Abstract
Chronic exposure to cocaine causes long-lasting behavioral changes associated with cocaine reinforcement and addiction. An important neural substrate for cocaine addiction is the nucleus accumbens (NAc), which receives dopaminergic input from the ventral tegmental area. Although the neural circuit of the NAc is controlled by several other neurotransmitters, their involvement in cocaine addiction remains elusive. In this investigation, we ablated cholinergic interneurons from the adult NAc with immunotoxin-mediated cell targeting and examined the role of acetylcholine transmitter in adaptive behavioral changes associated with cocaine reinforcement and addiction. Acute exposure to cocaine induced abnormal rotation in unilaterally cholinergic cell-eliminated mice. This abnormal turning was enhanced by repeated exposure of cocaine. In bilaterally cholinergic cell-eliminated mice, chronic cocaine administration induced a prominent and progressive increase in locomotor activity. Moreover, these mice showed robust conditioned place preference with a lower dose of cocaine, compared with wild-type littermates. This investigation demonstrates that acetylcholine in the NAc plays a key role in both acute and chronic actions of cocaine.
The mesolimbic dopaminergic system serves as a vital and fundamental role in behavioral adaptations that occur with repeated cocaine exposure (1–4). The mesolimbic dopaminergic pathway originates in the ventral tegmental area and projects to the nucleus accumbens (NAc), the ventral part of the striatum, as well as other brain regions (5, 6). Numerous studies have indicated that the NAc is a key neural substrate that is implicated in cocaine reinforcement and addiction (2–4). Cocaine blocks the activity of dopamine transporters and increases dopamine levels in the NAc (7). The predominant actions of dopamine in the NAc lead to neural adaptation that underlies reinforcement and addiction of cocaine (2, 3, 8). The neural circuit of the NAc, however, is controlled by several other neurotransmitters (3–5). Acetylcholine (ACh) is released from cholinergic interneurons within the NAc (5, 9) and acts concertedly but oppositely to dopamine on the NAc neural circuit (10–12). However, little is known about the involvement of ACh in acute and chronic actions of cocaine.
Cell ablation using the immunotoxin-mediated cell targeting technology is useful for selectively eliminating specific neuronal cells in the adult neural network (13–16). In this technology, transgenic mice are generated, in which human IL-2 receptor α-subunit (hIL-2Rα) fused in-frame to the jellyfish green fluorescent protein (GFP) is expressed under control of a neuron-specific promoter. hIL-2Rα/GFP-expressing neuronal cells are then ablated by injecting the recombinant immunotoxin (IT) that is composed of the specific hIL-2Rα antibody fused to a bacterial toxin (13). In this investigation, we have applied IT-mediated cell targeting to eliminate cholinergic neurons from the adult NAc and have examined the role of ACh in acute and chronic actions of cocaine. Here, we report that elimination of cholinergic cells in living adult NAc markedly enhances sensitivity to cocaine in both acute and long-lasting behavioral changes associated with cocaine addiction.
Materials and Methods
Animals and IT Treatment.
The IG17 line of heterozygous transgenic mice and their wild-type littermates (14) were deeply anesthetized with sodium pentobarbital at the ages of 9–13 weeks. A glass needle was introduced into one or both sides of the NAc with stereotaxic techniques. The anti-Tac(Fv)-PE38 IT (10 ng in 0.5 μl of PBS) was injected over 3 min as described (15). An injection coordinate was according to the atlas of Franklin and Paxinos (17) using the bregma as a reference: anterior + 1.5 mm, lateral + or −0.8 mm and ventral + 3.5 mm. All procedures were performed according to the guidelines of the Kyoto University Faculty of Medicine.
Immunostaining Analysis.
Two weeks after IT injection into the left NAc, mice were deeply anesthetized with diethylether and perfused with 0.01 M PBS, followed by 4% paraformaldehyde fixation. Coronal sections of 40-μm thickness were prepared as free-floating sections. Immunostaining was performed as described (18). The primary antibodies were obtained from the following sources and diluted as indicated in parentheses: mouse mAbs against choline acetyltransferase (ChAT) (1:100; Biogenesis, Poole, U.K.), calbindin D-28k (1:5,000; Swant, Bellinzona, Switzerland), and parvalbumin (1:1,000; Sigma), rabbit polyclonal antibodies against tyrosine hydroxylase (1:60; Chemicon) and preprotachykinin (1:100; a gift from T. Kaneko, Kyoto University), and guinea pig polyclonal preproenkephalin antibody (1:30; a gift from T. Kaneko). Immunoreactive cells at the IT-injected and uninjected sides of the NAc and the dorsal striatum were counted from three sections of transgenic and wild-type mice. Tyrosine hydroxylase immunoreactivity was assessed by measuring its optical density.
