Abstract
Background:
Physical activity has the potential to improve physical function in patients with Alzheimer’s disease (AD) and may contribute to modify disease processes and cognitive function.
Objective:
The aim of this study was to investigate 1) the effect of moderate-high-intensity aerobic exercise on cardiorespiratory fitness, i.e., peak oxygen uptake (VO2peak) determined by direct breath-by-breath cardiopulmonary exercise test, and 2) the association between changes in VO2peak and changes in cognition and neuropsychiatric symptoms in patients with mild AD.
Methods:
The study is based on secondary outcome analyses from the large single-blinded multi-center study ADEX (Preserving Cognition, Quality of Life, Physical Health and Functional Ability in Alzheimer’s Disease: The Effect of Physical Exercise). A preselected sub-group of 55 participants (age 52–83 years), 29 from the intervention group (IG) and 26 from the control group (CG), was included. IG performed 16 weeks of supervised moderate-to-high intensity aerobic exercise. Assessments of VO2peak, mental speed and attention (Symbol Digit Modalities Test, SDMT), and neuropsychiatric symptoms (Neuropsychiatric Inventory, NPI) were performed at baseline and at 16 weeks.
Result:
VO2peak increased 13% in the IG and a between-group difference in mean change (3.92 ml/kg/min, 95% CI 6.34–1.51, p = 0.003) was present in favor of the IG. Combined data from IG and CG showed positive associations between changes in VO2peak and changes in NPI (Rho = – 0.41, p = 0.042) and changes in SDMT (Rho = 0.36, p = 0.010), respectively.
Conclusion:
Aerobic exercise improves VO2peak in community-dwelling patients with mild AD. Furthermore, changes in VO2peak appear to be associated to changes in cognition and neuropsychiatric symptoms.
Keywords: Alzheimer’s disease, aerobic exercise, cardiorespiratory fitness, cognitive function, neuropsychiatric symptoms
INTRODUCTION
The benefits of physical activity on physical function and cognitive performance in patients with Alzheimer’s disease (AD) are receiving increased attention due to the lack of a disease modifying medical treatment in AD. Studies have shown that physical activity has the potential to improve physical function and helps to maintain activities of daily living in patients with dementia [1–3]. In addition, both animal studies and studies in humans suggest that physical activity may have a role in modifying the disease process and maintaining cognitive function in AD [4]. In patients with AD, a link between cardiorespiratory fitness (determined by peak oxygen uptake, VO2peak) and cognition has been suggested, and low VO2peak appears to be associated with brain atrophy in patients with mild AD [5–7]. However, the association between VO2peak and cognition in patients with AD is still unclear. Based on trials that were highly heterogeneous in terms of dementia type and severity, and exercise mode, two meta-analyses concluded that it is not yet established if physical exercise has a positive effect on cognitive function in patients with dementia [1, 8]. A third meta-analysis of 18 RCTs and 802 patients [8] concluded that physical activity interventions has a positive overall effect on cognitive function, and that the effect is driven by interventions that included aerobic exercises independent of the type of dementia [9].
The multi-center study ADEX (Preserving Cognition, Quality of Life, Physical Health and Functional Ability in Alzheimer’s Disease: the Effect of Physical Exercise) included 200 community-dwelling persons with mild AD and showed that 16 weeks of moderate-to-high intensity aerobic exercise had a positive effect on VO2peak (estimated by the Astrand test) and neuropsychiatric symptoms (using the 12-item Neuropsychiatric Inventory, NPI) [10, 11]. While the primary outcome Symbol Digit Modalities Test (SDMT), a test of mental speed and attention, did not improve in the intention-to-treat population, the per protocol analyses showed that results in SDMT were preserved in participants with high exercise attendance and high training intensity compared to those in the control group [10]. This suggests that a dose-response relation between aerobic exercise and cognition may be present. However, in the main study we were not able to detect whether the effects on SDMT and NPI were mediated by the exercise intervention itself or other factors including a generally higher physical activity level, the social interaction between participants and/or the interaction with health professionals.
Only one of the ADEX-centers had the gold standard equipment for measuring peak oxygen uptake (VO2peak), which was the reason why the Astrand test [12, 13] was chosen to estimate VO2peak in the main study. This test is relatively inexpensive, simple, easy to implement, appropriate for assessing change over time, and can be carried out in persons with mild-moderate dementia. However, the formula for calculating VO2peak uses the age predicted maximal heart rate (HR = 220-age), an estimate which is subject to some uncertainty [14]. In addition, valid test results cannot be obtained in persons who use beta-blockers or other medications that reduce the heart rate, because either the target HR cannot be achieved or the VO2peak is overestimated.
In patients with AD, only few studies have measured VO2peak by direct breath-by-breath cardiopulmonary exercise test (CPET) [5–7, 15–17], which is the gold standard for assessing VO2peak. Furthermore, to our knowledge no studies have investigated whether exercise can improve VO2peak measured by CPET in patients with mild AD. Based on secondary outcome analyses from a subset of the participants in the ADEX study, the aim of this study was to investigate 1) the effect of moderate-to-high intensity aerobic exercise on VO2peak in patients with mild AD using CPET, and 2) the association between changes in VO2peak and changes in cognition and neuropsychiatric symptoms.
