Abstract
All vertebrates require thyroid hormone (TH) for normal growth and development. Plasma TH enters cells and alters gene expression via nuclear receptors TRα and TRβ. In-vitro studies showed that TRs function as repressors of TH-inducible genes in the absence of TH and as activators of those same genes in the presence of TH. A dual function model was proposed to harmonize these molecular TR actions with the dynamic expression of TRs and peak in production of TH experienced during development. Conclusive tests of the repression activity of TRs early in development as predicted by the model awaited gene knockout technology targeting TRα. At the molecular level, active repression of genes involved in metamorphosis by TRα in the absence of TH was confirmed in whole bodies and intestine from TRα knockout studies. As a consequence of this reduced repression in TRα knockout animals, initiation of limb morphogenesis occurs precociously. However, subsequent limb development is retarded during rising plasma TH levels due to reduced TR-dependent responsivity to TH. In contrast to the limbs, intestine remodeling is delayed by one to two developmental stages in TRα knockout animals, despite de-repressed levels of TH-induced genes during premetamorphosis. Surprisingly, in the absence of TRα, hind limbs do not require gene induction by TH signaling to complete morphological growth and development, which is contrary to prediction by the dual function model. Full evaluation of the dual function model for all organs awaits the production of TRα and TRβ double knockout frogs.
Keywords: Xenopus tropicalis, tadpole, metamorphosis, gene knockout
Introduction
Thyroid hormone (TH) plays critical roles in all vertebrates during development and adult physiology (Braverman and Utiger, 2005; Yen, 2001). All vertebrates experience a peak in plasma levels of TH, often at a life history transition, such as birth in humans, weaning in mice, and metamorphosis in amphibians (Buchholz et al., 2006; Laudet, 2011). Lack of TH during development leads to severe growth and neurological impairments in humans, death around weaning in mice, and complete lack o f metamorphosis in frogs. The actions of TH are mediated by TRs, which are members of the nuclear hormone receptor superfamily (Cheng et al., 2010). All vertebrates have two TR isoforms: TRα and TRβ (Laudet, 2011). TRα is more wide-spread among tissues and is typically expressed before thyroid gland development when TH is minimally available to tissues (Cheng et al., 2010; Forhead and Fowden, 2014; Ng and Forrest, 2006). TRs act by binding TREs in the enhancers of TH response genes to regulate their transcription. For TH-induced genes, TRs heterodimerize with retinoid X receptors (RXRs) to recruit corepressors in the absence of TH to actively repress genes, and TR / RXR heterodimers recruit coactivators in the presence of TH to induce gene expression (Buchholz et al., 2006; Cheng et al., 2010; Shi et al., 2012).
Based on these molecular actions of TR derived mainly from in-vitro cell culture studies, a dual function model to describe the role of TR in development during pre- vs. metamorphosis was first proposed upon cloning of the frog TRs and analyzing their developmental expression (Yaoita and Brown, 1990). As elaborated in subsequent reviews (Buchholz et al., 2006; Sachs et al., 2000; Shi et al., 1996; Shi, 2009), the dual function model states that unliganded TR represses genes during premetamorphosis when TH is virtually absent to allow larval growth and that TR induces those same genes when TH is present to bind TR to enable TH-dependent metamorphosis. Given the low levels of TH during early stages of metamorphosis and the different levels of sensitivity to TH among tissues (Choi et al., 2015a; Leloup and Buscaglia, 1977; Shi et al., 1996), the transition from unliganded to liganded TR is not simultaneous among tissues but is achieved at different developmental time points. For instance, it is likely that at the beginning of metamorphosis TR is an activator in limbs while still a repressor in tail due to different levels of free intracellular TH.
The dual function model also addresses the developmental consequences of the molecular actions of TR, where TR-mediated gene repression allows premetamorphic animals to grow and prevents precocious metamorphosis until plasma TH becomes available to induce TH-response genes and stimulate development to proceed through metamorphosis. Numerous experiments in wild-type and transgenic animals supported the dual function model (Shi, 2013). However, the evidence for TR-mediated repression remained equivocal and the requirement for induced levels of TH-response gene expression for developmental progression remained untested (Choi et al., 2015b). Direct evaluation of the dual function model using TR gene knockout became possible with the advent of TALEN and CRISPR gene disruption technology in frogs (Blitz et al., 2013; Guo et al., 2014; Ishibashi et al., 2012; Lei et al., 2012; Nakayama et al., 2013; Suzuki et al., 2013).
