Abstract
Background:
Guidelines recommend a single postoperative instillation of intravesical chemotherapy within 24 hours of transurethral resection of bladder tumors (TURBT) in patients with low- and intermediate-risk non-muscle invasive bladder cancer (NMIBC) to reduce recurrence risk.
Objective:
To evaluate the 2-year recurrence rate (2-YRR) of bladder cancer in randomized patients with Ta, G1-G2 histology who receive TURBT plus apaziquone versus TURBT plus placebo.
Methods:
Two nearly identical Phase 3, multinational, randomized, double-blind, placebo-controlled trials were conducted in patients with histologically confirmed Ta, G1-G2 NMIBC (Target Population) to evaluate the efficacy/safety of a single instillation of apaziquone post-TURBT. A single intravesical instillation of apaziquone (4 mg/40 mL) or placebo was administered within 6 hours post-TURBT. The primary and secondary efficacy endpoints were 2-YRR and time to recurrence (TTR) respectively.
Results:
Overall, 1614 patients were enrolled, including 1146 patients in the Target Population. Individually, the two studies did not meet statistical significance for 2-YRR (38.0% vs 44.6% ; 39.7% vs. 46.3%). Because apaziquone is rapidly metabolized in blood, a post hoc subgroup analysis was performed by time window of drug instillation post-TURBT. Patients who had drug instilled in the time window 60±30 minutes post-TURBT demonstrated 20.3% and 20.8% reduction in 2-YRR and 56% (HR = 0.44) and 45% (HR = 0.55) reduction in hazards for TTR in two studies respectively. Apaziquone was well tolerated with minimal toxicity.
Conclusions:
Two identical Phase 3 studies supported the safety of apaziquone (4 mg/40 mL) administered as a single intravesical instillation post-TURBT and identified efficacy when instilled within 60±30-minutes time interval which requires further study.
Keywords: Apaziquone, E09, bladder, bladder cancer, chemotherapy, non-muscle invasive bladder cancer, TURBT, intravesical instillation
INTRODUCTION
Approximately 70% of newly diagnosed cases of bladder cancer are categorized as non-muscle invasive bladder cancer (NMIBC), which is initially treated with transurethral resection of bladder tumor (TURBT) [1]. Although the survival rate for the majority of patients with NMIBC is favorable [2], about 50% of patients with low-grade Ta disease are expected to have a recurrence within 5 years [1].
All international guidelines [1–5] recommend that patients with low-risk NMIBC and some with intermediate-risk NMIBC receive a single intravesical dose of chemotherapy immediately post- TURBT to reduce the risk of recurrence. To date, there has been no FDA-approved drug for this indication. Mitomycin C (MMC), epirubicin, and other antineoplastics, such as doxorubicin, are used “off-label,” but none of these drugs are specifically formulated for intravesical use.
Apaziquone is a fully synthetic bioreductive alkylating agent. It is a pro-drug that is enzymatically activated by DT diaphorase (DTD) and other reductases to generate cytotoxic species that lead to apoptosis and cell death. Several tumor types, including bladder tumors, have elevated DTD levels. Apaziquone has demonstrated potent antitumor activity in both in vitro and in vivo tumor models [6]. Moreover, apaziquone has demonstrated 30 to 100 times more potent activity than MMC in bladder cancer cells [7]. Based on encouraging preclinical activity, initial clinical development of apaziquone was undertaken by the European Organization for Research and Treatment of Cancer (EORTC). However, apaziquone did not show any appreciable activity when administered intravenously, due to rapid elimination of the drug from systemic circulation and poor drug delivery to tumors [8].
Direct evidence of the antitumor activity of apaziquone in NMIBC was provided in two Phase 1/2 marker lesion studies, in which patients received an intravesical instillation of apaziquone (4 mg in 40 mL) once a week for 6 weeks, starting 2 to 4 weeks after TURBT. In these marker lesion studies, apaziquone was well tolerated, and in each study, 67% of patients achieved a complete response [9, 10]. This compared favorably to studies of other intravesical agents in which only 26% to 46% of patients reported complete response [11, 12]. Given the evidence of efficacy in NMIBC in the marker lesion studies, two pivotal, Phase 3, multicenter, randomized, double-blind, placebo-controlled studies were conducted to evaluate the efficacy and safety of a single intravesical instillation of apaziquone immediately post-TURBT in the treatment of patients with Ta, G1-G2 NMIBC. This paper presents findings from the two pivotal studies with the hypothesis that administration of apaziquone reduces the 2-year recurrence rate(2-YRR) in NMIBC patients.
