Abstract
Neonatal hypoxia-ischemia (HI) is the most common cause of brain injury in neonates, which leads to high neonatal mortality and severe neurological morbidity in later life (Vannucci, 2000; Volpe, 2001). Yet the molecular mechanisms of neuronal death and brain damage induced by neonatal HI remain largely elusive. Herein, using both in vivo and in vitro models, we determine an endogenous neuroprotectant role of c-type natriuretic peptide (CNP) in preserving neuronal survival after HI brain injury in mouse pups. Postnatal day 7 (P7) mouse pups with CNP deficiency (Nppclbab/lbab) exhibit increased brain infarct size and worsened long-term locomotor function after neonatal HI compared with wildtype control (Nppc+/+). In isolated primary cortical neurons, recombinant CNP dose-dependently protects primary neurons from oxygen-glucose deprivation (OGD) insult. This neuroprotective effect appears to be mediated through its cognate natriuretic peptide receptor 2 (NPR2), in that antagonization of NPR2, but not NPR3, exacerbates neuronal death and counteracts the protective effect of CNP on primary neurons exposed to OGD insult. Immunoblot and confocal microscopy demonstrate the abundant expression of NPR2 in neurons of the neonatal brain and in isolated primary cortical neurons as well. Moreover, similar to CNP deficiency, administration of NPR2 antagonist P19 via intracerebroventricular injection prior to HI results in exacerbated neuronal death and brain injury after HI. Altogether, the present study indicates that CNP and its cognate receptor NPR2 mainly expressed in neurons represent an innate neuroprotective mechanism in neonatal HI brain injury.
Keywords: C-type natriuretic peptide, Natriuretic peptide receptor 2, Neonatal hypoxic-ischemic brain injury, Oxygen-glucose deprivation, Neuronal death
1. Introduction
Hypoxia-ischemia (HI) is the most common cause of neonatal brain injury, which results from systemic asphyxia that may occur during the perinatal period, and survivors often suffer from cognitive impairment, seizures, learning disabilities and motor impairment in their later life (Fatemi et al., 2009; Graham et al., 2008; Lee et al., 2013; Vannucci, 2000; Volpe, 2001). To date, only hypothermia treatment has been proven to provide some degree of clinical success in alleviation of neonatal HI-induced brain damage (Koenigsberger, 2000; Zanelli et al., 2009). The HI insult induces a series of neurotoxic events, such as cytotoxic reaction, oxidative stress, proinflammatory response, etc., and consequently results in neuronal death. However, the underlying mechanisms and pathways associated with HI brain injury remain largely elusive. Thus, it has become crucial to deepen our understanding of the pathogenesis of neonatal HI brain injury, especially regarding the innate mechanisms of neuronal protection and regeneration, so as to develop novel and effective treatment plans for HI brain injury in neonates.
C-type natriuretic peptide (CNP) is a potent neuropeptide (Kaneko et al., 1993; Stingo et al., 1992), which is released by vascular endothelial cells and brain cells in various regions of the rodent brain such as hippocampal subfields CA1–3, limbic cortices, dorsal endopiriform nucleus, etc. (Langub Jr et al., 1995; Maack, 1992; Potter et al., 2006). Considerable amounts of CNP have also been detected throughout the human brain the same as rodents (Komatsu et al., 1991). CNP belongs to a natriuretic peptide (NP) family and works locally as a neuronal growth hormone (Schmidt et al., 2009; Xia et al., 2013; Zhao and Ma, 2009), while other NP family members atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) mainly function as cardiac hormones (Potter et al., 2006). The physiological function of CNP is mediated by its receptors and NPR3 (Bennett et al., 1991; Koller et al., 1991; Lumsden et al., 2010; Suga et al., 1992). It has been reported that the CNP/NPR2 system controls axonal development of sensory neurons in the dorsal root ganglion via activation of the cGMP-PrkG1 pathway (Zhao and Ma, 2009; Zhao et al., 2009b), as well as regulates neuro-genesis in the developing brain (Muller et al., 2009). The brain concentration of CNP is significantly higher than that in peripheral tissues (Minamino et al., 1993), suggesting an important role of CNP in the brain. Indeed, it has been reported that CNP provides neuroprotective effect on retinal ganglion cells by reducing apoptotic damage induced in both in vitro and in vivo injury models (Ma et al., 2010). However, there is still a lack of evidence supporting a putative neuroprotectant role of CNP in neurological diseases, such as neonatal hypoxic-ischemic brain injury.
Herein, we reveal a novel role of CNP as an endogenous neuroprotectant in neonatal HI brain injury. We demonstrate that CNP deficiency increases the vulnerability of the neonatal brain to HI insult and leads to worsened neurological deficits. In primary cortical neurons, recombinant CNP treatment dose-dependently reduces neuronal death after oxygen-glucose deprivation (OGD) insult. In addition, the neuro-protective effect of CNP is mediated by its cognate receptor NPR2 that is abundantly expressed in neurons. Moreover, we find that NPR2 antagonist increases brain infarct size and neuronal death in response to HI brain injury, demonstrating that CNP/NPR2 system is an endogenous neuronal survival pathway in neonatal HI brain injury.
2. Material and methods
2.1. Neonatal mouse model of hypoxia-ischemia (HI)
A modified Rice-Vannucci model was produced in postnatal day 7 (P7) C57BL/6J mouse pups modified from the rat model as described previously (Ferriero et al., 1996; Ma et al., 2016; Rice 3rd et al., 1981; Ten et al., 2004). Briefly, mouse pups (Charles River Laboratories) were fully anesthetized with inhalation of 2–3% isoflurane. The right common carotid artery (CCA) in the neck was exposed, double ligated with an 8.0 silk surgical suture, and then cut between two ligation sites. After surgery, pups were recuperated on a heating pad for 1 h at 37 °C, and then placed in a hypoxic incubator containing humidified 8% oxygen balanced with 92% nitrogen for 20 min at 37 °C. At the end of hypoxia, pups were returned to their dams for recovery. Mouse pups of mixed males and females were randomly assigned into each experimental group. There is no significant difference in weights or sex composition in the different groups. Nppclbab/lbab (long bone abnormality;Nppc: gene encodes CNP precursor) or wildtype littermate control (WT, Nppc+/+) mice were provide by Dr. Zhen Zhao (Zhao et al., 2009b). All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Intracerebroventricular (i.c.v.) injection of NPR2 antagonist in mice
The stock solution of NPR2 antagonist P19 (Phoenix Pharmaceuticals Inc.) was prepared in 0.1M phosphate-buffered saline (PBS, pH 7.4) according to the manufacturer’s instruction. A total volume of 2 μl NPR2 antagonist solution (500 pmol/pup) were stereo-taxically injected into the ipsilateral hemisphere of P7 mouse pups intracerebroventricularly (placement coordinates: 0.8 mm lateral, 1.5 mm below the skull surface) with a flow rate of 0.5 μl/min as described previously (Sadakata et al., 2007). Then the HI brain injury was induced about 2 h after i.c.v. injection. For vehicle group, the same volume of PBS was injected into the mouse brains prior to HI operation.