TRα knockout (TRαKO) animals were analyzed in two papers by Choi et al (Choi et al., 2015b; Choi et al., 2017), two papers by Wen et al (Wen and Shi, 2015; Wen et al., 2017), a review by Wen and Shi (Wen and Shi, 2016), and 3 News & Views (Sachs, 2015; Schreiber, 2017; Yen, 2015). In both laboratories, the TRα DNA binding domain was targeted using TALENs. Frameshift mutations at this location were produced and expected to result in a truncated protein with no known activity. In all papers, overall growth and development as well as gene expression in whole body or tissues were analyzed, but various methods and experimental aims were present in each paper (Table 1).
Table 1.
Comparison of Methods and Experimental Aims among TRαKO articles.
| Article | Genotyping Method | Source | Developmental Stage/Organs |
|---|---|---|---|
| Choi et al 2015* | limb phenotype | F1 | premetamorphosis/limb, gills |
| Choi et al 2017* | limb phenotype | F1, F2 | metamorphosis/intestine |
| Wen et al 2015 | PCR typing | founders | pre- and prometamorphosis/limb |
| Wen et al 2017 | PCR typing | F2 line | metamorphosis, limb, tail, intestine |
Methimazole was used in Choi et al 2015, 2017.
Molecular Level Analysis of TRαKO
Expectations for altered regulation of gene expression in TRαKO tadpoles were based on the knowledge that before metamorphosis TRα is the predominant TR expressed in cells (Baker and Tata, 1990; Eliceiri and Brown, 1994; Yaoita and Brown, 1990). TRβ has low expression at this time due to an alternate promoter (Shi et al., 1992), but most TRβ expression is due to it being a direct TH response gene (Kanamori and Brown, 1992; Machuca et al., 1995; Ranjan et al., 1994). Consistent with premetamorphic expression of TR binding to TH response elements (TREs) both in the absence and presence of ligand, it was found that in premetamorphic TRαKO tadpoles, ChIP analysis using anti-TR and anti-NCoR (nuclear corepressor) antibodies showed a large reduction in TR binding to TRβ and TH/bZIP TREs associated with reduced NCoR recruitment, as was expected due to lack of wild-type TRα (Wen et al., 2017). Residual significant binding by TR and NCoR to TREs above background levels in TRαKO animals is likely due to wild-type TRβ which is also recognized by the anti-TR antibodies and recruits NCoR (Sachs and Shi, 2000; Sachs et al., 2002). After 18 hrs. of TH treatment which induces TRβ expression by greater than 10 fold in wild-type animals, recruitment of TRs is increased in wild-type and TRαKO tadpoles (Wen et al., 2017), but the degree of increase is less in TRαKO compared to wild-type tadpoles, likely due to reduced level of TRE occupancy in the absence of TRα.
Expected changes in gene expression due to reduced TRE occupancy and coregulator recruitment in TRαKO tadpoles were reduced repression in premetamorphosis as well as reduced activation of TH-response genes when given exogenous TH. Indeed, higher expression levels, i.e., de-repressed expression levels, of TRβ, TH-induced bZIP protein (TH/bZIP), Krüppel-like factor 9 (klf9), and stromelysin 3 (ST3) were observed in whole bodies of TRαKO premetamorphic animals (Choi et al., 2015b; Wen and Shi, 2015; Wen et al., 2017). Similarly, higher expression levels of the direct response genes TRβ, KLF9, ST3, and sonic hedgehog (xhh) were observed in intestines isolated from untreated premetamorphic TRαKO tadpoles (Choi et al., 2017). In response to exogenous TH, gene induction is expected to be reduced in TRαKO animals because of reduced overall TR binding to TREs for recruiting coactivators and inducing transcription. For whole bodies and intestines in premetamorphic animals, the degree of induction by exogenous TH of TRβ, TH/bZIP, klf9, ST3, and xhh was reduced in TRαKO tadpoles (Choi et al., 2015b; Choi et al., 2017; Wen and Shi, 2015; Wen et al., 2017). The reduced but still significant induction in TRα mutants is likely due to low levels of TRβ expression and autoregulation of TRβ available to induce TH-response genes. Non-genomic actions of TH may also contribute to TH-response gene induction (Cheng et al., 2010).
