Abstract
Diabetic nephropathy is a major risk factor for end-stage renal disease and cardiovascular diseases and has a marked genetic component. A common variant (D allele) of the angiotensin Iconverting enzyme (ACE) gene, determining higher enzyme levels, has been associated with diabetic nephropathy. To address causality underlying this association, we induced diabetes in mice having one, two, or three copies of the gene, normal blood pressure, and an enzyme level range (65–162% of wild type) comparable to that seen in humans. Twelve weeks later, the three-copy diabetic mice had increased blood pressures and overt proteinuria. Proteinuria was correlated to plasma ACE level in the three-copy diabetic mice. Thus, a modest genetic increase in ACE levels is sufficient to cause nephropathy in diabetic mice.
The vast extent of human genetic variability is becoming increasingly obvious as the human genome project progresses (1, 2). Accordingly, much effort is being placed on uncovering genetic variations that influence the risk for or progression of the common but complex disorders of humankind, such as atherosclerosis, hypertension, diabetes, and cancer. Identification of genetic variants associated with these conditions is expected to lead to improved diagnostic capabilities and more selective treatments, and even to avoidance of the conditions (3). A major difficulty already encountered in this type of study in humans is the difficulty of establishing causation, as distinct from association and genetic linkage. Useful practical options can often be devised from associations without proof or knowledge of causation, but definitive advances depend on establishing causation. A valuable approach to establishing causative links between human genetic variants and conditions of interest is to introduce functionally similar genetic variations into mice, combine these variations with any other necessary predisposing factors, and determine whether the candidate genetic variation directly causes the condition of interest. Once proof of causality is established, further studies, often impossible in humans, become practical in animal models and can lead to an ascertainment of molecular mechanisms. Here we apply this approach to a putative causal link between a common genetic polymorphism in the gene coding for the angiotensin I-converting enzyme (ACE, kininase II) and diabetic nephropathy.
Diabetic nephropathy develops in 30–50% of type 1 and in about 10% of type 2 patients who have had diabetes mellitus for 10–20 years (4). This disease is the most common cause of end-stage renal disease in the United States and Europe, and the presence of elevated microalbuminuria, an early marker of diabetic nephropathy, or overt proteinuria further increases the risk for cardiovascular disease in patients with diabetes by at least 3-fold (5, 6). The etiology of diabetic nephropathy is poorly understood, but family studies have suggested a genetic predisposition (7, 8), and numerous studies have sought associations between its incidence and a variety of genetic polymorphisms. Of these, the most robust has been an association between nephropathy in type 1 diabetics and the D allele of an insertion/deletion (I/D) polymorphism in intron 16 of the ACE gene (9–12). This polymorphism, first described in 1990 by Rigat et al. (13), is itself associated with differences in the plasma and tissue levels of ACE (I/I, 76%; I/D, 100%; D/D, 126% of average). These associations suggest that a high constitutive level of ACE is deleterious to the kidney in the setting of chronic hyperglycemia, but a causal link between higher levels of ACE and kidney malfunction in association with diabetes has not been established. To address this issue, we therefore used streptozotocin to induce diabetes in mice with modest genetically determined differences in their plasma ACE levels already caused by their having one, two, or three functional copies of the ACE gene at its normal chromosomal location (14).
Materials and Methods
Generation of Mice with One-Three Copies of the ACE Gene.
Genetically engineered mice carrying either an inactivation or a duplication of the ACE gene on chromosome 11 were used for the study (14). Heterozygous mice of each strain were crossed for eight generations with wild-type C57BL/6 mice (IFFA CREDO, Les Oncins, France) and among themselves to generate the experimental groups. Genotyping of each animal was performed by PCR and Southern blotting as described (14). Mice carrying either one (one-copy), two (two-copy), or three (three-copy) functional ACE gene copies were studied. We were unable to obtain, for unknown reasons, sufficient numbers of viable mice homozygous for the ACE gene duplication to include in the study.
Induction of Diabetes and Follow-Up of Animals.
