Abstract
Viruses of extreme thermophiles are of great interest because they serve as model systems for understanding the biochemistry and molecular biology required for life at high temperatures. In this work, we report the discovery, isolation, and preliminary characterization of viruses and virus-like particles from extreme thermal acidic environments (70–92°C, pH 1.0–4.5) found in Yellowstone National Park. Six unique particle morphologies were found in Sulfolobus enrichment cultures. Three of the particle morphologies are similar to viruses previously isolated from Sulfolobus species from Iceland and/or Japan. Sequence analysis of their viral genomes suggests that they are related to the Icelandic and Japanese isolates. In addition, three virus particle morphologies that had not been previously observed from thermal environments were found. These viruses appear to be completely novel in nature.
Viruses of extreme thermophiles are of great interest because they serve as model systems for understanding the biochemistry and molecular biology required for life at high temperatures. Of the three domains of life, Eukarya, Bacteria, and Archaea, organisms belonging to the archaeal domain are well represented in extreme environments (1). Because of their recent discovery, our understanding of archaeal organisms is in its infancy. Unlike the other domains of life, very few viruses of Archaea have been characterized to date. Only 12 different virus morphologies have been described from the Archaea (2). Most of these viruses have been isolated from extreme halophiles (especially Halobacterium spp.) or methanogens (Methanobacterium spp.). The majority of these viruses, with two exceptions, have similar head and tail morphologies to T phages and lambdoid phages belonging to the Myoviridae and Siphoviridae groups. In contrast, four completely unique virus morphotypes, which have necessitated the formation of four unique taxonomic groups (described below; ref. 2), have been isolated from the thermophilic archaeal genera Sulfolobus and Thermoproteus.
Sulfolobus solfataricus is one of the best-characterized members of the Archaea. Sulfolobus spp. are aerobic acidophiles that grow at an optimum of 80°C (with a range of 70°C to 87°C) and pH 3 (with a range of 1.5 to 5.5) and have been isolated from acidic hot springs in Yellowstone National Park (YNP), Japan, Iceland, New Zealand, El Salvador, The Dominican Republic (3), Russia (unpublished results), and Italy (4). Sulfolobus is likely to be present in most hot springs of the world that maintain this temperature and pH range. S. solfataricus is emerging as a model organism for the study of Archaea, because it can be easily cultured in the laboratory, the sequence of its 3-MB genome is complete (5), and transformation systems have been developed that greatly facilitate its genetic analysis (6, 7).
The viruses of Sulfolobus are the best studied of the archaeal viruses and represent four additional viral families: Rudiviridae, Lipothrixviridae, Fuselloviridae, and Guttaviridae. Members of the family Fuselloviridae have been isolated from acidic hot springs in Japan [Sulfolobus shibatae virus 1 (SSV1) (8)] and Iceland [SSV2 and SSV3 (9)]. All members of this group are characterized by their unique 60 × 90-nm spindle shape virions that possess an ≈15.5-kb circular double-stranded DNA (dsDNA) genome with about 35 ORFs (10). Two members of the family Rudiviridae [Sulfolobus islandicus rod virus 1 (SIRV1) and SIRV2] have been isolated from Iceland. The SIRV virions are stiff 23 × 800- to 900-nm helical rods that possess linear dsDNA genomes with covalently closed ends 33–36 kb in length, containing about 45 ORFs (11). SIFV is a member of the family Lipothrixviridae, which is characterized by 24 × 1,980-nm flexible rods with putative attachment fibers at both ends. The linear dsDNA genome of SIFV is estimated to be 42 kb in length with about 74 ORFs (12). The fourth group of Sulfolobus viruses is represented by Sulfolobus neozealandicus droplet-shaped virus, which is characterized by its bearded droplet-like morphology and a circular and highly modified dsDNA genome estimated to be 20 kb. Surprisingly, with the exception of SIRV (D. Prangishvili, personal communication), very few ORFs from the viral genomes have any significant identity to sequences in the publicly available databases. Most of the members of these four groups of viruses are not lytic. The Fusselloviridae appear to propagate in a lysogenic manner.
Previously, no viruses of Sulfolobus had been isolated from YNP despite it being the original site of discovery for Sulfolobus (3). In this work, we report the discovery, isolation, and preliminary characterization of viruses and virus-like particles from Sulfolobus species found in diverse thermal environments of YNP.