MATERIALS AND METHODS
This study included data from a subset of participants in the large randomized controlled trial ADEX, a single-blinded multicenter RCT, which involved 8 Danish memory clinics and enrolled 200 participants from January 2012 to June 2014. Only 3 of the 8 memory clinics had the equipment for advanced cardiopulmonary exercise testing and expertise in testing. The 55 participants from these three memory clinics in the Metropolitan area (Danish Dementia Research Center, Rigshospitalet; Regional Dementia Research Center, Roskilde Hospital; and Memory Clinic, Glostrup Hospital) were offered to participate in the sub-study. 55 of 66 (29 from the intervention group and 26 from the control group) agreed to participate in the sub-study and after the assessments in the main study they completed a test of maximal oxygen uptake (VO2peak).
The study adhered to the Helsinki declaration and was approved by The Danish National Committee on Biomedical Research Ethics (H-3-2011-128) and informed consent was obtained from each participant. ClinicalTrials.gov no.: NCT01681602.
Participants and study procedures
Inclusion criteria were: a diagnosis of AD according to the NINCDS-ADRDA criteria [18]; age 50–90 years; a score of 20 or above on the Mini-Mental State Examination (MMSE); and having contact more than once monthly to a caregiver who accepted to take part in the study. Use of anti-dementia or mood stabilizing medication was permitted if the doses had been stable for at least three months preceding the enrolment.
Exclusion criteria were: unstable cardiac disease; any musculoskeletal problems, joint problems and neurological diseases that contraindicated aerobic exercise; participation in moderate to high intensity exercise twice or more weekly. The complete list of all inclusion and exclusion criteria has been published in a protocol paper [19].
Patients who agreed to participate and fulfilled the inclusion criteria completed the baseline assessments and were subsequently randomized in groups of 4–10 participants to an intervention group (IG) or a control group (CG) at each of the contributing memory clinics. The randomization was done using a computerized random-number generator in favor of the exercise program if an odd number of participants were included.
Intervention
The participants in the IG exercised 1 h 3 times weekly for 16 weeks in groups of 2–5 participants supervised by an experienced physiotherapist. In week 1–4, the priority was on strength training of the lower extremity muscles (twice weekly) and introduction to aerobic exercise (once weekly). The following 12 weeks comprised 10 min of warm up followed by 3 times 10 min of moderate-to-high intensity aerobic exercise with small breaks of 2–5 min in between. The exercise was conducted on an ergometer bicycle, cross trainer, or treadmill and the target intensity was 70–80% of maximal hearth rate (HR, i.e., 220 minus the person’s age). The average HR was monitored during the aerobic exercises (3×10 min of exercise plus the pauses, in total 34–40 min) and documented in a training logbook. For each participant, the exercise intensity was estimated as: average HR of all exercise sessions/maximal HR (%). To ensure that the planned training intensity was reached the Borg Scale of Perceived Exertion was used as a supplement [20]. The total number of attended exercise sessions/total number of offered exercise sessions (%) was used to determine the attendance rate of each participant. Low adherence did not lead to exclusion.
The participants in the CG received usual care during the 16 weeks of the exercise intervention.
Assessments
VO2peak was measured at baseline and after the 16-week intervention by a specialized physiotherapist at Bispebjerg Hospital who had extensive expertise in testing cardiac patients. Cognitive tests and neuropsychiatric scales were administered by raters at the memory clinics. All assessors were blinded to the allocation of the participants.
VO2peak
CPET was performed using a graded cycle ergometer (Monarch) with a pedaling frequency of >60 RPM and increment increasing load to exhaustion, and a completed test in 6–12 min [21]. The test was performed with a mask for measurement of peak oxygen uptake by indirect calorimetry and expired gases were collected online and analyzed with a metabolic measurement system (Jaeger, Master Screen CPX vers.5.21, Cardinal Health, Germany). Maximal effort was determined by the respiratory exchange ratio (RER), currently the best non-invasive indicator of exercise effort and a secondary criterion for having attained maximal oxygen uptake [22].
VO2peak was measured at termination of the test, caused by either cardiovascular exhaustion or onset of leg fatigue requiring termination of the test. Variables selected for this paper are VO2peak (ml/kg/min), absolute VO2peak (ml/min), RER (ratio) and maximal HR (beats/min).
CPET was self-terminated by the subjects when they claimed that they had achieved maximal effort. However, we considered maximal or nearly maximal effort to be achieved if the RER was ≥1.05 [23].
Cognitive tests and neuropsychiatric rating
The Symbol Digit Modalities Test (SDMT) [24]: The primary outcome measure in the ADEX study was the SDMT, which assesses mental speed and attention. Participants are asked to decode lines of symbols for 120 s using a number to symbol key. The test score is defined as the number of correct answers. A higher test score indicates a better performance.