2.3. Measurement of brain infarct size
Brain infarct size was determined 48 h after HI using 2, 3, 5-triphenyltetrazolium chloride monohydrate (TTC, Sigma-Aldrich) staining as described previously (Ma et al., 2016). Briefly, the brain was isolated from each pup, dissected into coronal sections (2 mm thickness, 4 slices per brain), and immersed into pre-warmed 2% TTC in PBS for 5 min at 37 °C against light. Sections were washed with PBS, and then fixed by 10% formaldehyde overnight. The caudal and the rostral surfaces of each slice were photographed using a digital camera, and the percentage of infarct area (average of both sides) in the ipsilateral hemisphere for each slice was traced and analyzed by the NIH Image J software.
2.4. Neurobehavioral assay
Rotarod test for locomotor function evaluation was performed one month after neonatal HI as described previously (Hartman et al., 2012; Ma et al., 2016). Briefly, the rotarod (Columbus Instruments) consists of a horizontal cylinder (7 cm diameter) divided into four lanes. Three consecutive block trials were administered, in which the rotarod rotated at a constant speed of 5 RPM for 2 trials, followed by 2 trials of acceleration by 3 RPM every 5 s, and finally 2 trials of acceleration by 5 RPM every 3 s. Latency to fall was recorded as the time of walking on the cylinder.
2.5. Primary cortical neuron isolation, culture and treatment
Primary cortical neurons were prepared from early postnatal (P0) mouse pups of either sex as described previously (Beaudoin 3rd et al., 2012). Briefly, the cerebral cortices from P0 mouse pups were removed into HBSS buffer (Fisher Scientific) and dissociated with 0.25% trypsin (Fisher Scientific) and DNAse (Sigma) for 15 min at 37.0 °C water bath. After trituration with fire-polished Pasteur pipettes, dissociated cells were suspended in Neurobasal medium (Invitrogen) supplemented with 2% B27 (Invitrogen), 1% GlutaMAX (100×; Invitrogen) and 100 units/ml penicillin/streptomycin (Invitrogen), and run through a 40 μm cell strainer (Fisher Scientific). Cell suspensions plated on poly-D-lysine solution (PDL, 0.1 mg/ml in boric acid buffer; Sigma-Aldrich)-coated 6-well plates (Corning) or 96-well plates (Corning) were used for biochemical assays; Cell suspensions plated on PDL-coated German 12 mm glass coverslips (Fisher Scientific) in 24-well plates (Corning) were used for immunocytochemistry staining at 200–400 cells/mm2. After being seeded, cells were maintained in a CO2 incubator (5% CO2, 21% O2) at 37 °C. At day 2 of in vitro culture (DIV2), 2 μM cytosine β-D-arabinofuranoside hydrochloride (Ara-C; Sigma), an inhibitor of DNA replication, was added into culture medium to inhibit non-neuronal cell proliferation. Half of the culture medium was replaced every 2–3 days. Experiments were conducted at DIV 5–7, when cultures consisted primarily of neurons (approximately 90% MAP2-positive cells by immunocytochemistry staining).
Recombinant ANP, BNP and CNP was purchased from Sigma. NPR3 antagonist AP811 was purchased from Tocris. The stock solution was prepared according to the manufacturer’s instruction. For the neuro-protective effect study, primary cortical neurons were incubated with CNP, BNP or ANP for 6 h at concentration of 0, 5, 25, 100, or 500 nM. For other experiments, primary cortical neurons were incubated with ANP, BNP or CNP for 6 h at concentration of 100 nM. For the effect of NPR2 antagonist P19 or NPR3 antagonist AP811, primary cortical neurons were incubated with P19 or AP811 alone, or with the presence of CNP for 6 h at concentration of 500 nM.
2.6. Oxygen-glucose deprivation (OGD)
Primary cortical neurons were subjected to OGD insult as reported previously (Frantseva et al., 1999; Newcomb-Fernandez et al., 2001; Yin et al., 2002; Zhao et al., 2009a). Briefly, the neuron culture media were replaced with pre-warmed glucose-deprived Neurobasal-A medium (Invitrogen) pre-equilibrated with 1% oxygen, and then primary neurons were cultured in an incubator filled with 1% oxygen (5% CO2/94% N2) for 0.5, 1 or 1.5 h at 37 °C. At the end of hypoxia, cells were transferred to normal culture medium, returned to normal culture condition and incubated for 24 h (reoxygenation).
2.7. Neuronal damage assays
The degree of cell injury at the end of reoxygenation was assessed by determining the amount of lactate dehydrogenase (LDH) released into the culture medium using the Pierce™ LDH Cytotoxicity assay kit (Fisher Scientific) according to the manufacturer’s instructions. The medium was mixed with equal volume of reaction solution in a 96-well plate and incubated for 30 min at room temperature. Then the reaction was stopped by loading of stop solution, and read with a microplate reader (BioTek Instruments). The background signal (absorbance at 630 nm) was subtracted from reaction signal (absorbance at 490 nm). At least three independent experiments in duplicate were used for LDH release assay. The results were shown as fold changes over normal controls.
Cell viability was further evaluated using the LIVE/DEAD™ Viability/Cytotoxicity Kit (Invitrogen) according to the manufacturer’s instructions. At the end of the experiment, primary neurons seeded on the coverslips were washed with warm medium and incubated with calcein AM (4 μM) and ethidium homodimer 2 μM) for 30 min at 37 °C. Living cells show green fluorescence because of the uptake of calcein AM (excitation/emission: 495/515 nm), while dying cells show red fluorescence because of the penetration of ethidium homodimer (excitation/emission: 495/635 nm) that interacts with nucleic acids. Primary neurons were then fixed with 4% paraformaldehyde (PFA) for 10 min and mounted on slides using fluorescent mounting media (Dako). Images were obtained with the EVOS Cell Imaging Systems (Invitrogen). The number of live (green) and dead (red) cells was counted using the NIH image J software. At least three independent experiments in duplicate were used for live/dead cell counting. The results were presented as the percentage of dead cells of total cells.
Neuronal death was detected using the Roche In Situ Cell Death Detection Kit (TUNEL assay) according to the manufacturer’s instructions. The nuclei were stained with DAPI (Invitrogen). After being washed, coverslips with primary neurons were mounted on slides using fluorescent mounting media (Dako). Images were obtained with the EVOS Cell Imaging Systems (Invitrogen) and analyzed using the NIH Image J software. Eight images from each coverslip were taken with 20× objective. At least three independent experiments in duplicate were used for TUNEL-positive cell counting. The data are presented as the percentage of TUNEL-positive cells in total cells.
2.8. Isolation of cerebrovascular endothelial cells
The primary brain endothelial cells were prepared from P5–6 mouse pups as described previously (Daneman et al., 2010; Watson et al., 2013; Wu et al., 2003; Zhao et al., 2015b). Briefly, the mouse brains were diced using a scalpel in a Petri dish on ice into maximum 2-mm pieces, and then suspended in ice-cold HBSS in Eppendorf tubes before the homogenization with a Dounce homogenizer. The homogenates were incubated with a pre-warmed digestion mix containing 1 mg/ml collagenase/dispase (Roche), 10 μg/ml DNAse I (Roche) and 0.147 μg/ml tosyl-lysyl-chloromethylketone (TLCK) for indicated time at 37 °C and then centrifuged (700 g, 2 min). The collected pellet was re-suspended in DMEM, supplemented with 20% FBS, 100 units/ml penicillin/streptomycin and 2.5 μg/ml amphotericin B (Gibco Laboratories) (Invitrogen) and transferred to culture dishes. The medium was replaced after 2–3 days after seeding. The purity of brain endothelial cells is detected by endothelial cell marker (von Willebrand factor) staining (Wu et al., 2003).
