1 expression was dominant within mouse skin tissues. Many studies have demonstrated that integrin β1 is required for the angiogenesis from pre‐existing vessels in wound repair as well as for granulation tissue formation (Tabatabai et al., 2011; Scully et al., 2016). Therefore, we examined the effects of Shh on mESC growth and migration to elucidate the effect of Shh on wound closure mediated by an up‐regulation of integrin β1.
The cadherin/catenin complexes of adherens junctions are involved with actin filaments for tissue development and homeostasis. The disruption of this cadherin/catenin complex is responsible for cell proliferation, cell–cell adhesion and migration (Maretzky et al., 2005; Klucky et al., 2007). MMPs are capable of degrading all kinds of ECMs as well as being involved in adherens junction disruption (Klucky et al., 2007), while http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5310) modulate E‐cadherin/β‐catenin interactions in cancer cells (Bourboulia et al., 2013). Several studies have demonstrated that deleting Shh triggers a decrease in E‐cadherin expression and localization of β‐catenin to the nucleus in vivo and in vitro (Xiao et al., 2010). In this study, the Shh‐induced increase in MMP2/9 was able to dissociate β‐catenin from E‐cadherin via its proteolytic activities. In this regard, the inhibition of Gli or MMP resulted in an increase in E‐cadherin/β‐catenin binding. In addition, Shh increased β‐catenin activation, whereas knocking down the β‐catenin decreased the Shh‐induced cell proliferation. Since previous reports demonstrated that Snail, which binds to the E‐box region of CDH‐1 gene promoter, is required for β‐catenin activation (Stemmer et al., 2008; Ryu and Han, 2015), our findings suggest that the reduction in E‐cadherin induced by Shh via Gli1 is mediated through transcriptional repression of β‐catenin activated by Snail expression. Although a previous report revealed that Gli2 is required for ectopic activation of Shh signalling (Bai et al., 2002), another report showed that Gli1 is positively correlated with MMP9 expression, while negatively correlated with E‐cadherin expression (Fan et al., 2014). Therefore, in the present study we showed that Shh‐induced increase in MMP and integrins β1, β4 and β6 are mainly regulated by Gli1 activation, but not Gli2. The β‐catenin phosphorylation at serine 33, 37 or threonine 41 (S33/37/T41) cannot interact with cadherin and can possibly lead to proteasomal degradation. Thus, β‐catenin phosphorylation at serine 45 or threonine 41 (T41/S45) can associate with cadherin (Maher et al., 2010), suggesting that phosphorylated β‐catenin at T41/S45 may be necessary for cell–cell adhesion while phosphorylated β‐catenin at S33/37/T41 leads to proteasomal degradation. β‐Catenin phosphorylation at T41/S45 has been reported to be associated with cell cycle regulation (Olmeda et al., 2003). Moreover, nuclear β‐catenin is predominantly phosphorylated at T41/S45 (Maher et al., 2010). Therefore, our results indicate that Shh‐dependent adherens junction disruption releases β‐catenin into the cytosol. The accumulated β‐catenin migrates to the nucleus and provides a possible mechanism for mESC proliferation through cell cycle activation.
Integrins regulate the dynamic interactions between the ECM and the actin cytoskeleton. The integrin‐activated Src‐focal adhesion kinase (FAK) autophosphorylated at tyrosine 397 mediates signal transduction in cell migration (Huttenlocher and Horwitz, 2011). Furthermore, FAK activation is dependent on integrin‐mediated cell adhesion. In particular, integrin β1 expression is essential for tissue repair in vivo (Brakebusch and Fassler, 2005; Liu et al., 2010). A recent study on the importance of Hh signalling in regulating integrin‐mediated FAK activation showed that down‐regulation of the Gli family is related to inhibited migration and invasion of ovarian cancer cells (Chen et al., 2014). Thus, we attempted to identify how Shh regulates mESC migration by investigating the effects of integrin β1 expression on cytoskeleton rearrangement. Although stem cells have emerged as a means to enhance cell homing and engraftment, their ability to migrate to wound sites for regeneration and tissue repair has not been fully elucidated.