PATIENTS AND METHODS
Study designs and eligibility
SPI-611 and SPI-612 were Phase 3, double-blind, placebo-controlled studies of nearly identical design and were conducted between April 2007 and January 2012. The SPI-611 study was conducted at 76 sites in the US and 7 sites in Poland; and the SPI-612 study was conducted at 23 sites in the US, 30 sites in Canada, and 20 sites in Poland. The protocols were approved by Local Research and Ethics Committees and signed informed consent was obtained from all study patients. Eligible patients were 18 years of age or older with clinically apparent stage Ta, G1-G2 (World Health Organization [WHO] 1973 classification [13]) urothelial cell carcinoma (UCC) of the bladder.
Patients who signed informed consent underwent cystoscopy to determine clinically apparent stage Ta, G1-G2 NMIBC. However, the analysis population to be studied (Ta, G1-G2 NMIBC) was based on the pathological confirmation of the resected tumor specimens from TURBT procedure performed after randomization. Eligible patients underwent a TURBT procedure and were randomized in a 1 : 1 ratio to receive either placebo or apaziquone. The Target Population was defined as patients having ≤4 histologically confirmed tumors to be Ta, G1-G2 by the central laboratory using the WHO 1973 classification, each ≤3.5 cm. Urine cytology was performed at time of follow-up cystoscopy but was not for eligibility. Patients in the Target Population were to receive no further treatment for NMIBC, while patients with tumor histology other than Ta, G1-G2 were treated per standard of care. All patients were evaluated cystoscopically every 3 months for 2 years for tumor recurrence. All recurrences were confirmed histologically by the central laboratory.
Treatments administered and instillation regimen
All visible lesions were removed by TURBT at study entry. Within 6 hours of TURBT, and if there was no reported or suspected bladder perforation, a single dose of either apaziquone (4 mg in 40 mL) or matching placebo (containing FD&C red dye #40, sodium chloride and mannitol and supplied in identical vials) was instilled into the bladder via an indwelling Foley catheter and was retained in the bladder for 60 minutes. Patients were monitored during medication retention. At the end of the retention period, the bladder was drained.
Statistical analysis
In both studies, the primary efficacy endpoint was the 2-YRR of bladder cancer, which was defined as the proportion of patients with a histologically confirmed bladder tumor any time after randomization and on or before the end of 2 years and was analyzed using odds ratio (OR) and Cochran-Mantel-Haenszel chi-squared test. The key secondary efficacy endpoint was time to recurrence (TTR), which was defined as time from randomization to the date of the first histologically confirmed recurrence of bladder tumor and was analyzed using hazard ratio (HR) and Kaplan-Meier estimates and log-rank test. Multivariate and post hoc subgroup analyses of efficacy endpoints (2-YRR and TTR) were performed to identify covariates and patient subgroups that affected the pre-specified efficacy measures. The covariates and subgroups were gender, age, smoking history, country, primary or recurrent tumor, prior intravesical therapy (IVT), number of tumors, tumor grade, size, and time window of instillation post-TURBT. All statistical analyses were performed using SAS software, v9.3. All p-values presented for this study are nominal p-values. Corresponding 95% confidence intervals (CI) for OR and HR are presented in Figures.
Sample-size calculations for these studies were based on the recurrence rates reported in a meta- analysis in 2004, which showed recurrence rates of 48.4% for TURBT alone and 36.7% for TURBT plus intravesical therapy [14]. The studies were designed to detect a 12% difference between apaziquone and placebo in 2-YRR at a 5% level of significance and 80% power.
RESULTS
Data from two Phase 3 studies are provided by study as well as pooled for baseline patient characteristics, primary analysis, and safety. Consolidated Standards of Reporting Trials Diagrams are provided for the individual trials (SPI-611 and SPI-612) (Supplementary Figure 1) and for the pooled data (Supplementary Figure 2). A total of 1614 patients from the two studies (apaziquone n = 808, placebo n = 806) were enrolled and included in the Safety Population. Among them, 1146 patients (apaziquone n = 577, placebo n = 569) with Ta, G1-G2 bladder tumors were identified as the Target Population for efficacy.