Significant differences in TH response gene expression were found throughout larval development in intestines in TRαKO compared to wild-type tadpoles (Choi et al., 2017). Gene induction for TRβ and KLF9 was delayed by at least three developmental stages, and maximal induction was significantly reduced for ST3. For xhh, its expression was not maintained for as many developmental stages in TRαKO tadpoles. Each gene had a unique alteration of its expression profile, likely due to gene-specific regulators of expression, but in general there was delayed initiation and/or reduced and broadened shape of the expression profile across larval intestine development and metamorphosis.
Growth and development in TRαKO animals
The significant effects on gene expression observed in TRαKO animals were expected to have significant effects on phenotype. Studies so far using TRαKO animals have examined hind limb, intestine, overall growth, and timing of developmental events (Choi et al., 2015b; Choi et al., 2017; Wen and Shi, 2015; Wen et al., 2017). A surprising finding, given the high TRα gene expression levels throughout the tadpole across developmental stages, was that TRαKO animals can achieve complete metamorphosis and become fertile adults (Choi et al., 2017; Wen et al., 2017). Significant TRαKO growth and development phenotypes during the metamorphic process have been detected, but they have so far been seemingly mild and transitory, reminiscent of phenotypes seen in TRα-mutant mice (Gauthier et al., 2001).
In general, the growth trajectories are very similar between wild-type and TRαKO animals (Fig. 1A) (Choi et al., 2017). However, during the first 2-3 weeks after fertilization, TRαKO animals are significantly larger than wild type (Choi et al., 2017; Wen et al., 2017). This growth difference is correlated with a difference in growth hormone mRNA expression level in 11 day old tadpoles, where the larger TRαKO tadpoles express more growth hormone as measured in whole bodies (Wen et al., 2017). Such an effect in TRαKO animals may be due to the role of TRα on growth hormone expression known from mammalian studies where growth hormone is a direct response gene, and lack of repression due to mutant TRα may allow higher expression of growth hormone in the absence of TH. During the remainder of the 6-7 week larval period through metamorphosis, snout-vent length was not statistically significantly different between TRαKO and wild-type tadpoles (Choi et al., 2017) but body weight was significantly reduced by about 10% at tail resorption at the end of metamorphosis in TRαKO animals (Wen et al., 2017).
Figure 1.
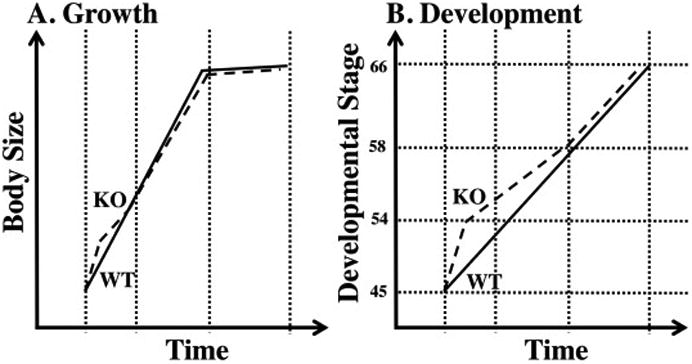
Diagrammatic representation of overall growth and development throughout the larval period and metamorphosis in wild-type and TRαKO tadpoles. A) TRαKO tadpoles (KO, dashed lines) initially grow faster than wild-type tadpoles (WT, solid lines) during premetamorphosis, but during the remainder of the larval period from the initiation of metamorphosis through to tail resorption, significant but small size differences are observed. B) TRαKO animals initially develop faster during premetamorphosis (< stage 54) (Nieuwkoop and Faber, 1994) than wild-type tadpoles based on hind limb criteria. Then, during prometamorphosis (stages 54 - 58) when TH levels begin to increase, TRαKO animals progress more slowly than wild-type tadpoles based on hind limb criteria. Then, during climax of metamorphosis (stages 58 - 66), when TH levels reach their peak, TRαKO and wild-type tadpoles develop at a similar rate. The vertical dotted lines from left to right in both graphs indicate initiation of feeding (stage 45), initiation of metamorphosis (stage 54), mid-point of plasma TH levels during metamorphosis (stage 58), and tail resorption (stage 66).