Shh signalling has been associated with the cell proliferation and activation involved in tissue regeneration and ECM deposition (Mobassarah et al., 2014). The activation of the Shh signalling pathway is reported to contribute to several parameters in wound healing, such as granulation tissue formation and angiogenesis, in various tissues (Le et al., 2008). Previous studies have already shown that Shh signalling induces cancer cell migration and invasion (hence metastasis) via epithelial‐mesenchymal transition (EMT) and MMP2/MMP9 expression in the tumour (Yoo et al., 2011; Chen et al., 2013). However, in therapeutic applications of stem cells, E‐cadherin complex disruption could be a major target for increasing mESC migration and stem cell therapeutic effects (Suh and Han, 2010; Suh and Han, 2015). For example, Shh leads to cell specification during skin development through the interaction between epigenetic and morphogen cues (Perdigoto et al., 2016). In addition, Shh accelerated the re‐epithelialization and dermal healing in diabetic mice via enhanced fibroblast activity (Asai et al., 2006). A recent study showed that Shh is required to restore cell functions as well as promote neovascularization in diabetic mice (Qin et al., 2016). Furthermore, several researchers have shown that Shh can up‐regulate angiogenic factors such as VEGF, which drive the formation of new blood vessels (Pola et al., 2001; Teng et al., 2012). Although Shh effectively regulates wound healing, the precise mechanism by which the Shh signalling pathway promotes ESC migration or proliferation in skin wounds remains unclear.
In this study, we showed that an Shh‐induced integrin β1 effect on cytoskeleton rearrangement is associated with cell migration and required for tissue repair in vivo by activating downstream signalling. Our observations demonstrate that Shh treatment improves mESC migration capacity and affects wound closure in a skin wound healing model. In our skin wound healing model with Shh pretreated mESC transplantation, large and thick microvasculature sprouting towards the wound site was observed. This indicates that Shh augmented the formation of mature vessels. When we knocked down integrin β1 expression, the phosphorylation of FAK and Src was inhibited. In addition, our results show that Shh‐induced integrin β1 increases the activation of Rac1 and Cdc42, which induces cell spreading, as well as lamellipodial and filopodial membrane protrusion, which results in cell motility. Our results indicate that Shh induces an overexpression of F‐actin, and knocking down Rac1, Cdc42 or PAK1 inhibits mESC migration. Taken together, our results suggest that Shh acts by increasing integrin β1 expression, which in turn, increases F‐actin formation through Rho small GTPase, resulting in cytoskeleton rearrangement and mESC migration.
The observations presented here indicate that Shh is an attractive candidate as a target with potential to promote tissue regeneration and wound repair in clinical applications. Moreover, Shh induces cell proliferation through adherens junction disruption and, in mice, enhances mESC migration through integrin β1‐dependent F‐actin formation by a process involving the Rac1/Cdc42 signalling pathway. Our results strongly suggest that skin wound healing is associated with the migration of ESCs to the wound site induced by adherens junction disruption and cytoskeleton rearrangements in the ESCs.
Author contributions
The study conception and design were undertaken by J.Y.O., H.N.S., C.K.L. and H.J.H. The collection and/or assembly of data were performed by J.Y.O., H.N.S., G.E.C., S.H.K., J.S.K. and C.W.C. The data analysis and interpretation were performed by J.Y.O., S.H.K., C.K.L. and H.J.H. J.Y.O., G.E.C., H.J.L. and Y.H.J. wrote the manuscript. J.Y.O. and H.J.H. did the final approval of the manuscript. H.J.H. gave financial support.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 mESCs were pretreated with Gant 61 prior to Shh treatment for 24 h. (A, B) The mRNA of the self‐renewal marker genes (Oct4 and Sox2), trophectoderm (Cdx2), endoderm (GATA4 and GATA6), mesoderm (Brachyury), and ectoderm (NeuroD) were measured by real‐time PCR. Each mRNA expression level was normalized to the β‐actin mRNA expression. The data are expressed as mean ± SEM for five independent experiments.
