Abstract
Superfund sites often consist of complex mixtures of polycyclic aromatic hydrocarbons (PAHs). It is widely recognized that PAHs pose risks to human and environmental health, but the risks posed by exposure to PAH mixtures are unclear. We constructed an environmentally relevant PAH mixture with the top 10 most prevalent PAHs (SM10) from a Superfund site derived from environmental passive sampling data. Using the zebrafish model, we measured body burden at 48 hours post fertilization (hpf) and evaluated the developmental and neurotoxicity of SM10 and the 10 individual constituents at 24 hours post fertilization (hpf) and 5 days post fertilization (dpf). Zebrafish embryos were exposed from 6–120 hpf to (1) the SM10 mixture, (2) a variety of individual PAHs: pyrene, fluoranthene, retene, benzo[a]anthracene, chrysene, naphthalene, acenaphthene, phenanthrene, fluorene, and 2-methylnaphthalene. We demonstrated that SM10 and only 3 of the individual PAHs were developmentally toxic. Subsequently, we constructed and exposed developing zebrafish to two sub-mixtures: SM3 (comprised of 3 of the developmentally toxicity PAHs) and SM7 (7 non-developmentally toxic PAHs). We found that the SM3 toxicity profile was similar to SM10, and SM7 unexpectedly elicited developmental toxicity unlike that seen with its individual components. The results demonstrated that the overall developmental toxicity in the mixtures could be explained using the general concentration addition model. To determine if exposures activated the AHR pathway, spatial expression of CYP1A was evaluated in the 10 individual PAHs and the 3 mixtures at 5 dpf. Results showed activation of AHR in the liver and vasculature for the mixtures and some individual PAHs. Embryos exposed to SM10 during development and raised in chemical-free water into adulthood exhibited decreased learning and responses to startle stimulus indicating that developmental SM10 exposures affect neurobehavior. Collectively, these results exemplify the utility of zebrafish to investigate the developmental and neurotoxicity of complex mixtures.
Keywords: passive sampling, polycyclic aromatic hydrocarbons, behavior, aryl hydrocarbon receptor, cytochrome P4501A, biomarker
Graphical Abstract

Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants formed from both anthropogenic and non-anthropogenic sources (Khalili et al., 1995; Yunker et al., 2002). There is growing recognition of the human and environmental health hazard potential of various of PAHs and PAH derivatives, and the limited data regarding their developmental toxicity, specifically on their effects on the developing nervous system. Prenatal PAH exposures have been associated with neurobehavioral effects in epidemiological studies (Perera et al., 1998; Perera et al., 2006; Perera et al., 2009; Perera et al., 2013). These studies have correlated developmental PAH exposure to decreased cognitive development and childhood IQ (Rauh et al., 2004; Perera et al., 2006; Perera et al., 2009; Edwards et al., 2010; Duarte-Salles et al., 2012; Jedrychowski et al., 2015), and increased rates of ADHD (Perera et al., 2014). Such findings are supported by vertebrate model studies demonstrating short and long-term neurodevelopmental effects of developmental PAH exposure, including rats (Chen et al., 2012; Peiffer et al., 2016), killifish (Brown et al., 2016a), and zebrafish (Incardona et al., 2004; Incardona et al., 2006; Vignet et al., 2014a; Vignet et al., 2014b; Geier et al., 2017; Knecht et al., 2017a; Knecht et al., 2017b).
PAHs are typically classified as hazardous to human health due to their carcinogenicity and/or mutagenicity, but the concern regarding the developmental toxicity of PAHs has increased in recent years. PAHs have been shown to cross the placenta during pregnancy in humans (Perera et al., 2004; Zhang et al., 2017), and developmental PAH exposure has been shown to cause oxidative stress and cardiovascular toxicity in humans (Burczynski et al., 1999) and fish (Incardona et al., 2004; Incardona et al., 2011b; Knecht et al., 2013; Brown et al., 2016b). Some developmental PAH toxicity is AHR dependent in mice (Kerley-Hamilton, 2012) and AHR2 dependent in zebrafish (Incardona et al., 2011a; Van Tiem and Di Giulio, 2011; Knecht et al., 2017b).
Most of these laboratory toxicity tests and risk assessments have focused on individual chemicals, rather than chemical mixtures (Carpenter et al., 2002; van Gestel et al., 2011). However, in the environment, organisms are generally exposed to complex chemical mixtures, rather than to individual chemicals (Altenburger and Greco, 2009; Beyer et al., 2014). Though knowledge of individual chemical toxicities is critically important, mixtures of chemicals may exhibit significantly different toxicities than their individual components for a variety of reasons, including competition for receptors, metabolic modulation, and altered bioavailability as described in several review articles (Carpenter et al., 2002; Altenburger et al., 2003; Cedergreen, 2014). Slotkin et al. demonstrated that the toxicity of an environmentally derived PAH mixture differed in both magnitude and direction from an individual PAH in an in-vitro neurodifferentiation model (Slotkin et al., 2017). Attempts to balance real-world complex chemical mixture exposures with available experimental methods have resulted in three main approaches (1) component based, (2) whole mixture, and (3) ‘sufficiently similar’ (U.S.EPA, 2000; van Gestel et al., 2011).
Component-based approaches utilize individual chemical toxicities to predict the toxicity of mixtures. This approach is only appropriate for groups of chemicals in which the mechanisms of toxicity are well established as either similar or independent, and are therefore limited in scope and considered the least comprehensive by the United States EPA (U.S.EPA, 2000; Altenburger and Greco, 2009). In the case of PAHs, toxic equivalency factors (TEFs) have been developed for carcinogenicity, but applying this same technique to developmental effects is not appropriate and does not adequately address the reality of multiple mechanisms leading to these effects (Billiard et al., 2008).
The most comprehensive approach to understanding complex chemical mixture toxicity is to directly assess the toxicity of whole chemical mixtures. Whole mixture approaches are financially and practically onerous and the seemingly infinite number of potential mixtures in the environment makes these approaches often unfeasible (Beyer et al., 2014). Perhaps most importantly, the identification of specific toxicants contributing to whole mixture toxicity and their mechanisms of action can be immensely challenging (Bergmann et al., 2017).
To combat these practicality issues, the US EPA conceptualized the third approach—representative, or “sufficiently similar” mixtures, defined as: “A mixture [where]…its components are not very different and are contained in about the same proportions as the mixture of concern.” This approach produces a simplified and well-defined mixture that allows for the direct comparison of concentration-response curves of the mixture with individual components (U.S.EPA, 2000). The foundation of this approach is a fixed ratio design which assumes that the relative ratios of chemicals from common sources are similar and that the ratios are vital to understanding the effects in the mixture (van Gestel et al., 2011). The sufficiently similar mixture approach may represent a compromise between the whole mixture and component-based approaches.
In the environment, PAHs exist primarily as complex mixtures with ratios reflective of their sources (Yunker et al., 2002; Incardona et al., 2006; Tobiszewski and Namiesnik, 2012). The Portland Harbor Superfund Site (PHSS) is a prime example of a location with multiple PAH sources resulting in exposure of humans and other organisms to complex PAH mixtures through contact with contaminated water and sediment (ATSDR, 2011) The PHSS is located on the Willamette River upstream of its confluence with the Columbia River in Portland, Oregon (U.S.EPA, 2016). Legacy sources (e.g. manufacturing and creosote operations), and modern (e.g. vehicle emissions and urban runoff contribute) to PAH load in the PHSS (Minick and Anderson, 2017).
In order to assess the developmental toxicity of a PAH mixture, a sufficiently similar PHSS mixture consisting of the 10 most abundant PAHs based on weight per volume from surface water (SM10) was constructed based on environmental passive sampling data from 2010 and 2015 (Allan et al., 2011; Minick and Anderson, 2017). We used the zebrafish model to investigate the toxicity of the individual PAHs compared to the SM10 mixture in developing larvae and to evaluate the long-term effects of developmental exposure in adult animals. Previous work has demonstrated that developmental exposure to benzo[a]pyrene resulted in persistent long-term deficits in adult zebrafish, such as learning and memory during an active avoidance test (Xu et al., 2007; Xu et al., 2012; Truong et al., 2014a; Knecht et al., 2017b). Here, we exposed embryonic zebrafish to both the individual 10 PAHs and SM10 from 6–120 hours post fertilization (hpf) and evaluated morphological and behavioral changes at 120 hpf. We quantitatively compared the developmental toxicity effect levels for individual PAHs and mixtures to determine if the effects followed the dose-additive concentration model. Additionally, PAH tissue concentrations were measured in embryos exposed to SM10 and the individual PAH components of SM10 from 6–48 hpf. To investigate the role of AHR in mediating these effects, we evaluated the expression of CYP1A in animals with AHR transiently knocked down. Finally, to assess if developmental exposure to a PAH mixture (SM10) leads to life-long behavioral effects, we exposed animals during development and raised them in chemical-free water into adulthood, and tested the behavioral deficits. Together this data demonstrates an approach using the zebrafish model to assess short and long-term developmental neurotoxicity of an environmentally relevant representative PAH mixture.