One of the most significant findings using TRαKO animals was the effect on hind limb development (Fig. 2) (Choi et al., 2015b; Wen and Shi, 2015; Wen et al., 2017). The dual function model predicted that lack of repression by TRs may allow increased expression levels of TH response genes important for metamorphosis thereby allowing precocious development to occur. Indeed, hind limb morphogenesis initiated much earlier in TRαKO tadpoles compared to their wild-type siblings. This finding is the clearest demonstration of a developmental role for unliganded TR in vertebrate development and is consistent with the dual function model.
Figure 2.
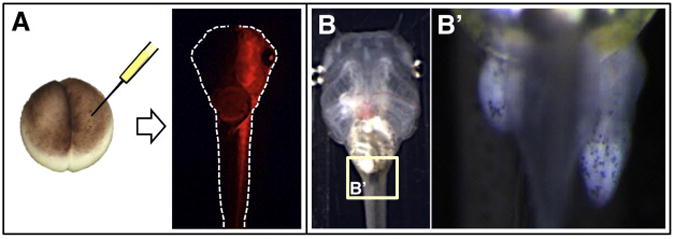
Mutations in TRα result in precocious initiation of hind limb morphogenesis. A) Injection of TALEN mRNAs targeting the DNA-binding domain of TRα was performed in one cell of a two-cell stage embryo of Xenopus tropicalis. mCherry mRNA was co-injected as a red fluorescent tracer to identify which side of the embryo (left in this case) was injected with TRα TALENs. B) Hind limbs of the resulting tadpole during premetamorphosis were examined for developmental progress on the injected and uninjected sides of the animal. As shown in B′, the hind limbs on the injected side are more advanced in size and stage, due to lack of TRα-mediated repression of genes important for metamorphic progression.
The limbs may be unique in the predominance of TRα compared to TRβ, where high levels of TRα are expressed throughout the limb bud and TRβ expression is limited in hind limbs to cartilage based on in-situ studies (Cai and Brown, 2004; Fairclough and Tata, 1997; Shi et al., 1996). Other organs are likely less affected by TRαKO because of their higher expression of TRβ which may in part or completely compensate for the loss of TRα (Wen et al., 2017). Thus, even though TRαKO animals initiate limb morphogenesis earlier in time (Fig. 1B), the other organs of the TRαKO tadpole may be in a state of developmental progression identical to wild-type tadpoles of the same age. On the other hand, in prometamorphosis (Nieuwkoop and Faber stages 54-58) (Nieuwkoop and Faber, 1994), there is a slow-down in developmental progression in TRαKO animals (Fig. 1B) (Choi et al., 2017; Wen et al., 2017). These stages are determined by the progress of limb morphogenesis, which may proceed more slowly due to the reduced rate of limb elongation from reduced responsivity to TH. Indeed, reduced limb elongation in response to exogenous TH treatment in TRαKO tadpoles is consistent with this possibility (Choi et al., 2015b). Later, during climax of metamorphosis (Nieuwkoop and Faber stages 58-66), the similarity in duration to progress through these stages in TRαKO and wild-type animals when plasma TH is highest may be due to compensation by sufficient induction of TRβ in other organs to mediate the TH signal (Fig. 1B).
Despite the effect of TRαKO on premetamorphic development (before stage 54) and prometamorphosis (stages 54-58), the total time from fertilization to the completion of metamorphosis (stages 1-66) in the TRαKO and WT animals was either the same (Wen et al., 2017) or slightly shorter (6 days out of 75 days) (Choi et al., 2017) (Fig. 1B). The somewhat different results may be explained by different methods and samples sizes. If TRαKO animals do metamorphose earlier, the mechanism is not clear and suggests that the opposing effects of TRαKO to initiate premetamorphosis earlier and delay progress through prometamorphosis fail to compensate for each other. Other possibilities include that perhaps TRαKO has the effect of increasing TH plasma levels, or derepression of TRβ prior to initiation of metamorphosis resulted in increased development rate in the presence of TH.
