Abstract
Background
Activation of AKT pathway attenuates brain damage and neuronal apoptosis during cerebral ischemia/reperfusion (I/R) injury. SC79 is a novel, selective and highly-efficient Akt activator. This study aimed to investigate the neuroprotective effect of SC79 against cerebral I/R injury in a rat model, and to explore the possible underlying mechanisms.
Material/Methods
Male Sprague-Dawley rats received cerebral ischemia for 1 hour, followed by brain reperfusion for 0.5–24 hours. The cerebral I/R injury animal model were treated with SC79 alone or SC79 in combination with LY294002. Western blots were used to detect the levels of expression of phosphatidylinositol AKT (p-Akt), Bax, and bcl-2. Twenty-four hours after cerebral I/R, the degree of brain injury was evaluated by detecting the neurological deficit score (NDS). The infarct rate of brain tissue was observed by TTC (2, 3, 5-triphenyltetrazolium chloride) staining. TUNEL (terminal deoxynucleotidyl transferase-mediated UTP nick end labeling) staining was used to detect cell apoptosis.
Results
p-Akt was activated during early cerebral I/R at 0.5 hours, and reached the highest levels at 4 hours, then gradually decreased from 6 hours, and reached and maintained the lowest levels at 12–24 hours. Bax expression was gradually increased from 6 hours and reached the highest level at 24 hours. However, bcl-2 expression was gradually increased and reached the highest levels at 4 hours, then gradually decreased from 6 hours, and reached the lowest levels at 24 hours. Administration of SC79 decreased infarct volumes and improved neurological function significantly. LY294002 in combination with SC79 lost the capability of SC79 to resist the cerebral I/R injury. SC79 treatment alone activated p-Akt and promoted anti-apoptotic bcl-2 and inhibited anti-apoptotic Bax expression in middle cerebral artery occlusion (MCAO) mice. However, combined SC79 and LY294002 treatment abolished SC79-induced p-Akt activity, inhibited anti-apoptotic bcl-2 and promoted anti-apoptotic Bax expression in MCAO mice. Furthermore, SC79 treatment alone attenuated apoptotic neuronal cell death, but abolished this effect in SC79 in combination with LY294002 treated groups.
Conclusions
SC79 significantly increased Akt activation and reduced infarct volume and subsequently improved neurological function in ischemic brain after cerebral I/R injury in rats. These findings suggested that SC79 may be as a neuroprotective drug to be potentially used in the clinic.
MeSH Keywords: Apoptosis, bcl-2-Associated X Protein, Oncogene Protein v-akt
Background
Obstruction of brain-artery blood flow results in cerebral ischemia/reperfusion (I/R) injury (ischemic stroke), leading to cell death and infarction [1]. However, the exact mechanism of cerebral I/R injury is still not completely understood. And effective treatment for cerebral I/R injury is needed. Cerebral I/R leads to neuronal injury and death involving in excitotoxicity, oxidative stress, programmed cell death (apoptosis), and inflammation [2]. Apoptosis, one of the main contributors to neuronal death, has been suggested to play fundamental pathogenic roles in cerebral I/R injury [3].
Many I/R animal models showed neuronal apoptosis [4], which is also evident in patients suffering from ischemic stroke [5]. Cerebral I/R induces the activation of a series of pro-apoptotic genes or inactivation of anti-apoptotic genes, leading to neuronal cell damage [6,7]. Therefore, searching for protective agents directed at apoptotic pathways may provide an attractive therapeutic approach for the therapy of cerebral I/R injury.
Akt is a well-established pro-survival signaling molecule. The acute activation of Akt is also beneficial to prevent apoptosis of cardiomyocytes upon ischemic injury [8]. A growing body of evidence suggests that showed that activation of phosphatidylinositol 3-kinase (PI3K)/AKT and pathways results in protection against apoptosis in rat models of transient focal cerebral I/R, and inhibition of the PI3K/Akt pathways reduce the protective effect [9–11]. Therefore, identifying the genuine activator of Akt for the therapeutic applications of cerebral I/R injury is needed.