Materials and Methods
Study Area and Mixture Construction
The Portland Harbor Superfund site (PHSS) is located on the Willamette River from river mile 1.8 to 11.2 as measured upstream of its confluence with the Columbia River in Portland, Oregon. The PHSS was added to the Superfund National Priorities List in 2000 due to high levels of PAHs, PCBs, dioxins, and metals in the sediment and overlying water. In two previously reported studies, low-density polyethylene passive sampling devices (PSDs) were deployed in the surface water in 2010 and 2015 at 7 different sites making up a total of 18 individual samples. Environmental concentrations are provided in the Supplemental Data (Table S1 and Table S2) and detailed study descriptions are provided in the previously published studies (Allan et al., 2011; Minick and Anderson, 2017). The surrogate mixture SM10 was constructed with relative ratios of PAHs, which fall within the range of ratios of the 10 PAHs with the highest average concentrations as measured in 2010 and 2015 (Table 1). These 10 PAHs from highest concentration to lowest are: pyrene (CAS 129-00-0), fluoranthene (CAS 206-44-0), retene (CAS 483-65-8), benzo[a]anthracene (CAS 56-55-3), chrysene (CAS 218-01-9), naphthalene (CAS 91-20-3), acenaphthene (CAS 83-32-9), phenanthrene (CAS 85-01-8), fluorene (CAS 86-73-7), and 2-methylnaphthalene (CAS 91-57-6). Supermix3 (SM3) was constructed with the three PAHs which caused morphological effects individually as determined in this study: pyrene, retene, and benz[a]anthracene. The remaining 7 PAHs were combined to make Supermix7 (SM7). The relative ratios in SM3 and SM7 were the same as SM10.
Table 1. Ratios of the 10 most abundant PAHs on average in surface waters of the Portland Harbor Superfund site based on passive sampling during 2010 and 2015, relative to the most abundant by mass PAHs (pyrene).
Surrogate mixtures were constructed based on the aquatic relative concentrations of PAHs in the Superfund site by mass. The highest initial exposure concentrations (1% DMSO) are reported for Supermix10 (SM10), Supermix3 (SM3), and Supermix7(SM7). Mixture ratio represents the ratio of that particular PAH in the mixture.
| PAH | Environmental Ratios | Mixture Ratios | Exposure concentration (μM) | ||
|---|---|---|---|---|---|
| SM10 | SM3 | SM7 | |||
| Pyrene | 0.50 – 1.0 | 1.0 | 24 | 24 | - |
| Fluoranthene | 0.45 – 1.0 | 1.0 | 24 | - | 48 |
| Retene | 0.083 – 1.0 | 0.60 | 13 | 12 | - |
| Benzo[a]anthracene | 0.053 – 0.15 | 0.21 | 4.2 | 4.4 | - |
| Chrysene | 0.068 – 0.26 | 0.20 | 4.4 | - | 8.8 |
| Naphthalene | 0.072 – 1.0 | 0.14 | 5.9 | - | 12 |
| Acenaphthene | 0.11 – 1.0 | 0.12 | 2.9 | - | 5.8 |
| Phenanthrene | 0.16 – 0.45 | 0.12 | 3.4 | - | 6.8 |
| Fluorene | 0.10 – 0.32 | 0.080 | 3.1 | - | 6.2 |
| 2-methylnaphthalene | 0.049 – 0.51 | 0.052 | 1.7 | - | 3.4 |
Zebrafish Husbandry
Wildtype Tropical 5D zebrafish were maintained at the Sinnhuber Aquatic Research Laboratory (SARL), at Oregon State University (Corvallis, OR, USA) under a 14:10 hour light/dark cycle. Fish were raised in densities of ~500 fish/50-gallon tank at 28°C in recirculating filtered water supplemented with Instant Ocean salts. Care for the adult zebrafish followed protocols previously published (Barton et al., 2016). Spawning funnels were placed in tanks the night prior, and the following morning embryos were collected, staged and maintained in plastic petri dishes in an incubator at 28°C in embryo media (EM) (Kimmel et al., 1995). Embryo media consisted of 15 mM NaCl, 0.5 mM KCl, 1 mM MgSO4, 0.15 mM KH2PO4, 0.05 mM Na2HPO4 and 0.7mM NaHCO3 (Westerfield, 2000). Adult care and reproductive techniques were conducted according to the Institutional Animal Care and Use Committee protocols at Oregon State University (OSU).
Exposures
To remove potential chemical barrier effects and to simplify endpoint assessments, the chorions of 4 hpf embryos were enzymatically removed using a custom automated dechorionator, and at 6 hpf embryos were placed one per well in round bottom 96-well plates prefilled with 100 μL EM, using automated embryo placement systems (Mandrell et al., 2012). A Hewlett Packard D300e chemical dispenser was used to dispense 100% DMSO stock into 4 replicate exposure plates. Final DMSO concentrations were normalized to 1% (vol/vol), a percentage previously demonstrated to not induce adverse morphological phenotypes (Knecht et al., 2013) and plates were gently shaken during dispensing. Plates were sealed to minimize evaporation, wrapped in aluminum foil to block external light, and shaken overnight on an orbital shaker at 235 rpm at 28°C to enhance solution uniformity (Truong et al., 2016). Embryos were statically exposed until 120 hpf and kept in a 28°C incubator for the duration of the exposure.
Initial exposure concentrations were increased by approximately six orders of magnitude compared to environmental concentrations to ensure detection of bioactivity. For the morphology screening experiments, the initial range-finding studies consisted of nominal exposure concentrations to identify a maximum tolerable dose to allow for a more precise estimate of effect levels. For the individual PAHs, 5 concentrations were tested (50, 35.6, 11.2, 5, and 1.00 μM), while for the mixtures (SM10, SM7, and SM3) exposures were a percentage of the prepared stock: 1.0, 0.91, 0.81, 0.72, 0.63, 0.53, 0.44, 0.34, 0.25, 0.16, and 0.063%. For SM10, a follow up study comprised of a percentage range of 1.0, 0.81, 0.63, 0.44, 0.33, 0.28, 0.23, 0.19, 0.14, 0.094, and 0.047% was performed. In the case of SM7, an additional exposure occurred at the following concentrations: 2.00, 1.82, 1.62, 1.44, 1.26, 1.06, 0.88, 0.68, 0.5, 0.32 and 0.126%. For all exposure concentration lists, each test plate contained at least 8 wells of each concentration. Exposure concentrations for EC50 determinations were the same as the morphology screening for SM10 and SM3. Although 3 PAHs caused adverse effects, only 2 (pyrene and retene) of the 10 individual PAHs’ developmental toxicity results yielded data where an EC50 value could be determined. The exposure concentrations for these experiments were as follows: retene, 22, 20, 18, 16, 14, 12, 10, 8, 6, 4, and 2 μM; pyrene, 50, 47, 44, 41, 38, 35, 32, 29, 26, 23, and 20 μM. EC50 values for SM7 and the remaining PAHs were not determined due to an inability to cause 50% effect within the concentrations tested.
Developmental Toxicity Screening
All exposure plates followed protocols described in Truong et al (Truong et al., 2014b). Briefly, at 24 hpf embryos were visually assessed for mortality, developmental progression, notochord formation, and spontaneous motion. At 120 hpf, larvae were further assessed for 18 developmental endpoints: mortality, yolk sac edema, pericardial edema, body axis, trunk length, caudal fin, pectoral fin, pigmentation, somite, eye, snout, jaw, otolith, brain, notochord and circulatory malformations, swim bladder presence and inflation, and touch response (Truong et al., 2011). Responses were recorded as a binary absence or presence of an abnormal morphology for each endpoint for each fish. To ensure all compounds with a measurable effect were identified in the screen, lowest effect levels (LELs) were calculated for each endpoint using a binomial test to estimate significance thresholds as previously described (p<0.05) (Truong et al., 2016).
Embryonic Photomotor Response
Embryonic photomotor response (EPR) was evaluated in 24 hpf embryos using a custom built photomotor response assay tool (PRAT) (Kokel et al., 2013; Noyes et al., 2015). After exposures, embryos were not exposed to light until administration of the EPR test. The test consisted of: 30 s of darkness (Background); first pulse of intense light; 9 s darkness (Excitation); second pulse of intense light (1000 LUX); 10 s darkness (Refractory). Pixel changes between video frames were recorded to quantify total movement for each embryo across the test. Before analysis, wells with any developmental delay or mortality observed at 24 hpf were removed. Statistical significance was assessed separately for each interval (Background, Excitation, and Refractory), using a Kolmogorov-Smirnov (KS) test (Bonferroni-corrected p-value threshold = 0.05) against the vehicle control animals (Reif et al., 2016).
Larval Photomotor Response
Larval photomotor response (LPR) was evaluated with a light-dark cycle in 120 hpf larvae using the ViewPoint Zebrabox system and video tracking software (ViewPoint Life Sciences, Lyon, France) (Saili et al., 2012). Fish with any observed morphology endpoints were excluded from behavioral analysis. There were 4 light cycles in total, each light cycle consisting of 3 minutes in the light (525 LUX), and 3 minutes in the dark. Statistical significance was quantified using a KS test (p<0.05) on measured Area Under the Curve, dividing the dark and light cycles into separate bins, and was further constrained by a 50% threshold of significance for hyper or hypoactivity (Knecht et al., 2017b).
