Abstract
Objectives:
To investigate the role of fluid intake from beverages before and after a diagnosis of bladder cancer in relation to the risk of developing bladder cancer recurrence.
Study Design:
Prospective cohort study.
Methods:
716 patients with non-muscle invasive bladder cancer (NMIBC), who received transurethral resection of a primary bladder tumour (TURBT) and completed self-administrated questionnaires on usual fluid intake from beverages at time of diagnosis (over the year before diagnosis) and during follow-up (over the year after diagnosis), were included. Multivariable Cox regression was used to calculate hazard ratios and 95% confidence intervals of developing recurrent bladder cancer in relation to the intake of total fluid, total alcohol, and individual beverages.
Results:
During 2,025 person-years of follow-up, 238 (33%) of the included 716 NMIBC patients developed one or more recurrences of bladder cancer. Total fluid intake before diagnosis was not associated with a first recurrence of bladder cancer when comparing the highest and lowest intake group (HR = 0.98, 95% C.I. 0.70–1.38, p = 0.91). Comparable results were obtained for total fluid intake pre-diagnosis and the risk of developing multiple recurrences of bladder cancer (HR = 1.01, 95% C.I. 0.87–1.19, p = 0.85). A total of 379 of the 716 patients reported on usual fluid intake within 1 year of diagnosis. No significant associations between total fluid intake 1 year after diagnosis and a first recurrence of bladder cancer were found when comparing the highest and lowest intake group (HR = 0.91; 95% C.I. 0.60–1.37, p = 0.65) or with multiple recurrences of bladder cancer (HR = 1.06; 95% C.I. 0.89–1.26, p = 0.54). In addition, total alcohol intake and individual beverages were not associated with bladder cancer recurrence.
Conclusions:
The results indicate that an individual’s fluid intake from beverages is unlikely to have an important role in bladder cancer recurrence.
Keywords: Fluid intake, bladder cancer, recurrence
INTRODUCTION
Non-muscle-invasive bladder cancer (NMIBC) is the most common malignancy of the urinary tract and has a high rate of recurrence despite adequate therapy. Identification of modifiable risk factors could reduce the risk of developing recurrences and improve prognosis. The urogenous contact theory hypothesizes that an increased voiding frequency may reduce bladder cancer risk [1, 2]. By increasing the intake of fluids, potential carcinogens present in the urine are diluted and the voiding frequency stimulated. By reducing the contact time of carcinogens with the bladder urothelium, the risk of bladder cancer decreases. On the contrary, it has been suggested that when the bladder wall is extended from a high volume of urine, carcinogens can come into contact with deeper layers of the bladder urothelium and increase bladder cancer risk [3]. In addition, in some parts of the world drinking water contaminated with a high concentration of arsenic could increase the risk of bladder cancer [4]. It is conceivable that the theories about fluid intake and the risk of developing bladder cancer represent a modifiable factor of importance in bladder cancer prognosis as well [5–7]; it would be a compelling strategy to simply increase or decrease fluid intake to decrease the risk of recurrence. To our knowledge, only Donat et al. [8] investigated the impact of total fluid intake on tumour recurrence in patients with NMIBC. At each visit, all patients undergoing surveillance for recurring tumours completed a self-administered fluid intake questionnaire that measured total fluid intake during a 24-hour period. Results of this study indicated that daily fluid intake levels did not affect recurrence and that the types of fluids imbibed may be more important than the total amount [8]. Therefore, the present study investigates the role of individual beverages, total alcohol and total fluid intake (over the year before and the year after diagnosis) and the risk of developing one or more recurrences of bladder cancer.
METHODS
The bladder cancer prognosis programme
This study is part of the Bladder Cancer Prognosis Programme (BCPP), a prospective cohort study in the West Midlands region of England. Details of the cohort have been published previously [9]. Briefly, during the enrolment period (December 2005–October 2011), a total of 1,550 male and female patients (age ≥18 years) were enrolled based on abnormal cystoscopic findings suggestive of bladder cancer. Transurethral resection of the primary bladder tumour (TURBT) was followed by cytoscopic surveillance. Optimal additional treatment comprised intravesical chemotherapy with mitomycin C within 24 hours of TURBT and/or a course of further mitomycin C or intravesical BCG, as per contemporary European Association of Urology guidelines. Bladder cancer recurrence was defined as the new occurrence of a non-muscle invasive bladder cancer (stage Ta, T1, or pTis) at the same or at a different site as the initial primary bladder tumour and excluding recurrence identified at the first check cystoscopy. Written informed consent was obtained from all participants. The study protocol was approved by the Nottingham Research Ethics Committee (06/MRE04/65) and registered on ClinicalTrials.gov (NCT00553566).