2.9. Western blotting
Protein extraction of cerebrovascular endothelial cells or primary cortical neurons was obtained using RIPA lysis buffer (Santa Cruz Biotechnology) with further centrifugation for 30min at 14,000g at 4 °C. The supernatant was collected, and the protein concentration was determined using a detergent compatible assay (Bio-Rad). Equal amounts of protein were loaded on an SDS-PAGE gel. After being electrophoresed and transferred to a nitrocellulose membrane, the membrane was blocked and incubated with the primary antibody overnight at 4 °C. The primary antibodies included: rabbit anti-NPR2 polyclonal antibody (Abcam) and mouse anti-β-actin monoclonal antibody (Sigma-Aldrich). Nitrocellulose membranes were incubated with secondary antibodies (Santa Cruz Biotechnology) for 1h at room temperature. Immunoblots were then probed with an ECL Plus chemiluminescence reagent kit (Fisher Scientific) and visualized with the imaging system (Bio-Rad, Versa Doc, model 4000). The images were analyzed using the NIH Image J software.
2.10. Immunofluorescence staining and TUNEL assay on brain slices
Mouse pups were transcardially perfused with ice-cold PBS followed by 4% PFA. The brains were post-fixed in 4% PFA overnight, dehydrated with 30% sucrose solution in PBS at 4°C, and allowed to completely sink to the bottom of the container. Then the dehydrated brains were dissected, embedded into Tissue-Tek optimal cutting temperature compound (OCT) (VWR) on dry ice, and cryosectioned at a thickness of 20 μm for immunofluorescence staining. Slices were blocked with 5% donkey serum (Jackson ImmunoResearch) containing 0.3% Triton X-100 (Sigma-Aldrich) at room temperature for 1 h, then incubated with mouse anti-NeuN antibody (Millipore, 1:500) and rabbit anti-NPR2 antibody (Abcam, 1:400) overnight at 4 °C and stained with Alexa Fluor 488-conjugated donkey anti-rabbit and Alexa Fluor 594-conjugated donkey anti-mouse secondary antibodies (Invitrogen, 1:500) for 1 h at room temperature. After being washed, tissue slices were mounted and coverslipped with fluorescent mounting media (Dako).
Cell death was detected using the In Situ Cell Death Detection Kit, Fluorescein (Roche) following neuron staining described above. The TUNEL staining was completed according to the manufacturer’s instructions. All slides were scanned with a Zeiss LSM 710 NLO confocal microscope (Zeiss) in Advanced Imaging and Microscopy Facility of Center of Perinatal biology and analyzed using the NIH Image J version 1.41 software.
2.11. Immunocytochemistry
Cortical neurons grown on PDL-coated glass coverslips were washed once with ice-cold PBS, and then fixed with 4% PFA for 10 min. After permeabilization with 0.3% Triton X-100 in PBS for 10 min and blocking with 5% donkey serum in PBS for 30 min, cells were incubated with primary antibodies in blocking solution overnight at 4 °C. The primary antibodies included: mouse anti-MAP2 polyclonal antibody (Biolegend) and rabbit anti-NPR2 polyclonal antibody (Abcam). After being washed with PBS, cells were incubated with Alexa Fluor 594-conjugated donkey anti-mouse and Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibodies for 1 h at room temperature. All secondary antibodies were purchased from Invitrogen. The nuclei were stained with DAPI (Invitrogen). After being washed, coverslips with cells were mounted on slides using fluorescent mounting media (Dako). All slides were scanned with a Zeiss LSM 710 NLO confocal microscope (Zeiss) in Advanced Imaging and Microscopy Facility of Center of Perinatal biology and analyzed using the NIH Image J software.
2.12. Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM).All graphs in this study were generated with GraphPad Prism 5. In experiments related to animals, experimental number (n) represents neonates from at least two different dams. All in vitro experiments were performed at least three times. Comparisons between two groups were analyzed using Student’s t-test (unpaired, two-tailed), and multiple comparisons were analyzed using one-way ANOVA followed by Newman-Keuls post hoc test. A p value < 0.05 was considered significant.
3. Results
3.1. CNP deficiency (Nppclbab/lbab) increased the vulnerability of the neonatal brain to HI insult
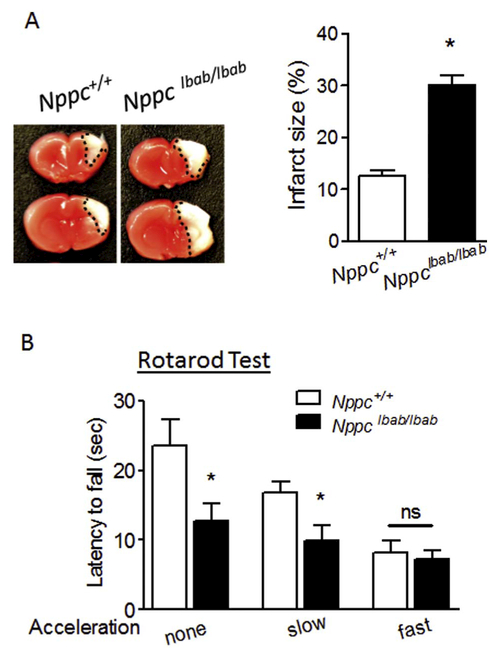
Neonatal HI brain injury was induced in P7 Nppclbab/lbab or wildtype littermate control (WT, Nppc+/+) mouse pups by the ligation of right common carotid artery followed with 20 min of hypoxic treatment (8% O2). The brain infarct size was detected 48 h after HI. As shown in Fig. 1A, CNP deficiency significantly exacerbated HI-induced brain injury by increasing about 2.3-fold in infarct size compared with WT. Rotarod test for the motor coordination function was performed one month after HI insult. The result showed that CNP deficient mice exhibited worsened performance by quickly falling off from the rotarod during the constant and slow acceleration trials compared with WT. There was no difference observed between CNP deficiency and WT during the more difficult fast acceleration trials (Fig. 1B).
Fig. 1.
CNP deficiency (Nppclbab/lbab) increases the vulnerability of the neonatal brain to HI insult. (A) CNP gene mutated or wildtype (Nppc+/+) mouse pups at postnatal day 7 (P7) were subjected to HI treatment (20 min of hypoxia, 8% O2), and brain infarct size was detected 48 h after HI. n = 8 pups/group. B) Rotarod test for locomotor function evaluation performed one month after HI. Data are expressed as mean ± SEM. n = 9–10 pups/group, *, p < 0.05 vs. Nppc+/+. ns, no significant difference. Two-tailed Student’s t-test.