Figure S2 Effect of Gli2 in Shh‐induced MMP2/9. (A) mESCs were transfected with Gli2 siRNA or NT siRNA for 24 h prior to Shh treatment and MMP2 and MMP9 were detected by Western blotting. β‐Actin was used as the loading control. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control of MMP2; &P < 0.05 versus control of MMP9. NS = non significant.
Figure S3 Effect of Shh‐induced Gli1 on Snail expression. (A) mESCs were pretreated with Gant 61 prior to Shh treatment and the expression of Snail were detected by Western blotting. β–Actin was used as the loading control. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; #P < 0.05 versus Shh + Gant 61. (B) mESCs were transfected with Snail siRNA (20 nM) or NT siRNA for 24 h prior to Shh treatment and E‐cadherin, MMP 2/9, p‐β‐catenin and integrin β1 were detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus NT siRNA; #P < 0.05 versus Shh + NT siRNA of E‐cadherin.
Figure S4 Effect of Shh‐induced Gli1/2 on Wnt and its signaling. (A) mESCs were transfected with Gli1, Gli2 siRNA (20 nM) or NT siRNA for 24 h prior to Shh treatment and Wnt1 and p‐GSK‐3β were detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus NT siRNA; #P < 0.05 versus Shh + NT siRNA.
Figure S5 Effect of MMP inhibitor on Shh‐phosphorylated β‐catenin at T41/S45. (A) mESCs were pretreated with MMP inhibitor prior to Shh treatment and the expression of p‐β‐catenin and active‐β‐catenin were detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; #P < 0.05 versus Shh. (B) mESCs were pretreated with MMP inhibitor prior to Shh treatment and the expression of E‐cadherin were detected by Western blotting. β–Actin was used as the loading control. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; #P < 0.05 versus Shh.
Figure S6 Effect of Gant 61 on Shh‐phosphorylated β‐catenin. (A) mESCs were pretreated with Gant 61 prior to Shh treatment and the expression of p‐β‐catenin and active‐β‐catenin were detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; #P < 0.05 versus Shh.
Figure S7 Relationship between integrin β1 and β‐catenin. (A) mESCs were transfected with β‐catenin siRNA or NT siRNA for 24 h prior to Shh treatment and integrin β1 was detected by Western blotting. β‐Actin was used as the loading control. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; NS = non significant. (B) mESCs were transfected with Integrin β1 siRNA or NT siRNA for 24 h prior to Shh treatment and the expressions of β‐catenin in nucleus and non‐nucleus fraction were detected by Western blotting. β–Actin was used as the cytosol marker and Lamin A/C was used as the nuclear marker. The data are expressed as mean ± SEM for five independent experiments. NS = non significant.
Figure S8 Effect of siRNA on E‐cadherin protein. (A) Cells were transfected with E‐cadherin or NT siRNA for 24 h prior to Shh treatment. The levels of E‐cadherin mRNA expression were measured by real‐time PCR. The data are expressed as mean ± SEM for ten independent experiments. *P < 0.05 versus NT siRNA; &P < 0.05 versus Shh + NT siRNA.
Figure S9 Effect of siRNA on target proteins. Cells were transfected for 24 h with siRNA specific for Gli1, E‐cadherin, β‐catenin, integrin β1, Rac1, and Cdc42 or NT siRNA using DharmaFECT transfection reagent. Proteins were analyzed by Western blotting with anti‐Gli1, anti‐E‐cadherin, and anti‐β‐catenin (A), anti‐integrin β1, anti‐Rac1, and anti‐Cdc42 (B) antibodies. The data are represented as mean ± SEM for five independent experiments. *P, &P, $P < 0.05 versus NT siRNA. NT, non‐targeting; ROD, relative optical density.
Table S1 The primers used for PCR.
Acknowledgements
This research was supported by National R&D Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF‐2013M3A9B4076541 and NRF‐2017R1A2B2008661) and BK21 PLUS Program for Creative Veterinary Science Research.