The 12-item Neuropsychiatric Inventory (NPI) [25]: Based on interviews with caregivers the NPI assesses symptoms and behavior common in patients with dementia. This includes hallucinations, agitation, depression, anxiety, apathy, etc., which is retrospectively assessed for the previous 4 weeks. The score range is 0–144 with higher ratings indicating worse symptoms and behavior.
Descriptive variables
Sex, age, comorbidities, general cognitive impairment (MMSE), co-habitation, and physical activity.
Statistical analyses
To assess the differences in baseline characteristics between the IG and the CG, Fisher’s exact test was used for categorical data, and Wilcoxon’s signed rank test for continuous data. The differences in outcome changes from baseline to the 16-week follow-up between the two randomization-groups were analyzed in linear regression models. The method of generalized estimating equations (GEE) was used to account for the longitudinal data; indicator variables were used to adjust for heterogeneity between clinics and training groups. Data on the outcomes at follow-up were weighted by the inverse of the probability of still being in the study; estimated in a logistic regression model with the patient’s baseline characteristics and the observed outcome at baseline as covariates. The associations between changes in SDMT, NPI, and VO2peak were assessed by Spearman’s rank correlation coefficient, rho. SAS 9.4 was used for all analyses. The level of significance was p≤0.05.
RESULTS
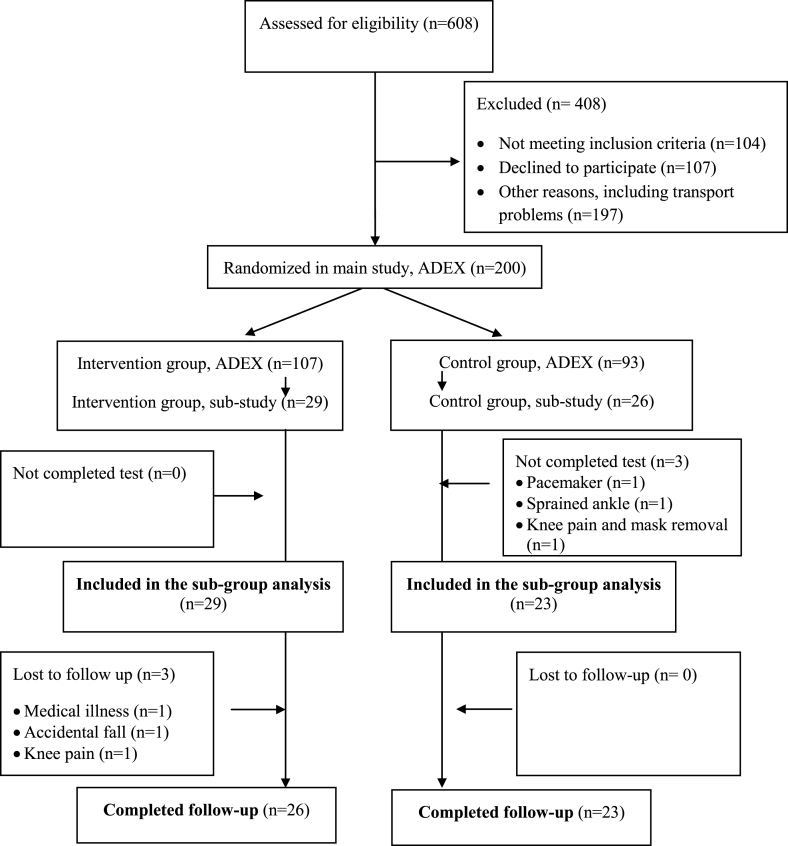
Of the 55 participants enrolled into the sub study, 52 completed the baseline test and 49 completed the test at follow-up (Fig. 1).
Fig.1.
Flowchart of the sub-group study in ADEX.
The median age was 69 years (range 52–83), which is similar to the median age in the whole ADEX patient group [11]. There were no significant between-group differences in any of the baseline characteristics shown in Table 1.
Table 1.