Materials and Methods
Sampling Locations.
Sampling sites were selected that had a range of temperature (70–92°C) and pH (1.0–4.5). Eight different thermal sites within YNP were selected for sample collection over a 12-month period (Table 1). Hot springs, mud pots, and soils were included in this sampling population.
Table 1.
YNP thermal features selected for sample collections
| Site name | X Position* | Y Position* | Temperature | pH | # of Samples collected | # of Cultures established |
|---|---|---|---|---|---|---|
| Amphitheater Springs | 521734.7 | 4960405.6 | 74–85 | 1.0–3.5 | 34 | 5 |
| Mud Volcano | 544849.3 | 4941373.9 | 70–85 | 2.0–4.0 | 25 | 8 |
| Norris Basin | 522734.3 | 4953262.0 | 70–84 | 1.5–4.5 | 60 | 5 |
| Nymph Lake | 521477.2 | 4955457.2 | 74–84 | 2.0–4.5 | 16 | 7 |
| Gibbon Basin | 518444.3 | 4949627.8 | 70–83 | 1.8–3.5 | 9 | 8 |
| Fountain Flats | 513307.2 | 4934500.0 | 74–87 | 3.0–4.1 | 8 | 4 |
| Rabbit Creek | 514867.9 | 4929918.2 | 72–92 | 2.9–3.9 | 16 | 11 |
| Crater Hills | 540943.3 | 4944686.8 | 73–81 | 2.5–3.6 | 15 | 3 |
Positions are UTM type projections, Datum type-NAD 83, zone 12.
Establishing Enrichment Cultures.
Two different methods were used to establish Sulfolobus enrichment cultures. The first method was based on previously described procedures (9). Briefly, liquid environmental samples were collected from the eight areas in sterile 50-ml centrifuge tubes. The pH of the samples was adjusted to 4.5–5.0 with solid CaCO3. Approximately 20 ml of the sample was subsequently injected with sterile 10-ml syringes into sealed 60-ml serum vials. Serum vials were prepared by adding 0.2 g elemental sulfur, 0.02 g resazurin as an oxygen indicator, and then replacing the ambient atmosphere with 1.1 atmosphere (atm; 1 atm = 101.3 kPa) CO2 and 0.1 atm of H2S to maintain anaerobicity. In the second method, samples were directly collected into 50-ml sterile centrifuge tubes. Soil samples from thermal sites also were collected directly into sterile 50-ml centrifuge tubes. All samples were transported at ambient temperature. Enrichment cultures of liquid environmental samples were established by withdrawing 1 ml with a sterile syringe and inoculating into long-necked Erlenmeyer flasks, to avoid excess evaporation, containing a salt base media previously developed for culturing Sulfolobus (9). Enrichment cultures from soil samples were established by addition of 1 g directly into liquid media in the flasks. Inoculated flasks were then incubated while shaking at 80°C for up to 24 days and monitored for microbial growth. Selected enrichment cultures positive for viruses (described below) were spread on solid media containing 0.6% gelrite (Kelco, San Diego) and incubated at 80°C to obtain single colony isolates.
16S rDNA Analysis.
Total DNA was extracted from single colony isolates replicating each virus type and representative enrichment cultures from each of the eight sampling sites by using previously described protocols (7). The 16S rDNA gene was amplified from the genomic DNA by standard PCR protocols using archaeal-specific primers 0023a forward (CTCCGGTTGATCCTGCC) or Sulfo1 (GCTATCGGGGTAGGGATAGC), a primer designed for amplification of Sulfolobus rDNA, and 1492 reverse (GGTTACCTTGTTACGACTT) (13). The resulting PCR products (≈1,500 bp in length) were subsequently cloned into pCR2.1 by using Topo-TA cloning protocols (Invitrogen). The complete 16S rDNA inserts were sequenced by Big Dye Termination protocols using an ABI 310 or 3700 automated capillary sequencer (Applied Biosystems). Multiple independent clones were sequenced. The complete sequence for each clone was determined. The resulting sequences were compared with sequences in the Ribosomal Database Project (http://rdp.cme.msu.edu/html/).
Virus Detection Methods.
Growth inhibition assay.