Oh, J. Y. , Suh, H. N. , Choi, G. E. , Lee, H. J. , Jung, Y. H. , Ko, S. H. , Kim, J. S. , Chae, C. W. , Lee, C.‐K. , and Han, H. J. (2018) Modulation of sonic hedgehog‐induced mouse embryonic stem cell behaviours through E‐cadherin expression and integrin β1‐dependent F‐actin formation. British Journal of Pharmacology, 175: 3548–3562. 10.1111/bph.14423.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C et al (2006). Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell‐mediated microvascular remodeling. Circulation 113: 2413–2424. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL (2002). Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129: 4753–4761. [DOI] [PubMed] [Google Scholar]
- Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W et al (2007). Cyclopamine‐mediated hedgehog pathway inhibition depletes stem‐like cancer cells in glioblastoma. Stem Cells 25: 2524–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourboulia D, Han H, Jensen‐Taubman S, Gavil N, Isaac B, Wei B et al (2013). TIMP‐2 modulates cancer cell transcriptional profile and enhances E‐cadherin/β‐catenin complex expression in A549 lung cancer cells. Regulation 14: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Fassler R (2005). β 1 integrin function in vivo: adhesion, migration and more. Cancer Metastasis Rev 24: 403–411. [DOI] [PubMed] [Google Scholar]
- Carlson TR, Hu H, Braren R, Kim YH, Wang RA (2008). Cell‐autonomous requirement for β1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice. Development 135: 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JS, Huang XH, Wang Q, Huang JQ, Zhang LJ, Chen XL et al (2013). Sonic hedgehog signaling pathway induces cell migration and invasion through focal adhesion kinase/Akt signaling‐mediated activation of matrix metalloproteinase (MMP)‐2 and MMP‐9 in liver cancer. Carcinogenesis 34: 10–19. [DOI] [PubMed] [Google Scholar]
- Chen Q, Xu R, Zeng C, Lu Q, Huang D, Shi C et al (2014). Down‐regulation of Gli transcription factor leads to the inhibition of migration and invasion of ovarian cancer cells via integrin β4‐mediated FAK signaling. PLoS One 9: e88386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement V, Sanchez P, De Tribolet N, Radovanovic I, Ruiz i Altaba A (2007). Hedgehog‐Gli1 signaling regulates human glioma growth, cancer stem cell self‐renewal, and tumorigenicity. Curr Biol 17: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA et al (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delloye‐Bourgeois C, Gibert B, Rama N, Delcros J‐G, Gadot N, Scoazec J‐Y et al (2013). Sonic hedgehog promotes tumor cell survival by inhibiting CDON pro‐apoptotic activity. PLoS Biol 11: e1001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI (2005). Alpha‐catenin is a molecular switch that binds E‐cadherin‐β‐catenin and regulates actin‐filament assembly. Cell 123: 903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HX, Wang S, Zhao H, Liu N, Chen D, Sun M et al (2014). Sonic hedgehog signaling may promote invasion and metastasis of oral squamous cell carcinoma by activating MMP‐9 and E‐cadherin expression. Med Oncol 31: 41. [DOI] [PubMed] [Google Scholar]
- Forbes SJ, Rosenthal N (2014). Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med 20: 857–869. [DOI] [PubMed] [Google Scholar]
- Guenou H, Nissan X, Larcher F, Feteira J, Lemaitre G, Saidani M et al (2009). Human embryonic stem‐cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet 374: 1745–1753. [DOI] [PubMed] [Google Scholar]
- Guo SA, DiPietro LA (2010). Factors affecting wound healing. J Dent Res 89: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS guide to pharmacology in 2018: updates and expansion to encompass the new guide to immunopharmacology. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson DM, Behfar A, Zingman LV, Kane GC, Perez‐Terzic C, Alekseev AE et al (2004). Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol 287: H471–H479. [DOI] [PubMed] [Google Scholar]