Diabetes was induced in groups of 10–12 3-month-old female mice of each genotype by 4–7 daily i.p. injections of streptozotocin (Sigma Aldrich, L'Isle d'Abeau, France), at a dose of 80 μg/g body weight in 8 μl of sterile 0.05 M sodium citrate (pH 4.5). Control groups of eight animals of each genotype received an equal volume of vehicle. Blood glucose levels (One Touch II glucometer, LifeScan, Mountain View, CA) and urinary glucose and ketonuric acid concentrations (Keto-Diabur-Test 5000, Roche Molecular Biochemicals) were monitored daily during streptozotocin or vehicle treatment and every 2 weeks thereafter. Approximately 80% of the streptozotocin-treated mice in each group developed stable hyperglycemia accompanied by mild ketonuria. Two diabetic and one control mice died during follow-up.
Mice were studied for 12 weeks without insulin treatment. They were fed ad libitum standard mice chow containing 0.4% of Na and 26% of mixed protein including 6% fish protein (Usine d'Alimentation Rationnelle, Paris) and had free access to tap water. Before diabetes induction and at 2 weeks of diabetes, a 20 μl blood sample was obtained by orbital puncture for the determination of plasma ACE activity. Mice were placed in metabolic cages (Marty Technology, Paris, France) every 2 weeks for the determination of albumin excretion in 24-hour urine. At 12 weeks, 3 days after a last urine collection, animals were killed (0.1 mg/g ketamine and 0.01 mg/g xylazine, i.p.), and blood was collected by orbital puncture. The left kidney and the two lungs were excised rapidly and frozen in liquid nitrogen for mRNA analyses. The right kidney was fixed by immersion in Bouins' solution for histological studies.
The procedures for the care and euthanasia of animals were in accordance with the European Community standards for the care and use of laboratory animals.
Blood Pressure Measurement.
Blood pressure was measured in conscious-trained mice on 3 consecutive mornings each month by the tail-cuff method, using a PowerLabo/S system connected to chart software (A. D. Instruments, Milford, MA) as described (15). Twenty consecutive measurements were averaged on each day, and the mean value of the 3 days was calculated for each mouse.
Biochemical and Hormonal Determinations.
Plasma and urine creatinine were measured by automated enzymatic methods (Kodak Biolyzer, Eastman Kodak). Urinary albumin concentration was measured by laser immunonephelometry (12), using a rabbit anti-mouse albumin Ab (ICN).
Plasma ACE activity was measured spectrophotometrically with hippuril-histidine-leucine as substrate (16). Plasma renin concentration (PRC) and urinary kallikrein excretion were determined by radioimmunoassays of the peptides generated in the presence of an excess of the corresponding substrates (15, 17).
Quantification of ACE mRNA in the Lung and Kidney.
Lung and kidney RNA extraction and Northern blot analyses of ACE mRNA, using a rat ACE cDNA probe, were carried out as described (16). The ACE mRNA signal was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA by using a human GAPDH cDNA probe (CLONTECH). Quantification of the hybridization signals was performed by solid-phase counting (InstantImager, Packard).
Histological and Morphometric Analyses of the Kidney.
Kidneys were cut transversally, fixed in alcoholic Bouins' solution, and embedded in paraffin. Sections, 5 μm thick of the entire kidney, were stained with Masson's trichrome for quantitative histology and with periodic acid-Schiff for morphometric analysis by using computerized image analysis (18). The following parameters were measured: total glomerular surface area delimited by the internal edge of the Bowman's capsule; glomerular tuft surface area defined by the total glomerular surface area minus the urinary space area and the urinary recesses; mesangial surface area defined by the glomerular tuft surface area minus the areas of the capillary lumens and the glomerular capillary free walls; and Bowman's capsule surface area and mean thickness of the Bowman's capsule.
Statistical Analysis.