Enrichment cultures, or cultures established from single colony isolates expressing virus, were subjected to a spot growth inhibition assay by spotting 1–2 μl of the culture directly onto lawns of S. solfataricus strain P1 (14), S. islandicus REN1H1 and HVE10/4, S. acidocaldarius DSM639, and two Sulfolobus isolates from YNP (14). S. islandicus culture REY15/4 (containing SSV2; ref. 15) was used as a positive control. Plates were incubated for 2–3 days at 80°C and scored for lawn growth inhibition.
Direct visualization by electron microscopy.
All enrichment cultures were processed for visualization by transmission electron microscopy. Briefly outlined, cultures were grown to late stationary phase (8–10 days, 80°C) followed by 2 days at 22°C to allow for a period of temperature stress. Cells were removed by centrifugation (6,000 × g for 10 min). The supernatants were filtered through Acrodisc PF 0.8/0.2 μm filters (Pall Gelman Laboratory, Ann Arbor, MI), and the remaining particles were pelleted by centrifugation at 100,000 × g for 2 h. Pellets were resuspended in 20 μl of ddH20, stained with 1% uranyl acetate, and examined with a Zeiss 100CA transmission electron microscope.
DNA Library Construction and Analysis from Virus and Virus-Like Particles.
Total DNA libraries were constructed from the concentrated supernatant of separate cultures, each containing a single virus particle morphology. Total DNA was extracted from each sample by a proteinase K, SDS, and phenol method (16), digested with either EcoRI, SacII, or BamHI restriction endonucleases, ligated into pBlueScript II SK+, and transformed into XL2 Blue MRF′ Escherichia coli (Stratagene). Recombinant plasmids were screened for insert size. Inserts of different size from each library were sequenced with M13 forward and reverse primers by using Big Dye Termination (Applied Biosystems) on ABI 310 or 3700 automated DNA sequencers. Sequences were compared with known Sulfolobus viral sequences, and multiple sequence alignments were performed by using gcg, biology workbench (http://workbench.sdsc.edu), and blast (17).
Results
Geographic Distribution of Sulfolobus.
YNP is estimated to have >10,000 geothermal features. Many of these thermal features are acidic and are likely to be favorable environments for Sulfolobus species and their viruses. A total of 183 hot springs, mud pots, and soils in eight different sites within YNP were sampled in this study. A total of 51 enrichment cultures, representing all of the geographically distributed sites, were successfully established (Table 1).
16S rDNA Analysis of Enrichment Cultures.
The sequences of the 16S rDNA genes were determined from single colony isolates replicating five of the six virus types (see below) and selected enrichment cultures representing the eight geographically distributed sites. A single 16S rDNA sequence was detected from each of the enrichment cultures, suggesting a single dominant archaeal species. The 16S rDNA sequences from seven of the eight enrichment cultures demonstrated >99% identity with S. solfataricus. However, one enrichment culture established from Gibbon Basin had a 16S rDNA gene sequence that was most similar to Metallosphaera prunae (93% identity). However, two different 16S rDNA sequences were identified from single colony clones harboring viruses. Comparison of 16S rDNA sequences with those in the Ribosomal Database Project showed that single colony clones replicating SSV-like, SIRV-like, and Sulfolobus islandicus filamentous virus (SIFV)-like viruses (described below) were nearly identical (>98% identity) to the 16S rDNA gene sequence of S. solfataricus previously determined for isolates from Italy (5). In contrast, two of the clones containing virus morphologies had 16S rDNA sequences that were nearly identical to S. acidocaldarius (18).
Detection of Virus-Like Particles.