Patient demographics and baseline characteristics for the target population are presented in Table 1. Patient demographics between treatment groups in both studies were similar. The majority of patients were male (71.6%) and White (97.2%) and the mean age was 66.7 years. In the Target Population, 62.2% patients had primary bladder cancer, 62.8% had only one lesion at baseline, 74.1% had G1 tumor and 84.6% had no prior intravesical therapy (IVT). About 40% of patients had drug instilled within 30 minutes post-TURBT, 18.9% between 31 and 90 minutes, and 41.3% after 90 minutes. Most patients retained the full 40 mL volume of drug (99.6%) in the bladder for the protocol specified duration of 60 minutes (86.5%). A full summary of patient demographics is presented in Supplementary Table 1 (Safety Population) and Supplementary Table 2 (Target Population). A full summary of tumor characteristics in the target population is presented in Supplementary Table 3.
Table 1.
Demographics and tumor characteristics for the target population
| Study SPI-611 | Study SPI-612 | Pooled Data | |||||
| Characteristic/Category | Apaziquone (n = 295) | Placebo (n = 271) | Apaziquone (n = 282) | Placebo (n = 298) | Apaziquone (n = 577) | Placebo (n = 569) | Total (n = 1146) |
| Gender, % | |||||||
| Female | 28.8 | 26.6 | 28.0 | 30.2 | 28.4 | 28.5 | 28.4 |
| Male | 71.2 | 73.4 | 72.0 | 69.8 | 71.6 | 71.5 | 71.6 |
| Race, % | |||||||
| White | 97.3 | 97.0 | 97.5 | 97.0 | 97.4 | 97.0 | 97.2 |
| Black or African American | 2.0 | 1.8 | 1.8 | 0.7 | 1.9 | 1.2 | 1.6 |
| Asian | 0 | 0.7 | 0.7 | 2.0 | 0.3 | 1.4 | 0.9 |
| Other | 0.7 | 0.4 | 0 | 0.3 | 0.3 | 0.4 | 0.3 |
| Age, years | |||||||
| Mean (SD) | 66.9 (11.3) | 67.7 (10.6) | 66.6 (11.9) | 65.9 (11.8) | 66.8 (11.6) | 66.7 (11.3) | 66.7 (11.4) |
| Bladder Cancer History, % | |||||||
| Primary | 63.7 | 60.9 | 61.3 | 62.8 | 62.6 | 61.9 | 62.2 |
| Recurrent | 36.3 | 39.1 | 38.7 | 37.2 | 37.4 | 38.1 | 37.8 |
| Prior Intravesical Therapies, % | |||||||
| Any Prior IVT | 17.6 | 15.1 | 15.6 | 14.0 | 17.0 | 13.9 | 15.4 |
| BCG | 14.6 | 12.9 | 8.2 | 5.7 | 11.4 | 9.1 | 10.3 |
| Mitomycin C | 7.8 | 4.4 | 6.0 | 6.7 | 6.9 | 5.6 | 6.3 |
| Other IVT | 0.7 | 2.6 | 5.0 | 4.0 | 2.8 | 3.3 | 3.1 |
| Tumor Lesion Number at Baseline, % | |||||||
| 1 lesion | 64.7 | 66.8 | 59.2 | 60.7 | 62.0 | 63.6 | 62.8 |
| 2 lesions | 17.3 | 19.9 | 23.0 | 17.8 | 20.1 | 18.8 | 19.5 |
| 3 lesions | 14.2 | 9.6 | 11.0 | 16.4 | 12.7 | 13.2 | 12.9 |
| 4 lesions | 3.7 | 3.7 | 6.7 | 5.0 | 5.2 | 4.4 | 4.8 |
| Tumor Grade at Baseline, % | |||||||
| G1 | 73.9 | 70.1 | 73.4 | 78.5 | 73.7 | 74.5 | 74.1 |
| G2 | 26.1 | 29.9 | 26.6 | 21.5 | 26.3 | 25.5 | 25.9 |
| Time from End of TURBT to Start of | |||||||
| Treatment Instillation, % | |||||||
| ≤30 minutes | 45.4 | 53.5 | 35.1 | 26.2 | 40.4 | 39.2 | 39.8 |
| 31–90 minutes | 20.3 | 14.4 | 20.2 | 20.5 | 20.3 | 17.6 | 18.9 |
| >90 minutes | 34.2 | 32.1 | 44.7 | 53.4 | 39.3 | 43.2 | 41.3 |
IVT = Intravesical Therapy; BCG = Bacillus Calmette–Guérin; SD = standard deviation.