SC79, a small molecule Akt activator, specially suppresses Akt membrane translocation while activating Akt in the cytosol [12]. It has shown cytoprotective effects in experimental ischemia-elicited neuronal death [12]. It protected against early brain injuries through the dual activities of antioxidation and anti-apoptosis [13]. In addition, SC79 has proved to have not significant side-effects in experimental mice and human cells [12], suggesting that the molecule could possibly be administered intravenously in patients.
In the present study, we investigate the effect of SC79 on cerebral I/R injury in vivo. We observed that Akt was activated at early 0.5 hours following cerebral I/R and reached the highest levels at 4 hours, then the Akt phosphorylation (pAkt) was gradually reduced and restored and maintained to the original level at 12–24 hours. SC79 significantly induced pAkt and bcl-2 expression and downregulated Bim in brain tissue. SC79 administration effectively reduced cerebral infarct area and decreased infarct volume following cerebral I/R. And the underlying mechanism of SC79 may relate to inhibition of neuron apoptosis SC79 by activate Akt signal and upregulate anti-apoptotic bcl-2 and to downregulate pro-apoptotic Bim expression.
Material and Methods
Ethics statement
The study was conducted in accordance with the ethical standards and the Declaration of Helsinki and according to the national and international guidelines and was approved by Shandong Provincial Hospital Affiliated to Shandong University
Establishing middle cerebral artery occlusion and reperfusion (MCAO/R) model
Male Sprague-Dawley rats weighing approximately 320–350 g (10–12 weeks) (n=16 per group) were obtained from Shanghai Lab Animal Research Center, Shanghai, China. The rats were under a 12 hours light cycle with food and drinking water available ad libitum. The experimental procedures were approved by Shandong Provincial Hospital Affiliated to Shandong University Animal Ethics Committee (2003-045). Transient middle cerebral artery occlusion (MCAO) using an intraluminal suture was induced under isoflurane anesthesia as previously described [14]. Reperfusion was accomplished by withdrawing the suture after 60 min of ischemia. The brain reperfusion was allowed for 0, 0.5, 2, 4, 6, 8, 12 and 24. Sham-operated rats underwent identical surgery except that the monofilament nylon suture was not inserted. After incision closure, the rats were placed into cages to recover, with free access to food and water. Rat rectal temperature was monitored and maintained at 37.0±0.5°C during the whole procedure.
Experimental groups
The rats were divided into sham, vehicle, MCAO, SC79, and SC79+LY294002 groups. 60 min prior to cerebral ischemia (60 min) followed by reperfusion, SC79 (S7863) (Selleck, Shanghai, China) was applied via intraperitoneal injection at a concentration of 0.04 mg/g of body weight. The mice were subjected to focal cerebral ischemia (60 min) followed by reperfusion (24 hours).
To determine the role of PI3K/Akt signaling in SC79-induced protection against cerebral I/R injury, the PI3K inhibitor LY294002 (Sigma, Shanghai, China) (1 mg/25 g body weight) was given to mice 15 min before SC79 administration according to the previous report [15]. Rats in the model and sham groups were injected with an equal volume of saline.
Western blot assay
As previously described [12], we carried out western blot assay to detect P-Akt (Ser473) (dilution 1: 1000), Bim (dilution 1: 1200) and bcl-2 (dilution 1: 1200) in brain tissues. Staining was visualized by ECL detection reagents.
The signals were visualized using the ECL detection system. The intensity (area × density) of individual bands was measured by densitometry (Thermo Scientific), and the background was subtracted from the calculated area. The protein level was reported as normalized β-actin loading control.
