Abstract
Background
Patients with systemic lupus erythematosus (SLE), especially with lupus nephritis (LN), undergo vascular damage and repair during the course of the disease. Since the recently identified angiogenic T cells (Tang) are involved in endothelial repair coupled with endothelial progenitor cells (EPCs), this study investigated the circulating Tang cells in LN patients and their potential correlations with disease features.
Material/Methods
Circulating Tang cells and EPCs were assessed by flow cytometry in peripheral blood samples from 67 SLE patients; of these, 32 had LN and 30 were matched healthy controls (HCs). The plasma levels of interleukin IL-17, IL-8, and vascular endothelial growth factor (VEGF) were quantified by immunoassays.
Results
The percentage of circulating Tang cells in LN patients was significantly increased as compared to the non-LN patients and HCs, and they were positively correlated with the level of EPC and VEGF. Additionally, circulating Tang cell percentages were positively correlated with the extent of proteinuria in LN patients.
Conclusions
The increased levels of circulating Tang cells in LN patients might play a role in the balance of endothelium dysfunction in these patients.
MeSH Keywords: Allergy and Immunology, Lupus Nephritis, T-Lymphocytes
Background
Systemic lupus erythematosus (SLE) is a common autoimmune disease characterized by the over-activation of lymphocytes and production of auto-antibodies that affect multiple organ systems and has a wide spectrum of clinical manifestations and severity. SLE is associated with accelerated atherosclerosis and increased cardiovascular disease that cannot be fully accounted for by the conventional cardiovascular risk factors [1]. Thus, in addition to chronic inflammation, adhesion molecules, and overexpression of pro-inflammatory cytokines, the dysregulated T cell subsets are proposed to play a critical role in the increased risk of cardiovascular diseases [2].
Therefore, a specific T cell subset and angiogenic T cells (Tang) that enhance endothelial repair function and the formation of new blood vessels by cooperating with the endothelial progenitor cells (EPC) are under intensive study [3]. EPC is a heterogeneous population involved in vasculogenesis and vascular repair [4]. Reportedly, the depletion of Tang population abrogates the functionality of EPC, and in vivo experiments showed that Tang cells play a major role in capillary formation [3]. Circulating Tang cells are characterized by the co-expression of CD3, CD31 (platelet endothelial cell adhesion molecule), and CXCR4 (receptor for stromal-derived factor-1) [3]. Furthermore, as a subset of CD3+ T cells, Tang cells also express CD4 (CD4+CD31+CXCR4+) or CD8 (CD8+CD31+CXCR4+). However, no significant differences were observed in the percentages of Tang and CD4+ Tang cells between SLE patients and healthy controls (HCs). Recently, we described an increase in the CD8+CD31+CXCR4+ T cells (CD8+ Tang) in SLE patients, especially in the patients with autoantibody [5].
As reported previously, the renal involvement, termed lupus nephritis (LN), is one of the most frequent manifestations of SLE and is associated with significant morbidity and mortality [6]. Furthermore, LN is considered a vital factor for endothelial dysfunction and accelerated atherosclerosis displayed by SLE patients [7,8]. Thus, the present study analyzed the percentages of Tang cells in LN patients, and their potential correlation to clinical and laboratory features associated with inflammation or endothelial balance in such patients.
Material and Methods
Participants
A total of 67 SLE patients and 30 age- and sex-matched HCs were included in the study. A total of 32 SLE patients were categorized with LN based on the results of renal biopsy and immunohistochemistry. All lupus patients fulfilled the American College of Rheumatology revised classification criteria for SLE [9], and all LN patients were confirmed according to the World Health Organization classification [10]. The disease activity was assessed using the SLE disease activity index (SLEDAI) [11]. All exclusion criteria of the subjects were combined with other autoimmune diseases and renal biopsy contraindications. The baseline characteristics of the patients and HCs are shown in Table 1. This study conformed to the recommendations of the Declaration of Helsinki and was approved by the Ethics Committee of Xijing Hospital. Informed consent was obtained from all subjects.
Table 1.