3.2. Recombinant CNP treatment protected primary cortical neurons from OGD insult
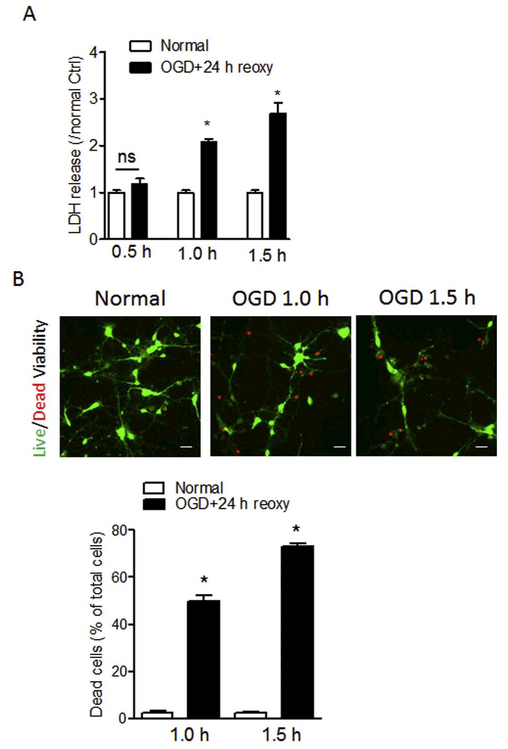
OGD model has been widely used to mimic hypoxic-ischemic events-induced neuron damage through optimizing the duration of OGD insult (Zhang et al., 2015; Zhao et al., 2015a). Primary cortical neurons at DIV5–7 were exposed to different durations (0.5 h, 1.0 h, or 1.5 h) of OGD insult followed by reoxygenation for 24 h. Control cells (normal) were cultured in normal condition for the same duration. Cell viability was detected by LDH assay and visualization of live/dead cells at the end of reoxygenation. As shown in Fig. 2A, OGD treatment for 1.0 h or 1.5 h significantly increased LDH release by about 2.0-fold and 2.7-fold in primary cortical neurons compared with normal controls, respectively, while there was no statistical difference of LDH release between 0.5 h of OGD treatment and normal control. In addition, OGD treatment remarkably increased the number of dead cells (red) compared with normal control (Fig. 2B). The subsequent quantification of dead cell numbers showed that OGD treatment for 1.0 h or 1.5 h significantly increased neuronal death by about 50% and 73% compared with normal controls, respectively (Fig. 2B). Due to the severe neuron loss induced by 1.5 h of OGD, in the rest of experiments the protocol of 1.0 h of OGD was applied.
Fig. 2.
Optimization of OGD treatment on primary neuron death. Primary cortical neurons were exposed to OGD treatment for 0.5, 1.0 or 1.5 h followed by reoxygenation for 24 h. Cells cultured in normal condition were used as control (Normal). Neuronal damage was determined by assessment of LDH release (A) and cell viability assay (B) at the end of reoxygenation. (B) Representative images of live (green) and dead (red) cells and quantification of the percentage of dead cells over total cells following the exposure of 1.0 or 1.5 h of OGD. Scale bar, 20 μm. Data are expressed as mean ± SEM. n = 3 independent experiments. *, p < 0.05 vs. Normal. ns, no significant difference. Two-tailed Student’s t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
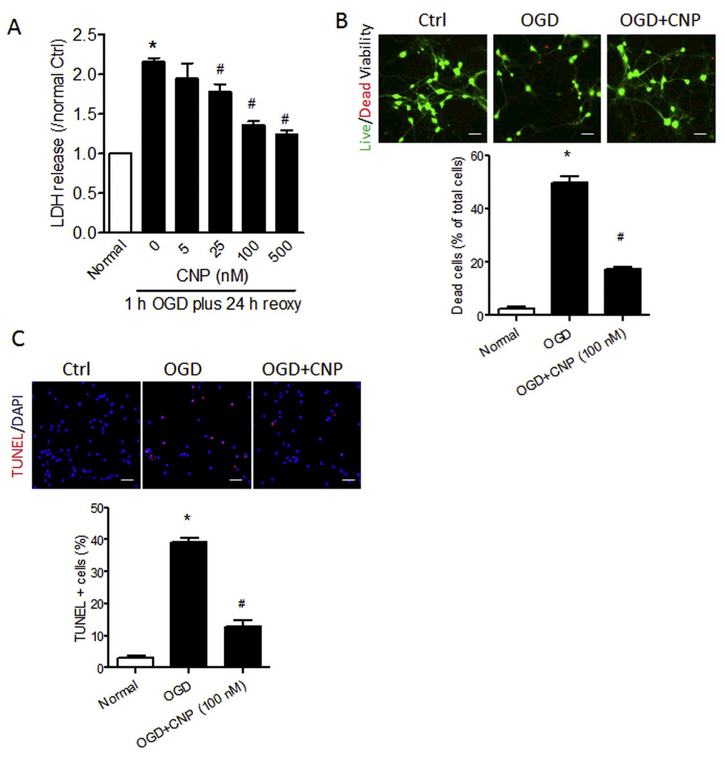
The isolated primary cortical neurons were incubated with multiple doses of recombinant CNP (0, 5, 25, 100 or 500 nM) for 6 h, and then exposed to OGD for 1.0 h followed by reoxygenation for 24 h. The result showed that CNP dose-dependently reduced LDH release into the medium (Fig. 3A). Moreover, recombinant CNP treatment significantly reduced neuronal death from approximate 50% to < 20% after OGD insult by Live/Dead viability assay (Fig. 3B), and also significantly reduced TUNEL-positive cells from approximate 40% to 12% (Fig. 3C) after OGD insult.
Fig. 3.
Recombinant CNP protects primary cortical neurons from OGD insult. Primary cortical neurons were incubated with 0, 5, 25, 100 or 500 nM of recombinant CNP for 6 h, and then exposed to OGD treatment for 1.0 h followed by reoxygenation for 24 h. LDH release (A), cell viability (B), and TUNEL staining (C) were performed at the end of reoxygenation. (B) Representative images of live (green) and dead (red) cells and quantification of the percentage of dead cells over total cells with recombinant CNP treatment (100 nM) after OGD/reoxygenation. Scale bar, 40 μm. (C) Representative images of TUNEL (red) staining and quantification of the TUNEL-positive cells over total cells with recombinant CNP treatment (100 nM) after OGD/reoxygenation. Data are expressed as mean ± SEM. n = 3 independent experiments. *, p < 0.05 vs. Normal. #, p < 0.05 vs. OGD. ANOVA following by Newman-Keuls post hoc test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. NPR2, but not NPR3, mediated the protective effect of CNP on primary neurons exposed to OGD insult
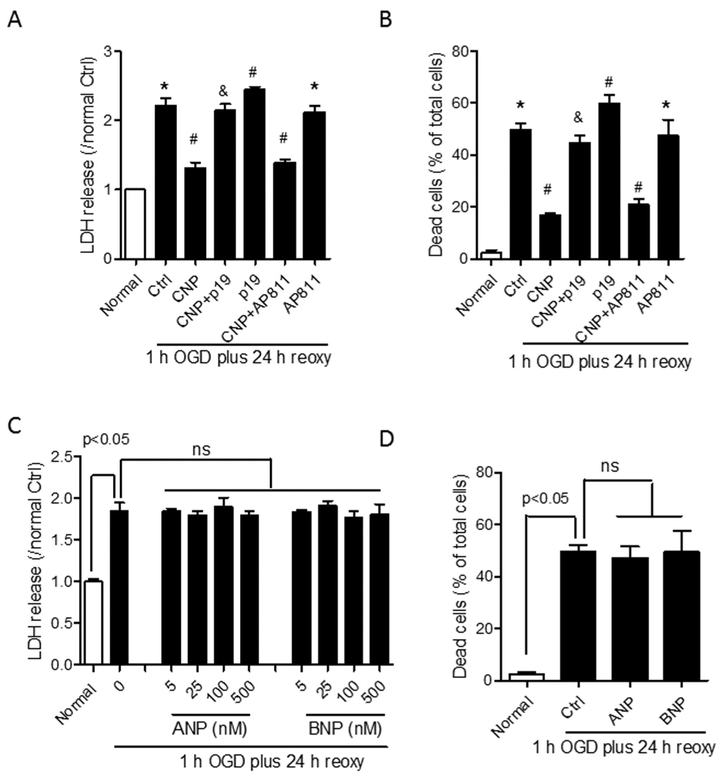
NPR2 antagonist P19 or NPR3 antagonist AP811 was used to treat primary cortical neurons prior to the exposure of OGD insult. The result showed that the treatment of NPR2 antagonist alone on primary neurons significantly intensified LDH release (Fig. 4A) and the percentage of dead cells (Fig. 4B) after OGD compared with OGD-treated control (Ctrl), and counteracted the neuroprotective effect of recombinant CNP treatment on primary neurons exposed to OGD. In contrast, NPR3 antagonist AP811 alone didn’t show effect on LDH release (Fig. 4A) and neuronal death induced by OGD insult (Fig. 4B), and didn’t affect the neuroprotective effect of recombinant CNP treatment. Other natriuretic peptides including ANP and BNP were also used to treat primary neurons with multiple doses. The result showed that neither ANP nor BNP showed neuroprotective effects on primary neurons exposed to OGD insult (Fig. 4C, D).