Data collection
Around the time of diagnosis, just prior to, or just post TURBT, data on medical history, socio-demographics, quality of life, and health-related lifestyle (including dietary intake) were collected by a trained research nurse using semi-structured face-to-face interviews and a questionnaire. The research nurse and patient went through the questionnaire page by page. Patients were asked about habitual dietary intake over the previous year. The developed version of the food-frequency questionnaire (FFQ) aims to assess the dietary intake, by asking the participants to report the frequency of consumption of approximately 16-line items over the last year. More specifically, the frequency of fluid intake from beverages asked in the questionnaire consisted of six levels: never or less than once per month, one to three times per month, once a week, two to four times per week, five to six times per week, or at least once per day. For each drink, a measure size was provided (e.g. cup, (small) glass, pub measure (2.5cl), or (half) pint glass). The frequency of intake of each beverage was multiplied by their measure size to calculate the millilitres of fluids consumed per day. Total fluid intake was computed as the sum of servings of all beverages in the questionnaire: wine-champagne, fortified wine, beer, cider, spirits, liqueurs, coffee, tea, hot chocolate, soup, ovaltine-horlicks, fizzy pop, pure fruit juice, fruit squash, milk, and water. Total alcohol intake included alcoholic beverages only: wine-champagne, fortified wine, beer, cider, spirits, and liqueurs. Repeated fluid intake from beverages was collected through a postal follow-up questionnaire one year after diagnosis.
Exclusion criteria
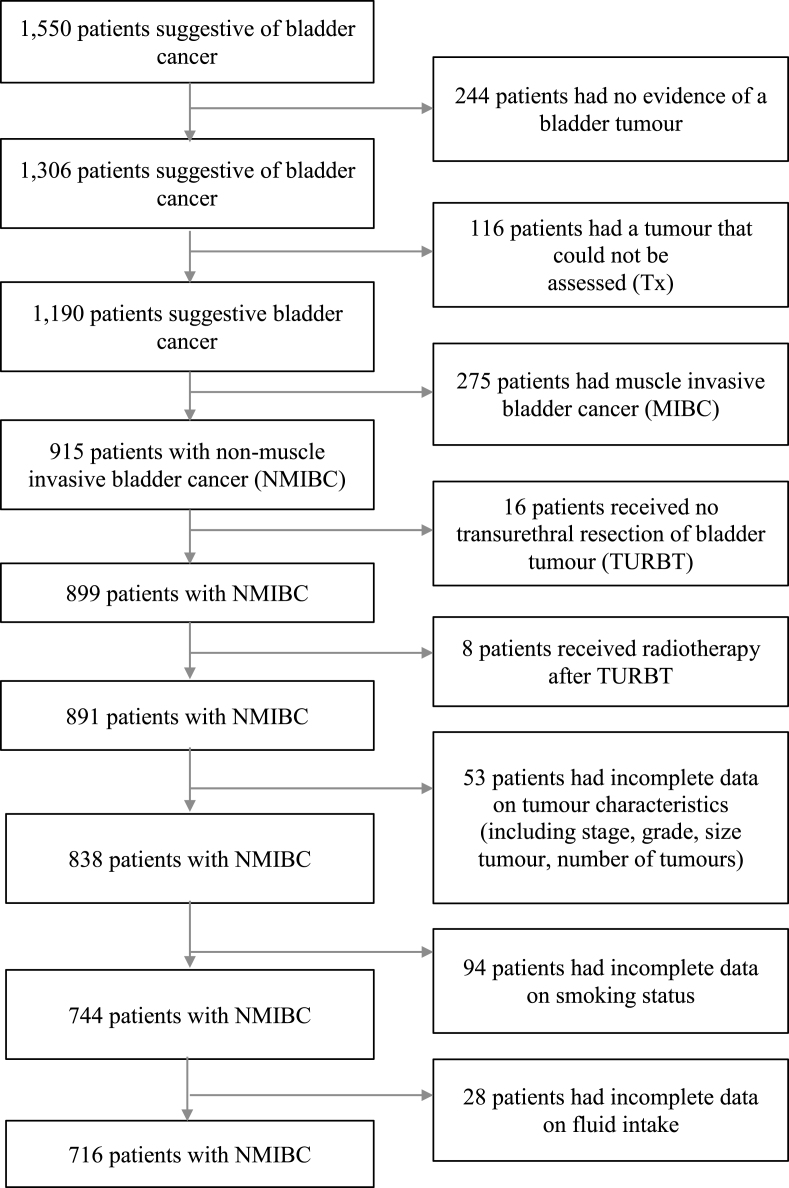
A total of 244 participants with no evidence of a bladder tumour (T0), patients who had a tumour that could not be assessed (Tx) (n = 116), who had muscle invasive bladder cancer (MIBC) (n = 275), who received no TURBT (n = 16), who had radiotherapy (on suspicion of MIBC) (n = 8), who had incomplete data on tumour characteristics (e.g. stage, grade, size, multiplicity) (n = 53) and smoking (n = 94), and had missing data on pre-diagnosis fluid intake (n = 28), were all excluded from this study (Fig. 1). The final analysis for fluid intake over the year before diagnosis comprised 716 patients. More than a third of these 716 patients did not complete a follow-up questionnaire one year after diagnosis (n = 278) or developed a recurrence of bladder cancer before completing the follow-up questionnaire (n = 59). Therefore, a total of 379 patients remained for investigating the association between fluid intake over the year after diagnosis and bladder cancer recurrence.
Fig.1.
Flow diagram of patients selection, with exclusion criteria.
Statistical analysis