Efficacy
2-Year Recurrence Rate and Time to Recurrence
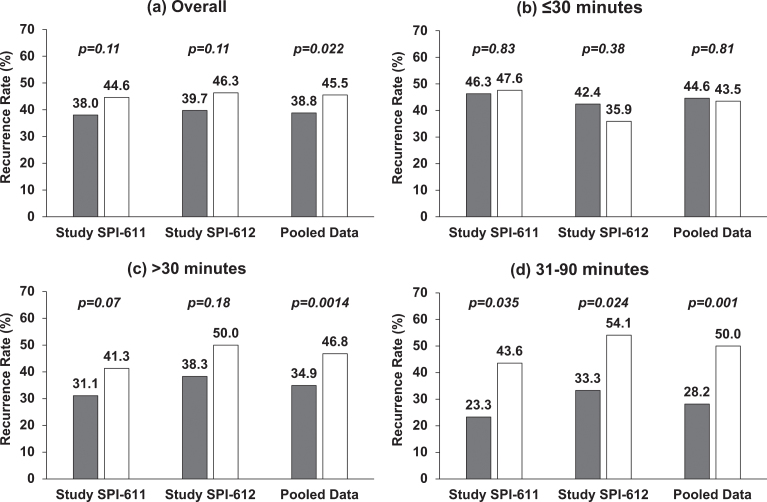
In both studies, the primary endpoints were not met although studies showed 6.7% (38.0% vs. 44.6%) and 6.6% (39.7% vs. 46.3%) reduction in 2-YRR in patients treated with apaziquone (Fig. 1a). The exploratory pooled analysis of the two identical studies showed significant reduction in 2-YRR of 6.7% (OR 0.76; p = 0.0218) (Figs. 1a, 3a).
Fig.1.
Two-year Recurrence Rate in Individual Studies and Pooled Data by Time from TURBT to Instillation. (a) Overall, treatment was instilled within 6 hours post-TURBT; (b) treatment was instilled within 30 minutes post-TURBT; (c) treatment was instilled after 30 minutes post-TURBT; (d) treatment was instilled between 31 and 90 minutes post-TURBT. Gray represents the recurrence rate in apaziquone group and white represents the recurrence rate in placebo group. Nominal p-value is based on Cochran-Mantel-Haenszel chi-square test.
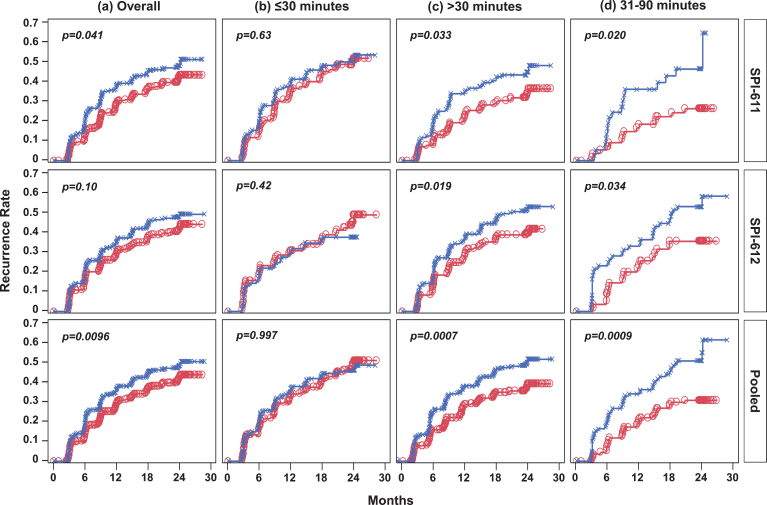
Similarly, the Kaplan-Meier curves showed improvements in TTR in the apaziquone group over placebo over the 2-year follow-up period in both studies (Fig. 2a). The improvement was significant in SPI-611 (HR 0.77; p = 0.0412) but was not significant in SPI-612 (HR 0.81; p = 0.1038) (Fig. 3a). The exploratory pooled analysis showed a significant improvement in TTR (HR 0.79; p = 0.0096). Since fewer than 50% of patients experienced a recurrence, a median TTR could not be calculated; however, using the first quartile (25%) of recurrence in both groups, the TTR was 3 to 5 months longer for patients receiving apaziquone compared to placebo.