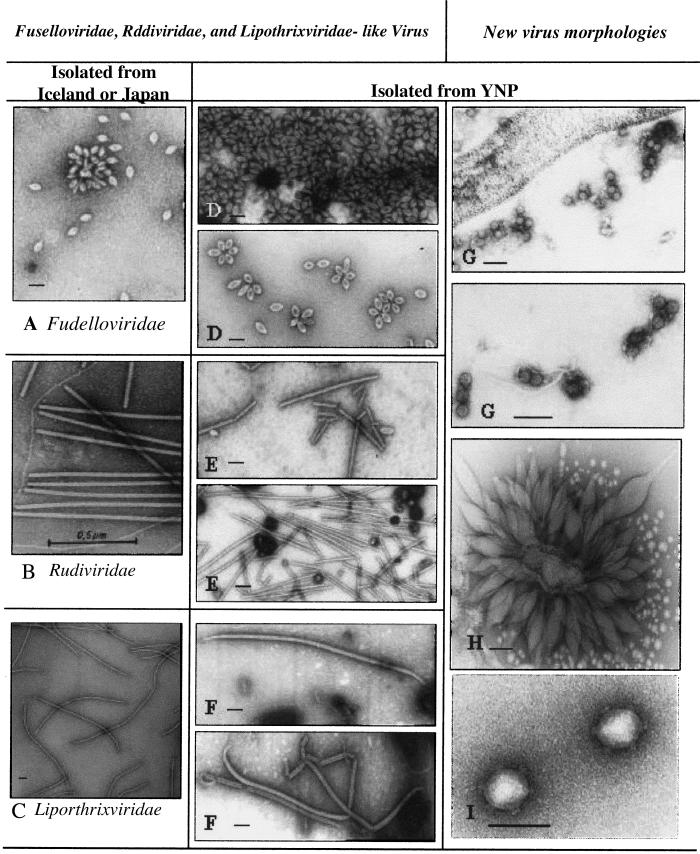
Six distinct virus and virus-like particle morphologies were detected in 22 of the 51 enrichment cultures (Fig. 1, Table 2). Single colony isolates replicating five of the six virus and virus-like particle morphologies were established. The virus or virus-like particle morphologies could be separated into two categories: those particles that have morphologies that appear similar to viruses isolated from Sulfolobus species from Japan and Iceland (Fig. 1 D–F), and those particles that have morphologies that have not been previously identified with viruses from Sulfolobus (Fig. 1 G–I). Twelve of the enrichment cultures contained multiple virus particle morphologies.
Figure 1.
Transmission electron micrographs of virus and virus-like particles isolated from YNP. (A) SSV1 Fusellovirus, (B) SIRV Rudivirus, and (C) SIFV Lipothrixvrus previously isolated from thermal areas of Japan or Iceland (kindly provided by W. Zillig, Max-Planck Institut für Biochemie, Martinsried, Germany). (D) SSV-like, (E) SIRV-like, and (F) SIFV-like particle morphologies isolated from YNP thermal features. (G–I) Virus-like P articles isolated from YNP thermal features. (Bars indicate 100 nm.)
Table 2.
YNP virus particle features
| Class | Morphology | Dimensions | # of Positive cultures |
|---|---|---|---|
| SSV-like | Lemon shaped | Variable 40 × 80 nm | 18 |
| SIRV-like | Rods | 25 × 900 nm | 6 |
| SIFV-like | Filaments | 50 × 900–1,500 nm | 2 |
| Unique | Spherical | 30 nm | 7 |
| Unique | A symmetrical spindle-shaped | 100 × 400 nm | 2 |
| Unique | Icosahedral | 52 nm | 2 |
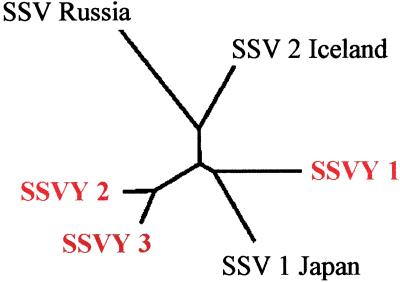
The most common virus morphology of the first category (18/51 of the enrichment cultures) appeared to be members of the Fuselloviridae. The virus particles were similar in size and shape to SSV-1 isolates from Japan (8) and SSV-2 isolates from Iceland (15). The 90 × 60-nm spindled-shape particles are often observed in rosette structures. These particles contain a ccc dsDNA genome of ≈15 kb. blast analysis of limited DNA sequence determined for three YNP isolates (SSVY1 from Amphitheater Springs, SSVY2 from Nymph Lake, and SSVY3 from Norris Basin) show similarity to SSV1, SSV2, and a SSV isolate that we recently isolated from Kamchatka, Russia (unpublished data). No other homologues were detected in the public databases. The coat protein gene (VP3) product of all isolates showed the most distinct sequence similarity (Table 3, Fig. 2; ref. 19). At the amino acid level there is a range of 36% to 91% identity between the SSV isolates. Phylogenetic comparison of the VP3 gene product indicates that two YNP isolates (SSVY2 and SSVY3) cluster together whereas SSVY1 is more closely related to SSV1 from Japan (Fig. 2). Although this limited analysis demonstrates that the YNP isolates are clearly related to the Japanese, Icelandic, and Russian isolates, there are differences associated with geographic location. However, one YNP isolate appears to be as distinct from the Icelandic, Japanese, and Russian isolates as the latter are from each other.