Terminal deoxynucleotidyl transferase-mediated UTP nick end labeling (TUNEL) staining
The terminal deoxynucleotidyl transferase-mediated UTP nick end labeling (TUNEL) was processed using an in-situ cell death detection kit (Roche, Hangzhou, China) according to the manufacturer’s instructions. Briefly, after xylene dewaxing, sections were rinsed 3 times in distilled water for 5 min, and they were washed in methanol containing 0.3% H2O2 at room temperature for 30 min to inhibit endogenous peroxidase activity. After rinsing in PBS 3 times at room temperature for 5 min, sections were treated with proteinase K at 37°C for 6 min. Section were rinsed in PBS 3 times at room temperature for 3 min, were soaked in TdT buffer for 10 min, and incubated in 50 μL TdT buffer containing TdT at 37°C for 60 min in a moist chamber (Roche). After 3 rinsing steps in PBS at room temperature for 5 min, the sections were incubated in 50 μL FITC (Roche) at 37°C for 40 min. After 3 further rinses in PBS for 3 min, the brain sections were incubated in DAB (Roche) at room temperature for 3 min. TUNEL-positive (green) and DAPI-positive (blue) staining patterns were acquired with a confocal laser scanning microscope (Fluoview FV 1000, Olympus, Japan). TUNEL-positive cells in the different regions of each slide were counted by an observer who was blinded to the treatment conditions. Cell apoptotic rate was defined as the percentage of TUNEL-positive nuclei to DAPI staining cells by examining the entire section with the 40× objective.
Evaluation of infarct volume
Twenty-four hours after reperfusion, each brain was quickly removed and sectioned coronally into 5 serial 2-mm slices. Tissue slices were immersed in a 0.25% solution of 2, 3, 5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich, Hangzhou, China) at 37°C for 30 minutes, and then fixed in 10% buffered formalin. The area of infarction was calculated using ImageJ computer software and determined by subtracting the uninfarcted tissue in the ischemic hemisphere from the volume of the contralateral hemisphere, which corrects for post-ischemic edema [16]. The percentage of the corrected infarct volume was calculated by dividing the infarct volume by the total contralateral hemispheric volume [17].
Evaluation of neurological score
The scoring system included 5 indexes [18]: 0 score was no deficit; 1 score was failure to extend left forepaw full, considered a mild focal neurologic deficit; 2 score was circling to the right; 3 score was falling to the left, considered a severe focal deficit; 4 score was unable to walk spontaneously.
Statistical analysis
Data in histograms are presented as mean ± standard error of the mean (SEM). The results were analyzed by unpaired Student’s t-test or by one-way ANOVA followed by Bonferroni’s post hoc tests when multiple experimental groups were compared. All statistical analyses were performed with SPSS software (version 13.0). A P value of P<0.05 was considered significant.
Results
Early Akt activation during cerebral I/R
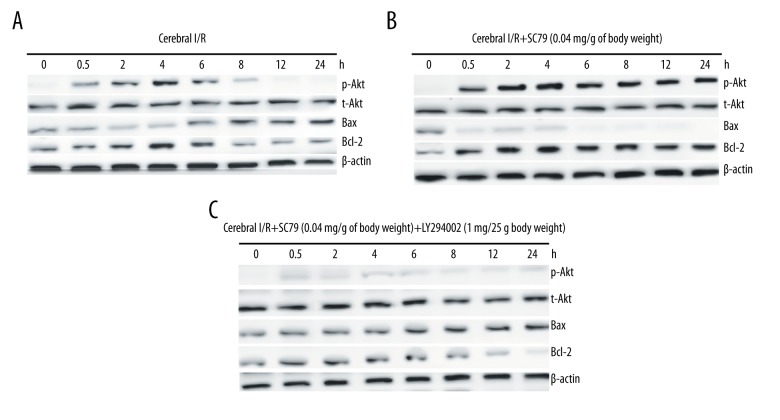
After cerebral I/R for 0.5–24 hours, we collected the ischemic hemisphere and detected the AKT phosphorylation [p-Akt(Ser473)], bax, and bcl-2 expression by western blot assay. Our results (Figure 1A) showed that AKT phosphorylation [p-Akt(Ser473)] began to increase at 0.5 hours after cerebral I/R and reached the highest level at 4 hours. Then the p-Akt(Ser473) expression was gradually decreased and restored and maintained to the original level at 12–24 hours. The anti-apoptotic Bcl-2 protein was gradually decreased from 6 hours after cerebral I/R and reached the lowest level at 24 hours; Pro-apoptotic Bim protein was gradually increased from 6 hours after cerebral I/R and reached the highest level at 24 hours (Figure 1A). The data showed that I/R induced p-Akt(Ser473) expression, subsequently increased Bim expression and decreased bcl-2 expression.