Baseline characteristics
| Intervention Group | Control Group | p | |
|---|---|---|---|
| n (%) | 29 (53.7) | 26 (47.3) | |
| Sex (male), n (%) | 14 (48.3) | 17 (65.4) | 0.278 |
| Age (y), mean (SD) | 69.2 (6.9) | 68.9 (7.2) | 0.854 |
| Caregiver living with patient, n (%) | 23 (79.3) | 18 (69.2) | 0.537 |
| PASE, median (IQR) | 76 (60–121) | 97 (55–143) | 0.458 |
| Comorbidities | |||
| Hypertension, n (%) | 13 (44.8) | 11 (42.3) | 0.850 |
| Diabetes, n (%) | 3 (10.3) | 2 (7.7) | 0.733 |
| Hypercholesterolemia, n (%) | 10 (34.5) | 8 (30.8) | 0.770 |
| Stroke, n (%) | 0 (0) | 0 (0) | |
| Acute myocardial infarction, n (%) | 0 (0) | 0 (0) | |
| Medicine | |||
| Hypertension medicines, n (%) | 13 (44.8) | 10 (61.5) | 0.785 |
| Depression medicines, n (%) | 6 (20.7) | 5 (19.2) | 0.893 |
| Alzheimer medicines, n (%) | 29 (100) | 23 (88.5) | 0.100 |
| Betablockers, n (%) | 2 (6.9) | 2 (7.7) | 0.910 |
| Cognitive performance | |||
| MMSE, mean (SD) | 25.1 (2.9) | 25.5 (3.3) | 0.607 |
| SDMT, mean (SD) | 35.0 (12.3) | 28.1 (14.6) | 0.074 |
| NPI, mean (SD) | 7.9 (8.1) | 9.2 (10.2) | 0.607 |
| Cardiorespiratory fitness, mean (SD) | |||
| VO2peak n = 52 (ml/kg/min)* | 21.9 (5.9) | 25.3 (6.9) | 0.068 |
| VO2peak RER≥1.05 (ml/kg/min)* | 23.4 (6.0) | 26.6 (6.6) | 0.134 |
| VO2peak absolute(ml/min)** | 1,563 (448) | 1,714 (574) | 0.297 |
| VO2peak absolute RER≥1.05, (l/min)** | 1,636 (449) | 1,821 (559) | 0.249 |
| Respiratory exchange ratio (RER) | 1.17 (0.14) | 1.15 (0.14) | 0.574 |
| Maximal heart rate (bpm) | 137 (24) | 140 (24) | 0.656 |
MMSE, Mini-Mental State Examination range from 0 (severe impairment) to 30 (no impairment); SDMT, Symbol Digit Modalities Test. Number of correct matches in 120 s are reported, with a higher score indicating a higher level of mental speed and attention; NPI, Neuropsychiatric Inventory range from 0 to 144, with higher scores indicating increased behavioral and psychological symptoms; PASE, Physical activity scale for the elderly, with higher scores indicating higher levels of habitual physical activity; VO2peak, Peak oxygen uptake measured by direct breath-by-breath cardiopulmonary exercise test (CPET); RER, respiratory exchange ratio.
Exercise attendance rate (mean(SD)) was 85.2% (16.8%) and exercise intensity 79.6% (10.6%) of maximal heart rate for participants not using beta-blockers in the intervention group (n = 24, 92%).
Training was safe with no serious adverse events related to the exercise intervention [11].
The test of VO2peak was not performed in three of the 55 included participants due to use of pacemaker (n = 1), a recently sprained ankle (n = 1), and knee pain (n = 1). RER of >1.05 was achieved in 37 (76%) out of 49 participants who completed the baseline and follow-up tests.
Effect of the 16 weeks exercise intervention on VO2peak
We found a 13% increase in VO2peak (2.9 ml/kg/min) in IG following the intervention period and a retained VO2peak in CG (– 0.8 ml/kg/min) (Table 2). The between-group differences in mean change from baseline was in favor of the IG following the intervention period for both VO2peak (p = 0.003) and absolute VO2peak (p = 0.001) (Table 2). There was no between-group difference in changes in body weight (p = 0.446) (Table 2).
Table 2.
Effect of the 16 weeks exercise intervention on VO2peak
| Intervention group (n = 29) | Control group (n = 23) | Between-group differences in | ||||
|---|---|---|---|---|---|---|
| mean change from baseline | ||||||
| Variables | Baseline | 16-week | Baseline | 16-week | Δ (95% CI) | p |
| follow-up | follow-up | |||||
| VO2peak (ml/kg/min) | 21.9 (5.9) | 24.8 (7.4) | 25.9 (6.5) | 25.1 (5.5) | – 3.9 (– 6.3; – 1.5) | 0.003 |
| VO2peak (ml/min) | 1,563 (448) | 1,771 (578) | 1,747 (564) | 1,635 (610) | – 330 (– 489; – 170) | 0.001 |
| RER | 1.17 (0.14) | 1.13 (0.13) | 1.16 (0.13) | 1.14 (0.13) | – 0.03 (– 0.06; 0.00) | 0.087 |
| HRmax (bpm) | 137 (24) | 136 (25) | 142 (21) | 142 (24) | – 3.4 (– 11.3; 4.6) | 0.402 |
| Body weight (kg) | 72.3 (14.9) | 72.1 (15.9) | 67.3 (12.0) | 68.9 (12.5) | – 0.6 (– 2.1; 0.9) | 0.446 |
Baseline and 16-week follow-up with unadjusted mean±SD and mean outcomes of the Intervention Group compared to mean outcomes in Control Group, beyond the difference already present at baseline, adjusted for clustering within training groups and centers, RER: respiratory exchange ratio, HRmax: maximal heart rate.
Excluding participants with an RER≤1.05 from the analyses did not change the results (Table 3).
Table 3.