Fig.2.
Kaplan-Meier Estimates of Time to Recurrence in Individual Studies and Pooled Data by Time from TURBT to Instillation. (a) Overall, treatment was instilled within 6 hours post-TURBT; (b) treatment was instilled within 30 minutes post-TURBT; (c) treatment was instilled after 30 minutes post-TURBT; (d) treatment was instilled between 31 and 90 minutes post-TURBT. Red line with circles represents the apaziquone group and blue line with Xs represents the placebo group. Nominal p-value is based onlog-rank test.
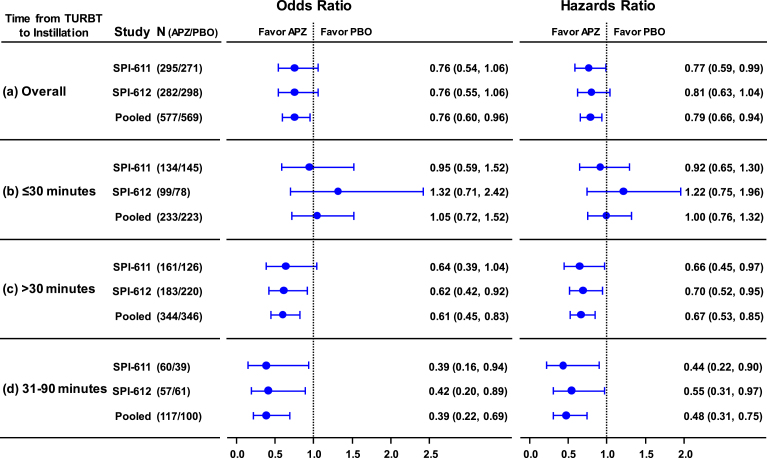
Fig.3.
Odds Ratio of 2-year recurrence rate (left panel) and Hazards Ratio of time to recurrence (right panel) and Corresponding 95% CI in Individual Studies and Pooled Data by Time from TURBT to Instillation. (a) Overall, treatment was instilled within 6 hours post-TURBT; (b) treatment was instilled within 30 minutes post-TURBT; (c) treatment was instilled after 30 minutes post-TURBT; (d) treatment was instilled between 31 and 90 minutes post-TURBT.
Time of instillation
Since apaziquone is known to be inactivated by red blood cells and TURBT is associated with varying degrees of postoperative hematuria, the impact of time of instillation was investigated during the multivariate and post hoc subgroup analyses. A threshold of 30 minutes from end of TURBT (≤30 and >30 minutes) was chosen for this investigation. Since this finding could influence the use of apaziquone in the clinic setting, the time stratified analysis is an important consideration in the analysis of efficacy.
It was found that apaziquone instilled within 30 minutes post-TURBT provided no benefit in reducing recurrence in both studies and overall (apaziquone n = 233, placebo n = 223; OR = 1.05; HR = 1) (Figs. 1b, 2b, 3b). However, when apaziquone was given after 30 minutes post-TURBT, the 2-YRR pooled analysis showed a reduction of 11.9% (25.5% relative reduction) with 34.9% (120/344) recurred in apaziquone and 46.8% (162/346) recurred in placebo (OR = 0.61, p = 0.0014) (Figs. 1c, 3c). For TTR, significant improvements in hazard ratios were demonstrated in both studies with a 33% reduction overall (HR = 0.67, p = 0.0007)(Figs. 2c, 3c).