Figure 1.
Akt activation with SC79 in the brain of rats subjected to cerebral ischemia/reperfusion (I/R). The rats were subjected to cerebral I/R (A) or pre-treatment with SC79 at a concentration of 0.04 mg/g of body weight (B) or pre-treatment with SC79 in combination of LY294002 (1 mg/25 g body weight) (C), then subjected to cerebral I/R for 0.5–24 hours. Cellular proteins were isolated from brain tissues. p-Akt, t-Akt, Bax, and bcl-2 was detected by western blot assay.
It has reported that activation of the PI3K/Akt signaling protects neuronal cells from cerebral I/R injury [19]. We next detect the effect of SC79 on the activation of PI3K/Akt signaling in the brain tissues. As shown in Figure 1B, treatment of SC79 at a concentration of 0.04 mg/g of body weight significantly increased the levels of p-Akt in the brain tissues of I/R. Furthermore, Bcl-2 protein was also significantly increased and Bax was significantly decreased (Figure 1B). The data showed that SC79 significantly increased p-Akt (Ser473) expression, subsequently decreased Bim expression and increased bcl-2 expression. However, LY294002 treatment prior to SC79 abolished the AKT phosphorylation, subsequently increased Bim expression and decreased bcl-2 expression (Figure 1C), indicating the active effect of SC79 on PI3K/AKT pathway.
SC79 administration decreased cerebral infarct volume following I/R
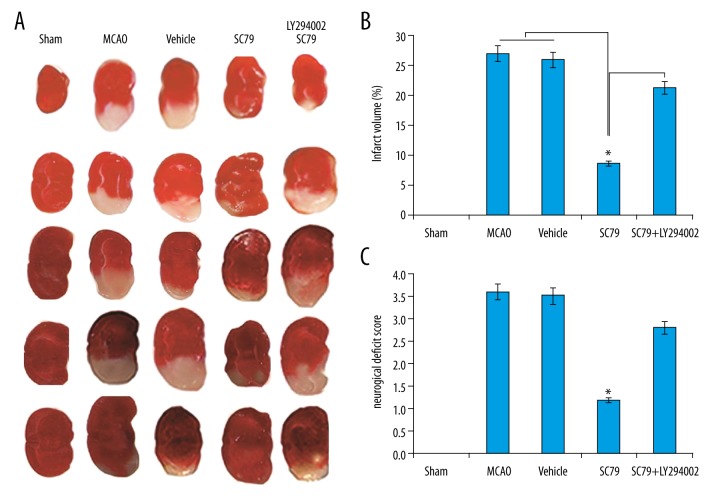
The results showed that the cortical hemisphere was stained deep red in the sham-operation group while pale grey in the untreated I/R groups (Figure 2A). The infarction areas were large and pale in the I/R untreated groups compared to the SC79 treatment alone groups (Figure 2A). In the SC79 in combination with LY294002 treatment groups, the infarction areas were large and pale compared to the SC79 treatment alone groups (Figure 2A). SC79 administration significantly reduced infarct volume compared to the untreated I/R group (Figure 2B). Combined LY294002 treatment reverse the neuroprotection of SC79 (Figure 2B). Administration of vehicle to mice did not alter I/R-induced cerebral infarct volume. Neurological deficits were examined. The scored on a 4-point scale was calculated. Mice subjected to untreated I/R showed significant motor behavior deficits. SC79 alone showed a significant reduction in I/R neurological deficits. LY294002 reverses the neuroprotection of SC79 as shown by the neurological deficit score (Figure 2C).