Effect of the 16 weeks exercise intervention on VO2peak if RER≥1.05
| Intervention group, n = 20 | Control group, n = 17 | Between-group differences in mean change from baseline | ||||
|---|---|---|---|---|---|---|
| Variables | Baseline | 16-week follow-up | Baseline | 16-week follow-up | Δ (95% CI) | p |
| VO2peak (ml/kg/min) | 23.4 (6.0) | 26.0 (7.0) | 26.6 (6.6) | 25.7 (6.1) | – 4.2 (– 6.7; – 1.8) | 0.001 |
| VO2peak (ml/min) | 1,636 (449) | 1,893 (533) | 1,821 (559) | 1,717 (650) | – 352 (– 609; – 94) | 0.007 |
| RER | 1.22 (0.11) | 1.18 (0.08) | 1.20 (0.10) | 1.20 (0.09) | 0.03 (– 0.02; 0.08) | 0.258 |
| HRmax (bpm) | 141 (22) | 141 (23) | 146 (19) | 150 (21) | 2.7 (– 6.3; 11.5) | 0.560 |
| Body weight (kg) | 73.6 (15.5) | 73.8 (15.8) | 68.2 (12.7) | 70.5 (10.4) | 1.2 (– 3.1; 5.5) | 0.583 |
Baseline and 16-week follow-up with unadjusted means±SD and mean outcomes of the Intervention Group compared to mean outcomes in Control Group, beyond the difference already present at baseline, adjusted for clustering within training groups and centers, RER: respiratory exchange ratio, HRmax: maximal heart rate.
Associations
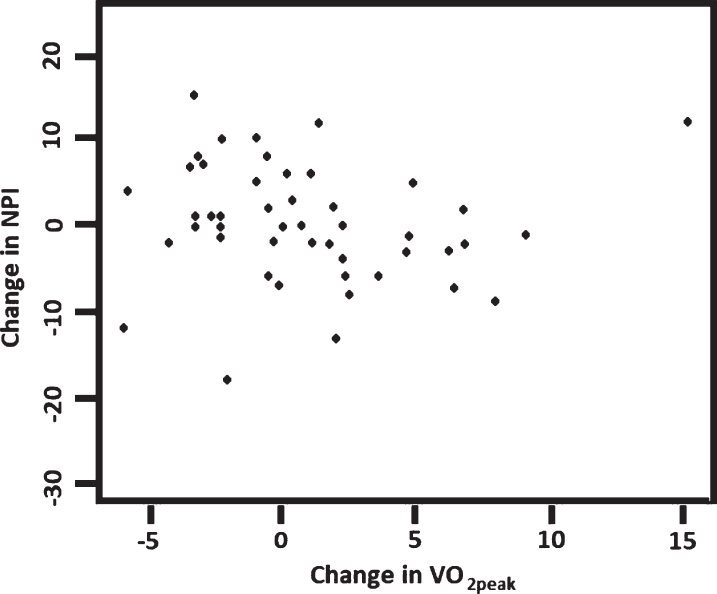
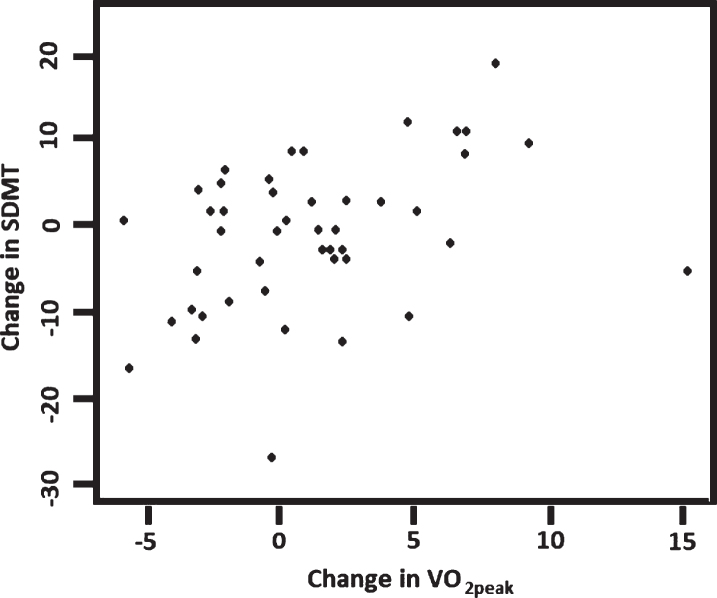
The relationship between changes over 16 weeks in VO2peak and the selected measures of cognition and neuropsychiatric symptoms were examined for participants with complete data sets. We found significant positive associations between changes in VO2peak and NPI (Rho = – 0.41, p = 0.042) and between changes in VO2peak and SDMT (Rho = 0.36, p = 0.010) (Figs. 2 and 3). Separate analyses of data from each group showed that there was a positive association between VO2peak and SDMT (rho = 0.74, p < 0.0001) in the control group but not in the intervention group (rho = 0.23; p = 0.26); and there was no correlation between NPI and VO2peak in any of the groups.