Turning Behavior.
Rotations were counted for a 5-min period by visual observation as described (15). One rotation was defined by the animal completing a 360° circle without turning back to the opposite direction. Apomorphine-HCl (1 mg/kg; Research Biochemicals, Natick, MA/Sigma) and different doses of cocaine (Takeda, Osaka) were injected 15 min before measuring rotations.
Locomotor Activity.
Locomotor activity was assessed for a 10-min period with an infrared activity monitor (Coulbourn Instruments, Allentown, PA) as described (19).
Conditioned Place Preference (CPP).
The CPP test was carried out in a three-chamber apparatus consisting of a small middle chamber that connected the two large side chambers (MED Associates, St. Albans, VT). The two large chambers differed in floor and wall conditions. At day 0, mice were allowed to move freely in the three chambers for 30 min. At days 1–3, mice were confined to one large chamber for 20 min immediately after they had received saline. Four hours later, they received cocaine and were confined to the other side chamber for 20 min. At day 4, mice were placed in the middle chamber and allowed to move freely in the three chambers for 30 min.
Statistical Analysis.
Immunostaining data were analyzed by Student's t test. Behavioral data were subjected to ANOVA, and posthoc comparisons were made with Scheffé test.
Results and Discussion
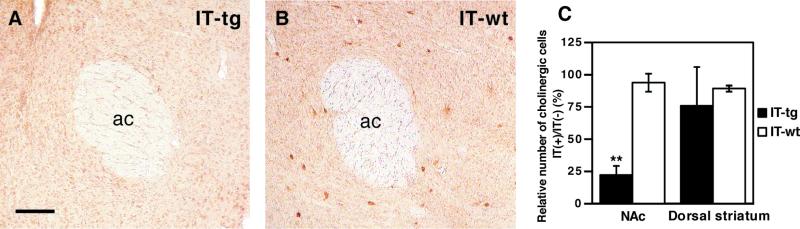
To study the role of ACh in acute and chronic actions of cocaine, we used transgenic mice that were previously generated with use of the mGluR2 promoter and the hIL-2Rα/green fluorescent protein (GFP) fusion protein (14). In these transgenic mice, hIL-2Rα/GFP was selectively expressed in cholinergic cells throughout the striatum including the NAc (15). To ablate cholinergic cells from the adult NAc, IT was stereotaxically injected into a single site of the NAc. ChAT immunostaining of serially dissected sections of the striatum showed that more than 70% of ChAT-positive neurons were eliminated from the NAc 2 weeks after IT injection (Fig. 1). No such reduction was observed in the dorsal striatum adjacent to the NAc of IT-treated transgenic mice, nor in the NAc of IT-treated wild-type mice (Fig. 1C). In addition, no abnormality of other NAc neurons occurred after IT treatment of transgenic mice, as evidenced by immunostaining of cell-specific markers; numbers of immunoreactive cells at the IT-injected side, relative to those at the IT-uninjected side, were as follows: calbindin D-28k (striatonigral and striatopallidal neurons, 95.6 ± 4.3%), preprotachykinin (striatonigral neurons, 101.9 ± 9.3%), preproenkephalin (striatopallidal neurons, 97.7 ± 9.1%), tyrosine hydroxylase (nigrostriatal neurons, 95.3 ± 5.2%), and parvalbumin (a subpopulation of interneurons, 97.5 ± 10.6%). The results indicate that a single injection of IT selectively eliminated cholinergic interneurons within the NAc of transgenic mice.
Figure 1.
IT-mediated ablation of NAc cholinergic interneurons. (A and B) ChAT immunostaining of the NAc of transgenic (IT-tg) and wild-type mice (IT-wt) 2 weeks after IT injection; ac, anterior commissure. (Scale bar, 100 μm.) (C) Columns indicate percentages of ChAT-immunoreactive cells at the IT-injected [IT(+)]/uninjected [IT(−)] side of both the NAc and dorsal striatum adjacent to the NAc 2 weeks after IT injection. Error bars represent ± SD (n = 5 each). **, P < 0.01; significantly reduced as compared with wild-type mice.