Fig. 4.
The neuroprotective effect of recombinant CNP is mediated by NPR2, but not NPR3. (A and B) Primary cortical neurons were incubated with 100 nM of recombinant CNP, NPR2 antagonist P19, or NPR3 antagonist AP811 alone, or combination of CNP with either P19 or AP811 for 6 h, and then exposed to OGD for 1.0 h followed by reoxygenation for 24 h. LDH release (A) and cell viability (B) were detected at the end of OGD/reoxygenation. (C and D) Primary cortical neurons were incubated with 0, 5, 25, 100 or 500 nm of ANP or BNP, and then exposed to OGD for 1.0 h followed by reoxygenation for 24 h. LDH release (C), and cell viability (D) were detected at the end of OGD/reoxygenation. Data are expressed as mean ± SEM. n = 4 independent experiments. *, p < 0.05 vs. Normal. #, p < 0.05 vs. Ctrl. &, p < 0.05 vs. CNP. ns, no significant difference. ANOVA following by Newman-Keuls post hoc test.
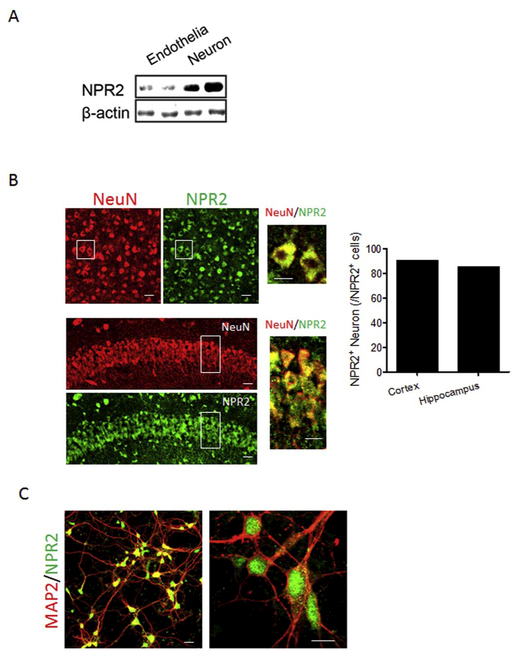
3.4. Determination of the NPR2 expression in neurons
The result of western blotting using isolated cerebrovascular endothelial cells and brain cortical neurons from mouse pups showed that NPR2 was abundantly expressed in cortical neurons with lowered expression in cortical endothelial cells (Fig. 5A). To visualize the expression of NPR2 in neurons, immunofluorescence staining was performed on brain slices and isolated primary cortical neurons. The confocal images showed that NPR2 (green) was broadly expressed in both cortex and hippocampus of the mouse brain and around 90% of NPR2-positive cells are neurons (NeuN, red) (Fig. 5B). A strong NPR2 expression (green) was also observed in isolated primary cortical neurons (MAP2, red) (Fig. 5C).
Fig. 5.
NPR2 is abundantly expressed in neuron cells. (A) the levels of NPR2 protein in cerebrovascular endothelial cells and neurons isolated from the cortices of mouse pups were detected by western blotting. (B) Representative confocal images of the colocalization of NPR2 (red) and neurons (NeuN, red) in the cerebral cortex (upper panel) and hippocampus (lower panel) in brain slices. Left, low magnification images; Right, high magnification images, scale bar, 20 μm. Bar graph shows the ratio of NPR2-positive neurons in total NPR2-positive cell in cortex and hippocampus. (C) Representative confocal images of the colocalization of NPR2 (green) and primary cortical neurons (MAP2, red). Left, low magnification images; Right, high magnification images, scale bar, 20 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
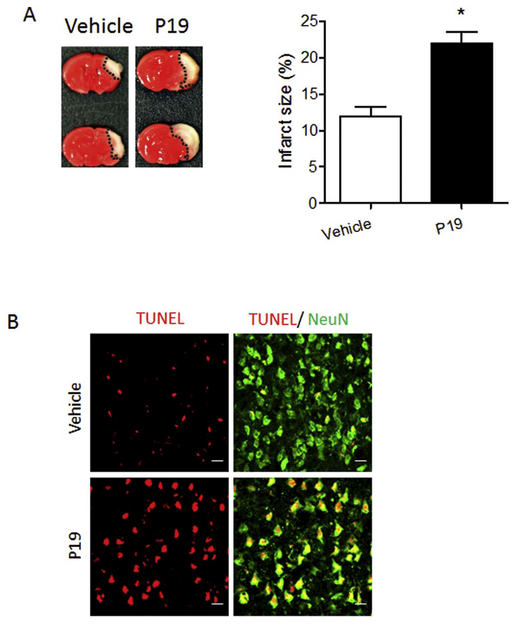
3.5. NPR2 antagonist increased the vulnerability of the neonatal brain to HI insult
To determine the role of NPR2 on HI brain injury in the pups, NPR2 antagonist P19 or vehicle was administered via i.c.v. injection into the ipsilateral hemisphere of P7 mouse brain followed by HI treatment (the ligation of right common carotid artery followed with 20 min of hypoxia in 8% O2). As shown in Fig. 6A, the NPR2 antagonist significantly exacerbated HI-induced brain injury by increasing approximate 1.8-fold in infarct size 48 h after HI, compared with the vehicle. In addition, the NPR2 antagonist treatment also increased the TUNEL-positive neurons in the cortex of the ipsilateral hemisphere 48 h after HI (Fig. 6B).
Fig. 6.