Results are presented as mean ± SEM. For parameters measured at different times after onset of diabetes, blood pressure, and albuminuria, statistical comparison was performed by using a multiway ANOVA test with diabetes, ACE genotype, and time as test factors (STATVIEW 4.5 software; Abacus Concepts, Berkeley, CA). For other parameters measured at the time of death at 12 weeks, a 2-way ANOVA test was performed with diabetes and ACE genotype as factors. The ANOVA tests were followed when appropriate by Fisher's tests for comparisons among multiple groups. Correlation between variables was tested by linear regression analysis. Statistical significance was achieved if P < 0.05.
Results and Discussion
Effect of Diabetes on Plasma ACE and ACE mRNA.
Diabetic levels of plasma glucose were present throughout the 12-week observational period in streptozotocin-treated mice of all 3 ACE genotypes but not in the untreated controls (Table1; P of treatment < 0.0001). The induction of diabetes by streptozotocin was not affected by ACE gene copy number (P of genotypic effect = 0.18).
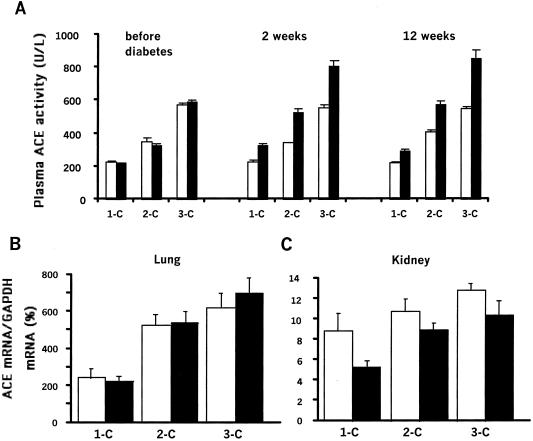
Fig. 1A summarizes the differences in plasma ACE activity in the one-, two-, and three-copy diabetic mice and the untreated controls. In agreement with published data (14), the plasma ACE activity of the untreated animals is directly related to their ACE gene copy number ranging from an average of 65% in the one-copy mice to 162% in the three-copy mice relative to the wild-type two-copy mice (P of genotypic effect < 0.0001). In humans, diabetes has been associated with elevated ACE activity (19), and we find this in our mice. Thus, induction of diabetes causes increases in plasma ACE activity of 70 ± 10, 174 ± 18, and 267 ± 32 milliunits/ml in the one-, two-, and three-copy mice, respectively (P of diabetes effect < 0.0001). Additionally, the present study shows that the extent of the increase is affected by the ACE genotype (P of interaction between diabetes and genotype < 0.001) with the effect being greatest in the three-copy and least in the one-copy animals.
Figure 1.
Plasma ACE level and ACE mRNA in control (□) and diabetic (■) mice having one (1-C), two (2-C), or three (3-C) copies of the ACE gene. (A) Plasma ACE activity was measured before diabetes induction and at 2 and 12 weeks. Results are means ± SEM. Effect of genotype or diabetes, P < 0.001; interaction between genotype and diabetes, P < 0.001. (B and C) ACE mRNA levels were measured in the lung and kidney at 12 weeks. Results are normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA level. (B) Lung. Effect of genotype, P < 0.0001; effect of diabetes, P = 0.6. (C) Kidney. Effect of genotype or diabetes, P < 0.01.
Possible explanations of this ACE genotype-dependent increase in the plasma ACE activity in the diabetic mice are that diabetes causes an increase in ACE production and/or a decrease in the clearance of ACE from the blood, which is influenced by the ACE genotype. To help distinguish between these possibilities, we determined ACE mRNA levels in the lung (the primary source of plasma ACE) and in the kidney, a quantitatively minor source. Fig. 1B shows that ACE mRNA in the lung parallels the plasma ACE activities and highly depends on the ACE gene copy number (P of genotype < 0.0001), but the lung ACE mRNA is not affected by the induction of diabetes (P = 0.6). The kidney ACE mRNA (Fig. 1C) also depends on gene copy number (P of genotype < 0.01), but in this tissue its amount is decreased slightly (≈25%) by the diabetes (P of treatment < 0.01), most probably by a decrease in ACE gene expression in the proximal tubule, the major site of ACE synthesis in the kidney (20). We conclude from these observations that the increase in plasma ACE activity accompanying the induction of diabetes is unlikely to be caused by an increased level of ACE production. The simplest hypothesis is that the clearance of ACE (21) is decreased by the effects of diabetes, and that these effects are rapid (already maximal at 2 weeks; Fig. 1A) and are greater when ACE plasma activities are higher. Thus, in diabetes, a genotype-dependent increase in circulating ACE can influence renal function.