- Huang C, Verhulst S, Shen Y, Bu Y, Cao Y, He Y et al (2016). Akr1b10 promotes breast cancer metastasis through integrin α5/δ‐catenin mediated FAK/Src/Rac1 signaling pathway. Oncotarget 7: 43779–43791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A, Horwitz AR (2011). Integrins in cell migration. Cold Spring Harb Perspect Biol 3: A005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, Fields AP (2015). Molecular pathways: novel approaches for improved therapeutic targeting of hedgehog signaling in cancer stem cells. Clin Cancer Res 21: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny FN, Connelly JT (2015). Integrin‐mediated adhesion and mechano‐sensing in cutaneous wound healing. Cell Tissue Res 360: 571–582. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MO, Ryu JM, Suh HN, Park SH, Oh YM, Lee SH et al (2015). Camp promotes cell migration through cell junctional complex dynamics and actin cytoskeleton remodeling: implications in skin wound healing. Stem Cells Dev 24: 2513–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke DJ 2nd, Horvath N, Cuppett V, Wu Y, Deng W, Kanj R (2015). Interlocked positive and negative feedback network motifs regulate β‐catenin activity in the adherens junction pathway. Mol Biol Cell 26: 4135–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucky B, Mueller R, Vogt I, Teurich S, Hartenstein B, Breuhahn K et al (2007). Kallikrein 6 induces E‐cadherin shedding and promotes cell proliferation, migration, and invasion. Cancer Res 67: 8198–8206. [DOI] [PubMed] [Google Scholar]
- Ko JY, Kim KI, Park S, Im GI (2014). In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 35: 3571–3581. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK et al (2007). Cardiomyocytes derived from human embryonic stem cells in pro‐survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25: 1015–1024. [DOI] [PubMed] [Google Scholar]
- Lane SW, Williams DA, Watt FM (2014). Modulating the stem cell niche for tissue regeneration. Nat Biotechnol 32: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H, Kleinerman R, Lerman OZ, Brown D, Galiano R, Gurtner GC et al (2008). Hedgehog signaling is essential for normal wound healing. Wound Repair Regen 16: 768–773. [DOI] [PubMed] [Google Scholar]
- Lee KB, Choi J, Cho SB, Chung JY, Moon ES, Kim NS et al (2011). Topical embryonic stem cells enhance wound healing in diabetic rats. J Orthop Res 29: 1554–1562. [DOI] [PubMed] [Google Scholar]
- Li X, Wang Z, Ma Q, Xu Q, Liu H, Duan W et al (2014). Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin Cancer Res 20: 4326–4338. [DOI] [PubMed] [Google Scholar]
- Liu S, Xu SW, Blumbach K, Eastwood M, Denton CP, Eckes B et al (2010). Expression of integrin β1 by fibroblasts is required for tissue repair in vivo . J Cell Sci 123: 3674–3682. [DOI] [PubMed] [Google Scholar]
- Maher MT, Mo R, Flozak AS, Peled ON, Gottardi CJ (2010). β‐Catenin phosphorylated at serine 45 is spatially uncoupled from β‐catenin phosphorylated in the GSK3 domain: implications for signaling. PLoS One 5: e10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E et al (2005). ADAM10 mediates E‐cadherin shedding and regulates epithelial cell‐cell adhesion, migration, and β‐catenin translocation. Proc Natl Acad Sci U S A 102: 9182–9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobassarah NJ, Choudhry Z, Rikani AA, Choudhry AM, Tariq S, Zakaria F et al (2014). Sonic hedgehog signalling pathway: a complex network. Ann Neurosci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmeda D, Castel S, Vilaro S, Cano A (2003). β‐Catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol Biol Cell 14: 2844–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A et al (2014). Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle) 3: 445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigoto CN, Dauber KL, Bar C, Tsai P‐C, Valdes VJ, Cohen I et al (2016). Polycomb‐mediated repression and sonic hedgehog signaling interact to regulate merkel cell specification during skin development. PLoS Genet 12: e1006151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisant M, Giorgetti‐Peraldi S, Gabrielson M, Loubat A, Dani C, Peraldi P (2011). Inhibition of hedgehog signaling decreases proliferation and clonogenicity of human mesenchymal stem cells. PLoS One 6: e16798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Pepinsky RB et al (2001). The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 7: 706–711. [DOI] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM (1998). Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell 9: 1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, He Y‐H, Hou N, Zhang G‐S, Cai Y, Zhang G‐P et al (2016). Sonic hedgehog improves ischemia‐induced neovascularization by enhancing endothelial progenitor cell function in type 1 diabetes. Mol Cell Endocrinol 423: 30–39. [DOI] [PubMed] [Google Scholar]