Table 3.
Amino acid sequence identity of viral coat protein 3 in SSVY1, SSVY2, SSVY3, SSV1, SSV2 and SSV Russia
| VP3 | SSV1 Japan | SSV2 Iceland | SSVY1 | SSVY2 | SSVY3 | SSV Russia |
|---|---|---|---|---|---|---|
| SSV1 | 100% | 66% | 69% | 68% | 64% | 36% |
| SSV2 | 100% | 57% | 66% | 65% | 48% | |
| SSVY1 | 100% | 64% | 71% | 37% | ||
| SSVY2 | 100% | 91% | 39% | |||
| SSVY3 | 100% | 39% | ||||
| SSV Russia | 100% |
Figure 2.
Dendrogram from sequence alignment of SSV VP3 protein sequences [phylip (19); biology work bench (http://workbench.sdsc.edu)].
The second virus particle morphology is characterized by rigid, helical 25 × 900-nm rods with characteristics similar to the Rudiviridae SIRV1 and SIRV2 from Iceland (20). The YNP SIRV-like particles were observed in six of the 51 enrichment cultures and were sometimes found in association with YNP SSV-like particles. The YNP SIRV-like particles possess dsDNA genomes 33–36 kb in length as estimated from viral DNA libraries. Sequence analysis indicates limited sequence similarity with SIRV-1 (D. Prangishvili, personal communication). The highest degree of similarity found in the analysis of 8 kb of sequence was 78% identical over a 393-nt span. Comparison of the predicted coat protein gene product revealed an 88% identity between the Icelandic and YNP isolates. Other regions of the genome had less than 50% sequence identity between the two isolates.
A third virus particle morphology is characterized by a long flexible rod with characteristics similar to those of the Lipothrixvirus, SIFV, previously isolated from Iceland (12). The YNP SIFV-like particles were observed in two of the 51 enrichment cultures. The YNP SIFV-like particles are 50 × 900- to 1,500-nm flexible rods with apparent attachment fibers at both ends. Partial sequence analysis (8.5 kb of noncontiguous sequence) showed a 45–65% range of identity with SIFV at the nucleotide level.
Three unique virus morphologies not previously associated with Sulfolobus were also observed (Fig. 1 G–I). Thirty-two-nanometer icosahedral virus-like particles were observed in seven of the 51 enrichment cultures from two separate sample sites (Fig. 1G). These virus-like particles do not appear to be enveloped. They do not form growth inhibition zones on any of the tested Sulfolobus lawns. Both high molecular weight dsDNA (>12 kb) and low molecular weight RNA (<0.5 kb) have been isolated from virus particle preparations. The extracted nucleic acid is infectious when electroporated into the virus-free host S. solfataricus P1. Virus particles identical to those originally observed from the YNP cultures appear 7–10 days after electroporation. This is also the case when virus-free S. solfataricus P1 is infected with the 32-nm particles themselves.
A unique morphology was observed in two of the 51 enrichment cultures (Fig. 1H). These virus-like particles have a large spindle-shaped center with appendages at each end of the central body. The dimensions of the central body are 100 × 200 nm. The appendixes have approximate dimensions of 50 × 100–200 nm. The overall tip-to-tip length is ≈400 nm. These virus-like particles are often observed in rosette-like structures and may be associated with the Sulfolobus S layer. The morphology of these virus-like particles does not resemble any known virus. The purified particles are infectious when mixed with the virus-free host S. solfataricus P2 strain. Virus particles identical to the inoculum were observed 5–10 days postinfection. dsDNA has been isolated from the virus particle preparations. Limited DNA sequence analysis shows only limited homology to regions of the SSV1 genome and reveals no significant sequence homology with other viruses isolated from Sulfolobus, the Sulfolobus genome, or any other sequences in the databases.