Demographic and clinical characteristics of SLE patients and healthy controls (HCs).
| Characteristics | LN | Non-LN | HC |
|---|---|---|---|
| Number of patients | 32 | 35 | 30 |
| Sex (Male/Female) | 4/28 | 5/30 | 4/26 |
| Age, median (IQR), (y) | 33.0 (23.5–40.8) | 33.0 (25.0–45.0) | 30.5 (24.5–34.0) |
| Disease duration, median (IQR), (y) | 4.0 (1.3–8.0) | 3.0 (1.0–8.0) | Na |
| Clinical features | |||
| SLEDAI, median (IQR) | 10.0 (5.3–13.0) | 11.0 (8.0–14.0) | Na |
| ESR, mm/hour, median (IQR) | 25.0 (15.3–46.8) | 30.0 (14.0–39.0) | Na |
| CRP, mg/dl, median (IQR) | 1.0 (0.4–1.8) | 1.2 (0.5–1.9) | Na |
| Positivity of anti-dsDNA, n (%) | 22 (68.8) | 21 (60.0) | Na |
| Positivity of anti-Sm, n (%) | 16 (50.0) | 18 (56.3) | Na |
| Positivity of anti-SSA/SSB, n (%) | 12 (37.5) | 11 (31.4) | Na |
| Cardiovascular risk factors, n (%) | |||
| Smoking | 3 (9.4) | 4 (11.4) | 2 (6.7) |
| Diabetes mellitus | 1 (3.1) | 1 (2.9) | 0 (0) |
| Hyperlipidemia | 4 (15.4) | 3 (8.6) | 3 (10.0) |
| Hypertension | 8 (25.0) | 9 (25.7) | 6 (20.0) |
| Treatment, n (%) | |||
| None or NSAIDs | 5 (15.6) | 7 (20.0) | Na |
| Antimalarial drugs | 11 (42.3) | 10 (35.7) | Na |
| Glucocorticoids | 20 (76.9) | 19 (67.8) | Na |
| Immunosuppressive drugs* | 8 (30.8) | 6 (21.4) | Na |
| Tripterygium glycosides | 15 (46.9) | 12(34.3) | Na |
LN – lupus nephritis patients; non-LN – SLE patients without lupus nephritis; IQR – interquartile range; n: number; SLEDAI – SLE disease activity index; ESR – erythrocyte sedimentation rate; CRP – C-reactive protein; NSAIDs – non-steroidal anti-inflammatory drugs; na – not applicable;
Azathioprine, mycophenolate mofetil.
Flow cytometry analysis
The percentages of circulating Tang cells and EPCs were measured by flow cytometry. Briefly, according to the manufacturer’s instructions, Tang cells were stained with peridinin chlorophyll protein (Percp)-conjugated CD3, phycoerythrin (PE)-conjugated CD31, and allophycocyanin (APC)-conjugated CXCR4 (BD Biosciences, San Diego, CA, USA). The EPCs were stained with fluorescein isothiocyanate (FITC)-conjugated CD34 (BD Bioscience), PE-conjugated VEGFR2 (BD Bioscience), and APC-conjugated CD133 (Miltenyi Biotech, Bergisch Gladbach, Germany). The appropriately conjugated IgG antibodies were used as isotype controls. The labeled cells were acquired on a FACS Calibur flow cytometer (BD Biosciences) and data were analyzed using Cell Quest software (BD Bioscience) and FlowJo 7.6.1 software (Tree Star, USA).
Quantification of cytokine plasma levels
The plasma samples were collected and preserved at −80°C until the evaluation of cytokines. The plasma levels of interleukin (IL)-17, IL-8, and vascular endothelial growth factor (VEGF) were quantified using an enzyme-linked immunosorbent assay (ELISA) kit (eBioscience, San Diego, CA, USA), according to the manufacturer’s instructions.
Statistical analysis
The comparison between the groups was evaluated by Mann–Whitney U test. The correlations were analyzed using Spearman’s rank correlation analysis. All analyses were performed using SPSS 17.0 software (SPSS, Chicago, IL, USA). A P value <0.05 was considered as statistically significant.
Results
Characteristics of subjects
A total of 32 LN patients, 35 patients without LN (non-LN patients), and 30 HCs were recruited in this study. All LN patients were confirmed biopsy-proven class III or IV. As illustrated in Table 1, the conventional cardiovascular risk factors, including smoking, diabetes, hyperlipidemia, and hypertension, which are negatively correlated with Tang cells [3], were comparable among the LN patients, non-LN patients, and HCs. In addition, no significant difference was observed in disease duration, SLEDAI, ESR, CRP, and positive rate of auto-antibodies, and current medications were similar between the LN and non-LN patients.