NPR2 antagonist administration increases brain infarct size and neuronal death after neonatal HI. NPR2 antagonist P19 was administered into the ipsilateral hemisphere via i.c.v. injection prior to HI, then HI insult was conducted. (A) Representative images of TTC staining and quantification of brain infarct size 48 h after HI. Data are expressed as mean ± SEM. n = 8 pups/group. *, p < 0.05 vs. vehicle. Two-tailed Student’s t-test. (B) Representative confocal images of the colocalization of TUNEL (red) with neurons (NeuN, green) in the cortex of the ipsilateral hemisphere of the brain 48 h after HI. Scale bar, 20 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Substantial neuronal death and subsequent functional deficits are the major consequences after neonatal HI insult (Hossain, 2008; Vasiljevic et al., 2011), thus, prevention of neuronal death is a major therapeutic goal for the treatment of neonatal HI brain injury (Han et al., 2001; Hossain, 2008). In the present study, we revealed an endogenous neuroprotectant role of CNP that produced an innate neuro-protective signal after neonatal HI brain injury. We found that mouse pups with CNP deficiency exhibited increased susceptibility to HI insult and exacerbated long-term neurological deficits. Using an in vitro OGD model in primary cortical neurons, we demonstrated that CNP reduced neuronal death induced by OGD insult, which was mediated by its cognate receptor NPR2, but not NPR3. Furthermore, we confirmed the abundant expression of NPR2 in neurons both in vivo and in vitro. Moreover, the role of NPR2 in neonatal HI brain was identified by administration of NPR2 antagonist that increased the brain infarct size and neuronal death after neonatal HI insult, indicating the innate neuroprotective role of CNP/NPR2 system in neurons after neonatal HI.
Different from other members of NP family, CNP mainly distributes in the brain and works locally as a neuropeptide (Kaneko et al., 1993; Schmidt et al., 2009; Xia et al., 2013). The neuroprotective effect of CNP has been delineated in both in vitro and in vivo neuronal injury models (Ma et al., 2010). To elucidate the role of endogenous CNP in neonatal HI brain injury, we used CNP spontaneous mutated mice Nppclbab/lbab (long bone abnormality; Nppc: gene encoding CNP precursor) that lose CNP activity because of Nppc gene mutation and develop axonal bifurcation defects in the embryonic spinal cord (Schmidt et al., 2009; Zhao and Ma, 2009). They are normal at birth, live up to several months, and do not develop neurological defects except dwarfism after 1 month of age (Chusho et al., 2001; Schmidt et al., 2009). Indeed, we didn’t find any effect caused by the skeletal development abnormality during the rotarod test. Therefore, it is a suitable loss-of-function model to investigate the role of innate CNP in neonatal HI brain injury. We conducted neonatal HI brain injury on Nppclbab/lbab mice by a perma nent unilateral common carotid artery ligation followed by systemic hypoxia (8% O2/92% N2). This model is the most commonly used rodent model to mimic the pathologies in infants with perinatal hypoxicischemic encephalopathy (HIE)/asphyxia, including neuronal cell death (Bolouri et al., 2014; Fang et al., 2013; Han et al., 2001; Ten et al., 2004; Wang et al., 2007). Of importance, the result showed that CNP deficient mouse pups exhibited increased infarct size and worsened neurological functions in response to HI, implicating that CNP is an innate neuroprotectant against neonatal HI brain injury. This role of CNP was further validated in vitro using primary cortical neurons exposed to OGD, in which we demonstrated that the recombinant CNP treatment protected primary neurons from OGD-induced neuron injury in a dose-dependent manner. Further study will directly overexpress or silence CNP gene in primary neurons to further confirm the role of CNP in neurons in response to OGD.
We then sought to uncover the receptors that mediated the effect of CNP in the neonatal HI brain injury. In general, three types of receptors, NPR1, 2, and 3, mediate the actions of NP family peptides. NPR1 and NPR2 are membrane bound guanylyl cyclase (GC)-coupled receptors, while NPR3 is a Gi-coupled receptor and mainly mediates the clearance of NPs (Cao and Yang, 2008; Murthy et al., 2000; Zhou and Murthy, 2003). It has been reported that NPR2 and NPR3, but not NPR1, mediate the physiological response of CNP (Bennett et al., 1991; Koller et al., 1991; Lumsden et al., 2010; Suga et al., 1992), while NPR1 and NPR3 have high binding affinity with ANP and BNP. Moreover, the distribution of NP receptors in the brain is different. In the postnatal and adult brain, NPR2 is widely expressed in the neurons of the cortex and hippocampal CA1–3 regions, including the granular and pyramidal cell layers (Barmashenko et al., 2014; Brackmann et al., 2005; Herman et al., 1996). NPR3 is abundantly expressed in vascular endothelial cells and mediates the action of CNP on vascular homeostasis (Maack, 1992; Moyes et al., 2014). In contrast, NPR1 is not found in the brain tissue (DiCicco-Bloom et al., 2004). In line with these findings, using an in vitro OGD model on primary cortical neurons, we demonstrated that the NPR2 antagonist P19 exacerbated neuronal death and counteracted the neuroprotection of CNP after OGD insult, while NPR3 antagonist didn’t show any effect. The result indicated that NPR2 was the major receptor that mediated the neuroprotective effect of CNP in primary cortical neurons. Moreover, the findings that other natriuretic peptides ANP and BNP didn’t show effect on primary neurons exposed to OGD further confirmed the paucity or nonfunctioning of their binding receptors NPR1 and NPR3 in primary neurons. One limitation of our in vitro system is that the isolated primary cortical neurons are about 90% pure, which are mixed with about 10% of glial cells such as astrocytes. Therefore, we cannot rule out the potential effect of glial cells on neuronal viability in this study.
To further confirm that neuronal NPR2 mediates the neuroprotective effect of CNP after neonatal HI, we validated the expression of NPR2 receptors in neurons. We first performed immunoblot using isolated cortical neurons and cerebrovascular endothelia cell, and found NPR2 abundantly expressed in cortical neurons, while a very low level of NPR2 expression was observed in endothelial cells. The confocal images in brain slices and isolated primary cortical neurons further confirmed the highly expressed NPR2 in neurons. These results were in agreement with previous reports regarding the distribution of NPR2 in the brain (Barmashenko et al., 2014; Brackmann et al., 2005; Herman et al., 1996). Further study will test the expression of NPR3 in neurons of the neonatal brain and isolated primary cells. In regards to the function of NPR2, the administration NPR2 antagonist caused increased brain infarct size and neuronal death, which is similar to the finding in animals with CNP deficiency. All the evidence suggests that CNP protects neuron survival from HI insult by acting on neuronal NPR2.
The activation of NPR2 by CNP releases cGMP that affects the function of multiple downstream effectors, such as the PrkG1 that is cGMP-dependent protein kinase G type 1 (Zhao and Ma, 2009), or some specific cyclic nucleotide phosphodiesterases (PDE) (Threlfell and West, 2013). The activation of PrkG1 has been demonstrated as a survival signal for neural cells (Andoh et al., 2002; Bonthius et al., 2004; Fiscus, 2002) by inhibition of proapoptotic pathways (Fiscus, 2002). PrkG1 also inhibits GSK3β pathway by increasing the phosphorylation levels (Zhao and Ma, 2009; Zhao et al., 2009b). It has been reported that inhibition of GSK3β reduced neuronal death in ischemic brain injury (Kelly et al., 2004). PDE is an enzyme that can hydrolyze cGMP to 5’GMP (Threlfell and West, 2013). Mounting evidence determined that the inhibition of PDE provided neuroprotective effect (Knott et al., 2017; Nakamizo et al., 2003).