A third type of particle was detected in two of 51 enrichment cultures (Fig. 1I). The particles are 44–70 nm in diameter, nonenveloped, and appear to be icosahedral in morphology. At each of the vertices there are 10- to 20-nm projections with unique mushroom-shaped ends. There appear to be 12 projections per virion, suggesting that these projections may be from the 5-fold axes of the particle. dsDNA has been isolated from purified virus particles. Limited DNA sequence analysis shows no significant homology to other Sulfolobus viruses, the Sulfolobus genome, or sequences in the databases.
Discussion
We report on viruses and virus-like particles isolated from high-temperature acidic environments in YNP. Six different particle morphologies were found in Sulfolobus enrichment cultures grown at 80°C and pH 3. Virus and virus-like particles were readily detected in a high proportion of enrichment cultures (43%), suggesting that viruses are a common feature of Sulfolobus species in YNP. Three of the particle morphologies are similar to viruses previously isolated from Sulfolobus species from Iceland and/or Japan. Three virus particle morphologies have not been previously observed from thermal environments. Some of these morphologies appear to be completely novel.
Despite the fact that the YNP SSV-like, SIRV-like, and SIFV-like particles have nearly identical morphologies to Fuselloviruses (SSVs), Rudiviruses (SIRVs), and Lipothrixviruses (SIFV) isolated from Japan and Iceland, limited analysis of their genomic sequence indicates that they are genetically distinct. This genomic diversity may reflect their long-term geographic isolation or it may be a function of adaptation to unique features of Sulfolobus species present in YNP.
It is likely that this initial survey has underestimated the total diversity of viruses that are present in YNP thermal environments. In this study, only a limited number of representative high-temperature acidic sites were examined. These sites represent a fraction of the more than 10,000 thermal features of varying pH and temperature. In addition, culturing conditions were selected that were likely to support Sulfolobus growth, thereby limiting the growth of other potential virus hosts. A large number of potential virus host organisms are known to exist in YNP's thermal sites (21). We failed to observe virus particles by direct filtration of hot spring water by using techniques that have been highly successful for detection of viruses in marine environments (22). This failure may be due at least in part to the reduced total biomass present in acidic extreme thermal hot springs or the harshness of the environment that selects for viruses that principally reside inside their hosts.
Nevertheless, the use of enrichment cultures proved to be successful in isolating unique virus morphologies and viruses related to known viruses from extreme thermophiles. The detection of these virus morphologies suggests that there are many more viruses yet to be discovered in thermal environments. The fact that the viral morphologies mentioned here do not resemble other known viruses from either thermal or nonthermal environments suggests that their analysis will provide future insights into structure and function. It is possible that further analysis of these viruses will indicate that they represent new virus families, similar to the first four families of viruses found in Sulfolobus (23). This diversity of unique virus types from a single host is truly remarkable.
16S rDNA analysis suggests that the range of virus types found in YNP are harbored by Sulfolobus species most closely related to S. solfataricus and S. acidocaldarius. Not surprisingly, the hosts of the three viruses from YNP that closely resemble the previously isolated viruses from Japan and Iceland are from hosts that are also closely related to S. solfataricus. In contrast, the three virus types from YNP are replicating in hosts most similar to S. acidocaldarius, a Sulfolobus species from which viruses had not been previously identified. A thorough analysis of viruses present in a diversity of Sulfolobus species is likely to provide unique insights into the biochemistry and molecular biology of life in extreme thermal environments. In addition, a detailed understanding of the viral replication cycle in Sulfolobus species will likely provide insight into cellular processes present in Archaea and lead to a more thorough understanding of this unique domain of life.
Acknowledgments
This investigation was supported by grants from the National Science Foundation (LexEn DEB 9809360) and the National Aeronautics and Space Administration for support of the Montana State University Center for Life in Extreme Environments Thermal Biology Institute.
Abbreviations
- YNP
Yellowstone National Park
- SSV
Sulfolobus shibatae virus
- SIRV
Sulfolobus islandicus rod virus
- SIFV
S. islandicus filamentous virus
- dsDNA
double-stranded DNA
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. 424246, 424250, 425595, 425579, 425591, 424256, 425581, 424252, 425599, 425587, 425593, 425603, 425589, 425597, 425611, and 425607).