According to the UK government recommendations on eating healthy and achieving a balanced diet, anybody living in a maritime climate should consume at least 1,200 mL of fluids from drinks a day [10]. Patients were divided into three groups: a group with an intake of 250 mL –850 mL of total fluid per day, a group consuming 850 mL –1,200 mL of total fluid per day, or a group with a total intake of more than the recommended 1,200 mL of fluid per day. Patients became at risk for a recurrence of bladder cancer from the date of TURBT and remained at risk until the earliest occurrence of a recurrence, cystectomy, death, the most recent surveillance cystoscopy, or study end (five years post-TURBT). Cox regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (95% C.I.) of developing a first recurrence of bladder cancer in relation to total fluid intake, total alcohol intake, and individual beverages. To identify possibly influential outliers in total fluid and alcohol intake, Cook’s Distance was used. The association of fluid intake with recurrent bladder cancer was examined in both crude and multivariate models. Confounders were considered a priori based on known prognostic factors for NMIBC recurrence from the European Association of Urology guidelines and included: age at diagnosis (continuous) [11], sex (male/female) [12], smoking status (never/former/current smoker) [13], and tumour characteristics including stage (pTa/pT1/pTis), grade (1/2/3), size of largest tumour (diameter <3 cm/≥3 cm), and tumour multiplicity (1/>1) [14–16].
Conditional risk set modelling was applied to investigate time to each recurrent event and analysis time was reset at each event [17]. For this analysis, re-resection of tumours (yes/no) was added as a confounder. The proportional hazards assumption was checked in all models using Schoenfeld residuals [18]. Cumulative incidence functions (CIF) accounting for competing risks (death and cystectomy) were made [19]. These models are conditional as the failure times are conditional on the occurrence of the prior recurrence, i.e. a second recurrence cannot occur before the first recurrence. P-values were 2-sided with a significance level of 0.05. All statistical analyses were performed using Stata softwareversion 14.
RESULTS
Baseline characteristics and number of recurrences
During 2,025 person-years of follow-up (mean (SD) follow-up 3.7 (1.5) years), 238 (33%) of the 716 NMIBC patients developed one or more recurrences of bladder cancer. More specifically, 80 (34%) of these 238 patients developed a second recurrence, 35 (15%) a third recurrence, 17 (8%) a fourth recurrence, five (3%) a fifth recurrence, two (1%) a sixth recurrence, and one (1%) a seventh recurrence. Patient characteristics at diagnosis and initial treatment are presented in Table 1. The median age at diagnosis was 71 years and most patients were male (80%), Caucasian (97%), and current or former smokers (86%). The average intake of fluid was relatively evenly distributed between patients who had a recurrence of bladder cancer and those who had not (mean total fluid intake 1,087 mL per day and 1,103 mL per day, respectively).
Table 1.