Detection of PAH body burden in zebrafish embryos
Ten individual PAHs and SM10 were exposed to embryos as described above from 6 to 48 hpf. For each PAH, embryos were exposed to 5.39, 11.6 and 25 μM, and embryos were exposed to 0.0625, 0.125, and 0.25% SM10. At 48 hpf, exposed plates were evaluated for dead embryos. Wells with dead embryos were excluded. The method to detect PAHs in zebrafish were as described in Goodale et al (Goodale et al., 2013). Briefly, for each concentration, 3 biological replicates consisting of 40 embryos, were removed from the exposure plates and transferred into glass 20 mL vials. These vials were placed on ice to reduce activity, and rinsed 4 times with cold fish water. Afterwards, the embryos were transferred into Eppendorf safe lock tube prefilled with 1mm glass beads. The tubes were kept on ice for 10 minutes, after which 500 μL of ethyl acetate was added, and the tubes were lightly vortexed, then homogenized with a bullet blender (Next Advance, Averill Park, NY) at speed 8 for 3 minutes. The tubes were then incubated at room temperature for 15 minutes, and centrifuged for 5 minutes at 16,000 RCF. Then, 400 μL of supernatant was transferred into labeled amber vials and stored at 4 °C until all samples were collected. For each sample day (3 total), a control vial was collected and set aside.
Zebrafish extracts were analyzed using an Agilent 5975B Gas Chromatograph-Mass Spectrometer (GC-MS) with a DB-5MS column (30 m × 0.25 mm × 0.25 μm) in electron impact mode (70 eV) using selective ion monitoring (SIM). The parameters were injection port maintained at 300 °C, 1.0 mL min−1 helium flow, 70 °C initial temperature, 1 min hold, 10 °C min−1 ramp to 300 °C, 4 min hold, 10 °C min−1 ramp to 310 °C, 4 min hold. The MS temperatures were operated at 150, 230 and 280 °C for the quadrupole, source and transfer line respectively. Standards for the 10 PAHs (≥97% purity) were purchased from Sigma-Aldrich or Santa Cruz Biotechnology. At least a five-point calibration curve (10 pg/μl to 10 ng/μl) was conducted to determine relative response ratios of PAHs to deuterated surrogate standards. Body burden (nmol/embryo) was calculated using average embryo weights of 0.3 mg at 48 hpf. To calculate body burden for embryos exposed to SM10, the ratio of each of the individual PAHs were scaled to the appropriate dilution factor and then used to calculate the nmol/embryo.
Concentration uptake ratio (CUR) was defined as the ratio of the PAH concentration inside an embryo measured at 48 hpf to the nominal concentration of the media. For each PAH, the mean and standard deviation of 9 CUR values for different nominal media concentrations (3 concentrations) and replicates (3 for each concentration value) were calculated and the log-transformed values were plotted against the corresponding log KOW values.
Adult Exposures
For adult studies, 288 embryos were statically exposed to 0.1% SM10 in 0.1% DMSO and a 0.1% DMSO vehicle control from 6 – 120 hpf in individual wells on 96 well plates as described above. The final DMSO concentration was 0.1% in the exposure wells. The concentration of 0.1% SM10 was chosen due to the lack of overt morphological effects at 120 hpf. At 120 hpf, exposed animals were rinsed with clean water and raised on the lab system as described in zebrafish husbandry. At 30 days, juvenile fish were split into 9L tanks with a density of ~45 fish per tank. At approximately 6 months, a subset of ~60 animals were randomly chosen for behavior and learning assessments.
Habituation to an audio startle stimulus was tested in adult fish using the zebrafish visual imaging system (zVIS) as previously described (Knecht et al., 2017a; Knecht et al., 2017b). Briefly, 48 adult fish (24 male, 24 female) per treatment group were individually placed into an array of 8 tanks (12cm × 12cm) filled with 750 mL of fish water. Taps were generated by an electric solenoid below each tank. Following a 10-minute acclimation period, a total of ten taps were delivered, with 20s following each tap, after which the distance moved was quantified for both SM10 and vehicle controls.
An active avoidance conditioning test was used to assess learning and performance differences in SM10 developmentally exposed zebrafish compared to controls. Custom built shuttleboxes, previously described (Truong et al., 2014b), were used with a modified protocol to test for learning deficiencies. Briefly, zebrafish were conditioned to leave a darkened side (conditioned stimulus), by using a shock pulse on this “incorrect side”, and swim to the non-shock blue-lighted, “correct side” compartment within an 8 sec “seek period” to avoid the mild shock. The shuttle box test consisted of a 10-minute acclimation period in the dark, followed by 30 consecutive trials. Each trial consisted of an 8 sec. seek period, initiated by the conditioned stimulus (CS), a blue LED light. If the fish did not swim to the correct side before the end of the seek period, or after the seek period was over crossed back to the incorrect side after it had occupied the correct side, the unconditioned stimulus (US), a mild shock (2.8V, 500ms duration, at a 1sec interval, for a maximum 16 sec duration), was administered until the fish returned to the correct side. After each 24 sec trial, there was a 60 sec inter-trial interval where both sides of the shuttlebox were lighted blue before the next trial began. If the zebrafish did not leave the incorrect side for the entire 24 sec trial, for 8 trials in a row, the fish would “fail out” and the test would stop. Conversely, if the fish left the incorrect side before the end of the seek period and did not cross back to the incorrect side for the entire 24 sec trial, the fish received no shock.
Immunohistochemistry
Immunohistochemistry (IHC) of cytochrome P450, family 1, subfamily A (CYP1A) protein localization was performed as previously described (Mathew et al., 2006). Briefly, wildtype embryos were exposed from 6 to 120 hpf to 0.43% for the mixtures, and the highest soluble concentration tested that did not cause significant mortality for the individuals. Two replicates of 8 larvae each were euthanized with tricaine at 120 hpf and fixed overnight in 4% paraformaldehyde at 4°C. Fixed embryos were permeablized 10 minutes on ice in 0.005% trypsin, rinsed with PBS+Tween 20 (PBST) and post-fixed in 4% paraformaldehyde for 10 minutes. Larvae were blocked with 10% normal goat serum (NGS) in PBS+0.5% Triton X-100 (PBSTx) for 1 hour at RT and incubated overnight in the primary antibody mouse α fish CYP1A monoclonal antibody (BiosenseLaboratories, Bergen, Norway) (1:500) in 1% NGS. Larvae were washed in PBST and incubated for 2 hours in secondary antibody (Fluor 594 goat anti-mouse, IgG). Eight embryos per treatment group were assessed by epifluorescence microscopy using a Keyance BZ-X700 microscope with 10× and 20× objectives and assessed for tissue-specific CYP1A expression patterns.
Morpholino Injections
Embryos were injected at the single cell stage with a fluorescein-tagged translation-blocking morpholino targeting AHR2 (AHR2-MO, 5′TGTACCGATACCCGCCGACATGGTT3′), splice-blocking morpholinos targeting AHR1A (AhR1a-MO, 5′CTTTTGAAGTGACTTTTGGCCCGCA3′), or AHR1B (AHR1B-MO, 5′ACACAGTCGTCCATGATTACTTTGC3′), or a standard nonsense control (c-MO, 5′CCTCTTACCTCAGTTACAATTTATA3′) purchased from Gene Tools (Philomath, Oregon) at a concentration of 0.6 mM. Injection volume was ~2 nl. Fertilized, normally developing embryos were screened for morpholino incorporation at 4 hpf by fluorescence microscopy. Embryos with evenly incorporated morpholino were then dechorionated. Following dechorionation, embryos were exposed to SM10 or individual PAHs using the same methods as in the developmental toxicity screening as described above.
Statistics
The statistical software JMP Pro version 13.0.0 was used to calculate EC50 values for SM10, SM3, pyrene, and retene. Each concentration-response curve consisted of 11 concentrations with n=32 fish at each concentration and were constructed with a binomial distribution and logistic regression. Confidence intervals for EC50 values were calculated based on n=4 and n=3 individual concentration-response curves for SM10 and SM3 respectively. Confidence intervals were not calculated for individual PAH concentration-response curves. Concentration-response curves can be found in the supplemental data (Figure S1 and S2). Body burden was calculated using custom R scripts where a linear model was fit to the data. Significance of adult startle response assay was assessed using a Single Factor Repeated Measure Analysis of Variance (ANOVA) and a Treatment by Subject ANOVA, with a Tukey Post Hoc Test, where significance was determined to be p<0.05.
Results/Discussion
Evaluating developmental toxicity of a chemical mixture
Laboratory experiments typically evaluate the toxicity of one chemical in isolation, which is unrealistic compared to what occurs in the environment. A sufficiently similar PAH mixture was constructed and assessed in the developmental zebrafish model to assess the short and long-term developmental neurotoxicity of an environmentally relevant representative PAH mixture.