- Reinchisi G, Parada M, Lois P, Oyanadel C, Shaughnessy R, Gonzalez A et al (2013). Sonic hedgehog modulates EGFR dependent proliferation of neural stem cells during late mouse embryogenesis through EGFR transactivation. Front Cell Neurosci 7: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo H‐W (2016). Targeting the sonic hedgehog signaling pathway: review of smoothened and Gli inhibitors. Cancer 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JM, Han HJ (2015). Autotaxin‐LPA axis regulates hMSC migration by adherent junction disruption and cytoskeletal rearrangement via LPAR1/3‐dependent PKC/GSK3β/β‐catenin and PKC/Rho GTPase pathways. Stem Cells 33: 819–832. [DOI] [PubMed] [Google Scholar]
- Scully KM, Skowronska‐Krawczyk D, Krawczyk M, Merkurjev D, Taylor H, Livolsi A et al (2016). Epithelial cell integrin β1 is required for developmental angiogenesis in the pituitary gland. Proc Natl Acad Sci U S A 113: 13408–13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer V, De Craene B, Berx G, Behrens J (2008). Snail promotes Wnt target gene expression and interacts with β‐catenin. Oncogene 27: 5075–5080. [DOI] [PubMed] [Google Scholar]
- Suh HN, Han HJ (2010). Laminin regulates mouse embryonic stem cell migration: involvement of Epac1/Rap1 and Rac1/Cdc42. Am J Physiol Cell Physiol 298: C1159–C1169. [DOI] [PubMed] [Google Scholar]
- Suh HN, Han HJ (2015). Sonic hedgehog increases the skin wound‐healing ability of mouse embryonic stem cells through the microRNA 200 family. Br J Pharmacol 172: 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai G, Tonn JC, Stupp R, Weller M (2011). The role of integrins in glioma biology and anti‐glioma therapies. Curr Pharm Des 17: 2402–2410. [DOI] [PubMed] [Google Scholar]
- Takeo M, Lee W, Ito M (2015). Wound healing and skin regeneration. Cold Spring Harb Perspect Med 5: A023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Chopp M, Hozeska‐Solgot A, Shen L, Lu M, Tang C et al (2012). Tissue plasminogen activator and plasminogen activator inhibitor 1 contribute to sonic hedgehog‐induced in vitro cerebral angiogenesis. PLoS One 7: e33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS et al (1998). Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147. [DOI] [PubMed] [Google Scholar]
- Wang S‐J, Cui H‐Y, Liu Y‐M, Zhao P, Zhang Y, Fu Z‐G et al (2015). Cd147 promotes Src‐dependent activation of Rac1 signaling through Stat3/Dock8 during the motility of hepatocellular carcinoma cells. Oncotarget 6: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ge J, Tredget EE, Wu Y (2013). The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat Protoc 8: 302–309. [DOI] [PubMed] [Google Scholar]
- Wu SM, Choo AB, Yap MG, Chan KK (2010). Role of sonic hedgehog signaling and the expression of its components in human embryonic stem cells. Stem Cell Res 4: 38–49. [DOI] [PubMed] [Google Scholar]
- Xiao C, Ogle SA, Schumacher MA, Orr‐Asman MA, Miller ML, Lertkowit N et al (2010). Loss of parietal cell expression of sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology 138: 550–561 561.e1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan GN, Lv YF, Yang L, Yao XH, Cui YH, Guo DY (2013). Glioma stem cells enhance endothelial cell migration and proliferation via the hedgehog pathway. Oncol Lett 6: 1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK, Kim HK et al (2011). Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP‐9 pathway in gastric cancer. Cancer Res 71: 7061–7070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 mESCs were pretreated with Gant 61 prior to Shh treatment for 24 h. (A, B) The mRNA of the self‐renewal marker genes (Oct4 and Sox2), trophectoderm (Cdx2), endoderm (GATA4 and GATA6), mesoderm (Brachyury), and ectoderm (NeuroD) were measured by real‐time PCR. Each mRNA expression level was normalized to the β‐actin mRNA expression. The data are expressed as mean ± SEM for five independent experiments.