Patient characteristics at diagnosis for 716 NMIBC patients treated with transurethral resection of a primary bladder tumour
| Number (%) | ||
| Age at time of diagnosis (years) median (25th–75th percentile) | 71 (63–77) | |
| Sex | Male | 573 (80%) |
| Female | 143 (20%) | |
| Smoking status | Current smokers | 335 (47%) |
| Former smokers | 281 (39%) | |
| Never smokers | 100 (14%) | |
| Alcohol consumption | Drinkers | 540 (75%) |
| Non-drinkers | 176 (25%) | |
| Educational level | High | 89 (12%) |
| Middle | 91 (13%) | |
| None/Low | 163 (23%) | |
| BCG intravesical immunotherapy | Yes | 111 (15%) |
| No | 275 (39%) | |
| Mitomycin C intravesical chemotherapy | Yes | 337 (47%) |
| No | 73 (10%) | |
| Tumour stage | pTa | 478 (67%) |
| pT1 | 232 (32%) | |
| pTis | 6 (1%) | |
| Tumour grade | 1 | 206 (29%) |
| 2 | 261 (36%) | |
| 3 | 249 (35%) | |
| Size largest tumour (cm) | <3 | 449 (63%) |
| ≥3 | 267 (37%) | |
| Tumours multiplicity | 1 | 427 (59%) |
| >1 | 289 (41%) | |
| No of recurrences over 5 years | 0 | 478 (67%) |
| 1 | 158 (22%) | |
| >1 | 80 (11%) |
Where the data contains missing values the percentages do not add up to 100%.
Total fluid intake and bladder cancer recurrence
Table 2 presents HRs with corresponding 95% C.I. for total fluid intake over the year before diagnosis from beverages and time to a first bladder cancer recurrence and multiple bladder cancer recurrences among 716 NMIBC patients. Total fluid intake the year before diagnosis did not affect the recurrence of a first bladder tumour (HR = 0.98, 95% C.I. 0.70–1.38, p = 0.91) when adjusted for age, sex, smoking status, stage, grade, and tumour size and multiplicity. The association between total fluid intake the year before diagnosis and time to multiple recurrences showed similar results (HR = 1.01, 95% C.I. 0.87–1.19, p = 0.85) when adjusted for the same confounders and tumour re-resection (second transurethral resection).
Table 2.
Hazard ratios (HR) and 95% confidence intervals (95% C.I.) for Cox proportional hazard models predicting a first and multiple recurrences of bladder cancer, based on total fluid intake over the year before diagnosis, in 716 NMIBC patients
| Model 1* | Model 2** | Model 3*** | |||||||
| n | events | HR (95% C.I.) | p-value | HR (95% C.I.) | p-value | HR (95% C.I.) | p-value | p for trend | |
| Time to first recurrence | |||||||||
| 250–850 mL | 205 | 64 | ref | ref | ref | 0.86 | |||
| 850–1200 mL | 257 | 98 | 1.24 (0.90–1.69) | 0.19 | 1.26 (0.92–1.72) | 0.16 | 1.17 (0.85–1.62) | 0.33 | |
| >1200 mL | 254 | 76 | 0.92 (0.66–1.28) | 0.62 | 0.93 (0.67–1.31) | 0.70 | 0.98 (0.70–1.38) | 0.91 | |
| Time to multiple recurrences | |||||||||
| 250–850 mL | 205 | 92 | ref | ref | ref | 0.78 | |||
| 850 –1200 mL | 257 | 171 | 0.95 (0.83–1.09) | 0.48 | 0.96 (0.84–1.10) | 0.57 | 0.95 (0.83–1.08) | 0.42 | |
| >1200 mL | 254 | 115 | 1.05 (0.90–1.22) | 0.51 | 1.05 (0.90–1.23) | 0.54 | 1.01 (0.87–1.19) | 0.85 | |
*Model 1 is unadjusted, **Model 2 is adjusted for age, sex, and smoking status, ***Model 3 is adjusted for age, sex, smoking status, tumour stage, grade, size and multiplicity. In the time to multiple recurrences analyses, Model 2 and Model 3 were additionally adjusted for the variable re-resection of a bladder tumour (second transurethral resection).