Construction of Mixture
Supermix10 (SM10) was constructed from the top 10 most abundant PAHs in the freely dissolved phase of the water column in the Portland Harbor Superfund site, as measured by passive sampling devices during the summer of 2010 and 2015 (Allan et al., 2011; Minick and Anderson, 2017). These 10 PAHs accounted for 87%, by mass, of Σ33 PAH in 2010 and 76%, by mass, of Σ62 PAH in 2015. The environmental representativeness of SM10 over time is demonstrated by the fact that in both 2010 and 2015, the top 10 most abundant PAHs were the same. Despite the representativeness of this mixture, it is important to note that several known bioactive PAHs were detected in one or both years and are not included in SM10 including: benzo[a]pyrene, benzo[c]fluorene, benzo[k]fluoranthene, and benzo[j]fluoranthene (U.S.EPA, 2010; Geier et al., 2017).
The constructed SM10 used in this study consisted of concentrations with approximately 6 orders of magnitude higher than environmental levels. This ensured bioactivity was detectable and allowed more detailed interrogation of pathways of toxicity involved in the mixture (Table 1).
Developmental Toxicity of Individual PAHs
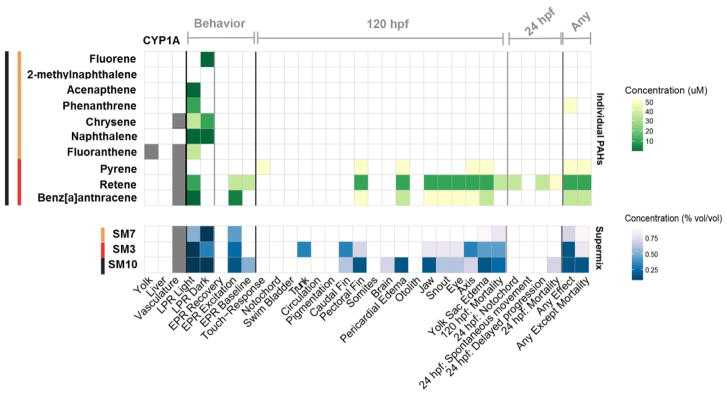
To assess the potential roles of the 10 components of SM10 in its developmental toxicity, we defined the developmental toxicity profile for each of the individual compounds, including morphological and behavioral effects, and the specific tissue expression of CYP1A. All compounds except 2-methylnaphthalene were bioactive in at least one assay (Figure 1). The remaining 9 compounds had effects in at least one phase of the LPR. Retene and benzo[a]anthracene had effects in the excitation phase of EPR, and retene also had significant effects during the baseline phase. Abnormalities in either phase have been demonstrated to be highly predictive of morphological effects at 5 dpf (Reif et al., 2016). Accordingly, only pyrene, retene, and benzo[a]anthracene had morphological effects. Several other studies have shown these compounds to cause developmental toxicity in zebrafish (Billiard et al., 1999; Incardona et al., 2006; Scott et al., 2011; Geier et al., 2017). The developmental toxicity profiles shared several common endpoints between these three compounds, including yolk sac, pericardial edema, axial and pectoral fin malformations. Pyrene additionally caused an abnormal touch response, while retene and benzo[a]anthracene produced craniofacial malformations. Retene was the most toxic of the 10 compounds, with lower LELs than the other compounds and significant mortality at 24 and 120hpf. Because retene is primarily derived from the combustion of wood, this indicates that sources of PAHs outside of the Superfund site may be important contributors to developmental toxicity within the Superfund site (Stout and Graan, 2010).
Figure 1. Heatmap of Lowest Effect Levels (LEL) and CYP1A tissue expression the 10 individual PAH constituents (top) and the mixtures (bottom).
The LEL individual components of SM10 (in μM), while the mixtures are in percent (vol/vol). A total of 27 endpoints were evaluated at either 24 or 120 hpf for all PAHs and mixtures. The darker green or blue indicates lower concentration and higher potency. Gray indicates the spatial CYP1A expression pattern. Orange bar indicates components of SM 7, red indicates components of SM3 and black indicates components of SM10.
CYP1A expression is a biomarker for activation of the AhR pathway. Of the ten PAHs, five induced CYP1A expression in the tissue. All the compounds that expressed CYP1A had expression in the vasculature, which is associated with AhR2 mediated toxicity (Goodale et al., 2012). Fluoranthene additionally had CYP1A expression in the yolk, which has been previously observed, but the mechanism for this expression pattern remains unknown (Goodale et al., 2015).
Developmental Toxicity of Constructed Mixtures
Zebrafish embryos developmentally exposed to SM10 elicited both morphological and neurobehavioral endpoints (Figure 1). Ten of the 18 morphological endpoints were adversely affected, and at 120 hpf, the larval exhibited abnormal swimming behavior in both the light and dark phase. The comparison of the toxicity profile for SM10 and the individual PAHs revealed 3 PAHs that were likely driving the mixture toxicity. To test this hypothesis, we constructed two mixtures: (1) SM3, which consisted of the 3 constituents (pyrene, retene, and benzo[a]anthracene) in the same ratios as in SM10 and (2) SM7 which consisted of the same ratios for the 7 non-bioactive PAHs.
Overall, the morphological endpoints affected by SM10 and SM3 were very similar to each other and to the endpoints of the individual components. The components of SM7 did not cause morphological effects and the range of exposure concentrations for SM7 did not exceed the individual exposure concentrations. Therefore, morphological effects were not expected following SM7 exposure. However, developmental exposure of SM7 caused both yolk sac edema and mortality at five days suggesting alternate affected pathways and/or synergistic effects, which cannot be easily teased apart in this model to potential metabolite toxicity. Significant LPR effects were observed even at the lowest concentrations tested for all three mixtures, possibly due to LPR being the most common endpoint across the individual components. Significant effects were also observed in the excitation period of the EPR for all three mixtures. While this was expected for SM10 and SM3, because individual constituents of these mixtures had EPR effects, these effects would not have been expected in SM7. These results indicate the most robust endpoint for detecting individual bioactivity and predicting mixture activity in the zebrafish assay is the LPR (in only morphologically normal animals).
SM10 and SM3 morphology effects correlated well with effects observed in the individual compounds, with pectoral fin, pericardial and yolk sac edema, and craniofacial malformations dominating morphological effects at the lower concentrations. However, despite the fact that the higher mixture concentrations did not exceed those of the individual PAH exposures, there were several endpoints at these higher concentrations that only appeared in the mixture exposures, including caudal fin, swim bladder, trunk, somites, and notochord. While individually SM7’s components did not have morphology effects (except for the cumulative measure “any effect” for phenanthrene), the mixture resulted in 120 hpf mortality and yolk sac edema.
This underlines the need to consider mixtures effects in environmental exposures. These results are similar to previous findings, which showed that a complex PAH mixture can produce effects on neurodifferentiation that differ in magnitude and direction from a single PAH (Slotkin et al., 2017). Interestingly, when compared to SM10, SM3 generally had morphology effects at higher concentrations, but a higher proportion of the cumulative effects endpoints were attributable to mortality. This suggests that the seven additional compounds may play some role in tempering the lethality of SM10, but not the overall prevalence of the any effect endpoint.
Differential PAH tissue burdens in 48 hpf zebrafish embryos
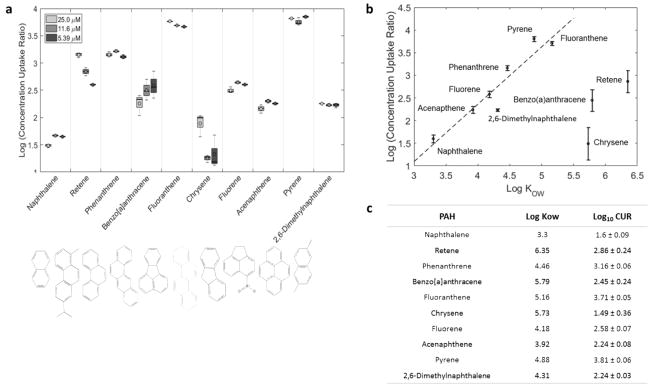
The internal body burdens of individual 10 PAHs in embryos were measured after exposure to 3 concentrations (5.39, 11.6, and 25 μM). The amount of PAHs detected in the embryos differed between individual PAHs (Figure S3). At all concentrations, fluoranthene, and pyrene body burden in embryos were the highest and had the highest concentration uptake rate of >3.5 on a log10 scale (CUR) (Figure 2A). To determine if the different CUR was due to solubility of the PAHs, we modeled the CUR values as a function of log KOW literature values (Figure 2B). For PAHs with a log KOW < 5.5, a linear relationship was observed with the lower CUR values correlating with low log Kow values. The model indicated a high correlation (R2 = 0.89). There was no trend evident for PAHs with higher log KOW and these PAHs were benzo[a]anthracene, chrysene, and retene.
Figure 2. PAH detected in embryos exposed to 10 PAHs from 6 to 48 hpf.
(A) Boxplots showing the distribution of concentration uptake ratios (CUR) for different PAHs at three different concentrations (5.39, 11.6 and 25 μM). (B) Relationship between concentration uptake ratio (CUR) and Log KOW for different PAHs. The points on the plot represent the mean of the 9 CUR values (3 concentrations X 3 replicates at each concentration) and the error bars represent the standard deviations. The PAHs with log KOW < 5.5 were it with a linear model (dotted line). (C) A table of average CUR ± SD and published log KOW.