It was further identified that when apaziquone was instilled within 31 to 90 minutes, the reduction in recurrence and improvements in TTR were significant in both studies. The reduction in 2-YRR was 20.3% (23.3% vs. 43.6%) in SPI-611 and 20.8% (33.3% vs. 54.1%) in SPI-612 (Figs. 1d, 3d). The first quartile of TTR was extended for 8.4 months from 6.8 months in the placebo group to 15.2 months in the apaziquone group, and the reduction in hazards for TTR was 56% and 45% respectively in two studies, and 52% overall (HR = 0.48, p = 0.0009) (Figs. 2d, 3d).
Other subgroup analyses
As presented in Supplementary Figure 3 using OR and HR with 95% CI, the subgroup analyses showed that apaziquone was favored, regardless of patient demographics or country of enrollment, whether the tumor was primary or recurrent, single or multiple, and Grade 1 or Grade 2.
Additional subgroup analysis indicated reductions in 2-YRR were similar in low and intermediate AUA risk. The reductions in apaziquone group were 6.9% (p = 0.1756) and 6.7% (p = 0.2080) in low risk and 8.7% (p = 0.1674) and 7.6% (p = 0.2038) in intermediate risk groups in two studies respectively. Similarly, the hazard rate reductions were 30% (p = 0.0857) and 24% (p = 0.1781) in low risk and 23% (p = 0.1225) and 18% (p = 0.2166) in intermediate risk group in two studies respectively. The reductions in apaziquone group were 6.3% and 6.4% in pTa G1 and 5.7% and 10.2% in pTa G2 patients in two studies respectively.
Safety
The incidence of adverse events (AE) was similar between the two treatment groups, with the most common treatment-related AEs in the apaziquone and placebo groups being dysuria (4.6% vs 4.1%), bladder spasm (1.4% vs 1.1%), micturition urgency (1.4% vs 2.0%), bladder pain (1.2% vs 0.5%), hematuria (1.0% vs 1.7%), urinary tract infection (1.0% vs 0.1%), and pollakiuria (0.7% vs. 1.9%). All the other treatment-related AEs were reported in <1% patients in both groups.
DISCUSSION
Current guidelines for treatment of patients with low-grade Ta NMIBC support instillation of chemotherapy into the bladder following TURBT [15, 16]. Physicians’ low adoption of the use of a single post-TURBT chemotherapy treatment may be due not only to the perceived toxicity associated with currently available agents, such as MMC, but also, in large part, to the lack of FDA-approved drugs for this indication [11]. It should be noted that none of the existing antineoplastic drugs are formulated for intravesical use.
Two large pivotal Phase 3 trials, SPI-611 and SPI-612, were conducted to evaluate the treatment effect of apaziquone compared with placebo. The treatment effect of a 12% reduction in 2-YRR was used to power the pivotal studies based on a meta-analysis that included seven studies in which the treatment effects were highly variable, as observed by a significant test of heterogeneity (χ2 = 14, df = 6, p = 0.03) [14]. Our studies were not successful in meeting the pre-specified 12% improvement in 2-YRR due to the potential underpowering of the study for lack of effect in patients receiving drug within 30 minutes ofinstillation.
When apaziquone was instilled within 30 minutes post-TURBT, the treatment effect was no different from placebo. The lack of efficacy observed in patients who were instilled within 30 minutes post-TURBT may be explained by inactivation of apaziquone by blood that is present after surgery. Similarly, lack of efficacy of apaziquone when drug was administered intravenously was attributed to inactivation of the drug by blood. Approximately 40% of the patients in these studies were treated within 30 minutes and, therefore, reduced the statistical power of the study due to diminished efficacy. In contrast, when apaziquone was instilled after 30 minutes and before 6 hours post-TURBT, the difference in the 2-YRR, although underpowered, was 10.2% and 11.7% in both studies, almost the same as the effect originally proposed in the study design.
When instilled between 31 and 90 minutes post-TURBT, the difference was significant and was further expanded to 20.3% and 20.8% in both studies. The HR for TTR was also improved to 0.67 when treatment instilled after 30 minutes and to 0.48 if instilled between 31 to 90 minutes post-TURBT in the pooled analysis. The benefit of apaziquone instillation after 30 minutes post-TURBT was consistent in individual studies and throughout the various patient subgroups within each study. The even larger benefit of apaziquone within 31–90 minutes was also consistent between the two studies.