Percentages of Tang cells in SLE patients with or without LN
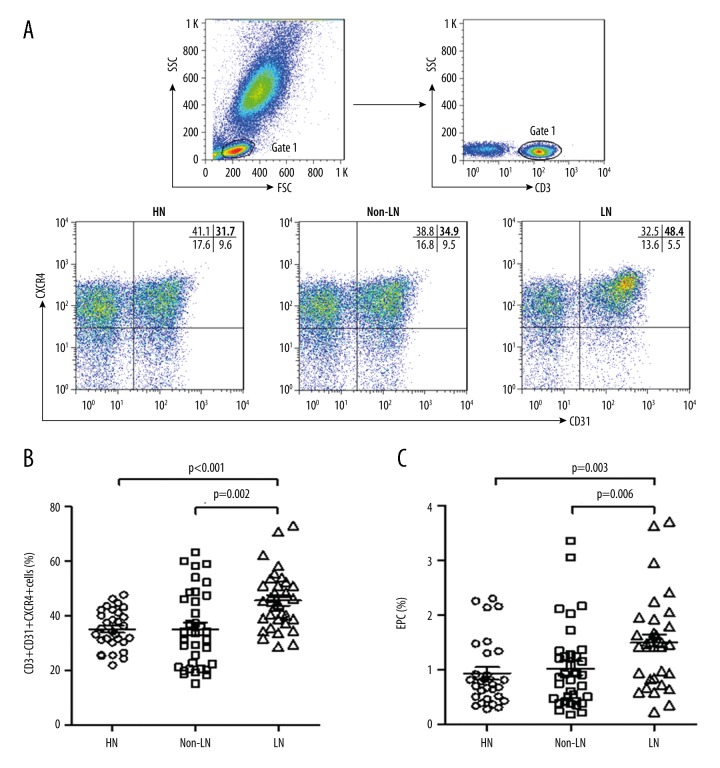
To evaluate the prevalence of the circulating Tang cells in SLE patients with or without LN, the percentages of CD31+CXCR4+ cells in CD3+ T cells were analyzed by flow cytometry. As shown in Figure 1, the percentages of circulating CD31+CXCR4+ cells in total CD3+ T cells (Tang) in LN patients were significantly increased as compared to the non-LN patients and HCs. In addition, the levels of circulating EPCs were also increased in LN patients as compared to the non-LN patients and HCs. We also observed that the percentages of circulating Tangs were significantly positively correlated with the EPC levels in LN and non-LN patients (Table 2).
Figure 1.
Presence of circulating Tang cells in SLE patients and HCs. (A) Flow cytometric dot-plots of circulating Tang cells (CD31+CXCR4+ cells in CD3+ T cells) obtained from 1 representative healthy control (HC), and SLE patients with lupus nephritis (LN) and without lupus nephritis (non-LN). Percentages of circulating Tang cells (B) and EPCs (CD34+CD133+VEGFR2+ cells) (C) in HC, LN, and non-LN patients.
Table 2.
Correlations between the percentages of Tang cells and EPC or cytokines in SLE patients.