Table 3 presents the results for the remaining 379 NMIBC patients on total fluid intake after diagnosis –no significant associations were observed between total fluid intake and the development of a first bladder cancer recurrence (HR = 0.91; 95% C.I. 0.60–1.37, p = 0.65) or multiple recurrences (HR = 1.06; 95% C.I. 0.89–1.26, p = 0.54) when corrected for known prognostic factors for NMIBC recurrence provided in the European Association of Urology guidelines (including age, sex, smoking status, tumour characteristics and tumour re-resection).
Table 3.
Hazard ratios (HR) and 95% confidence intervals (95% C.I.) for Cox proportional hazard models predicting a first and multiple recurrences of bladder cancer, based on total fluid intake over the year after diagnosis, in 379 remaining NMIBC patients
| Model 1* | Model 2** | Model 3*** | |||||||
| n | Events | HR (95% C.I.) | p-value | HR (95% C.I.) | p-value | HR (95% C.I.) | p-value | p for trend | |
| Time to first recurrence | |||||||||
| 250–850 mL | 116 | 45 | ref | ref | ref | 0.65 | |||
| 850–1200 mL | 124 | 48 | 1.08 (0.72–1.63) | 0.71 | 1.04 (0.69–1.57) | 0.86 | 1.07 (0.71–1.61) | 0.76 | |
| >1200 mL | 139 | 46 | 0.81 (0.54–1.21) | 0.31 | 0.80 (0.53–1.20) | 0.27 | 0.91 (0.60–1.37) | 0.65 | |
| Time to multiple recurrences | |||||||||
| 250–850 mL | 116 | 67 | ref | ref | ref | 0.53 | |||
| 850–1200 mL | 124 | 71 | 1.07 (0.91–1.25) | 0.41 | 1.06 (0.91–1.24) | 0.43 | 1.08 (0.91–1.27) | 0.38 | |
| >1200 mL | 139 | 68 | 1.05 (0.87–1.26) | 0.63 | 1.04 (0.86–1.26) | 0.67 | 1.06 (0.89–1.26) | 0.54 | |
*Model 1 is unadjusted, **Model 2 is adjusted for age, sex, and smoking status, ***Model 3 is adjusted for age, sex, smoking status, tumour stage, grade, size and multiplicity. In the time to multiple recurrences analyses, Model 2 and Model 3 were additionally adjusted for the variable re-resection of a bladder tumour (second transurethral resection).
Total alcohol and individual beverages intake and bladder cancer recurrence
Results of the Cox proportional hazard models predicting the development of recurrence based on total alcohol intake and individual beverages can be found in the online supplementary file (Tables S1–S8). Among 716 NMIBC patients, total alcohol intake before diagnosis (highest intakes >125 mL versus lowest intakes 0 mL) was not associated with a first bladder tumour (HR = 0.97; 95% CI 0.70–1.36) in the most extensive model (adjusted for age, sex, smoking status, stage, grade, and tumour size and multiplicity) (Table S1). Similar results were found for total alcohol intake prior to a diagnosis of bladder cancer and multiple recurrences of bladder cancer (HR = 0.97; 95% CI 0.84–1.11) when corrected for the same confounders as in the time to a first recurrence analysis plus re-resection of a bladder tumour (Table S2).
None of the individual alcoholic beverages including beer, cider, wine/champagne, fortified wine, spirits or liqueurs consumed prior to bladder cancer diagnosis influenced the risk of developing a first or multiple recurrences of bladder cancer when comparing the highest frequency of intake ‘at least 1 per day’ versus the lowest frequency of intake /never/less than 1 per month’ (Tables S1–S2). The warm beverages coffee, tea, hot chocolate, ovaltine/horlicks and soup were also investigated. No associations were found when comparing the frequencies of these warm beverages (at least one per day versus never/less than 1 per month before diagnosis) and a first or multiple recurrences of bladder cancer (Tables S3–S4). In addition, pre-diagnosis consumption of the cold beverages milk, water, fizzy pop, pure fruit juice, and fruit squash/cordial, did not affect the development of one or multiple recurrences of bladder cancer when comparing the highest versus the lowest frequency intakes.
Among the 379 NMIBC that remained for analyses one year after a diagnosis of bladder cancer, total alcohol intake was not associated with a first recurrence (HR = 1,01; 95% CI 0.65–1.58) or multiple recurrences (HR = 1,05; 95% CI 0.88–1.25) (Tables S5–S6). Consumption of alcoholic beverages one year after a diagnosis of bladder cancer was not related to the development of bladder cancer recurrences (Tables S5–S6). Finally, no associations were found for usual warm and hot non-alcoholic beverages consumption and one or more recurrence of bladder cancer (Tables S7–S8).