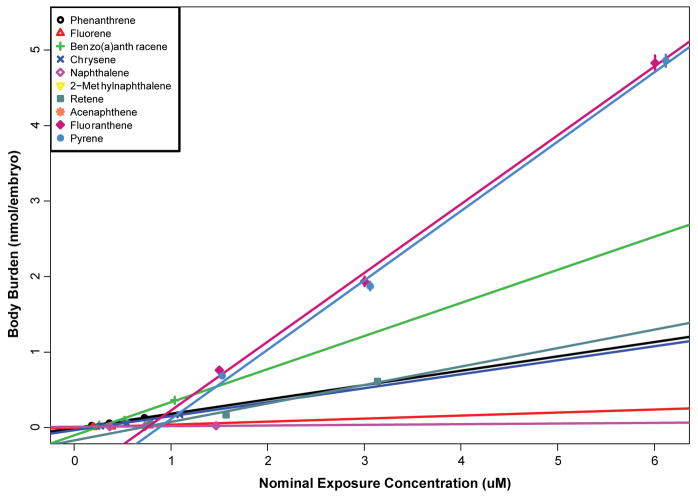
The body burdens of embryos exposed to 3 ratios of SM10 were measured at 48 hpf. Embryos were exposed to 0.0625, 0.125, and 0.25% SM10. The internal concentrations of PAHs were measured and compared to the nominal exposure concentrations in mixture (Figure 3). As might be expected from their relative abundance in SM10, fluoranthene and pyrene had the highest body burdens in the embryos (Table 1). Both naphthalene and 2-methylnaphthalene had very low to no body burden. The constituents of SM3 were the highest body burden in the embryos, which could be a partial explanation of the toxicity observed. The PAH with the highest detected concentration was fluoranthene, which did not induce any toxicity when exposed individually to embryos. Embryos exposed to only phenanthrene did not induce any morphological endpoints; however, the body burden levels were highly correlated (Figure S3). As these body burden measurements were obtained at 48 hpf, prior to the development of metabolism in the embryos, the effects of potential metabolites on observed toxicity at 120 hpf is unknown.
Figure 3. Body burden of embryos exposed to SM10.
Embryos were exposed to 6.25, 12.5, and 25% of the SM10 and the body burden of each PAH was measured. The nominal exposure concentration was corrected based on a PAHs ratio in the mixture (Table 1). Linear regression model was applied to each PAH.
Assessment of Assumption of Additivity
Risk assessment practices for PAHs are based upon individual PAH toxicities, developed for carcinogenicity, with the assumption that PAHs have similar mechanisms of action and therefore behave in an additive manner as mixtures (Altenburger and Greco, 2009). However, it has been well demonstrated that PAHs have dissimilar mechanisms of action in both their developmental toxicity and genotoxicity (Incardona et al., 2006; Billiard et al., 2008; Labib et al., 2016). In order to simplify the data and enable evaluation of the usefulness of a dose additivity model with PAHs with disparate modes of action, morphological effects of SM10 and SM3 in zebrafish were tested with the additivity model using the “any effect” endpoint, which is a summation of all individual morphological endpoints. The cumulative EC50 value of the “any effect” endpoint in SM10 was 0.24% with a confidence range of 0.13% to 0.29%. The cumulative EC50 value of SM3 was statistically the same (0.39%) ranging from 0.28% to 0.60%. However, the data demonstrates the remaining 7 PAH constituents were more toxic in the SM7 mixture, then when evaluated individually (Figure 1), thus suggesting possible interactions when combined in a mixture of more PAHs. This interaction may result in the lower EC50 observed in the SM10.
The assumption of additivity for both SM10 and SM3 was tested using Equation 1:
| (1) |
In this equation, c is the concentration of the individual component in the ECx of the mixture (x=50 in this case), EC is the ECx of the individual compound, and i is the ith component in an n-compound mixture. If mixture toxicity was purely additive, a value of 1 would be expected. Here, the average value was 0.82 for SM10 and SM3 with a confidence interval of 0.34 to 1.25. These data are therefore not statistically different than 1 and we cannot reject the premise of additivity for these mixtures.
Previous work has demonstrated that concentration addition can yield an accurate prediction of combination effects with mixtures of PAHs that operate diverse modes of action (Gonçalves et al., 2008; van Gestel et al., 2011). Here, despite the known diverse modes of action for PAH developmental toxicity and the disparate individual PAH toxicity profiles presented in this study, we cannot reject the use of the concentration addition model for assessing the developmental toxicity of this Superfund PAH mixture when considering the any effect endpoint. However, the assumption of additivity may not be appropriate for all PAH mixtures and warrants further investigation with other mixtures. Additionally, the any effect endpoint doesn’t represent the full resolution of the zebrafish model, and might not be adequate to fully distinguish the developmental hazard posed by environmental mixtures.
AHR-mediated toxicity observed in 3 mixtures
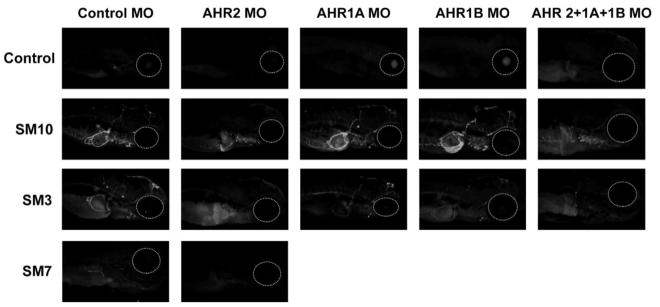
In SM10 and SM3, CYP1A expression was primarily in the vasculature, while in SM7, there was no appreciable CYP1A tissue expression. Knockdown of AHR2 eliminated vasculature expression and produced liver expression in SM3, but in SM10 vasculature expression persisted along with liver expression (Figure 4). When the different isoforms of AHR were transiently knocked down using morpholinos, then exposed to SM3, CYP1A tissue expression was obliterated, while those exposed to SM10 still had some CYP1A expression. Liver expression has previously been associated with AHR1A dependent PAH toxicity (Goodale et al., 2012), but has not been previously observed following AHR2 knockdown. Although the reason for this change in tissue expression is not completely clear, there are potential explanations. First, it is possible that in the absence of AHR2, AHR1A may become the primary receptor for some ligands (Goodale et al., 2012). Additionally, PAH metabolism may be altered in the absence of AHR2 and produces a ligand with a higher affinity for AHR1A. It is suspected that AHR1A, though unable to bind may large parent PAHs, may be able to bind smaller PAH metabolites (Garner et al., 2013). Finally, altered PAH metabolism may modulate oxidative stress response which modulates the expression of CYP1A directly (Barouki and Morel, 2001). These hypotheses should be pursued in future work.
Figure 4. Spatial expression of AHR activation biomarker, CYP1A using immunohistochemistry.
Zebrafish have 3 forms of aryl hydrocarbon receptor: AHR2, AHR1A and AHR2B. Each isoform was transiently knocked down using morpholinos (MOs) individually and in combination and the injected larvae were exposed at 6 hpf to 0.43% SM10, 0.43% SM3 or 1% DMSO. At 120 hpf, the CYP1A expression was observed in SM10 and SM3 (control MO). Exposure to SM7 did not produce CYP1A expression. The combined AHR Morpholino injected larvae had some CYP1A expression, when exposed to SM10, while those exposed to SM3 did not. Larvae were imaged from right side to better visualize liver expression. Dashed lines indicate eye position.
Although individually knocking down AHR1A and AHR1B did not have an appreciable effect on the vasculature expression of CYP1A in SM3 and SM10, knockdown of all three isoforms eliminated CYP1A expression in SM3. However, in SM10, while it reduced the intensity of expression patterns overall, knockdown of all three AHR isoforms did not completely eliminate CYP1A expression. It is possible that combined, the five AHR active PAHs present in SM10 overpowered the incomplete knockdown of the receptors.
Developmental exposure results in long-term neurobehavioral effects
To assess if developmental exposure to the SM10 leads to life-long behavioral effects, we exposed animals during development and raised them in chemical-free water into adulthood, and tested the adults for learning and memory deficits. Forty-eight to sixty-four animals (1:1 ratio of each gender) were evaluated in the suite of behavioral assays. Individual animals were not tracked through each assay, nor was gender considered a factor. The objective was to identify whether developmental exposure results in any adverse response in adulthood. The developmentally exposed adult zebrafish were assessed for distance moved in response to successive mechanical taps (Figure 5). Over the course of repeated taps, control fish increasingly habituate to the stimulus and decrease their total distance moved. In SM10 exposed fish, the response to the first tap was not significantly different compared to carrier control fish (p=0.48). Both treatment groups significantly habituated to successive taps starting on the 3rd tap (p<0.001), however, the rate of habituation was greater for control animals than SM10 exposed animals (p<0.001), and SM10 exposed animals moved significantly greater distances over the course of the test than control animals.
Figure 5. Increased distance moved after mechanical stimulus in developmentally exposed adult zebrafish.