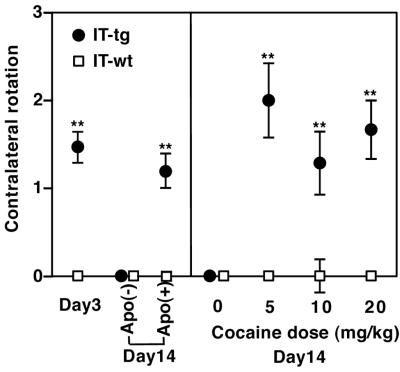
Our previous study showed that elimination of striatal cholinergic cells significantly reduced intrastriatal ACh levels without affecting dopamine levels and perturbed a convergent action of dopamine and ACh in the striatal circuit (15). We first examined whether ablation restricted to cholinergic cells in the NAc impairs the dynamic balance of motor movement. When cholinergic cells were eliminated at one side of the NAc, these mice showed abnormal contralateral rotation on a hemispherical container at day 3 and then a recovery at day 14 (Fig. 2). Furthermore, similar to animals with unilateral cholinergic cell elimination at whole ranges of the striatum (15), excess stimulation of dopamine by s.c. injection of the dopamine agonist apomorphine induced contralateral rotation at day 14 after NAc cholinergic cell elimination (Fig. 2). We then examined acute effects of i.p. cocaine injection on motor balance at day 14 after unilateral cholinergic cell elimination at the NAc (Fig. 2). Three different doses of cocaine all induced contralateral rotation at the chronic phase of cholinergic cell-eliminated mice (Fig. 2). IT-treated wild-type mice never showed contralateral or ipsilateral rotation at the acute phase or after cocaine administration at the chronic phase (Fig. 2). The results indicate that cholinergic cells in the NAc are critical for controlling motor balance and that the adaptive recovery after persistent cell elimination is disturbed by cocaine administration.
Figure 2.
Abnormal rotation of unilaterally IT-treated mice. Numbers of rotation were counted for a 5-min period at days 3 and 14 after IT injection into the left NAc (IT-tg, n = 31; IT-wt, n = 30). At day 14, rotations were measured before and 15 min after i.p. injection of saline or cocaine or after s.c. injection of apomorphine (Apo) (n = 5–8). Data points and error bars represent mean ± SEM, respectively. **, P < 0.01.
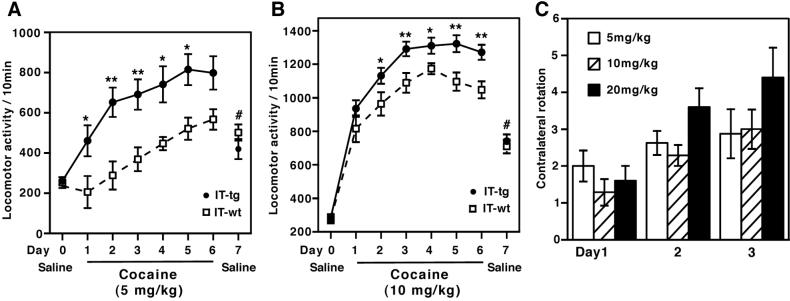
The addictive potential of cocaine has been related to its tendency to evoke a progressive increase in locomotor activity in rodents, called locomotor sensitization (20, 21). We assessed whether elimination of cholinergic cells at the NAc affects locomotor sensitization with repeated cocaine administration. IT was injected at a single site of both sides of the NAc, and locomotor activity was measured 2 weeks after IT injection. IT-treated transgenic and wild-type mice, when placed in a new chamber, showed a high initial locomotion and its subsequent habituation but with no statistical difference in these locomotor responses (data not shown). Remarkably, cholinergic cell-eliminated mice exhibited a striking increase in locomotor responses as compared with wild-type mice not only at initial cocaine exposure but also throughout the course of repeated cocaine administration (Fig. 3 A and B). The enhanced locomotor activity seemed to be manifested more in response to a low dose (5 mg/kg) (Fig. 3A) than to higher doses (10 mg/kg and 20 mg/kg) of cocaine administration (Fig. 3B and data not shown).
Figure 3.