| Tang (%) | |||
|---|---|---|---|
| r | p | ||
| Non-LN | |||
| EPC (%) | 0.577 | <0.001 | |
| IL-17 (pg/ml) | 0.214 | 0.216 | |
| IL-8 (pg/ml) | −0.128 | 0.465 | |
| VEGF (pg/ml) | 0.162 | 0.352 | |
| LN | |||
| EPC (%) | 0.433 | 0.013 | |
| IL-17 (pg/ml) | −0.102 | 0.577 | |
| IL-8 (pg/ml) | −0.101 | 0.582 | |
| VEGF (pg/ml) | 0.431 | 0.014 | |
Plasma cytokine levels in SLE patients with or without LN
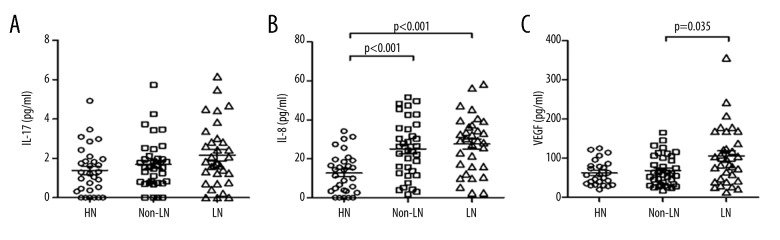
To evaluate the possible relationship between Tang cells and cytokines involved in vasculogenesis and vascular repair, the plasma levels of IL-17, IL-8, and VEGF were assessed in SLE patients and HCs. As shown in Figure 2, no significant differences were observed in the levels of IL-17 between the 3 groups. Although the LN and non-LN patients exhibited higher levels of IL-8 as compared to HCs, the differences were not significant between the LN and non-LN patients (Figure 2). Moreover, significantly higher VEGF levels were observed in LN patients as compared to the non-LN patients (Figure 2). Additionally, the levels of VEGF, but not IL-17 and IL-8, were positively correlated with circulating Tang cells in LN patients (Table 2).
Figure 2.
Plasma levels of cytokines in SLE patients and HCs. The plasma levels of IL-17 (A), IL-8 (B), and VEGF (C) in SLE patients with lupus nephritis (LN), SLE patients without lupus nephritis (non-LN), and HCs.
Correlations of Tang cells with characteristics in SLE patients with or without LN
Subsequently, we searched for clinical features of LN patients that might be associated with Tang cells. As shown in Table 3, no significant correlation was observed among the analyzed clinical features between Tang cells and SLEDAI, ESR, CRP, or disease duration in LN and non-LN patients. In line with our previous study [5], the percentages of circulating Tang cells were negatively correlated with age. As most of the non-LN patients did not have urinary protein, a correlation was established between the levels of Tang cells and the extent of proteinuria in LN patients. The circulating Tang cells showed a positive correlation with the extent of proteinuria in LN patients (Table 3).
Table 3.
Correlations between the percentages of Tang cells and clinical features in SLE patients.
| Tang (%) | |||
|---|---|---|---|
| r | p | ||
| Non-LN | |||
| Age (y) | −0.470 | 0.012 | |
| Disease duration (y) | −0.069 | 0.694 | |
| ESR (mm/hour) | −0.109 | 0.532 | |
| CRP (mg/dl) | −0.199 | 0.251 | |
| Complement C3 (g/L) | −0.017 | 0.923 | |
| Complement C4 (g/L) | −0.118 | 0.500 | |
| SLEDAI | −0.212 | 0.221 | |
| LN | |||
| Age (y) | −0.497 | 0.004 | |
| Disease duration (y) | 0.289 | 0.108 | |
| ESR (mm/hour) | −0.182 | 0.318 | |
| CRP (mg/dl) | 0.176 | 0.335 | |
| Complement C3 (g/L) | 0.038 | 0.837 | |
| Complement C4 (g/L) | −0.152 | 0.406 | |
| SLEDAI | 0.072 | 0.695 | |
| Proteinuria (g/day) | 0.658 | 0.000 | |
Discussion
SLE is a chronic autoimmune disease with catastrophic clinical and pathological manifestations. Previous studies reported that approximately 30% of SLE patients with vascular disease and 60% of SLE patients present LN [12–14]. Furthermore, LN plays a major role in endothelial dysfunction and accelerated atherosclerosis, and is related to a higher risk of cardiovascular disease than with other forms of organ involvement in SLE patients [7,8,15]. Reportedly, circulating Tang cells enhanced the endothelial repair function by cooperating with EPC, which might be used as a biomarker for endothelial function and cardiovascular risk [3,5]. Therefore, in this study, the percentages of circulating Tang cells and their putative correlation to clinical and laboratory features were assessed in SLE patients with or without LN. Accordingly, our data showed that the percentages of circulating Tang cells in LN patients were significantly increased as compared to the non-LN patients and HCs.
As reported previously, LN patients have an increased risk of cardiovascular diseases [8]. This phenomenon might be partially explained by the high prevalence of conventional risk factors and immunological abnormalities [16] and the elevated serum creatinine and proteinuria in LN patients [17]. Therefore, the present data support that LN patients are associated with endothelial damage and high cardiovascular risk, and the increased levels of circulating Tang cells may be related to increased cardiovascular risk and renal involvement.