The effect of TRαKO on intestinal remodeling reveals a different picture compared to the effect on hind limbs. The initiation of intestinal shrinkage occurred at a later developmental stage in TRαKO vs. wild-type animals and did not progress to the same extent during natural or TH-induced metamorphosis (Choi et al., 2017; Wen et al., 2017). Importantly, despite derepression of TH response genes in premetamorphic TRαKO intestines, intestinal remodeling was delayed rather than being precocious as in hind limb (Choi et al., 2017). Specifically, larval epithelial cell apoptosis was delayed by two stages, and the number of intestinal folds into the lumen was significantly reduced in TRαKO animals. However, no differences in cell proliferation or cell size or intestinal diameter were detected. These intestinal phenotypes may reflect a permanent deficit in intestinal remodeling which may explain the slower growth observed after 4 weeks post-metamorphosis (Choi et al., 2017). However, as in TRαKO mice, the effect on growth and development and intestinal morphology appears to be minimal (Gauthier et al., 2001).
A surprising finding in the TRαKO animals was their morphology after prolonged treatment with methimazole, which blocks TH synthesis (Fig. 3). Continuous treatment with methimazole at the beginning of feeding when the thyroid gland is being formed can virtually eliminate the production TH in treated tadpoles (Buchholz et al., 2004; Buckbinder and Brown, 1993). Wild-type animals kept in methimazole stop developmental progression around stage 53-54, when the hind limbs are around the paddle stage. These animals continue to grow indefinitely in size, but they do not advance in stage, whereas their untreated siblings undergo metamorphosis after 5-7 weeks. TRαKO tadpoles treated with methimazole for over 10 weeks have a mixed phenotype (Choi et al., 2017). Specifically, when TRαKO tadpoles are reared in methimazole, the limbs, which express predominantly TRα and little TRβ, experience a lack of TH signaling yet achieve complete morphogenesis (Fig. 3). This result indicates that derepressed levels, rather than induced levels, of response gene expression are sufficient to complete limb morphogenesis. The skin of methimazole-reared TRαKO tadpoles also appeared to complete development, except in skin covering gill and tail areas where adult skin does not form (Fig. 3) (Suzuki et al., 2009). Other parts of their external morphology remain larval, such as mouth parts, gills, and tail. Thus, in the complete absence of TH signaling, hind limbs and adult skin can form if TRα is absent, although the rate of limb and skin development may be affected. This result is surprising because according to the dual function model, TH response gene induction is required for full development to the adult form. Derepression is predicted to be most complete in hind limbs because they are dominated by TRα. The formation of adult skin in methimazole-treated TRαKO animals predicts that skin development is also predominantly dependent on TRα. The tail has a high proportion of TRβ, and thus TRβ in tail may be sufficient to maintain TH-response gene repression. Alternatively, tail may require TH response gene induction above levels of gene expression achieved by derepression. These findings using methimazole further suggest that, at least for the limb, the role of TH signaling is not to determine cell fate during development but rather to permit development that is already specified. Thus, a major action of TRs in development appears to be to control the timing of developmental initiation and rate of the change from the larval to adult type.
Figure 3.
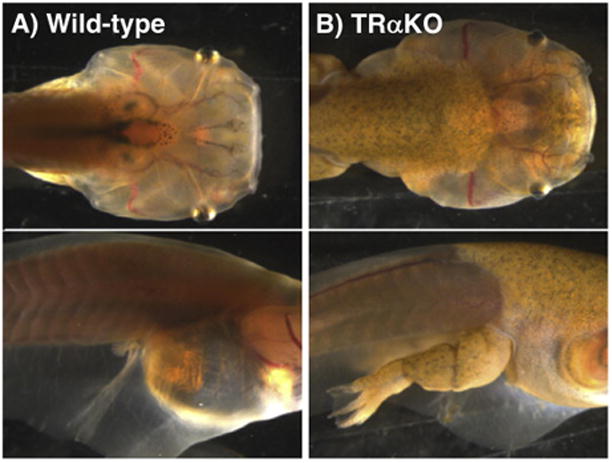
Hind limb and skin morphogenesis in the absence of TH signaling in TRαKO tadpoles. Wild-type (A) and TRαKO (B) tadpoles shown in dorsal and side views were reared in 1 μM methimazole (inhibits TH synthesis) for 10 weeks starting at the initiation of feeding when the thyroid gland is forming. Development in the methimazole-treated wild-type tadpole is blocked at the beginning of metamorphosis, at the point when plasma TH levels begin to rise in untreated tadpoles. Notably, hind limb morphogenesis has stalled just after the paddle stage, and the skin is still thin and transparent as it is before it remodels to adult skin. In the methimazole-treated TRαKO tadpole, hind limb morphogenesis is complete, and in locations where adult skin replaces larval skin (i.e., not skin covering gills and tail), the skin has become opaque obscuring view of internal structures.