Effect of ACE Gene Copy Number on Blood Pressure.
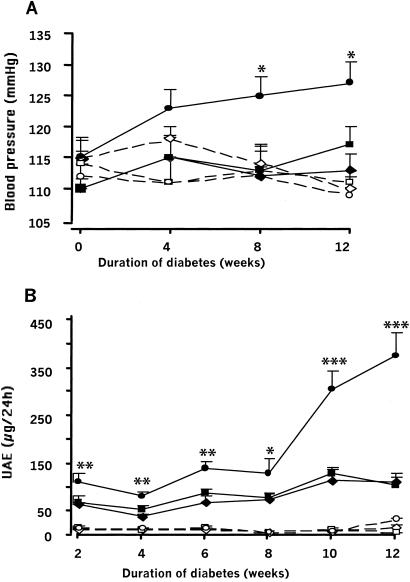
Fig. 2A compares the changes with time of the blood pressures, measured by tail cuff, of one-, two-, and three-copy mice after streptozotocin treatment with the pressures of the untreated controls. The blood pressures of the untreated mice are not affected by ACE gene copy number (P of genotype effect in control groups = 0.63), which is in agreement with previous observations (14). Nor are the blood pressures of the wild-type (two-copy) mice and the one-copy mice with genetically lower ACE activity significantly affected by induction of diabetes. However, the blood pressures of the three-copy diabetic mice with genetically higher ACE activity increase with time, and 12 weeks after the induction of diabetes are 10–20 mmHg higher than the blood pressures of the one and two copy diabetic mice or the control mice of all three genotypes (P of genotype effect in diabetic groups < 0.001). We conclude that, although the higher level of ACE gene function in the three-copy mice does not normally affect their blood pressure, it causes blood pressure to increase in the presence of diabetes.
Figure 2.
Evolution of blood pressure and UAE during diabetes in mice having one, two, or three copies of the ACE gene. (A) Blood pressure was measured in conscious-trained mice. Results are means ± SEM. Groups are one- (◊), two- (□), or three- (○) copy control nondiabetic mice; and one-(⧫), two-(■), or three-(●) copy diabetic mice. Effect of diabetes, P < 0.05; interaction between genotype and diabetes, P < 0.01 (multiway ANOVA). Effect of genotype in control groups, P = 0.63; and in diabetic groups, P < 0.001 (two-way ANOVA). *, P < 0.05, three-copy diabetic group compared with other diabetic groups. (B) UAE was measured on 24-h urine. Presentation of data is the same as in A. Effect of diabetes, genotype, or time, P < 0.0001. Interaction between genotype and diabetes, P < 0.0001. Effect of genotype in control groups, P = 0.30; and in diabetic groups, P < 0.001. *, P < 0.05; **, P < 0.01; ***, P < 0.0001, three-copy diabetic group compared with other diabetic groups.
Effect of ACE Gene Copy Number on Urinary Albumin Excretion (UAE) and Other Renal Parameters.