DISCUSSION
The results of this study indicate that there is no evidence of an association between total fluid, total alcohol, or individual beverages (before and after diagnosis) and recurrence of a bladder tumour in patients diagnosed for NMIBC. These results are consistent with the findings of Donat et al. [8] who also concluded it remains unclear whether increased total fluid intake is beneficial against the development of bladder cancer recurrence. With regard to fluid intake and the risk of developing bladder cancer for the first time, the results of a recent case-control study have shown that there was no association with total water intake from both beverages and foods [20]. Also the results of a meta-analysis demonstrated no association between total fluid intake and the risk of developing primary bladder cancer [21]. However, subgroup analyses indicated that high fluid intake could increase the risk of bladder cancer in European men (and possibly American men) and decrease the risk of bladder cancer in Asian men [21].
It seems plausible, however, that substances in beverages could be involved in carcinogenesis in the bladder as they come into contact with the bladder urothelium when excreted via the urine. The numerous substances may react differently with cells of the bladder urothelium and be involved in several different pathways associated with cancer pathogenesis including inflammation, cell survival and self-renewal of cancer stem cells [22, 23]. A possible explanation for the lack of an association between fluid intake from beverages and bladder cancer recurrence could be that tumour recurrence is more influenced by tumour biology, field cancerization, and cancer treatment than continued exposure of potential carcinogens with the bladder urothelium [24–26]. Incomplete resection of the primary tumour and tumour cell re-implantation remain to be the most influential factors in the development of NMIBC recurrence [26]. Finally, it is inevitable that fluid intake was not measured without error - recall bias and measurement errors in dietary intake cannot be excluded and are a common limitation of epidemiological studies.
CONCLUSIONS
The results of this study indicate that fluid intake from beverages is unlikely to have a dominant role in influencing the risk of subsequent recurrence(s). Proposed strategies aimed at decreasing contact time of carcinogens with the urothelium by increasing fluid intake are unlikely to delay or prevent the development of bladder cancer recurrence in NMIBC patients.
CONFLICT OF INTEREST STATEMENT
All authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest in the subject matter or materials discussed in this manuscript.
FUNDING
BCPP was supported by Cancer Research UK, the Institute of Applied Health Research (IAHR), and the Institute of Cancer and Genomic Sciences of the University of Birmingham.
Supplementary Material
SUPPLEMENTARY MATERIAL
Online Supplemental Tables S1-S8. Hazard ratios (HR) and 95% confidence intervals (95% C.I.) for Cox proportional hazard models predicting a first and multiple recurrences of bladder cancer, based on alcohol intake from beverages and individual beverages consumed in the year before and in the year after diagnosis.
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-180172.
REFERENCES
- [1]. Silverman DT, Hartge P, Morrison AS, Devesa SS. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 1992;6:1–30. [PubMed] [Google Scholar]
- [2]. McDonald DF, Lund RR. The role of the urine in vesical neoplasm. I. Experimental confirmation of the urogenous theory of pathogenesis. J Urol. 1954;71:560–70. [DOI] [PubMed] [Google Scholar]
- [3]. Claude J, Kunze E, Frentzel-Beyme R, Paczkowski K, Schneider J, Schubert H. Life-style and occupational risk factors in cancer of the lower urinary tract. Am J Epidemiol. 1986;124:578–89. [DOI] [PubMed] [Google Scholar]
- [4]. World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: Diet, Nutrition, Physical Activity and Bladder Cancer. 2015. n.d.
- [5]. Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Curhan GC, Willett WC, et al. Fluid intake and the risk of bladder cancer in men. N Engl J Med. 1999;340:1390–7. doi: 10.1056/NEJM199905063401803 [DOI] [PubMed] [Google Scholar]
- [6]. Zhou J, Smith S, Giovannucci E, Michaud DS. Reexamination of total fluid intake and bladder cancer in the health professionals follow-up study cohort. Am J Epidemiol. 2012;175: 696–705. doi: 10.1093/aje/kwr359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50:167–78. doi: 10.3109/0284186X.2010.529822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Donat SM, Bayuga S, Herr HW, Berwick M. Fluid intake and the risk of tumor recurrence in patients with superficial bladder cancer. J Urol. 2003;170: 1777–80. doi: 10.1097/01.ju.0000091803.35049.da. [DOI] [PubMed] [Google Scholar]
- [9]. Zeegers MP, Bryan RT, Langford C, Billingham L, Murray P, Deshmukh NS, et al. The West Midlands Bladder cancer prognosis programme: Rationale and design. BJU Int. 2010;105: 784–8. doi: 10.1111/j.1464-410X.2009.08849.x. [DOI] [PubMed] [Google Scholar]
- [10]. Public Health England in association with the Welsh government, Food Standards Scotland and the Food Standards Agency in Northern Ireland. The Eatwell Guide. n.d.