Based on the findings from the two pivotal studies and pooled data, a new Phase 3, multinational, randomized, double-blind, placebo-controlled trial (NCT03224182) to evaluate the efficacy of apaziquone instilled within the potentially optimal window of 60±30 minutes post-TURBT is currently recruiting. The new study will assess the importance of the time window for adjuvant treatment post-TURBT for apaziquone [17–19].
CONCLUSIONS
Given the current challenges in the management of NMIBC, including rising incidence, especially among the elderly with comorbidities, high recurrence rates, and concerns regarding global supplies of current therapies, new safe and effective therapies specifically formulated and approved for the intravesical treatment of low- and intermediate-risk NMIBC represent an unmet need [20, 21].
Although two Phase 3 global studies did not individually meet the pre-specified efficacy, two studies individually showed similar treatment effect with the single intravesical instillation of apaziquone (4 mg in 40 mL) post-TURBT and the drug was well tolerated in patients with Ta, G1-G2 NMIBC. Furthermore, there were larger reductions in the 2-YRR and improvements in TTR in the subset of patients who received apaziquone between 31 and 90 minutes post-TURBT. This suggests that the optimal time for apaziquone administration may be 60±30 minutes after TURBT which requires further study.
AUTHOR CONTRIBUTIONS
Study concept and design: Gajanan Bhat, Guru Reddy, Alfred Witjes
Acquisition of data: Gajanan Bhat
Analysis and interpretation of data: Szu-Yun Leu, Gajanan Bhat
Drafting of the manuscript: Gajanan Bhat
Critical revision of the manuscript for important intellectual content: Lawrence Karsh, Neal Shore, Mark Soloway, Gajanan Bhat, Guru Reddy, Alfred Witjes
Statistical analysis: Gajanan Bhat, Szu-Yun Leu
Obtaining funding: Spectrum Pharmaceuticals, Inc.
Other (specify): None
Financial disclosures: Gajanan Bhat, Guru Reddy, and Szu-Yun Leu are employees of Spectrum Pharmaceuticals; Alfred Witjes, Lawrence Karsh, and Neal Shore are advisors to Spectrum Pharmaceuticals.
FUNDING/SUPPORT AND ROLE OF THE SPONSOR
Spectrum Pharmaceuticals, Inc. supported conduct of the trials and provided funding for the trials.
Supplementary Material
ACKNOWLEDGMENTS
Pamela Hsu and Steve Hasal from Spectrum Pharmaceuticals have contributed to various data analyses and clinical study reports.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-180166.
REFERENCES
- [1]. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Bladder Cancer (Version 5.2017).. http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed October 18, 2017.
- [2]. Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Guideline. J Urol. 2016;196(4):1021–9. doi: 10.1016/j.juro.2016.06.049. Epub 2016 Jun 16. [DOI] [PubMed] [Google Scholar]
- [3]. Babjuk M. Optimized management in patients with bladder cancer. Cent European J Urol. 2015;68(1):15–6. doi: 10.5173/ceju.2015.01.e93. Epub 2014 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Bellmunt J, Orsola A, Leow JJ, Wiegel T, De Santis M, Horwich A; ESO Guidelines Working Group Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii40–48. doi: 10.1093/annonc/mdu223. Epub 2014 Aug 5. [DOI] [PubMed] [Google Scholar]
- [5]. Kassouf W, Aprikian A, Black P, Kulkarni G, Izawa J, Eapen L, et al. Recommendations for the improvement of bladder cancer quality of care in Canada: A consensus document reviews and endorsed by Bladder Cancer Canada (BCC), Canadian Urological Oncology Group; (CUOG), and Canadian Urological Association (CUA), December 2015. Can Urol Assoc J. 2016;10(1–2):e46–e80. doi: 10.5489/cuaj.3583. Epub 2016 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Phillips RM, Hendriks HR, Peters GJ. EORTC-Pharmacology and Molecular Mechanism Group. EO9 (Apaziquone): From the clinic to the laboratory and back again. Br J Pharmacol. 2013;168(1):11–8. doi: 10.111.j.1476-5381.2012.01996.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. van der Heijden AG, Verhaegh G, Jansen CF, Schalken JA, Witjes JA. Effect of the hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: An in vitro study. J Urol. 2005;173(4):1375–80. [DOI] [PubMed] [Google Scholar]