The earliest indicator of renal involvement in diabetes is an increase in the level of UAE (4). Fig. 2B compares the UAE of one-, two-, and three-copy mice after streptozotocin treatment with that of their untreated controls. The one-, two-, and three-copy control mice have very low levels of UAE (≈10 μg/24 h), which do not change significantly with time. Two weeks after induction of diabetes, the one- and two-copy mice have UAE levels (66 ± 5 and 70 ± 7 μg/24 h) ≈7× higher than the control mice (P vs. untreated < 0.0001), which increases slowly to 113 ± 19 and 107 ± 16 μg/24 h 10 weeks later. In marked contrast, the UAE of the three-copy diabetic mice, already nearly twice that of the one- and two-copy diabetic mice, increases rapidly over the next 10 weeks, reaching a level of 380 ± 48 μg/24 h. At the 12-week time point, UAE was significantly correlated with plasma ACE levels in the three-copy diabetic mice (r = 0.89, P < 0.01). Thus, the modestly higher ACE level of the three-copy mice causes the three-copy mice when diabetic to develop overt albuminuria early in the course of the disease. In contrast, the UAE of the one- and two-copy diabetic mice progresses much less rapidly.
We compared various biochemical parameters in plasma and urine and kidney morphometry in the normal and diabetic one-, two-, and three-copy mice with two aims: first, to determine how similar the induced changes are to those seen in human diabetics, and second, to uncover the effects of ACE genotype, of diabetes, and of any interactions between the effects of diabetes and the ACE genotype. The results of these comparisons are presented in Table 1. Body weight, plasma glucose, urine volume, and creatinine clearance are all significantly affected (P < 0.01–0.001) in the expected directions by the induction of diabetes, but not by the ACE genotype. Absolute and relative kidney weights are increased by diabetes (P < 0.0001), but we found no evidence in the kidney weights of any interaction between diabetes and the ACE genotype (P = 0.25). Plasma renin concentration was increased in the ACE one-copy animals (P of genotype 0.03), as reported (14), and tended to decrease with diabetes (P = 0.07) but with no evidence of interaction (P = 0.57). Urinary kallikrein significantly increased with increase in ACE copy number (P < 0.01) and tended to increase with diabetes (P = 0.06), but again with no evidence of interaction (P = 0.13). Light microscopy observations revealed glomerular hypertrophy without overt glomerulosclerosis in the diabetic mice, and only slight and sporadic interstitial fibrosis and tubular atrophy. Diabetes significantly increased (P < 0.0001) the total glomerular surface area, the glomerular tuft surface area, and the surface area of Bowman's capsule. These lesions are similar to those observed at the earliest stage of diabetes in humans (22). Mesangial morphology was not altered. There was a significant positive relationship between Bowman's capsule surface area and ACE genotype in both control and diabetic mice (P < 0.01). However, no interaction between the genotype and diabetes was observed for any of the morphological parameters. We conclude that the changes in kidney morphology observed in our diabetic mice are similar to those seen in the initial stages of human diabetes, but that these kidney structural changes are not affected by the ACE genotype. Thus, the proteinuria observed in the three-copy diabetic mice must arise from an increased glomerular permeability without structural injury that can be detected by light microscopy.
Table 1.
Main biochemical parameters in plasma and urine and renal morphometric data 12 weeks after induction of diabetes
| One-copy
|
Two-copy
|
Three-copy
|
P values*
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| CT (n = 8) | DM (n = 11) | CT (n = 8) | DM (n = 6) | CT (n = 7) | DM (n = 7) | Genotype | Diabetes | Interaction | |
| Body weight, g | 22.5 ± 0.5 | 18.3 ± 1.1 | 21.8 ± 0.5 | 21.0 ± 0.6 | 22.7 ± 0.7 | 20.3 ± 0.3 | 0.27 | <0.001 | 0.08 |