Fig.2.
Correlation between changes in VO2peak and changes in NPI (n = 49), Rho = – 0.41, p = 0.042. NPI, Neuropsychiatric Inventory, score range 0–144, with higher scores indicating increased behavioral and psychological symptoms.
Fig.3.
Correlation between changes in VO2peak and changes in SDMT (n = 47), Rho = 0.36, p = 0.010. SDMT, Symbol Digit Modalities Test are the number of correct matches in 120 s, with a higher score indicating a higher level of mental speed and attention.
DISCUSSION
To our knowledge, this study is the first randomized controlled trial to find a significant effect of an exercise intervention on VO2peak (ml/kg/min) when using CPET in patients with mild AD. When pooling data from the IG and the CG our results show that there is a positive association between changes in VO2peak and changes in cognitive test results and ratings of neuropsychiatric symptoms in patients with mild AD.
VO2peak can be improved by exercise in patients with mild cognitive impairment (MCI) [26] but the feasibility of improving VO2peak in patients with AD has so far not been established. In older healthy adults VO2peak is related to overall physical function [27], and in patients with early stage AD a correlation between VO2peak and physical functional performance, e.g., 50-foot walk and chair rise, has been reported [17]. Because high physical capacity helps maintain activities of daily living in patients with dementia [1–3], improving VO2peak may have important implications for decreasing the risk of dependency in patients with AD. A meta-analysis [9] suggests that aerobic exercises positively influence cognitive function in patients with dementia, and in line with this we found a correlation between changes in VO2peak and NPI and SDMT, respectively, when we combined the data from the IG and the CG. The physiological mechanism behind our findings is unknown. However, an association between VO2peak, memory composite score and hippocampal volume in patients with MCI and AD has been reported [28].
Results from previous studies suggest that it is feasible to measure and detect changes in VO2peak using CPET in patients with mild AD [7, 16, 28, 29]. Of the 55 participants, only 2 participants were unable to perform the CPET due to comorbidities not related to AD, and only one person was unable to complete the test due to knee pain. Further, the proportion of participants with an RER≥1.05 in our study complies with a reliability study (n = 14) finding 71% of patients with mild AD reaching an RER≥1.05 and 93% reaching an RER>1.0 in a treadmill exercise test [29]. In that study, an excellent reliability (ICC = 0.92, CI 0.78–0.97) was reported, when VO2peak was measured 3 days apart [29]. We chose a cycle ergometer test because all Danes learn to bike at an early age and biking for transportation is very common throughout life. One could also speculate that patients with AD may feel safer on a cycle ergometer, which is not as challenging with regards to balance as a treadmill, especially for those who have gait impairment, motor coordination complications, and/or are afraid of falling [30].
A general improvement in neuropsychiatric symptoms (NPI) following the exercise intervention was present in the main study, and SDMT improved in participants with the highest exercise dose [10]. Our results showed a positive association between changes in VO2peak and neuropsychiatric symptoms, which suggests a mutual effect of cognition on neuropsychiatric symptoms, and potentially imply a positive effect of exercise training upon AD cognitive capacity and symptoms.
Although the analyses suggest causal effects, this cannot be ascertained, and furthermore the analyses cannot detect potential mediating effects. Also, in our study, the correlation between cognition/neuropsychiatric symptoms and VO2peak was not straightforward: when correlations were performed separately on control group and intervention group, the association was only evident in the control group for SDMT. There could be numerous explanations for this unexpected finding, but obviously, the small sample size makes interpretations difficult. In particular, we cannot exclude that other factors not accounted for may have caused changes in symptoms and fitness in both groups. However, the association is noteworthy and calls for more research into the area.
The link between VO2peak and symptomatology of AD may in theory be mediated through effects on the pathophysiology associated with AD [4]. Cross-sectional studies have found an association between VO2peak and brain atrophy in AD [5, 6], and in a longitudinal study VO2peak was associated with progression of dementia severity and brain atrophy over 2 years in patients with AD [7]. These results suggest that high VO2peak may have an impact on the rate of neurodegeneration. A direct effect on specific AD pathology (amyloid-β and tau accumulation) is still uncertain [31], although an exercise program similar to ours documented a reduction in tau in cerebrospinal fluid as a result of exercise in older adults with MCI [32]. Multiple other mechanisms that could be induced in the relatively short time interval of 4 months may more likely be involved. These include improved cerebrovascular and endothelial function, reduction in oxidative stress and neuroinflammation, and improved metabolic function, which may contribute to improved neuronal function [4, 33, 34]. In healthy persons using resting-state functional magnetic resonance imaging, exercise increased the functional connectivity of neural networks [35], but this mechanism has not yet been established in AD. In addition, studies have found exercise associated increases in levels of brain-derived neurothropic factor (BDNF) and insulin-like growth factor 1 (IGF-1) to be important factors behind the possible protective mechanism of physical activity [4, 36], but evidence for these pathways is scarce in AD [31].