Similar to recent studies in SLE patients [18,19], our data show that the levels of circulating EPC were also increased in LN patients. Furthermore, the circulating Tang cell percentages were significantly positively associated with EPC levels in non-LN and LN patients. EPCs originate from hematopoietic stem cells and have a physiological role in vascular repair and vasculogenesis [4]. Moreover, Tang cells are critical in the formation of EPC colonies that can enhance EPC differentiation and angiogenesis by secreting angiogenic cytokines [3]. Consequently, these findings are in agreement with the theory that enhanced EPC and Tang cell populations serve as repair responses to the increased impairment of endothelial cells present in SLE patients [20].
As reported previously [21–23], several pro-inflammatory and immunoregulatory cytokines, including VEGF, IL-8, and IL-17A, are involved in angiogenesis. By secreting these proangiogenic cytokines, Tang cells can facilitate the differentiation of EPCs and stimulate the resident endothelial cells [3]. Moreover, these cytokines are known to be dysregulated in autoimmune diseases, which could be a relevant immune-mediated mechanism underlying the vascular damage. In the present study, we did not find a significant difference in the levels of IL-17 and IL-8 between LN and non-LN patients. As compared to the non-LN patients, increased levels of plasma VEGF were observed in LN patients. Moreover, as the main angiogenic cytokine secreted by Tang cells, VEGF was positively correlated with the percentages of circulating Tang cells in LN patients. These data demonstrate that VEGF plays a critical role in vasculogenesis and vascular repair due to its role in endothelial proliferation and initiation of vessel sprouting [24,25].
We did not find statistically significant correlations between Tang cells and clinical features, including SLEDAI, ESR, CRP, Complement C3, Complement C4, and disease duration, in non-LN and LN patients. Similar to the data from previous studies [3,5,26,27] and the present study, the levels of circulating Tang cells were inversely correlated with age in non-LN and LN patients. We also showed that the percentages of circulating Tang cells were positively correlated with the extent of proteinuria in LN patients. Similar results were also found in patients with systemic sclerosis (SSc), a common autoimmune disease, showing that circulating Tang cells are selectively increased in SSc patients with severe peripheral vascular complications like digital ulcers, and indicating that Tang cells may be a biomarker reflecting vascular damage severity [28]. Consistently, our data support the theory that Tang cells are a potential biomarker to identify and monitor patients with renal involvement in SLE.
LN is known to contribute to high mortality in SLE patients, and the identification of a noninvasive biomarker might predict lupus nephritis as early diagnosis, and then be used to monitor LN relapses during follow-up. However, our results may be influenced by medication history of patients and the single pathological type; therefore, further studies with larger sample sizes and longer follow-up observation are required to confirm the present findings.
Conclusions
In conclusion, we demonstrated that an increase in the circulating Tang cells was observed in patients with LN. However, no obvious correlation was observed between Tang cells and SLEDAI. The correlation with the extent of proteinuria suggests that the assessment of circulating Tang cells is a potentially useful biomarker to recognize and monitor LN. The observed relationships among increased circulating Tang cells, EPCs, and VEGF levels in LN patients support the hypothesis that Tang cells play a critical role in the repair of damaged endothelium in LN patients during the treatment period.
Footnotes
Conflicts of interest
None.