Our result also showed that a > 2.3-fold increase in infarct size was observed in CNP mutated mouse pups, while that is about 1.8-fold in NPR2 antagonized mouse pups, after HI insult. This finding suggests that multiple mechanisms other than NPR2 may exist to mediate the effect of CNP in neonatal brain after HI. Indeed, CNP plays dual action on both neuronal and vascular cells through NPR2 or NPR3 (Bennett et al., 1991; Koller et al., 1991; Lumsden et al., 2010; Suga et al., 1992). It has been reported that CNP/NPR3 can preserves the integrity of the blood vessel wall and disruption of CNP leads to endothelial dysfunction (Khambata et al., 2011; Moyes et al., 2014). Therefore, in addition to neuronal injury, the increased vulnerability of the neonatal brain to HI insult in CNP mutated mice may be partially attributable to the damage of cerebrovascular system because of the disruption of CNP/NPR3 system, which is worthy of future study.
5. Conclusion
In conclusion, the present study provides novel evidence of an endogenous neuroprotectant role of CNP in the neonatal brain. We demonstrate that CNP deficiency results in increased vulnerability of the neonatal brain to HI insult in mice. Using in vitro OGD treatment on cultured primary cortical neurons, we reveal the neuroprotective effect of recombinant CNP on neuronal death induced by OGD, which is counteracted by the antagonist of NPR2, but not NPR3. Furthermore, the NPR2 antagonist also exacerbates neuronal death and brain infarct size after neonatal HI. Taken together, the study provides clear evidence that CNP and its cognate receptor NPR2 in neuron cells represent an innate protective mechanism in neonatal HI brain injury and other neurological disorders.
Acknowledgments
A portion of this research used the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core, a facility supported in part by the National Science Foundation through the Major Research Instrumentation program of the Division of Biological Infrastructure Grant No. 0923559 and the Loma Linda University School of Medicine. The authors acknowledge Dr. Zhen Zhao for providing the Nppclbab/lbabmice.
Sources of funding
This work was supported by the American Heart Association WSA winter 2015 Beginning Grant-in-Aid 15BGIA25750063 to Q.M. and the National Institutes of Health grants HL118861 and NS103017 to L.Z.
Footnotes
Disclosures
None.
References
- Andoh T, Chock PB, Chiueh CC, 2002. Preconditioning-mediated neuroprotection: role of nitric oxide, cGMP, and new protein expression. Ann. N. Y. Acad. Sci 962, 1–7. [DOI] [PubMed] [Google Scholar]
- Barmashenko G, et al. , 2014. Regulation of hippocampal synaptic plasticity thresholds and changes in exploratory and learning behavior in dominant negative NPR-B mutant rats. Front. Mol. Neurosci 7, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin GM 3rd, et al. , 2012. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc 7, 1741–1754. [DOI] [PubMed] [Google Scholar]
- Bennett BD, et al. , 1991. Extracellular domain-IgG fusion proteins for three human natriuretic peptide receptors. Hormone pharmacology and application to solid phase screening of synthetic peptide antisera. J. Biol. Chem 266, 23060–23067. [PubMed] [Google Scholar]
- Bolouri H, et al. , 2014. Innate defense regulator peptide 1018 protects against perinatal brain injury. Ann. Neurol 75, 395–410. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Karacay B, Dai D, Hutton A, Pantazis NJ, 2004. The NO-cGMP-PKG pathway plays an essential role in the acquisition of ethanol resistance by cerebellar granule neurons. Neurotoxicol. Teratol 26, 47–57. [DOI] [PubMed] [Google Scholar]
- Brackmann M, et al. , 2005. Neuronal Ca2+ sensor protein VILIP-1 affects cGMP signalling of guanylyl cyclase B by regulating clathrin-dependent receptor recycling in hippocampal neurons. J. Cell Sci 118, 2495–2505. [DOI] [PubMed] [Google Scholar]
- Cao LH, Yang XL, 2008. Natriuretic peptides and their receptors in the central nervous system. Prog. Neurobiol 84, 234–248. [DOI] [PubMed] [Google Scholar]
- Chusho H, et al. , 2001. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc. Natl. Acad. Sci. U. S. A 98, 4016–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, et al. , 2010. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One 5, e13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCicco-Bloom E, et al. , 2004. Embryonic expression and multifunctional actions of the natriuretic peptides and receptors in the developing nervous system. Dev. Biol 271, 161–175. [DOI] [PubMed] [Google Scholar]
- Fang AY, et al. , 2013. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia-ischemia. Pediatr. Res 73, 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi A, et al. , 2009. Hypoxic-ischemic encephalopathy in the term infant. Clin.Perinatol 36 (vii), 835–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM, et al. , 1996. Neonatal mice lacking neuronal nitric oxide synthase are less vulnerable to hypoxic-ischemic injury. Neurobiol. Dis 3, 64–71. [DOI] [PubMed] [Google Scholar]
- Fiscus RR, 2002. Involvement of cyclic GMP and protein kinase G in the regulation of apoptosis and survival in neural cells. Neurosignals 11, 175–190. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, et al. , 1999. A submersion method to induce hypoxic damage in organotypic hippocampal cultures. J. Neurosci. Methods 89, 25–31. [DOI] [PubMed] [Google Scholar]
- Graham EM, et al. , 2008. A systematic review of the role of intrapartum hypoxiaischemia in the causation of neonatal encephalopathy. Am. J. Obstet. Gynecol 199, 587–595. [DOI] [PubMed] [Google Scholar]
- Han BH, et al. , 2001. Clusterin contributes to caspase-3-independent brain injury following neonatal hypoxia-ischemia. Nat. Med 7, 338–343. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Kamper JE, Goyal R, Stewart JM, Longo LD, 2012. Motor and cognitive deficits in mice bred to have low or high blood pressure. Physiol. Behav 105, 1092–1097. [DOI] [PubMed] [Google Scholar]
- Herman JP, et al. , 1996. Localization of natriuretic peptide-activated guanylate cyclase mRNAs in the rat brain. J. Comp. Neurol 369, 165–187. [DOI] [PubMed] [Google Scholar]
- Hossain MA, 2008. Hypoxic-ischemic injury in neonatal brain: involvement of a novel neuronal molecule in neuronal cell death and potential target for neuroprotection. Int. J. Dev. Neurosci 26, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, et al. , 1993. C-type natriuretic peptide (CNP) is the major natriuretic peptide in human cerebrospinal fluid. Brain Res. 612, 104–109. [DOI] [PubMed] [Google Scholar]
- Kelly S, Zhao H, Hua Sun G, Cheng D, Qiao Y, Luo J, Martin K, Steinberg GK, Harrison SD, Yenari MA, 2004. Glycogen synthase kinase 3beta inhibitor Chir025 reduces neuronal death resulting from oxygen-glucose deprivation, glutamate excitotoxicity, and cerebral ischemia. Exp. Neurol 188, 378–386. [DOI] [PubMed] [Google Scholar]