In the main effect study (n = 200), we did not find any significant association between changes in estimated VO2peak and changes in results in cognitive tests and in neuropsychiatric ratings [11]. One of the reasons for this discrepancy in results could be the aforementioned problem with the Astrand test, a method that is less valid than direct breath-by-breath CPET measurement [12–14].
The strength of our study includes a meticulous design including highly supervised moderate to high aerobic exercise with a high adherence rate and the use of the gold standard CPET measurement of VO2peak in a well characterized group of community-dwelling patients with mild AD. A weakness of the study is that the control group was not offered any social activity. It cannot be excluded that the social interaction in the training group may have had a positive impact on changes in NPI, and that the social interaction in the training group may have contributed to the high adherence rate and thus to the improvement in fitness. Finally, our sample is fairly small and our participants were physically well-functioning [37], which may limit the generalizability of our findings to all patients with mild AD.
Conclusion
Our study shows that it is possible to improve cardiorespiratory fitness in community-dwelling patients with mild AD. Moreover, the results suggest that this improvement may have a positive effect on mental speed, attention and neuropsychiatric symptoms in patients with mild AD. However, studies are needed to confirm the findings from our study and determine, which mechanisms that may explain potential causal relations between cardiorespiratory fitness and cognition and neuropsychiatric symptoms, respectively.
ACKNOWLEDGMENTS
The ADEX study is supported by a grant from the Innovation Fund Denmark (j. no.: 10-092814) and this work is supported by the Lundbeck Foundation (grant number FP 73/2012). The Danish Dementia Research Centre is supported by grants from the Danish Ministry of Health (J.no: 2007-12143-112, project 59506/J.no: 0901110, project 34501) and the Danish Health Foundation (J.no.: 2007B004).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0253r2).
REFERENCES
- [1]. Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S (2015) Exercise programs for people with dementia. Cochrane Database Syst Rev 4, CD006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Blankevoort CG, van Heuvelen MJ, Boersma F, Luning H, de Jong J, Scherder EJ (2010) Review of effects of physical activity on strength, balance, mobility and ADL performance in elderly subjects with dementia. Dement Geriatr Cogn Disord 30, 392–402. [DOI] [PubMed] [Google Scholar]
- [3]. Littbrand H, Stenvall M, Rosendahl E (2011) Applicability and effects of physical exercise on physical and cognitive functions and activities of daily living among people with dementia: A systematic review. Am J Phys Med Rehabil 90, 495–518. [DOI] [PubMed] [Google Scholar]
- [4]. Phillips C, Akif Baktir M, Das D, Lin B, Salehi A (2015) The link between physical activity and cognitive dysfunction in Alzheimer disease. Phys Ther 95, 1046–1060. [DOI] [PubMed] [Google Scholar]
- [5]. Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH (2008) Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology 71, 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM (2009) Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord 23, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Vidoni ED, Honea RA, Billinger SA, Swerdlow RH, Burns JM (2012) Cardiorespiratory fitness is associated with atrophy in Alzheimer’s and aging over 2 years. Neurobiol Aging 33, 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Ohman H, Savikko N, Strandberg TE, Pitkala KH (2014) Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: A systematic review. Dement Geriatr Cogn Disord 38, 347–365. [DOI] [PubMed] [Google Scholar]
- [9]. Groot C, Hooghiemstra AM, Raijmakers PG, van Berckel BN, Scheltens P, Scherder EJ, van der Flier WM, Ossenkoppele R (2016) The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res Rev 25, 13–23. [DOI] [PubMed] [Google Scholar]
- [10]. Hoffmann K, Sobol NA, Frederiksen KS, Beyer N, Vogel A, Vestergaard K, Brændgaard H, Gottrup H, Lolk A, Wermuth L, Jacobsen S, Laugesen LP, Gergelyffy RG, Høgh P, Bjerregaard E, Andersen BB, Siersma V, Johannsen P, Cotman CW, Waldemar G, Hasselbalch SG (2016) Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: A randomized controlled trial. J Alzheimers Dis 50, 443–453. [DOI] [PubMed] [Google Scholar]
- [11]. Sobol NA, Hoffmann K, Frederiksen KS, Vogel A, Vestergaard K, Braendgaard H, Gottrup H, Lolk A, Wermuth L, Jakobsen S, Laugesen L, Gergelyffy R, Hogh P, Bjerregaard E, Siersma V, Andersen BB, Johannsen P, Waldemar G, Hasselbalch SG, Beyer N (2016) Effect of aerobic exercise on physical performance in patients with Alzheimer’s disease. Alzheimers Dement 12, 1207–1215. [DOI] [PubMed] [Google Scholar]
- [12]. Astrand PO, Ryhming I (1954) A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. J Appl Physiol 7, 218–221. [DOI] [PubMed] [Google Scholar]