- [11]. Taylor JA, Kuchel GA. Bladder cancer in the elderly: Clinical outcomes, basic mechanisms, and future research direction. Nat Clin Pract Urol. 2009; 6:135–44. doi: 10.1038/ncpuro1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Fajkovic H, Halpern JA, Cha EK, Bahadori A, Chromecki TF, Karakiewicz PI, et al. Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol. 2011;29:457–63. doi: 10.1007/s00345-011-0709-9. [DOI] [PubMed] [Google Scholar]
- [13]. Wyszynski A, Tanyos SA, Rees JR, Marsit CJ, Kelsey KT, Schned AR, et al. Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer. 2014;120:408–14. doi: 10.1002/cncr.28394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Stein JP, Grossfeld GD, Ginsberg DA, Esrig D, Freeman JA, Figueroa AJ, et al. Prognostic markers in bladder cancer: A contemporary review of the literature. J Urol. 1998;160:645–59. [DOI] [PubMed] [Google Scholar]
- [15]. Yan Y, Andriole GL, Humphrey PA, Kibel AS. Patterns of multiple recurrences of superficial (Ta/T1) transitional cell carcinoma of bladder and effects of clinicopathologic and biochemical factors. Cancer. 2002;95: 1239–46. doi: 10.1002/cncr.10822. [DOI] [PubMed] [Google Scholar]
- [16]. Ather MH, Nazim SM. New and contemporary markers of prognosis in nonmuscle invasive urothelial cancer. Korean J Urol. 2015;56:553. doi: 10.4111/kju.2015.56.8.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Amorim LDAF, Cai J. Modelling recurrent events: A tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44:324–33. doi: 10.1093/ije/dyu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Schoenfeld D. Partial residuals for the proportionnal hazards regression model. Biometrika. 1982;69:239–41. [Google Scholar]
- [19]. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- [20]. Di Maso M, Bosetti C, Taborelli M, Montella M, Libra M, Zucchetto A, et al. Dietary water intake and bladder cancer risk: An Italian case–control study. Cancer Epidemiol. 2016;45:151–6. doi: 10.1016/j.cane2016.09.015. [DOI] [PubMed] [Google Scholar]
- [21]. Liu Q, Liao B, Tian Y, Chen Y, Luo D, Lin Y, et al. Total fluid consumption and risk of bladder cancer: A meta-analysis with updated data. Oncotarget. 2017;8:55467–77. doi: 10.18632/oncotarget.18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Prasad S, Phromnoi K, Yadav V, Chaturvedi M, Aggarwal B. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010;76:1044–63. doi: 10.1055/s-0030-1250111. [DOI] [PubMed] [Google Scholar]
- [23]. Kim YS, Farrar W, Colburn NH, Milner JA. Cancer stem cells: Potential target for bioactive food components. J Nutr Biochem. 2012;23:691–8. doi: 10.1016/j.jnutbio.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Takahashi T, Habuchi T, Kakehi Y, Mitsumori K, Akao T, Terachi T, et al. Clonal and chronological genetic analysis of multifocal cancers of the bladder and upper urinary tract. Cancer Res. 1998;58:5835–41. [PubMed] [Google Scholar]
- [25]. Simon R, Eltze E, Schäfer KL, Bürger H, Semjonow A, Hertle L, et al. Cytogenetic analysis of multifocal bladder cancer supports a monoclonal origin and intraepithelial spread of tumor cells. Cancer Res. 2001;61:355–62. [PubMed] [Google Scholar]
- [26]. Bryan RT, Collins SI, Daykin MC, Zeegers MP, Cheng K, Wallace DMA, et al. Mechanisms of recurrence of Ta/T1 bladder cancer. Ann R Coll Surg Engl. 2010;92:519–24. doi: 10.1308/003588410X12664192076935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.