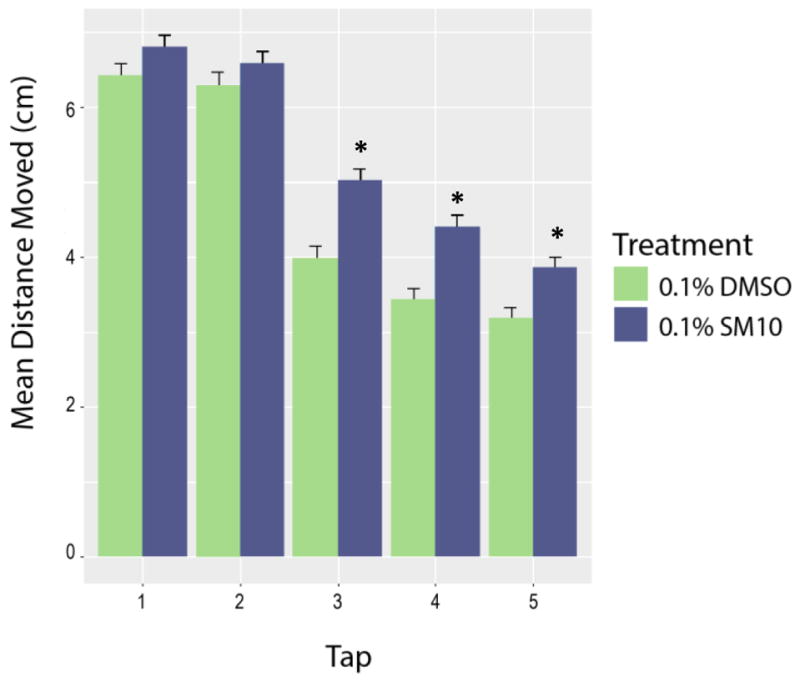
Embryos developmentally exposed to 0.1% SM10 or vehicle control (DMSO) from 6–120 hpf, and raised in chemical-free water until adulthood. Adults were evaluated for response to 5 taps using a mechanical stimulus with 20s inter-tap period. By the 3rd tap, the SM10 exposed zebrafish moved more (* denotes p<0.05, Single Factor Repeated Measure Analysis of Variance (ANOVA) and a Treatment by Subject ANOVA, with a Tukey Post Hoc Test)
Fish were also assessed for learning by being conditioned to move to the dark side of a two-chamber box. Developmental SM10 exposure resulted in differences in learning in adult zebrafish. Fish exposed to SM10 took longer to make their first move to the correct side, with a higher intercept and lower slope than the DMSO controls (Table 3), and a significant difference between treatment and control (p=0.005). Additionally, treated fish spent less time overall on the correct side than controls (p=0.001), and were less likely to learn over the course of the trial.
Table 3. Performance in the active avoidance test for 3 parameters: Total shocked time, time to accept, and total time on correct side.
Animals that failed out of the test (continuously shocked for 6 consecutive trials) were removed from analysis. These are reported along with those animals which successfully learned over the course of the trials, and are reported as percentages. The intercept and slope for the assay, and the differences between vehicle control (0.1% DMSO) and SM10 treated animals are reported.
| Time Shocked | ||||
|---|---|---|---|---|
| Treatment | Failed Out | Learned | Intercept, Slope | Diff, p-value |
| Vehicle Control | 0% | 42.5% | 1.47, −0,041 | - |
| 0.1% SM10 | 2.80% | 40% | 2.03, −0.032 | 0.21, 0.06 |
| Decision Time | ||||
| Treatment | Failed Out | Learned | Intercept, Slope | Diff, p-value |
| Vehicle Control | 0% | 35% | 4.945, −0.067 | - |
| 0.1% SM10 | 2.80% | 22.9% | 5.119, −0.013 | 0.44, 0.005* |
| Time on Correct Side | ||||
| Treatment | Failed Out | Learned | Intercept, Slope | Diff, p-value |
| Vehicle Control | 0% | 47.5% | 18.164, 0.102 | - |
| 0.1% SM10 | 2.80% | 29.2% | 17.611, 0.063 | 0.55, 0.001* |
p<0.05 repeated measures ANOVA and Tukey’s post hoc test.
These results indicate that developmental exposure to SM10 can result in long-term behavioral effects, including a decreased ability to habituate to environmental stimuli and a decreased learning capacity. This is consistent with previous epidemiological studies reporting the neurological effects of PAH exposure during development (Perera et al., 2006; Perera et al., 2009; Perera et al., 2014) and effects on neurodifferentiation and neurobehavior (Crepeaux et al., 2012; Slotkin et al., 2017). While it has been previously demonstrated that developmental exposure to chlorpyrifos can significantly increase overall startle response in adult zebrafish (Eddins et al., 2010), startle response effects have not been previously observed for adult zebrafish developmentally exposed to PAHs. However, startle response effects have been observed in the progeny of zebrafish developmentally exposed to benzo[a]pyrene (Knecht et al., 2017a). Collectively, this demonstrates exposure to SM10 results in neurobehavioral defects as larvae and adults.
Limitations of mixture study interpretation
There are several important limitations to the interpretations of the results of this study. Exposure concentrations in the present study are higher than reported environmental concentrations in the PHSS (Allan et al., 2011; Minick and Anderson, 2017). Additionally, PAHs are known to sorb to the walls of 96 well polystyrene exposure plates (Chlebowski et al., 2016) which creates uncertainty in the actual exposure concentrations of this study. This study is, therefore, a hazard assessment and not an assessment of the risk posed by environmentally relevant concentrations. Further work is also needed to quantify exposure and tissue burden during the course of the study. It is noteworthy that in our assays phenanthrene did not elicit cardiovascular effects previously reported in several studies (Incardona et al., 2004; Zhang et al., 2013a; Zhang et al., 2013b). However, this discrepancy can be explained by differences in exposure methods, because the previous studies all employed repeated exposures, rather than the static exposures used in this study.
Conclusions
The representative mixture approach demonstrated in this study may offer a more comprehensive and practical alternative to component-based and whole mixture approaches respectively. With this approach, we were able to identify the mixture components that drive toxicity, provided insight into the potential mechanisms of toxicity for these mixtures, and show that these environmentally relevant mixtures have behavioral effects in adult fish following developmental exposure. Additionally, while uncertainty exists due to a lack of exposure concentrations over time, the developmental effects caused by these PAH mixtures appear to behave in an additive manner. However, we also showed that endpoints caused by chemical mixtures may not be predictable from single chemical effects alone. Further work is needed to improve understanding of the dosimetry, pharmacokinetics, and metabolism of this mixture. This approach should also be applied using other representative chemical mixtures from various locations and matrices including other classes of chemicals. Data of this kind would allow for comparisons between representative mixtures and allow for the determination of sufficient similarity while providing needed additional insight into toxicities of chemical mixtures.
Supplementary Material
Table 2. Experimentally determined EC50 values (μM) for individual PAHs where the EC50 was less than 50 μM.
EC50 values were experimentally determined for Supermix10 (SM10) and Supermix3 (SM3) and the concentrations of the individual PAHs at the mixture EC50 values are reported.
| PAH | Individual EC50 (μM) | SM10 EC50 (μM) | SM3 EC50 (μM) |
|---|---|---|---|
| Pyrene | 27 | 5.9 | 9.5 |
| Fluoranthene | * | 5.8 | - |
| Retene | 5.1 | 3.0 | 4.9 |
| Benzo[a]anthracene | * | 1.0 | 1.6 |
| Chrysene | * | 1.0 | - |
| Naphthalene | * | 1.4 | - |
| Acenaphthene | * | 0.81 | - |
| Phenanthrene | * | 0.70 | - |
| Fluorene | * | 0.74 | - |
| 2-methylnaphthalene | * | 0.41 | - |
EC50 values greater than 50μM.
– Compound not in mixture.
Highlights.
Passive sampling was used to construct a representative Superfund PAH mixture.
Individual and mixture toxicities were assessed in the embryonic zebrafish model.
Individual toxic endpoints differed for the mixture compared to individual PAHs.
Developmental effects in zebrafish could be explained through the additivity model.
Developmental exposure of the mixture caused adult behavioral effects.