| Plasma | |||||||||
| Glucose, mmol/l | 7.2 ± 0.3 | 27.5 ± 1.2 | 7.4 ± 0.3 | 23.5 ± 1.0 | 6.9 ± 0.3 | 24.4 ± 1.6 | 0.18 | <0.0001 | 0.07 |
| Hematocrit, % | 43.1 ± 0.8 | 44.9 ± 1.8 | 43.2 ± 0.8 | 44.5 ± 1.7 | 43 ± 0.6 | 42.9 ± 2.3 | 0.82 | 0.25 | 0.65 |
| Creatinine, μmol/l | 13.1 ± 1.1 | 16.3 ± 1.3 | 11.5 ± 1.1 | 15.5 ± 0.8 | 14.7 ± 1.4 | 16.7 ± 0.8 | 0.21 | <0.01 | 0.71 |
| PRC, μgAI/ml/h | 1.29 ± 0.14 | 0.94 ± 0.17 | 0.82 ± 0.13 | 0.78 ± 0.18 | 0.86 ± 0.12 | 0.59 ± 0.11 | 0.03 | 0.07 | 0.57 |
| Urine | |||||||||
| Urine volume, ml/24 h | 0.5 ± 0.1 | 19.3 ± 3.5 | 0.5 ± 0.1 | 13.0 ± 1.5 | 0.7 ± 0.1 | 19.4 ± 2.7 | 0.14 | <0.0001 | 0.18 |
| Creatinine clearance, μl/min | 127 ± 32 | 224 ± 33 | 142 ± 26 | 187 ± 16 | 139 ± 22 | 237 ± 21 | 0.72 | <0.001 | 0.59 |
| UKE, μgBK/min/24 h urine | 1.49 ± 0.39 | 1.48 ± 0.23 | 1.59 ± 0.31 | 2.10 ± 0.39 | 2.94 ± 0.76 | 3.31 ± 0.32 | <0.01 | 0.06 | 0.13 |
| Kidney weight | |||||||||
| Absolute, mg | 120 ± 4 | 164 ± 7 | 112 ± 3 | 140 ± 7 | 124 ± 13 | 176 ± 3 | <0.01 | <0.0001 | 0.25 |
| Relative, mg/g BW | 5.4 ± 0.1 | 9.2 ± 0.5 | 5.1 ± 0.1 | 6.7 ± 0.3 | 5.4 ± 0.6 | 8.7 ± 0.1 | <0.01 | <0.0001 | 0.16 |
| Morphometric data | |||||||||
| Glomerular tuft surface area, μm2 | 2226 ± 58 | 2785 ± 103 | 2284 ± 67 | 2553 ± 80 | 2077 ± 71 | 2555 ± 93 | 0.11 | <0.0001 | 0.08 |
| Mesangial surface area, μm2 | 531 ± 33 | 571 ± 32 | 598 ± 19 | 571 ± 26 | 585 ± 27 | 630 ± 31 | 0.20 | 0.63 | 0.23 |
| Bowman's capsule surface area, μm2 | 207 ± 8 | 304 ± 41 | 292 ± 9 | 336 ± 14 | 291 ± 14 | 377 ± 20 | 0.01 | <0.0001 | 0.56 |
| Mean thickness of Bowman's capsule, μm | 2.56 ± 0.12 | 2.01 ± 0.22 | 2.07 ± 0.07 | 1.70 ± 0.04 | 2.03 ± 0.10 | 1.69 ± 0.09 | 0.03 | <0.0001 | 0.9 |
CT, control groups; DM, diabetic groups; Creat, creatinine; PRC, plasma renin concentration; UKE, urinary kallikrein excretion. Results are means ± SEM; AI, angiotensin I; BK, bradykinin; BW, body weight.
Two-way ANOVA.
Mechanism of ACE-Mediated Renal Dysfunction in Diabetes.
Some potentially useful inferences can be drawn from our present studies when combined with previous observations on the marked differences in the effects of changes in ACE activity on the steady-state levels of the substrates of ACE (angiotensin I and bradykinin; ref. 23) and its products (angiotensin II and bradykinin 1–7). Experimental data from rats (24) and computer simulations and experimental data from mice (25) have indicated that modest changes in ACE activity in either direction affect the level of the substrates of the enzyme (including bradykinin), but have little effect on the level of its products (including angiotensin II), which are only significantly affected when ACE function is more substantially decreased. These considerations together with our present data lead us to suggest that bradykinin levels, which decrease when ACE levels are genetically increased, may be more immediately important than angiotensin II levels in the causal chain between genetically higher ACE levels and the development of diabetic proteinuria in mice. This suggestion is supported by the close anatomic juxtaposition of the kallikrein-producing cells in the late distal tubule with renal glomerular arterioles (26), and by experiments showing that the kinin system markedly affects glomerular hemodynamics in diabetic animals (27). Direct tests of the idea should be possible in mice by determining the effects on diabetic nephropathy of experimentally manipulating the kinin system. Nevertheless, it is also important to consider the possibility that increased intrarenal formation of angiotensin II, a potent regulator of glomerular hemodynamics (28), mediates the deleterious effect of the ACE gene, given the unique features of the renal circulation with regard to both high renin and angiotensin I concentration and low ACE content, and experimental evidence showing that the renal distribution of ACE protein is markedly affected by diabetes (20, 29).