Figure 2.
SC79 reduced cerebra I/R injury. SC79 or/and LY294002 was administered to mice as before cerebral I/R was induced. (A) Representative photographs of brain sections stained with TTC. (B) Summary of cerebral infarct size in brains. Infarct size was determined by TTC staining and the infarct volume was expressed as the percentage of the contra lateral hemispheric area. Data were expressed as mean ± SD, * P<0.05. I/R – ischemia/reperfusion; TUNEL – terminal deoxynucleotidyl transferase-mediated UTP nick end labeling; SD – standard deviation.
Effect of SC79 on cerebral apoptosis in ischemic brain
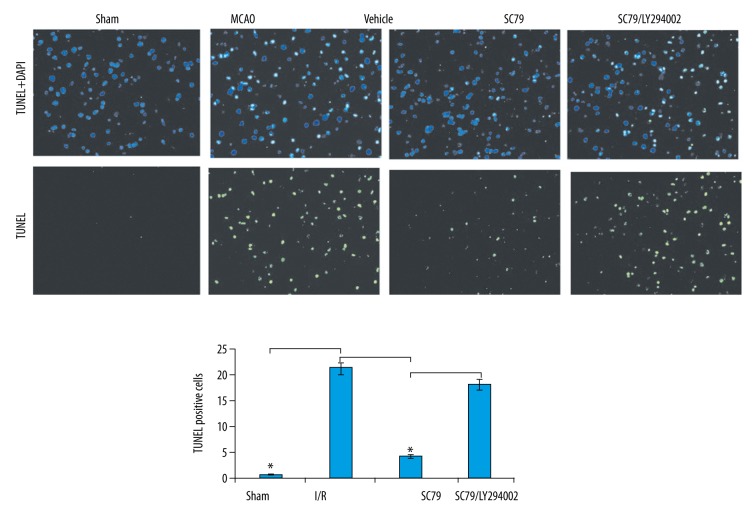
To understand the cellular mechanisms underlying the neuroprotective effect of SC79, we used the TUNEL staining to evaluate the degree of apoptotic death during cerebral I/R. Relative to the sham operation animals, the untreated I/R animals showed significantly increased neuronal apoptotic rate (Figure 3). The neuronal apoptotic rate was significantly decreased in SC79 alone treated animals compared with vehicle-treated animals at 24 hours in rat brain subjected to I/R (Figure 3). However, the decrease in neuronal apoptosis was markedly increased in the groups treated with SC79 in combination with LY294002 compared to the SC79 alone (Figure 3). These results suggested that the treatment with SC79 effectively prevented apoptotic cell death in I/R model.
Figure 3.
Effect of SC79 on I/R-induced cerebral apoptosis determined by TUNEL. The rats were subjected to cerebral I/R or pre-treatment with SC79 at a concentration of 0.04 mg/g of body weight or pre-treatment with SC79 in combination of LY294002 (1 mg/25 g body weight), then subjected to cerebral I/R for 0.5–24 hours. Apoptosis was measured by TUNEL staining in ischemic brain. * P<0.01. I/R – ischemia/reperfusion; TUNEL – terminal deoxynucleotidyl transferase-mediated UTP nick end labeling.
Discussion
In our study, we demonstrated that SC79 efficiently activated the AKT phosphorylation (p-Akt) and confer neuroprotection after transient cerebral I/R injury in a rat model. 24 hours after cerebral I/R could induce apoptosis of cortical neurons. Pre-treatment with SC79 significantly inhibited cerebral neuron apoptosis. The possible underlying mechanisms of neuroprotective effects of SC79 is related to its ability to upregulate anti-apoptotic bcl-2 and to downregulate anti-apoptotic Bim expression.