Enhanced sensitization to cocaine in IT-treated transgenic mice. (A and B) Transgenic and wild-type mice 2 weeks after bilateral IT injection into the NAc received, by i.p., saline once a day and were then habituated in a novel chamber for 3 days. Doses of 5 mg/kg (n = 8–10) and 10 mg/kg (n = 13–19) of cocaine were i.p. injected once a day from day 1 to day 6, and immediately after cocaine injection, locomotor activity was counted for a 10-min period. Data points and bars represent mean ± SEM, respectively. Repeated measure ANOVA shows that IT-treated transgenic mice maintained a higher locomotor activity with repeated cocaine injection (A, F1,16 = 8.73, P < 0.01; B, F1,30 = 8.42, P < 0.01); **, P < 0.01 and *, P < 0.05, significantly different on each day. At day 7, locomotor activity immediately after saline injection was counted. The activity of both groups at day 7 significantly increased as compared with their activity at day 0 (#, P < 0.01). No difference was observed between the two groups at day 7. (C) Two weeks after IT injection into one side of the NAc, numbers of rotation were counted for a 5-min period after cocaine administration at days 1–3 (n = 5–8). Columns and error bars represent mean ± SEM, respectively. Repeated measure ANOVA shows that IT-treated transgenic mice progressively increased abnormal rotation at all three doses of cocaine administration (F2,36 = 16.7, P < 0.01). IT-injected wild-type mice never showed abnormal rotation.
Repeated cocaine exposure results in a distinct response, termed conditioned locomotor activity, which is elicited by association with a cocaine-injecting environment (21, 22). To test this response, animals were conditioned with repeated cocaine administration and then examined for locomotor activity in the cocaine-injecting chamber by saline injection. Both cholinergic cell-eliminated and wild-type mice showed a conditioned locomotor activity but with no difference between groups (Fig. 3 A and B). This finding suggests that the cocaine-induced locomotor abnormality in cholinerigic cell-eliminated mice was not caused primarily by changes in sensory, motivational, or motor functions (2).
We also examined whether an abnormal contralateral turning is sensitized by repeated cocaine exposure in unilaterally cholinergic cell-eliminated mice. Repeated administration with three different doses of cocaine all showed a progressive increase in contralateral rotation in mutant mice (Fig. 3C). In contrast, abnormal turning was never seen at any stages of cocaine administration in IT-treated wild-type mice (data not shown). The two different paradigms of behavioral sensitization provide strong evidence that ACh greatly contributes to cocaine reinforcement at the NAc circuit.
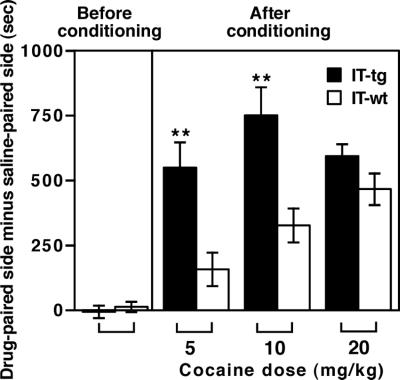
Cocaine can establish preference for an environment associated with repeated cocaine exposure. The CPP paradigm serves as a model of rewarding and abusive effects of repeated cocaine administration (23). Animals were conditioned with repeated cocaine administration in one of two chambers that differed visually and texturally (Fig. 4). Before conditioning, both IT-treated wild-type and transgenic mice visited the two chambers with no preference. After conditioning with repeated cocaine exposure for 3 days, both groups exhibited CPP. However, cholinergic cell-eliminated mice showed a dramatic enhancement in CPP to low doses of cocaine (5 mg/kg and 10 mg/kg). A high dose of cocaine (20 mg/kg) showed equivalent CPP in the two groups of animals. The results indicate that ACh in the NAc plays an important role in the rewarding and addictive effects of repeated cocaine exposure.
Figure 4.
Effect of cholinergic cell elimination on cocaine-induced CPP. CPP developed by repeated cocaine administration was analyzed in animals 2 weeks after bilateral IT injection into the NAc (n = 8–14). Before and after conditioning was performed with indicated doses of i.p. cocaine injection for 3 days; the time difference was calculated by subtracting time mice spent in the saline-paired side from time they spent in the cocaine-paired side. Columns and error bars represent mean ± SEM, respectively. Cholinergic cell-eliminated transgenic mice spent significantly more time in the cocaine-paired side after conditioning with 5 mg/kg and 10 mg/kg of cocaine (**, P < 0.01).
Previous pharmacological studies failed to indicate the role of ACh in cocaine actions because of its global effects on many other brain regions such as the cerebral cortex and hippocampus (23–25). The present investigation has provided compelling evidence that ACh from cholinergic neurons plays a pivotal role in neural responses and adaptation that underlie cocaine reinforcement and addiction. Cholinergic interneurons represent only a few percent of the NAc neuronal population (26). Therefore, it is striking that elimination of these cells confers a profound effect on both acute actions of cocaine and long-lasting behavioral changes associated with cocaine addiction. Importantly, cholinergic neurons possess highly radiating dendritic trees and a dense plexus of axonal branches (9, 27). Therefore, these cells fill the NAc and provide a rich innervation to medium-sized spiny neurons. In the striatum, ACh and dopamine convergently but antagonistically regulate the activity of principal medium-sized spiny neurons (15). The most straightforward interpretation of this investigation is that depletion of ACh by cholinergic cell elimination fortifies cocaine-induced dopamine actions. This predominance of dopamine actions in the NAc is thought to be responsible for cocaine reinforcement and addiction. More specifically, cholinergic cells frequently produce spontaneous firing and can tonically modulate principal spiny neurons (28). ACh from cholinergic neurons thus contributes to homeostatic regulation in the mesolimbic dopaminergic pathway.