- [13]. Cink RE, Thomas TR (1981) Validity of the Astrand-Ryhming nomogram for predicting maximal oxygen intake. Br J Sports Med 15, 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Robergs RA, Landwehr R (2002) The surprising history of the “HRmax=220-age” equation. J Exerc Physiol Online 5, 1–10. [Google Scholar]
- [15]. Burns JM, Mayo MS, Anderson HS, Smith HJ, Donnelly JE (2008) Cardiorespiratory fitness in early-stage Alzheimer disease. Alzheimer Dis Assoc Disord 22, 39–46. [DOI] [PubMed] [Google Scholar]
- [16]. Billinger SA, Vidoni ED, Honea RA, Burns JM (2011) Cardiorespiratory response to exercise testing in individuals with Alzheimer’s disease. Arch Phys Med Rehabil 92, 2000–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Vidoni ED, Billinger SA, Lee C, Hamilton J, Burns JM (2012) The physical performance test predicts aerobic capacity sufficient for independence in early-stage Alzheimer disease. J Geriatr Phys Ther 35, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- [19]. Hoffmann K, Frederiksen KS, Sobol NA, Beyer N, Vogel A, Simonsen AH, Johannsen P, Lolk A, Terkelsen O, Cotman CW, Hasselbalch SG, Waldemar G (2013) Preserving cognition, quality of life, physical health and functional ability in Alzheimer’s disease: The effect of physical exercise (ADEX trial): Rationale and design. Neuroepidemiology 41, 198–207. [DOI] [PubMed] [Google Scholar]
- [20]. Borg G (1970) Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2, 92–98. [PubMed] [Google Scholar]
- [21]. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA (2013) Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 128, 873–934. [DOI] [PubMed] [Google Scholar]
- [22]. Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J (2012) EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 126, 2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Magri D, Corra U, Di Lenarda A, Cattadori G, Maruotti A, Iorio A, Mezzani A, Giannuzzi P, Mantegazza V, Gondoni E, Sinagra G, Piepoli MF, Fiorentini C, Agostoni P (2014) Cardiovascular mortality and chronotropic incompetence in systolic heart failure: The importance of a reappraisal of current cut-off criteria. Eur J Heart Fail 16, 201–209. [DOI] [PubMed] [Google Scholar]
- [24]. Smith A (1982) Symbol Digit Modalities Test (SDMT) Manual(revised). Western Psychological Services, Los Angeles. [Google Scholar]
- [25]. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. [DOI] [PubMed] [Google Scholar]
- [26]. Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S (2010) Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol 67, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Arnett SW, Laity JH, Agrawal SK, Cress ME (2008) Aerobic reserve and physical functional performance in older adults. Age Ageing 37, 384–389. [DOI] [PubMed] [Google Scholar]
- [28]. Billinger SA, Vidoni ED, Greer CS, Graves RS, Mattlage AE, Burns JM (2014) Cardiopulmonary exercise testing is well tolerated in people with Alzheimer-related cognitive impairment. Arch Phys Med Rehabil 95, 1714–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Anderson HS, Kluding PM, Gajewski BJ, Donnelly JE, Burns JM (2011) Reliability of peak treadmill exercise tests in mild Alzheimer disease. Int J Neurosci 121, 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Bronas UG, Salisbury D, Kelly K, Leon A, Chow L, Yu F (2017) Determination of aerobic capacity via cycle ergometer exercise testing in Alzheimer’s disease. Am J Alzheimers Dis Other Demen 32, 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Jensen CS, Hasselbalch SG, Waldemar G, Simonsen AH (2015) Biochemical markers of physical exercise on mild cognitive impairment and dementia: Systematic review and perspectives. Front Neurol 6, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Baker LD, Skinner JS, Craft S, Sink KM, Montine T, Hansen A, Wilson VM, Jung Y, Johnston M, Cholerton B, Callaghan M (2015) Aerobic exercise reduces phosphorylated tau protein in cerebrospinal fluid in older adults with mild cognitive impairment. Alzheimers Dementia 11(Suppl), P324. [Google Scholar]
- [33]. Intlekofer KA, Cotman CW (2013) Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis 57, 47–55. [DOI] [PubMed] [Google Scholar]
- [34]. Lange-Asschenfeldt C, Kojda G (2008) Alzheimer’s disease, cerebrovascular dysfunction and the benefits of exercise: From vessels to neurons. Exp Gerontol 43, 499–504. [DOI] [PubMed] [Google Scholar]
- [35]. Rajab AS, Crane DE, Middleton LE, Robertson AD, Hampson M, MacIntosh BJ (2014) A single session of exercise increases connectivity in sensorimotor-related brain networks: A resting-state fMRI study in young healthy adults. Front Hum Neurosci 8, 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Bherer L, Erickson KI, Liu-Ambrose T (2013) A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res 2013, 657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Sobol NA, Hoffmann K, Vogel A, Lolk A, Gottrup H, Hogh P, Hasselbalch SG, Beyer N (2016) Associations between physical function, dual-task performance and cognition in patients with mild Alzheimer’s disease. Aging Ment Health 20, 1139–1146. [DOI] [PubMed] [Google Scholar]