Acknowledgments
This work was supported by National Institute of Health [P42 ES016465, T32 ES07060, and P30 ES000210]. The content is solely the responsibility of the authors and does not necessarily represent the official views of any funding organization. We are highly appreciative of the staff at the Sinnhuber Aquatic Research Laboratory including Carrie Barton for fish husbandry, Jane LaDu for morpholino injections, Greg Gonnerman for screening assistance and Eric Johnson for adult behavioral assessment. In addition, we are thankful for environmental data collected by Sarah Allan and the analytical expertise from Richard Scott, and Gary Points. We also thank Sean Carver for his graphical design expertise.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan SE, Sower GJ, Anderson KA. Estimating risk at a Superfund site using passive sampling devices as biological surrogates in human health risk models. Chemosphere. 2011;85:920–927. doi: 10.1016/j.chemosphere.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenburger R, Greco WR. Extrapolation concepts for dealing with multiple contamination in environmental risk assessment. Integrated Environmental Assessment and Management. 2009;5:62–68. doi: 10.1897/ieam_2008-038.1. [DOI] [PubMed] [Google Scholar]
- Altenburger R, Nendza M, Schüürmann G. Mixture toxicity and its modeling by quantitative structure-activity relationships. Environmental Toxicology and Chemistry. 2003;22:1900–1915. doi: 10.1897/01-386. [DOI] [PubMed] [Google Scholar]
- ATSDR. Porland Harbor: Recreational Use 2011 [Google Scholar]
- Barouki R, Morel Y. Repression of cytochrome P450 1A1 gene expression by oxidative stress: mechanisms and biological implications. Biochemical Pharmacology. 2001;61:511–516. doi: 10.1016/s0006-2952(00)00543-8. [DOI] [PubMed] [Google Scholar]
- Barton CL, Johnson EW, Tanguay RL. Facility Design and Health Management Program at the Sinnhuber Aquatic Research Laboratory. Zebrafish. 2016;13:S-39–S-43. doi: 10.1089/zeb.2015.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann AJ, Tanguay RL, Anderson KA. Using passive sampling and zebrafish to identify developmental toxicants in complex mixtures. Environmental Toxicology and Chemistry. 2017;36:2290–2298. doi: 10.1002/etc.3802. [DOI] [PubMed] [Google Scholar]
- Beyer J, Petersen K, Song Y, Ruus A, Grung M, Bakke T, Tollefsen KE. Environmental risk assessment of combined effects in aquatic ecotoxicology: A discussion paper. Marine Environmental Research. 2014;96:81–91. doi: 10.1016/j.marenvres.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT. Nonadditive effects of PAHs on Early Vertebrate Development: mechanisms and implications for risk assessment. Toxicol Sci. 2008;105:5–23. doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiard SM, Querbach K, Hodson PV. Toxicity of retene to early life stages of two freshwater fish species. Environmental Toxicology and Chemistry. 1999;18:2070–2077. [Google Scholar]
- Brown DR, Bailey JM, Oliveri AN, Levin ED, Di Giulio RT. Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicol Teratol. 2016a;53:55–63. doi: 10.1016/j.ntt.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Thompson J, Chernick M, Hinton DE, Di Giulio RT. Later Life Swimming Performance and Persistent Heart Damage Following Subteratogenic PAH Mixture Exposure in the Atlantic Killifish (Fundulus heteroclitus) Environmental Toxicology and Chemistry. 2016b doi: 10.1002/etc.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burczynski M, Lin H, Penning T. Isoform-specific Induction of a Human Aldo-Keto Reductase by Polycyclic Aromatic Hydrocarbons (PAHs), Electrophiles, and Oxidative Stress: Implications for the Alternative Pathway of PAH Activation Catalyzed by Human Dihydrodiol Dehydrogenase. Cancer Research. 1999;59:8. [PubMed] [Google Scholar]
- Carpenter DO, Arcaro K, Spink DC. Understanding the human health effects of chemical mixtures. Environ Health Perspect. 2002;110(Suppl 1):25–42. doi: 10.1289/ehp.02110s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedergreen N. Quantifying Synergy: A Systematic Review of Mixture Toxicity Studies within Environmental Toxicology. PLOS ONE. 2014;9:e96580. doi: 10.1371/journal.pone.0096580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Tang Y, Jiang X, Qi Y, Cheng S, Qiu C, Peng B, Tu B. Early Postnatal Benzo(a)pyrene Exposure in Sprague-Dawley Rats Causes Persistent Neurobehavioral Impairments that Emerge Postnatally and Continue into Adolescence and Adulthood. Toxicological Sciences. 2012;125:248–261. doi: 10.1093/toxsci/kfr265. [DOI] [PubMed] [Google Scholar]
- Chlebowski AC, Tanguay RL, Simonich SL. Quantitation and prediction of sorptive losses during toxicity testing of polycyclic aromatic hydrocarbon (PAH) and nitrated PAH (NPAH) using polystyrene 96-well plates. Neurotoxicol Teratol. 2016;57:30–38. doi: 10.1016/j.ntt.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepeaux G, Bouillaud-Kremarik P, Sikhayeva N, Rychen G, Soulimani R, Schroeder H. Late effects of a perinatal exposure to a 16 PAH mixture: Increase of anxiety-related behaviours and decrease of regional brain metabolism in adult male rats. Toxicol Lett. 2012;211:105–113. doi: 10.1016/j.toxlet.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Duarte-Salles T, Mendez MA, Morales E, Bustamante M, Rodríguez-Vicente A, Kogevinas M, Sunyer J. Dietary benzo (a) pyrene and fetal growth: Effect modification by vitamin C intake and glutathione S-transferase P1 polymorphism. Environment international. 2012;45:1–8. doi: 10.1016/j.envint.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol Teratol. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SC, Jedrychowski W, Butscher M, Camann D, Kieltyka A, Mroz E, Flak E, Li Z, Wang S, Rauh V. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environmental health perspectives. 2010;118:1326. doi: 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner LVT, Brown DR, Di Giulio RT. Knockdown of AHR1A but not AHR1B exacerbates PAH and PCB-126 toxicity in zebrafish (Danio rerio) embryos. Aquatic Toxicology. 2013;142–143:336–346. doi: 10.1016/j.aquatox.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier MC, Chlebowski AC, Truong L, Massey Simonich SL, Anderson KA, Tanguay RL. Comparative developmental toxicity of a comprehensive suite of polycyclic aromatic hydrocarbons. Archives of Toxicology. 2017 doi: 10.1007/s00204-017-2068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves R, Scholze M, Ferreira AM, Martins M, Correia AD. The joint effect of polycyclic aromatic hydrocarbons on fish behavior. Environmental Research. 2008;108:205–213. doi: 10.1016/j.envres.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Goodale BC, La Du J, Tilton SC, Sullivan CM, Bisson WH, Waters KM, Tanguay RL. Ligand-Specific Transcriptional Mechanisms Underlie Aryl Hydrocarbon Receptor-Mediated Developmental Toxicity of Oxygenated PAHs. Toxicol Sci. 2015;147:397–411. doi: 10.1093/toxsci/kfv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale BC, La Du JK, Bisson WH, Janszen DB, Waters KM, Tanguay RL. AHR2 mutant reveals functional diversity of aryl hydrocarbon receptors in zebrafish. PLoS One. 2012;7:e29346. doi: 10.1371/journal.pone.0029346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale BC, Tilton SC, Corvi MM, Wilson GR, Janszen DB, Anderson KA, Waters KM, Tanguay RL. Structurally distinct polycyclic aromatic hydrocarbons induce differential transcriptional responses in developing zebrafish. Toxicol Appl Pharmacol. 2013;272:656–670. doi: 10.1016/j.taap.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Oil spills and fish health: exposing the heart of the matter. Journal of exposure science & environmental epidemiology. 2011a;21:3–4. doi: 10.1038/jes.2010.51. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Linbo TL, Scholz NL. Cardiac toxicity of 5-ring polycyclic aromatic hydrocarbons is differentially dependent on the aryl hydrocarbon receptor 2 isoform during zebrafish development. Toxicol Appl Pharmacol. 2011b;257:242–249. doi: 10.1016/j.taap.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Majewska R, Mrozek-Budzyn D, Mroz E, Roen EL, Sowa A, Jacek R. Depressed height gain of children associated with intrauterine exposure to polycyclic aromatic hydrocarbons (PAH) and heavy metals: the cohort prospective study. Environ Res. 2015;136:141–147. doi: 10.1016/j.envres.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerley-Hamilton JS, Trask Heidi W, Ridley Christian JA, DuFour Eric, Lesseur Corina, Ringelberg Carol S, Moodie Karen L, Shipman Samantha L, Korc Murray, Gui Jiang, Shworak Nicholas W, Tominson Craig R. Inherent and Benzo[a]pyrene-Induced Differential Aryl Hydrocarbon Receptor Signaling Greatly Affects Life Span, Atherosclerosis, Cardiac Gene Expression, and Body and Heart Growth in Mice. Toxicological Sciences. 2012;126:391–404. doi: 10.1093/toxsci/kfs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili NR, Scheff PA, Holsen TM. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmospheric environment. 1995;29:533–542. [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Knecht AL, Goodale BC, Truong L, Simonich MT, Swanson AJ, Matzke MM, Anderson KA, Waters KM, Tanguay RL. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol Appl Pharmacol. 2013;271:266–275. doi: 10.1016/j.taap.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht AL, Truong L, Marvel SW, Reif DM, Garcia A, Lu C, Simonich MT, Teeguarden JG, Tanguay RL. Transgenerational inheritance of neurobehavioral and physiological deficits from developmental exposure to benzo [a] pyrene in zebrafish. Toxicology and Applied Pharmacology. 