Conclusions
The dual function model was largely supported using TRαKO animals by studies reviewed here (Choi et al., 2015b; Choi et al., 2017; Wen and Shi, 2015; Wen et al., 2017). Molecular analyses from ChIP and gene expression supported functional gene repression by unliganded TR in early premetamorphosis. Reduced gene induction during metamorphosis and after exogenous TH treatment supported the role of gene activation by liganded TR. These effects on gene regulation are consistent with the dual function model and explain precocious initiation of hind limb development in TRαKO animals as well as delayed completion of limb morphogenesis. The relationship between the dual function model and initial faster growth but reduced weight at tail resorption is not as clear but may relate to TH regulation of pituitary hormones. The early intestine gene derepression followed by subsequent delay in intestine remodeling is also not explained but may be due to low levels of TRβ to accomplish repression and reduced TRβ autoregulation to reduce the rate of remodeling. The requirement for induced levels of TH-response gene expression for developmental progression as proposed by the dual function model was found to be not accurate for the limbs and in need of further evaluation in other organs. It appears that levels of TH-response gene expression equivalent to de-repressed levels when TRα is absent, rather than induced levels of these genes, are sufficient to achieve complete metamorphosis.
Our understanding of the TRα knockout phenotype would benefit from knowing when plasma TH becomes available in TRαKO animals and what level plasma TH achieves. However, early TH production does not explain precocious hind limb development, because TRαKO animals reared in methimazole, which blocks TH synthesis, exhibit the same precocious hind limb phenotype. Because hind limb development and nothing else seems to be precocious in TRαKO animals, it may not be appropriate to compare stage 54 wild-type animals to stage 54 TRαKO animals because the rest of the organs besides the limb in TRαKO stage 54 animals are not expected to be in an accelerated state of development.
TRβ is expressed at least to some degree in most if not all tissues, such that full evaluation of the dual function model will require TRα/β double knockout animals. It will be important to determine the degree to which other organs besides limb may exhibit precocious initiation of metamorphosis and be able to complete development in the complete absence of TR-mediated repression and activation. In this regard, it is interesting to note that TR α/β double knockout mice have reduced viability and significant morphological and physiological defects, even though mouse development is less dependent on TH than frog metamorphosis (Gothe et al., 1999). Given the complete limb morphogenesis without TH signaling and despite the extraordinary dependence of metamorphosis on TH, it is an open question whether completion of metamorphosis through tail resorption in the absence of TRs will occur.
Highlights.