References
- 1.Stetter K. FEBS Lett. 1999;452:22–25. doi: 10.1016/s0014-5793(99)00663-8. [DOI] [PubMed] [Google Scholar]
- 2.Zillig W, Arnold H P, Holz I, Prangishvili D, Schweier A, Stedman K, She Q, Phan H, Garrett R, Kristjansson J K. Extremophiles. 1998;2:131–140. doi: 10.1007/s007920050052. [DOI] [PubMed] [Google Scholar]
- 3.Brock T D, Brock K M, Belly R T, Weiss R L. Archiv Mikrobiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 4.Zillig W, Stetter K O, Wunderl S, Schulz W, Priess H, Holz I. Archiv Microbiol. 1980;125:259–269. [Google Scholar]
- 5.She Q, Singh R K, Confalonieri F, Zivanovic Y, Allard G, Awayez M J, Chan-Weiher C C, Clausen I G, Curtis B A, De Moors A, et al. Proc Natl Acad Sci USA. 2001;98:7835–7840. doi: 10.1073/pnas.141222098. . (First Published June 26, 2001; 10.1073/pnas.141222098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannio R, Contursi P, Rossi M, Bartolucci S. J Bacteriol. 1998;180:3237–3240. doi: 10.1128/jb.180.12.3237-3240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stedman K, Schleper C, Rumpf E, Zillig W. Genetics. 1999;152:1397–1405. doi: 10.1093/genetics/152.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin A, Yeats S, Janekovic D, Reiter W D, Aicher W, Zillig W. EMBO J. 1984;3:2165–2168. doi: 10.1002/j.1460-2075.1984.tb02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zillig W, Kletzin A, Schleper C, Holz I, Janekovic D, Hain J, Lanzendoerfer M, Kristjansson J K. Syst Appl Microbiol. 1994;16:609–628. [Google Scholar]
- 10.Palm P, Schleper C, Grampp B, Yeats S, McWilliam P, Reiter W D, Zillig W. Virology. 1991;185:242–250. doi: 10.1016/0042-6822(91)90771-3. [DOI] [PubMed] [Google Scholar]
- 11.Blum H, Zillig W, Mallok J, Domaley H, Prangishvili D. Virology. 2001;281:6–9. doi: 10.1006/viro.2000.0776. [DOI] [PubMed] [Google Scholar]
- 12.Arnold H P, Zillig W, Ziese U, Holz I, Crosby M, Utterback T, Weidmann J F, Kristjanson J K, Klenk H P, Nelson K E, Fraser C M. Virology. 2000;267:252–266. doi: 10.1006/viro.1999.0105. [DOI] [PubMed] [Google Scholar]
- 13.Achenbach L, Woese C. In: Thermophiles. Robb F T, Place A R, editors. Vol. 3. Plainview, NY: Cold Spring Harbor Lab. Press; 1995. pp. 201–203. [Google Scholar]
- 14.Schleper C, Kubo K, Zillig W. Proc Natl Acad Sci USA. 1992;89:7645–7649. doi: 10.1073/pnas.89.16.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold H P, She Q, Phan H, Stedman K, Prangishvili D, Holz I, Kristjanson J K, Garrett R A, Zillig W. Mol Microbiol. 1999;34:217–226. doi: 10.1046/j.1365-2958.1999.01573.x. [DOI] [PubMed] [Google Scholar]
- 16.Maniatis T, Fritsch E F, Sambrook J. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1982. [Google Scholar]
- 17.Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W, Lipman D. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takayanagi S, Kawasaki H, Sugimori K, Yamada T, Sugai A, Ito T, Yamasato K, Shioda M. Int J Sys Bacteriol. 1996;46:377–382. doi: 10.1099/00207713-46-2-377. [DOI] [PubMed] [Google Scholar]
- 19.Felsenstein J. Cladistics. 1989;5:164–166. [Google Scholar]
- 20.Prangishvili D. Genetics. 1999;152:1387–1396. doi: 10.1093/genetics/152.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barns S M, Fundyga R E, Jeffries M W, Pace N R. Proc Natl Acad Sci USA. 1994;91:1609–1613. doi: 10.1073/pnas.91.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suttle C A. Natural History. 1999;108:48–51. [Google Scholar]
- 23.Prangishvili D, Stedman K, Zillig W. Trends Microbiol. 2001;9:39–43. doi: 10.1016/s0966-842x(00)01910-7. [DOI] [PubMed] [Google Scholar]