We conclude that the observations reported here establish a causal link between a genetically determined modest increase in ACE gene function and the development of proteinuria in diabetic mice. This causal link has considerable relevance to the effects of the insertion/deletion ACE polymorphism in human diabetics, in that it establishes that higher ACE levels are sufficient to initiate the chain of events leading to diabetic nephropathy. The three-copy mice should provide a valuable model for helping determine the molecular, microvascular, and other physiological steps involved in this process and in determining how best to intervene.
Acknowledgments
This work was supported by the Institut National de la Santé et de la Recherche Médicale and the National Institutes of Health (HL49277) and by a grant from the Bristol-Myers Squibb Institute for medical research. W.H. was the recipient of a fellowship from the French Hypertension Society. We are grateful to Lise Bankir, Tom Coffman, Ariel Gomez, Joel Menard, Mary Osborne-Pellegrin, and Nobuyuki Takahashi for critically reading our manuscript.
Abbreviations
- ACE
angiotensin I-converting enzyme
- UAE
urinary albumin excretion
References
- 1.Lander E S, Linton L M, Birren B, Nusbaum C, Zody M C, Baldwin J, Devon K, Dewar K, Doyle M, Fitzhugh W, et al. Nature (London) 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 2.Venter J C, Adams M D, Myers E W, Li P W, Mural R J, Sutton G G, Smith H O, Yandell M, Evans C A, Holt R A, et al. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 3.Collins F S, McKusick V A. JAMA. 2001;285:540–544. doi: 10.1001/jama.285.5.540. [DOI] [PubMed] [Google Scholar]
- 4.Mogensen C E, Keane W F, Bennett P H, Jerums G, Parving H H, Passa P, Steffes M W, Striker G E, Viberti G C. Lancet. 1995;346:1080–1084. doi: 10.1016/s0140-6736(95)91747-0. [DOI] [PubMed] [Google Scholar]
- 5.Borch-Johnsen K, Kreiner S. Br Med J (Clin Res Ed) 1987;294:1651–1654. doi: 10.1136/bmj.294.6588.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messent J W, Elliott T G, Hill R D, Jarrett R J, Keen H, Viberti G C. Kidney Int. 1992;41:836–839. doi: 10.1038/ki.1992.128. [DOI] [PubMed] [Google Scholar]
- 7.Earle K, Walker J, Hill C, Viberti G. N Engl J Med. 1992;326:673–677. doi: 10.1056/NEJM199203053261005. [DOI] [PubMed] [Google Scholar]
- 8.Seaquist E R, Goetz F C, Rich S, Barbosa J. N Engl J Med. 1989;320:1161–1165. doi: 10.1056/NEJM198905043201801. [DOI] [PubMed] [Google Scholar]
- 9.Marre M, Bernadet P, Gallois Y, Savagner F, Guyene T T, Hallab M, Cambien F, Passa P, Alhenc-Gelas F. Diabetes. 1994;43:384–388. doi: 10.2337/diab.43.3.384. [DOI] [PubMed] [Google Scholar]
- 10.Doria A, Warram J H, Krolewski A S. Diabetes. 1994;43:690–695. doi: 10.2337/diab.43.5.690. [DOI] [PubMed] [Google Scholar]