Akt phosphorylation and activation is a crucial mediator of cell survival. Enhancement of Akt activity exerts pro-survival effect in neuronal injury and neurodegenerative diseases [20]. It also serves as a therapeutic target for treatment of early brain injury following subarachnoid hemorrhage (SAH) [13]. Deactivation of Akt is implicated in various stress-induced pathological cell death and degenerative diseases [20,21]. In the present study, p-Akt began to be activated at 0.5 hours after cerebral I/R and reached the highest levels at 4 hours, then gradually decreased and reached the lowest levels at 12–24 hours. Cerebral I/R did not change the expression of total Akt. We therefore suggest that early transient Akt activation during I/R may contribute to the neuroprotective role, which may be the stress response of the cells responds to external stimuli. The gradually decreasing process of Akt phosphorylation contributes to pathogenesis of cerebral I/R injury. Therefore, cerebral I/R injury can be protected by activation of Akt using a specific Akt activator.
SC79 is a novel and safe small molecule compound, which can be as the selective, highly-efficient and cell-permeable Akt activator [12]. It has reported that SC79 activated Akt and protected H9c2 myocardial cells and primary murine myocardiocytes from OGD/re-oxygenation in vitro [22], but not in vivo of rat hearts [23]. In our study, administration of SC79 (0.04 mg/g of body weight) significantly increased the levels of p-Akt compared to the untreated mice at 24 hours after I/R. Importantly, SC79 significantly reduced the infarction volume and neurologic deficits, and protected cerebral neuron from I/R injury. However, PI3K inhibition with LY29004 (1 mg/25 g body weight) largely attenuated SC79-induced Akt phosphorylation after cerebral I/R, and abolished the protection by SC79, suggesting that SC79-induced protection against cerebral is mediated via the PI3K/Akt signal pathway dependent mechanisms.
Cerebral I/R induces neuronal apoptosis, which contributes to brain injury in response to I/R. It has previously been reported that acute cerebral I/R injury is related to the inhibition of apoptosis through suppression of NF-κB/PUMA signaling pathway [24]. Activating PI3K/Akt signaling has been demonstrated to protect cells from apoptosis induced by I/R [25,26]. Activated PI3K/Akt can phosphorylates pro-apoptotic molecule Bax, leading to dissociation of Bax from anti-apoptotic molecule Bcl-2 [27]. In our study, we found that I/R could indeed induce Bim expression and decrease bcl-2 expression in the brain tissues. And SC79 administration significantly reduced I/R-induced apoptosis and I/R-induced upregulation of Bax, increased bcl-2 expression in the brain tissues, indicating that PI3K/Akt signaling activation contribute to the SC79-induced protective effect against cerebral I/R injury. However, treating mice with LY294002 before SC79 administration increased I/R-induced apoptosis and I/R-induced upregulation of Bax, decreased bcl-2 expression in the brain tissues, and completely abolished SC79-induced protection against cerebral I/R injury, suggesting the anti-apoptotic effect of SC79 may be one of the mechanisms by which SC79 attenuated cerebral I/R injury.
Conclusions
SC79 significantly decreased I/R-induced infarct volume and improved neurological score following cerebral I/R. We also found that activation of PI3K/Akt signaling was involved in the effect of SC79 against cerebral I/R injury. SC79 may provide a new therapeutic strategy for treating cerebral I/R injury.