Source of support: This study was funded by the National Basic Research Program of China (no. 2015CB553700-04) and the National Natural Science Foundation of China (no. 81401338)
References
- 1.Gorman C, Isenberg D. Atherosclerosis and lupus. Rheumatology (Oxford) 2004;43:943–45. doi: 10.1093/rheumatology/keh217. [DOI] [PubMed] [Google Scholar]
- 2.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–37. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Hur J, Yang HM, Yoon CH, et al. Identification of a novel role of T cells in postnatal vasculogenesis: Characterization of endothelial progenitor cell colonies. Circulation. 2007;116:1671–82. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–67. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Miao J, Qiu F, Li T, et al. Circulating angiogenic T cells and their subpopulations in patients with systemic lupus erythematosus. Mediators Inflamm. 2016;2016:2842143. doi: 10.1155/2016/2842143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82:299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 7.Karsh J, Klippel JH, Balow JE, Decker JL. Mortality in lupus nephritis. Arthritis Rheum. 1979;22:764–69. doi: 10.1002/art.1780220712. [DOI] [PubMed] [Google Scholar]
- 8.Serikova S, Kozlovskaia NL, Shilov EM. [Lupus nephritis as a factor of atherosclerosis risk in patients with systemic lupus erythematosus]. Ter Arkh. 2008;80:52–58. [in Russian] [PubMed] [Google Scholar]
- 9.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz GS, D’Agati VD. Classification of lupus nephritis. Curr Opin Nephrol Hypertens. 2009;18:220–25. doi: 10.1097/mnh.0b013e328327b379. [DOI] [PubMed] [Google Scholar]
- 11.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 12.Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;49:2407–15. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 13.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 14.Cameron JS. Lupus nephritis. J Am Soc Nephrol. 1999;10:413–24. doi: 10.1681/ASN.V102413. [DOI] [PubMed] [Google Scholar]
- 15.Wang XY, Tang XQ, Huang YJ, et al. Frequency of established cardiovascular disease and its risk factors in Chinese patients with systemic lupus erythematosus. Clin Rheumatol. 2012;31:669–75. doi: 10.1007/s10067-011-1910-3. [DOI] [PubMed] [Google Scholar]
- 16.Font J, Ramos-Casals M, Cervera R, et al. Cardiovascular risk factors and the long-term outcome of lupus nephritis. QJM. 2001;94:19–26. doi: 10.1093/qjmed/94.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Tao J, Tang X, et al. Prevalence and correlation of conventional and lupus-specific risk factors for cardiovascular disease in Chinese systemic lupus erythematosus patients. J Eur Acad Dermatol Venereol. 2012;26:95–101. doi: 10.1111/j.1468-3083.2011.04211.x. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Carrio J, Prado C, de Paz B, et al. Circulating endothelial cells and their progenitors in systemic lupus erythematosus and early rheumatoid arthritis patients. Rheumatology (Oxford) 2012;51:1775–84. doi: 10.1093/rheumatology/kes152. [DOI] [PubMed] [Google Scholar]
- 19.Robak E, Kierstan M, Cebula B, et al. Circulating endothelial cells and angiogenic proteins in patients with systemic lupus erythematosus. Lupus. 2009;18:332–41. doi: 10.1177/0961203308097572. [DOI] [PubMed] [Google Scholar]
- 20.Clancy R, Marder G, Martin V, et al. Circulating activated endothelial cells in systemic lupus erythematosus: Further evidence for diffuse vasculopathy. Arthritis Rheum. 2001;44:1203–8. doi: 10.1002/1529-0131(200105)44:5<1203::AID-ANR204>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 21.Bernardini G, Ribatti D, Spinetti G, et al. Analysis of the role of chemokines in angiogenesis. J Immunol Methods. 2003;273:83–101. doi: 10.1016/s0022-1759(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 22.O’Neill TJ, 4th, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97:1027–35. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 23.Moran EM, Connolly M, Gao W, et al. Interleukin-17A induction of angiogenesis, cell migration, and cytoskeletal rearrangement. Arthritis Rheum. 2011;63:3263–73. doi: 10.1002/art.30582. [DOI] [PubMed] [Google Scholar]
- 24.Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell. 2009;17:377–86. doi: 10.1016/j.devcel.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–36. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez P, Rodriguez-Carrio J, Martinez-Zapico A, et al. Senescent profile of angiogenic T cells from systemic lupus erythematosus patients. J Leukoc Biol. 2016;99:405–12. doi: 10.1189/jlb.5HI0215-042R. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Carrio J, Alperi-Lopez M, Lopez P, et al. Angiogenic T cells are decreased in rheumatoid arthritis patients. Ann Rheum Dis. 2015;74:921–27. doi: 10.1136/annrheumdis-2013-204250. [DOI] [PubMed] [Google Scholar]
- 28.Manetti M, Pratesi S, Romano E, et al. Angiogenic T cell expansion correlates with severity of peripheral vascular damage in systemic sclerosis. PLoS One. 2017;12(8):e0183102. doi: 10.1371/journal.pone.0183102. [DOI] [PMC free article] [PubMed] [Google Scholar]