- 11.Parving H H, Jacobsen P, Tarnow L, Rossing P, Lecerf L, Poirier O, Cambien F. BMJ. 1996;313:591–594. doi: 10.1136/bmj.313.7057.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadjadj S, Belloum R, Bouhanick B, Gallois Y, Guilloteau G, Chatellier G, Alhenc-Gelas F, Marre M. J Am Soc Nephrol. 2001;12:541–549. doi: 10.1681/ASN.V123541. [DOI] [PubMed] [Google Scholar]
- 13.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krege J H, Kim H S, Moyer J S, Jennette J C, Peng L, Hiller S K, Smithies O. Hypertension. 1997;29:150–157. doi: 10.1161/01.hyp.29.1.150. [DOI] [PubMed] [Google Scholar]
- 15.Meneton P, Bloch-Faure M, Hagege A A, Ruetten H, Huang W, Bergaya S, Ceiler D, Gehring D, Martins I, Salmon G, et al. Proc Natl Acad Sci USA. 2001;98:2634–2639. doi: 10.1073/pnas.051619598. . (First Published February 20, 2001; 10.1073/pnas.051619598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costerousse O, Allegrini J, Huang H, Bounhik J, Alhenc-Gelas F. Am J Physiol. 1994;267:E745–E753. doi: 10.1152/ajpendo.1994.267.5.E745. [DOI] [PubMed] [Google Scholar]
- 17.Menard J, Catt K J. Endocrinology. 1972;90:422–430. doi: 10.1210/endo-90-2-422. [DOI] [PubMed] [Google Scholar]
- 18.Heudes D, Michel O, Chevalier J, Scalbert E, Ezan E, Bariety J, Zimmerman A, Corman B. Am J Physiol. 1994;266:R1038–R1051. doi: 10.1152/ajpregu.1994.266.3.R1038. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman J, Sastre A. Ann Intern Med. 1980;93:825–826. doi: 10.7326/0003-4819-93-6-825. [DOI] [PubMed] [Google Scholar]
- 20.Anderson S, Jung F F, Ingelfinger J R. Am J Physiol. 1993;265:F477–F486. doi: 10.1152/ajprenal.1993.265.4.F477. [DOI] [PubMed] [Google Scholar]
- 21.Casarini D E, Boim M A, Stella R C, Krieger-Azzolini M H, Krieger J E, Schor N. Am J Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 22.Osterby R. Diabetologia. 1992;35:803–812. doi: 10.1007/BF00399925. [DOI] [PubMed] [Google Scholar]
- 23.Erdos E G. Hypertension. 1990;16:363–370. doi: 10.1161/01.hyp.16.4.363. [DOI] [PubMed] [Google Scholar]
- 24.Campbell D J, Kladis A, Duncan A M. Hypertension. 1994;23:439–449. doi: 10.1161/01.hyp.23.4.439. [DOI] [PubMed] [Google Scholar]
- 25.Smithies O, Kim H S, Takahashi N, Edgell M H. Kidney Int. 2000;58:2265–2280. doi: 10.1046/j.1523-1755.2000.00411.x. [DOI] [PubMed] [Google Scholar]
- 26.Barajas L, Powers K, Carretero O, Scicli A G, Inagami T. Kidney Int. 1986;29:965–970. doi: 10.1038/ki.1986.94. [DOI] [PubMed] [Google Scholar]
- 27.Jaffa A A, Rust P F, Mayfield R K. Diabetes. 1995;44:156–160. doi: 10.2337/diab.44.2.156. [DOI] [PubMed] [Google Scholar]
- 28.Navar L G, Harrison-Bernard L M, Imig J D, Wang C T, Cervenka L, Mitchell K D. J Am Soc Nephrol. 1999;10:S266–S272. [PubMed] [Google Scholar]
- 29.Metzger R, Bohle R M, Pauls K, Eichner G, Alhenc-Gelas F, Danilov S M, Franke F E. Kidney Int. 1999;56:1442–1454. doi: 10.1046/j.1523-1755.1999.00660.x. [DOI] [PubMed] [Google Scholar]