Figure S2 Effect of Gli2 in Shh‐induced MMP2/9. (A) mESCs were transfected with Gli2 siRNA or NT siRNA for 24 h prior to Shh treatment and MMP2 and MMP9 were detected by Western blotting. β‐Actin was used as the loading control. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control of MMP2; &P < 0.05 versus control of MMP9. NS = non significant.
Figure S3 Effect of Shh‐induced Gli1 on Snail expression. (A) mESCs were pretreated with Gant 61 prior to Shh treatment and the expression of Snail were detected by Western blotting. β–Actin was used as the loading control. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; #P < 0.05 versus Shh + Gant 61. (B) mESCs were transfected with Snail siRNA (20 nM) or NT siRNA for 24 h prior to Shh treatment and E‐cadherin, MMP 2/9, p‐β‐catenin and integrin β1 were detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus NT siRNA; #P < 0.05 versus Shh + NT siRNA of E‐cadherin.
Figure S4 Effect of Shh‐induced Gli1/2 on Wnt and its signaling. (A) mESCs were transfected with Gli1, Gli2 siRNA (20 nM) or NT siRNA for 24 h prior to Shh treatment and Wnt1 and p‐GSK‐3β were detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus NT siRNA; #P < 0.05 versus Shh + NT siRNA.
Figure S5 Effect of MMP inhibitor on Shh‐phosphorylated β‐catenin at T41/S45. (A) mESCs were pretreated with MMP inhibitor prior to Shh treatment and the expression of p‐β‐catenin and active‐β‐catenin were detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; #P < 0.05 versus Shh. (B) mESCs were pretreated with MMP inhibitor prior to Shh treatment and the expression of E‐cadherin were detected by Western blotting. β–Actin was used as the loading control. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; #P < 0.05 versus Shh.
Figure S6 Effect of Gant 61 on Shh‐phosphorylated β‐catenin. (A) mESCs were pretreated with Gant 61 prior to Shh treatment and the expression of p‐β‐catenin and active‐β‐catenin were detected by Western blotting. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; #P < 0.05 versus Shh.
Figure S7 Relationship between integrin β1 and β‐catenin. (A) mESCs were transfected with β‐catenin siRNA or NT siRNA for 24 h prior to Shh treatment and integrin β1 was detected by Western blotting. β‐Actin was used as the loading control. The data are expressed as mean ± SEM for five independent experiments. *P < 0.05 versus control; NS = non significant. (B) mESCs were transfected with Integrin β1 siRNA or NT siRNA for 24 h prior to Shh treatment and the expressions of β‐catenin in nucleus and non‐nucleus fraction were detected by Western blotting. β–Actin was used as the cytosol marker and Lamin A/C was used as the nuclear marker. The data are expressed as mean ± SEM for five independent experiments. NS = non significant.
Figure S8 Effect of siRNA on E‐cadherin protein. (A) Cells were transfected with E‐cadherin or NT siRNA for 24 h prior to Shh treatment. The levels of E‐cadherin mRNA expression were measured by real‐time PCR. The data are expressed as mean ± SEM for ten independent experiments. *P < 0.05 versus NT siRNA; &P < 0.05 versus Shh + NT siRNA.
Figure S9 Effect of siRNA on target proteins. Cells were transfected for 24 h with siRNA specific for Gli1, E‐cadherin, β‐catenin, integrin β1, Rac1, and Cdc42 or NT siRNA using DharmaFECT transfection reagent. Proteins were analyzed by Western blotting with anti‐Gli1, anti‐E‐cadherin, and anti‐β‐catenin (A), anti‐integrin β1, anti‐Rac1, and anti‐Cdc42 (B) antibodies. The data are represented as mean ± SEM for five independent experiments. *P, &P, $P < 0.05 versus NT siRNA. NT, non‐targeting; ROD, relative optical density.
Table S1 The primers used for PCR.