2017a doi: 10.1016/j.taap.2017.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht AL, Truong L, Simonich MT, Tanguay RL. Developmental benzo[a]pyrene (B[a]P) exposure impacts larval behavior and impairs adult learning in zebrafish. Neurotoxicol Teratol. 2017b;59:27–34. doi: 10.1016/j.ntt.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokel D, Dunn TW, Ahrens MB, Alshut R, Cheung CY, Saint-Amant L, Bruni G, Mateus R, van Ham TJ, Shiraki T, Fukada Y, Kojima D, Yeh JR, Mikut R, von Lintig J, Engert F, Peterson RT. Identification of nonvisual photomotor response cells in the vertebrate hindbrain. J Neurosci. 2013;33:3834–3843. doi: 10.1523/JNEUROSCI.3689-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib S, Williams A, Kuo B, Yauk CL, White PA, Halappanavar S. A framework for the use of single-chemical transcriptomics data in predicting the hazards associated with complex mixtures of polycyclic aromatic hydrocarbons. Archives of Toxicology. 2016:1–18. doi: 10.1007/s00204-016-1891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell D, Truong L, Jephson C, Sarker MR, Moore A, Lang C, Simonich MT, Tanguay RL. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J Lab Autom. 2012;17:66–74. doi: 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew LK, Andreasen EA, Tanguay RL. Aryl hydrocarbon receptor activation inhibits regenerative growth. Molecular pharmacology. 2006;69:257–265. doi: 10.1124/mol.105.018044. [DOI] [PubMed] [Google Scholar]
- Minick DJ, Anderson KA. Diffusive flux of PAHs across sediment-water and water-air interfaces at urban superfund sites. Environ Toxicol Chem. 2017 doi: 10.1002/etc.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes PD, Haggard DE, Gonnerman GD, Tanguay RL. Advanced morphological-behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicological Sciences. 2015:145. doi: 10.1093/toxsci/kfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer J, Grova N, Hidalgo S, Salquèbre G, Rychen G, Bisson JF, Appenzeller BM, Schroeder H. Behavioral toxicity and physiological changes from repeated exposure to fluorene administered orally or intraperitoneally to adult male Wistar rats: A dose–response study. Neurotoxicology. 2016;53:321–333. doi: 10.1016/j.neuro.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Perera FP, Chang HW, Tang D, Roen EL, Herbstman J, Margolis A, Huang TJ, Miller RL, Wang S, Rauh V. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PLoS One. 2014;9:e111670. doi: 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, Rauh V. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124:e195–202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, Hoepner L, Barr D, Tu YH, Camann D, Kinney P. Effect of Prenatal Exposure to Airborne Polycyclic Aromatic Hydrocarbons on Neurodevelopment in the First 3 Years of Life among Inner-City Children. Environmental Health Perspectives. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Tu YH, Cruz LA, Borjas M, Bernert T, Whyatt RM. Biomarkers in Maternal and Newborn Blood Indicate Heightened Fetal Susceptibility to Procarcinogenic DNA Damage. Environmental Health Perspectives. 2004;112:1133–1136. doi: 10.1289/ehp.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Wang S, Rauh V, Zhou H, Stigter L, Camann D, Jedrychowski W, Mroz E, Majewska R. Prenatal exposure to air pollution, maternal psychological distress, and child behavior. Pediatrics. 2013 doi: 10.1542/peds.2012-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Whyatt RM, Jedrychowski W, Rauh V, Manchester D, Santella RM, Ottman R. Recent Developments in Molecular Epidemiology: A Study of the Effects of Environmental Polycyclic Aromatic Hydrocarbons on Birth Outcomes in Poland. American Journal of Epidemiology. 1998;147:309–314. doi: 10.1093/oxfordjournals.aje.a009451. [DOI] [PubMed] [Google Scholar]
- Rauh V, Whyatt R, Garfinkel R, Andrews H, Hoepner L, Reyes A, Diaz D, Camann D, Perera F. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicology and teratology. 2004;26:373–385. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif DM, Truong L, Mandrell D, Marvel S, Zhang G, Tanguay RL. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch Toxicol. 2016;90:1459–1470. doi: 10.1007/s00204-015-1554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saili KS, Corvi MM, Weber DN, Patel AU, Das SR, Przybyla J, Anderson KA, Tanguay RL. Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology. 2012;291:83–92. doi: 10.1016/j.tox.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Incardona JP, Pelkki K, Shepardson S, Hodson PV. AhR2-mediated, CYP1A-independent cardiovascular toxicity in zebrafish (Danio rerio) embryos exposed to retene. Aquat Toxicol. 2011;101:165–174. doi: 10.1016/j.aquatox.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Card J, Giulio RTD, Seidler FJ. In vitro models reveal differences in the developmental neurotoxicity of an environmental polycylic aromatic hydrocarbon mixture compared to benzo[a]pyrene: Neuronotypic PC12 Cells and embryonic neural stem cells. Toxicology. 2017;377:49–56. doi: 10.1016/j.tox.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout SA, Graan TP. Quantitative Source Apportionment of PAHs in Sediments of Little Menomonee River, Wisconsin: Weathered Creosote versus Urban Background. Environmental Science & Technology. 2010;44:2932–2939. doi: 10.1021/es903353z. [DOI] [PubMed] [Google Scholar]
- Tobiszewski M, Namiesnik J. PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut. 2012;162:110–119. doi: 10.1016/j.envpol.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Truong L, Bugel SM, Chlebowski A, Usenko CY, Simonich MT, Simonich SL, Tanguay RL. Optimizing multi-dimensional high throughput screening using zebrafish. Reprod Toxicol. 2016;65:139–147. doi: 10.1016/j.reprotox.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Harper SL, Tanguay RL. Evaluation of embryotoxicity using the zebrafish model. Drug Safety Evaluation: Methods and Protocols. 2011:271–279. doi: 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Mandrell D, Mandrell R, Simonich M, Tanguay RL. A rapid throughput approach identifies cognitive deficits in adult zebrafish from developmental exposure to polybrominated flame retardants. Neurotoxicology. 2014a;43:134–142. doi: 10.1016/j.neuro.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL. Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci. 2014b;137:212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S.EPA. Supplementary guidance for conducting health risk assessment of chemical mixtures 2000 [Google Scholar]
- U.S.EPA. Development of a relative potency factor (RPF) approach for polycyclic aromatic hydrocarbon (PAH) mixtures 2010 [Google Scholar]
- U.S.EPA. Portland Harbor Superfund Site: proposed plan 2016 [Google Scholar]
- van Gestel CAM, Jonker MJ, Kammenga JE, Laskowski R, Svendsen C. Mixture toxicity: linking approaches from ecological and human toxicology. CRC Press; New York, New York: 2011. [Google Scholar]
- Van Tiem LA, Di Giulio RT. AHR2 knockdown prevents PAH-mediated cardiac toxicity and XRE- and ARE-associated gene induction in zebrafish (Danio rerio) Toxicol Appl Pharmacol. 2011;254:280–287. doi: 10.1016/j.taap.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignet C, Devier MH, Le Menach K, Lyphout L, Potier J, Cachot J, Budzinski H, Begout ML, Cousin X. Long-term disruption of growth, reproduction, and behavior after embryonic exposure of zebrafish to PAH-spiked sediment. Environ Sci Pollut Res Int. 2014a;21:13877–13887. doi: 10.1007/s11356-014-2585-5. [DOI] [PubMed] [Google Scholar]
- Vignet C, Le Menach K, Lyphout L, Guionnet T, Frere L, Leguay D, Budzinski H, Cousin X, Begout ML. Chronic dietary exposure to pyrolytic and petrogenic mixtures of PAHs causes physiological disruption in zebrafish--part II: behavior. Environ Sci Pollut Res Int. 2014b;21:13818–13832. doi: 10.1007/s11356-014-2762-6. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish. 2000 http://zfin.org/zf_info/zfbook/zfbk.html.
- Xu X, Scott-Scheiern T, Kempker L, Simons K. Active avoidance conditioning in zebrafish (Danio rerio) Neurobiol Learn Mem. 2007;87:72–77. doi: 10.1016/j.nlm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Xu X, Weber D, Carvan MJ, 3rd, Coppens R, Lamb C, Goetz S, Schaefer LA. Comparison of neurobehavioral effects of methylmercury exposure in older and younger adult zebrafish (Danio rerio) Neurotoxicology. 2012;33:1212–1218. doi: 10.1016/j.neuro.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S. PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic geochemistry. 2002;33:489–515. [Google Scholar]
- Zhang X, Li X, Jing Y, Fang X, Zhang X, Lei B, Yu Y. Transplacental transfer of polycyclic aromatic hydrocarbons in paired samples of maternal serum, umbilical cord serum, and placenta in Shanghai, China. Environ Pollut. 2017;222:267–275. doi: 10.1016/j.envpol.2016.12.046. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang L, Wang C, Gao D, Zuo Z. Phenanthrene exposure produces cardiac defects during embryo development of zebrafish (Danio rerio) through activation of MMP-9. Chemosphere. 2013a;93:1168–1175. doi: 10.1016/j.chemosphere.2013.06.056. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang L, Zuo Z, Chen Y, Wang C. Phenanthrene exposure causes cardiac arrhythmia in embryonic zebrafish via perturbing calcium handling. Aquatic toxicology. 2013b;142:26–32. doi: 10.1016/j.aquatox.2013.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.