Derepression of TH response genes occurs in premetamorphosis in TRαKO tadpoles
TRαKO hind limb morphogenesis initiates precociously in premetamorphosis
TRαKO hind limb morphogenesis is slower during prometamorphosis
TRαKO intestine remodeling is delayed, despite TH-response gene derepression
TH signaling is not required to complete hind limb morphogenesis in TRαKO tadpoles
Acknowledgments
This work was supported in part by the Intramural Research Program of NICHD, NIH (YS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker BS, Tata JR. Accumulation of proto-oncogene c-erb-A related transcripts during Xenopus development: association with early acquisition of response to thyroid hormone and estrogen. Embo j. 1990;9:879–885. doi: 10.1002/j.1460-2075.1990.tb08185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz IL, Biesinger J, Xie X, Cho KWY. Biallelic Genome Modification in F0 Xenopus tropicalis Embryos Using the CRISPR/Cas System. Genesis. 2013;51:827–834. doi: 10.1002/dvg.22719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman LE, Utiger RD, editors. Werner and Ingbar's The Thyroid. Lippincott, Williams, and Wilkins; Philadelphia: 2005. [Google Scholar]
- Buchholz DR, Paul BD, Fu L, Shi YB. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol. 2006;145:1–19. doi: 10.1016/j.ygcen.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Tomita A, Fu L, Paul BD, Shi YB. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol. 2004;24:9026–9037. doi: 10.1128/MCB.24.20.9026-9037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckbinder L, Brown DD. Expression of the Xenopus laevis prolactin and thyrotropin genes during metamorphosis. Proc Natl Acad Sci U S A. 1993;90:3820–3824. doi: 10.1073/pnas.90.9.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Brown DD. Expression of type II iodothyronine deiodinase marks the time that a tissue responds to thyroid hormone-induced metamorphosis in Xenopus laevis. Dev Biol. 2004;266:87–95. doi: 10.1016/j.ydbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Cheng S, Leonard JL, Davis PJ. Molecular Aspects of Thyroid Hormone Actions. Endocr Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Atsuko Ishizuya-Oka A, Buchholz DR. Growth, development, and intestinal remodeling occurs in the absence of thyroid hormone receptor alpha in tadpoles of Xenopus tropicalis. Endocrinol. 2017;158:1623–1633. doi: 10.1210/en.2016-1955. [DOI] [PubMed] [Google Scholar]
- Choi J, Moskalik CL, Ng A, Matter SF, Buchholz DR. Regulation of thyroid hormone-induced development in vivo by thyroid hormone transporters and cytosolic binding proteins. Gen Comp Endocrinol. 2015a;222:69–80. doi: 10.1016/j.ygcen.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Choi J, Suzuki KT, Sakuma T, Shewade L, Yamamoto T, Buchholz DR. Unliganded thyroid hormone receptor alpha regulates developmental timing via gene repression as revealed by gene disruption in Xenopus tropicalis. Endocrinol. 2015b;156:735–744. doi: 10.1210/en.2014-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri BP, Brown DD. Quantitation of endogenous thyroid hormone receptors alpha and beta during embryogenesis and metamorphosis in Xenopus laevis. J Biol Chem. 1994;269:24459–24465. [PubMed] [Google Scholar]
- Fairclough L, Tata JR. An immunocytochemical analysis of the expression of thyroid hormone receptor alpha and beta proteins during natural and thyroid hormone-induced metamorphosis in Xenopus. Dev Growth Differ. 1997;39:273–283. doi: 10.1046/j.1440-169x.1997.t01-2-00003.x. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. J Endocrinol. 2014;221:R87–R103. doi: 10.1530/JOE-14-0025. [DOI] [PubMed] [Google Scholar]
- Gauthier K, Plateroti M, Harvey CB, Williams GR, Weiss RE, Refetoff S, Willott JF, Sundin V, Roux J, Malaval L, Hara M, Samarut J, Cha ssande O. Genetic analysis reveals different functions for the products of the thyroid hormone receptor alpha locus. Mol Cell Biol. 2001;21:4748–4760. doi: 10.1128/MCB.21.14.4748-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennstrom B, Forrest D. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhang T, Hu Z, Zhang Y, Shi Z, Wang Q, Cui Y, Wang F, Zhao H, Chen Y. Efficient RNA/Cas9-mediated genome editing in Xenopus tropicalis. Development. 2014;141:707–714. doi: 10.1242/dev.099853. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Cliffe R, Amaya E. Highly efficient bi-allelic mutation rates using TALENs in Xenopus tropicalis. Biology Open. 2012;1:1273–1276. doi: 10.1242/bio.20123228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori A, Brown DD. The regulation of thyroid hormone recepotor ß genes by thryoid hormone in Xenopus laevis. J Biol Chem. 1992;267:739–745. [PubMed] [Google Scholar]