Because the NAc is a key neural substrate for many categories of drugs that are abused (2–4), cholinergic cell-eliminated mice may respond more to other addictive drugs. Furthermore, all available pharmacological treatments for drug addiction are ineffective in most cases of drugs of abuse. The cholinergic cell-eliminated mice will serve as an animal model not only for an understanding of the cellular basis of drug addiction but also for development of a medical treatment of patients who abuse drugs.
Acknowledgments
We thank Kumlesh K. Dev for valuable advice and Takeshi Kaneko for a gift of antibodies. This work was supported in part by research grants from the Ministry of Education, Science, and Culture of Japan and the International Resource Program of the National Cancer Institute.
Abbreviations
- NAc
nucleus accumbens
- ACh
acetylcholine
- hIL-2Rα
human IL-2 receptor α-subunit
- IT
immunotoxin
- ChAT
choline acetyltransferase
- CPP
conditioned place preference
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on May 2, 2000.
References
- 1.Koob G F, Le Moal M. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 2.Nestler E J. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 3.Koob G F, Sanna P P, Bloom F E. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 4.Wise R A. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 5.Pennartz C M A, Groenewegen H J, Da Silva F H L. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 6.Oades R D, Halliday G M. Brain Res Rev. 1987;12:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 7.Di Chiara G, Imperato A. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White F J, Kalivas P W. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi Y, Wilson C J, Augood S J, Emson P C. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 10.Di Chiara G, Morelli M, Consolo S. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 11.Nisenbaum L K, Kitai S T, Gerfen C R. Neuroscience. 1994;63:435–449. doi: 10.1016/0306-4522(94)90541-x. [DOI] [PubMed] [Google Scholar]
- 12.Wang J Q, McGinty J F. Neuroscience. 1996;75:43–56. doi: 10.1016/0306-4522(96)00277-1. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, Fujita K, Kreitman R J, Pastan I, Nagatsu T. Proc Natl Acad Sci USA. 1995;92:1132–1136. doi: 10.1073/pnas.92.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R, Hirano T, Toyama K, Kaneko S, Yokoi M, et al. Cell. 1998;95:17–27. doi: 10.1016/s0092-8674(00)81779-1. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko S, Hikida T, Watanabe D, Ichinose H, Nagatsu T, Kreitman R J, Pastan I, Nakanishi S. Science. 2000;289:633–637. doi: 10.1126/science.289.5479.633. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- 17.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 18.Ohishi H, Ogawa-Meguro R, Shigemoto R, Kaneko T, Nakanishi S, Mizuno N. Neuron. 1994;13:55–66. doi: 10.1016/0896-6273(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 19.Masugi M, Yokoi M, Shigemoto R, Muguruma K, Watanabe Y, Sansig G, van der Putten H, Nakanishi S. J Neurosci. 1999;19:955–963. doi: 10.1523/JNEUROSCI.19-03-00955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koob G F. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- 21.Robinson T E, Berridge K C. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 22.Nestler E J. Nat Genet. 2000;26:277–281. doi: 10.1038/81570. [DOI] [PubMed] [Google Scholar]
- 23.Tzschentke T M. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 24.Heidbreder C A, Shippenberg T S. Synapse. 1996;24:182–192. doi: 10.1002/(SICI)1098-2396(199610)24:2<182::AID-SYN10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Itzhak Y, Martin J L. Psychopharmacology. 2000;152:216–223. doi: 10.1007/s002130000537. [DOI] [PubMed] [Google Scholar]
- 26.Phelps P E, Houser C R, Vaughn J E. J Comp Neurol. 1985;238:286–307. doi: 10.1002/cne.902380305. [DOI] [PubMed] [Google Scholar]
- 27.Wilson C J. In: The Synaptic Organization of the Brain. Shepherd G M, editor. New York: Oxford Univ. Press; 1998. pp. 329–375. [Google Scholar]
- 28.Bennett B D, Callaway J C, Wilson C J. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]