- [8]. Loadman PM, Bibby MC, Phillips RM. Pharmacological approach towards the development of indolequinone bioreductive drugs based on the clinically inactive agent EO9. Br J Pharmacol. 2002;137(5):701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Puri P, Palit V, Loadman PM, Flannigan M, Shah T, Choudry GA, et al. Phase I/II pilot study of intravesical apaziquone (EO9) for superficial bladder cancer. J Urol. 2006;176(4 Pt 1):1344–8. [DOI] [PubMed] [Google Scholar]
- [10]. van der Heijden AG, Moonen PM, Cornel EB, Vergunst H, de Reijke TM, van Boven E, et al. Phase II marker lesion study with intravesical instillation of apaziquone for superficial bladder cancer: Toxicity and marker response. J Urol. 2006;176(4 Pt 1):1349–53; discussion 1353. [DOI] [PubMed] [Google Scholar]
- [11]. Cookson MS, Chang SS, Oefelein MG, Gallagher JR, Schwartz B, Heap K. National practice patterns for immediate postoperative instillation of chemotherapy in nonmuscle invasive bladder cancer. J Urol. 2012;187(5):1571–6. doi: 10.1016/j.juro.2011.12.056. Epub 2012 Mar 14. [DOI] [PubMed] [Google Scholar]
- [12]. Newling DW, Hetherington J, Sundaram SK, Robinson MR, Kisbenedek L. The use of valrubicin for the chemoresection of superficial bladder cancer –a marker lesion study. Eur Urol. 2001;39(6):643–47. [DOI] [PubMed] [Google Scholar]
- [13]. Mostofi FK, Sobin LH, Torloni H. Histological Typing of Urinary Bladder Tumours., Geneva, Switzerland: World Health Organization; 1973. [Google Scholar]
- [14]. Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: A meta-analysis of published results of randomized clinical trials. J Urol. 2004;171(6 Pt 1):2186–90, quiz 2435. [DOI] [PubMed] [Google Scholar]
- [15]. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur Urol. 2013;64(4):639–53. doi: 10.1016/j.eururo.2013.06.003. Epub 2013 Jun 12. [DOI] [PubMed] [Google Scholar]
- [16]. Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178(6):2314–30. [DOI] [PubMed] [Google Scholar]
- [17]. Bouffioux C, Kurth KH, Bono A, Oosterlinck W, Kruger CB, De Pauw M, et al. Intravesical adjuvant chemotherapy for superficial transitional cell bladder carcinoma: Results of 2 European Organization for Research and Treatment of Cancer randomized trials with mitomycin C and doxorubicin comparing early versus delayed instillations and short-term versus long-term treatment. European Organization for Research and Treatment of Cancer Genitourinary Group. J Urol. 1995;153(3 Pt 2):934–41. [PubMed] [Google Scholar]
- [18]. Kaasinen E, Rintala E, Hellstrom P, Viitanen J, Juusela H, Rajala P, et al. Factors explaining recurrence in patients undergoing chemoimmunotherapy regimens for frequently recurring superficial bladder carcinoma. Eur Urol. 2002;42(2):167–74. [DOI] [PubMed] [Google Scholar]
- [19]. Pan JS, Slocum HK, Rustum YM, Greco WR, Gaeta JF, Huben RP. Inhibition of implantation of murine bladder tumor by thiotepa in cauterized bladder. J Urol. 1989;142(6):1589–93. [DOI] [PubMed] [Google Scholar]
- [20]. Lerner SP, Bajorin DF, Dinney CP, Efstathiou JA, Groshen S, Hahn NM, et al. Summary and Recommendations from the National Cancer Institute’s Clinical Trials Planning Meeting on Novel Therapeutics for Non-Muscle Invasive Bladder Cancer. Bladder Cancer. 2016;2(2):165–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Malmstrom P-U, Babjuk M, Ohlmann C, Malavaud B, Kamat A. Managing patients with non-muscle invasive bladder cancer: Old disease, new ideas. Eur Med J. 2016;4(1):36–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.