Footnotes
Source of support: Departmental sources
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2010 update: A report from the American Heart Association. Circulation. 2010;121(7):e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Turley KR, Toledo-Pereyra LH, Kothari RU. Molecular mechanisms in the pathogenesis and treatment of acute ischemic stroke. J Invest Surg. 2005;18:207–18. doi: 10.1080/08941930591004449. [DOI] [PubMed] [Google Scholar]
- 3.Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):267–82. doi: 10.1016/S0278-5846(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 4.Fujimura M, Morita-Fujimura Y, Kawase M, et al. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci. 1999;19:3414–22. doi: 10.1523/JNEUROSCI.19-09-03414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sairanen T, Karjalainen-Lindsberg ML, Paetau A, et al. Apoptosis dominant in the periinfarct area of human ischaemic stroke – a possible target of antiapoptotic treatments. Brain. 2006;129:189–99. doi: 10.1093/brain/awh645. [DOI] [PubMed] [Google Scholar]
- 6.Lv Z, Liu C, Zhai M, et al. LPS pretreatment attenuates cerebral ischaemia/reperfusion injury by inhibiting inflammation and apoptosis. Cell Physiol Biochem. 2018;45:2246–56. doi: 10.1159/000488170. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Yan D, Liu X, et al. U0126 protects hippocampal CA1 neurons against forebrain ischemia-induced apoptosis via the ERK1/2 signaling pathway and NMDA receptors. Neurol Res. 2018;40:318–23. doi: 10.1080/01616412.2018.1441693. [DOI] [PubMed] [Google Scholar]
- 8.Harvey PA, Leinwand LA. The cell biology of disease: Cellular mechanisms of cardiomyopathy. J Cell Biol. 2011;194:355–65. doi: 10.1083/jcb.201101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Zhang X, Cui H, et al. Apelin-13 protects the brain against ischemia/reperfusion injury through activating PI3K/Akt and ERK1/2 signaling pathways. Neurosci Lett. 2014;568:44–49. doi: 10.1016/j.neulet.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Zhang H, Xu X, et al. bFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol Lett. 2012;212:137–46. doi: 10.1016/j.toxlet.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Yu Z, Cai M, Li X, et al. Neuroprotective effects of Tongxinluo on focal cerebral ischemia and reperfusion injury in rats associated with the activation of the MEK1/2/ERK1/2/p90RSK signaling pathway. Brain Res. 2018;1685:9–18. doi: 10.1016/j.brainres.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Jo H, Mondal S, Tan D, et al. Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc Natl Acad Sci USA. 2012;109(26):10581–86. doi: 10.1073/pnas.1202810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D, Zhang H, Hao S, et al. Akt specific activator SC79 protects against early brain injury following subarachnoid hemorrhage. ACS Chem Neurosci. 2016;7(6):710–18. doi: 10.1021/acschemneuro.5b00306. [DOI] [PubMed] [Google Scholar]
- 14.McCullough LD, Zeng Z, Blizzard KK, et al. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–12. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 15.Lu C, Ha T, Wang X, et al. The TLR9 ligand, CpG-ODN, induces protection against cerebralischemia/reperfusion injury via activation of PI3K/Akt signaling. J Am Heart Assoc. 2014;3(2):e000629. doi: 10.1161/JAHA.113.000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara H, Huang PL, Panahian N, et al. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J Cereb Blood Flow Metab. 1996;16:605–11. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Hara H, Friedlander RM, Gagliardini V, et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA. 1997;94:2007–12. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa M, Sekizuka E, Yamaguchi N, et al. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2007;292:H2306–15. doi: 10.1152/ajpheart.00601.2006. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–70. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- 20.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–9. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 21.Dudek H. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–65. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 22.Zheng K, Zhang Q, Lin G, et al. Activation of Akt by SC79 protects myocardiocytes from oxygen and glucose deprivation (OGD)/re-oxygenation. Oncotarget. 2017;8:14978–87. doi: 10.18632/oncotarget.14785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira JB, Wohlwend M, Alves MN, et al. A small molecule activator of AKT does not reduce ischemic injury of the rat heart. J Transl Med. 2015;13:76. doi: 10.1186/s12967-015-0444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Song C, Feng B, Fan W. Neuroprotection by triptolide against cerebral ischemia/reperfusion injury through the inhibition of NF-κB/PUMA signal in rats. Ther Clin Risk Manag. 2016;12:817–24. doi: 10.2147/TCRM.S106012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujio Y, Nguyen T, Wencker D, et al. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101(6):660–67. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Li X-M, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol. 2000;151:483–94. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrmann JL, Beham AW, Sarkiss M, et al. Bcl-2 suppresses apoptosis resulting from disruption of the NF-kappa B survival pathway. Exp Cell Res. 1997;237(1):101–9. doi: 10.1006/excr.1997.3737. [DOI] [PubMed] [Google Scholar]