- Khambata RS, et al. , 2011. Natriuretic peptide receptor-3 underpins the disparate regulation of endothelial and vascular smooth muscle cell proliferation by C-type natriuretic peptide. Br. J. Pharmacol 164, 584–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott EP, Assi M, Rao SN, Ghosh M, Pearse DD, 2017. Phosphodiesterase inhibitors as a therapeutic approach to neuroprotection and repair. Int. J. Mol. Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberger MR, 2000. Advances in neonatal neurology: 1950–2000. Rev. Neurol 31, 202–211. [PubMed] [Google Scholar]
- Koller KJ, et al. , 1991. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 252, 120–123. [DOI] [PubMed] [Google Scholar]
- Komatsu Y, Nakao K, Suga S, Ogawa Y, Mukoyama M, Arai H, Shirakami G, Hosoda K, Nakagawa O, Hama N, et al. , 1991. C-type natriuretic peptide (CNP) in rats and humans. Endocrinology 129, 1104–1106. [DOI] [PubMed] [Google Scholar]
- Langub MC Jr., et al. , 1995. Distribution of natriuretic peptide precursor mRNAs in the rat brain. J. Comp. Neurol 356, 183–199. [DOI] [PubMed] [Google Scholar]
- Lee AC, et al. , 2013. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res 74 (Suppl. 1), 50–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden NG, et al. , 2010. C-type natriuretic peptide (CNP): cardiovascular roles and potential as a therapeutic target. Curr. Pharm. Des 16, 4080–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, et al. , 2010. Neuroprotective effects of C-type natriuretic peptide on rat retinal ganglion cells. Invest. Ophthalmol. Vis. Sci 51, 3544–3553. [DOI] [PubMed] [Google Scholar]
- Ma Q, et al. , 2016. Inhibition of microRNA-210 provides neuroprotection in hypoxicischemic brain injury in neonatal rats. Neurobiol. Dis 89, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maack T, 1992. Receptors of atrial natriuretic factor. Annu. Rev. Physiol 54, 11–27. [DOI] [PubMed] [Google Scholar]
- Minamino N, et al. , 1993. Distribution of C-type natriuretic peptide and its messenger RNA in rat central nervous system and peripheral tissue. Biochem. Biophys. Res. Commun 197, 326–335. [DOI] [PubMed] [Google Scholar]
- Moyes AJ, et al. , 2014. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J. Clin. Invest 124, 4039–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, et al. , 2009. Expression of guanylyl cyclase (GC)-A and GC-B during brain development: evidence for a role of GC-B in perinatal neurogenesis. Endocrinology 150, 5520–5529. [DOI] [PubMed] [Google Scholar]
- Murthy KS, et al. , 2000. G(i-1)/G(i-2)-dependent signaling by single-transmembrane natriuretic peptide clearance receptor. Am. J. Physiol. Gastrointest. Liver Physiol 278, G974–80. [DOI] [PubMed] [Google Scholar]
- Nakamizo T, Kawamata J, Yoshida K, Kawai Y, Kanki R, Sawada H, Kihara T, Yamashita H, Shibasaki H, Akaike A, Shimohama S, 2003. Phosphodiesterase inhibitors are neuroprotective to cultured spinal motor neurons. J. Neurosci. Res 71, 485–495. [DOI] [PubMed] [Google Scholar]
- Newcomb-Fernandez JK, et al. , 2001. Concurrent assessment of calpain and caspase-3 activation after oxygen-glucose deprivation in primary septo-hippocampal cultures. J. Cereb. Blood Flow Metab 21, 1281–1294. [DOI] [PubMed] [Google Scholar]
- Potter LR, et al. , 2006. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev 27, 47–72. [DOI] [PubMed] [Google Scholar]
- Rice JE 3rd, et al. , 1981. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol 9, 131–141. [DOI] [PubMed] [Google Scholar]
- Sadakata T, et al. , 2007. Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J. Clin. Invest 117, 931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, et al. , 2009. C-type natriuretic peptide (CNP) is a bifurcation factor for sensory neurons. Proc. Natl. Acad. Sci. U. S. A 106, 16847–16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingo AJ, et al. , 1992. Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am. J. Phys 263, H1318–21. [DOI] [PubMed] [Google Scholar]
- Suga S, et al. , 1992. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 130, 229–239. [DOI] [PubMed] [Google Scholar]
- Ten VS, et al. , 2004. Late measures of brain injury after neonatal hypoxia-ischemia in mice. Stroke 35, 2183–2188. [DOI] [PubMed] [Google Scholar]
- Threlfell S, West AR, 2013. Review: modulation of striatal neuron activity by cyclic nucleotide signaling and phosphodiesterase inhibition. Basal Ganglia 3, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci RC, 2000. Hypoxic-ischemic encephalopathy. Am. J. Perinatol 17, 113–120. [DOI] [PubMed] [Google Scholar]
- Vasiljevic B, et al. , 2011. New insights into the pathogenesis of perinatal hypoxic-ischemic brain injury. Pediatr. Int 53, 454–462. [DOI] [PubMed] [Google Scholar]
- Volpe JJ, 2001. Perinatal brain injury: from pathogenesis to neuroprotection. Ment. Retard. Dev. Disabil. Res. Rev 7, 56–64. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. , 2007. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxicischemic brain injury. Ann. Neurol 61, 263–271. [DOI] [PubMed] [Google Scholar]
- Watson PM, et al. , 2013. Modelling the endothelial blood-CNS barriers: a method for the production of robust in vitro models of the rat blood-brain barrier and blood-spinal cord barrier. BMC Neurosci 14, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, et al. , 2003. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J. Neurosci. Methods 130, 53–63. [DOI] [PubMed] [Google Scholar]
- Xia C, et al. , 2013. CNP/cGMP signaling regulates axon branching and growth by modulating microtubule polymerization. Dev. Neurobiol 73, 673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HZ, et al. , 2002. Blockade of Ca2+-permeable AMPA/kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J. Neurosci 22, 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelli G, et al. , 2009. Reorientation ability of adults and healthy children submitted to whole body horizontal rotations. Cogn. Process 10 (Suppl. 2), S346–50. [DOI] [PubMed] [Google Scholar]
- Zhang W, et al. , 2015. n-3 Polyunsaturated fatty acids reduce neonatal hypoxic/ischemic brain injury by promoting phosphatidylserine formation and Akt signaling. Stroke 46, 2943–2950. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ma L, 2009. Regulation of axonal development by natriuretic peptide hormones. Proc. Natl. Acad. Sci. U. S. A 106, 18016–18021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. , 2009a. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J. Neurosci 29, 6186–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, et al. , 2009b. Regulate axon branching by the cyclic GMP pathway via inhibition of glycogen synthase kinase 3 in dorsal root ganglion sensory neurons. J. Neurosci 29, 1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. , 2015a. Neuronal Interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J. Neurosci 35, 11281–11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, et al. , 2015b. Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance. Nat. Neurosci 18, 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Murthy KS, 2003. Identification of the G protein-activating sequence of the single-transmembrane natriuretic peptide receptor C (NPR-C). Am. J. Phys. Cell Physiol 284, C1255–61. [DOI] [PubMed] [Google Scholar]