- Laudet V. The Origins and Evolution of Vertebrate Review Metamorphosis. Curr Biol. 2011;21:R726–R737. doi: 10.1016/j.cub.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Lei Y, Guo X, Liu Y, Cao Y, Deng Y, Chen X, Cheng CHK, Dawid IG, Chen Y, Zhao H. Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs) Pnas. 2012;109:17484–17489. doi: 10.1073/pnas.1215421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup J, Buscaglia M. La triiodothyronine, hormone de la metamorphose des Amphibies. C R Acad Sci Paris, Ser D. 1977;284:2261–2263. [Google Scholar]
- Machuca I, Esslemont G, Fairclough L, Tata JR. Analysis of structure and expression of the Xenopus thyroid hormone receptor-beta gene to explain its autoinduction. Mol Endocrinol. 1995;9:96–107. doi: 10.1210/mend.9.1.7760854. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Fish MB, Fisher M, Oomen-Hajagos J, Thomsen GH, Grainger RM. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis. 2013;51:835–843. doi: 10.1002/dvg.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Forrest D. Developmental roles of thyroid hormone receptor a and b genes. Adv Dev Biol. 2006;16:1–31. [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Garland Publishing; New York: 1994. [Google Scholar]
- Ranjan M, Wong J, Shi YB. Transcriptional repression of Xenopus TR beta gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem. 1994;269:24699–24705. [PubMed] [Google Scholar]
- Sachs LM. Unliganded Thyroid Hormone Receptor Function: Amphibian Metamorphosis Got TALENs. Endocrinology. 2015;156:409–410. doi: 10.1210/en.2014-2016. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Shi YB. Targeted chromatin binding and histone aceylation in vivo by thyroid hormone receptor during amphibian develoment. Pnas. 2000;97:13138–13143. doi: 10.1073/pnas.260141297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs LM, Damjanovski S, Jones PL, Li Q, Amano T, Ueda S, Shi YB, Ishizuya-Oka A. Dual functions of thyroid hormone receptors during Xenopus development. Comp Biochem Physiol B Biochem Mol Biol. 2000;126:199–211. doi: 10.1016/s0305-0491(00)00198-x. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Jones PL, Havis E, Rouse N, Demeneix BA, Shi YB. Nuclear receptor corepressor recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol Cell Biol. 2002;22:8527–8538. doi: 10.1128/MCB.22.24.8527-8538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM. Unliganded TRa: A “Safety Lock” to Metamorphosis. Endocrinology. 2017;158:1577–1580. doi: 10.1210/en.2017-00259. [DOI] [PubMed] [Google Scholar]
- Shi YB, Matsuura K, Fujimoto K, Wen L, Fu L. Thyroid hormone receptor actions on transcription in amphibia: the roles of histone modification and chromatin disruption. Cell Biosci. 2012;2:42. doi: 10.1186/2045-3701-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Unliganded Thyroid Hormone Receptor Regulates Metamorphic Timing via the Recruitment of Histone Deacetylase Complexes. Curr Top Dev Biol. 2013;105:275–297. doi: 10.1016/B978-0-12-396968-2.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YB. Dual functions of thyroid hormone receptors in vertebrate development: the roles of histone-modifying cofactor complexes. Thyroid. 2009;19:987–999. doi: 10.1089/thy.2009.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YB, Wong J, Puzianowska-Kuznicka M, Stolow MA. Tadpole competence and tissue-specific temporal regulation of amphibian metamorphosis: roles of thyroid hormone and its receptors. Bioessays. 1996;18:391–399. doi: 10.1002/bies.950180509. [DOI] [PubMed] [Google Scholar]
- Shi YB, Yaoita Y, Brown DD. Genomic organization and alternative promoter usage of the two thyroid hormone receptor beta genes in Xenopus laevis. J Biol Chem. 1992;267:733–738. [PubMed] [Google Scholar]
- Suzuki K, Machiyama F, Nishino S, Watanabe Y, Kashiwagi K, Kashiwagi A, Yoshizato K. Molecular features of thyroid hormone-regulated skin remodeling in Xenopus laevis during metamorphosis. Develop Growth Differ. 2009;51:411–427. doi: 10.1111/j.1440-169X.2009.01100.x. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Isoyama Y, Kashiwagi K, Sakuma T, Ochiai H, Sakamoto N, Furuno N, Kashiwagi A, Yamamoto T. High efficiency TALENs enable F0 functional analysis by targeted gene disruption in Xenopus laevis embryos. Biology Open. 2013;2:448–452. doi: 10.1242/bio.20133855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Shi Y. Unliganded thyroid hormone receptor α controls developmental timing in Xenopus tropicalis. Endocrinol. 2015;156:721–734. doi: 10.1210/en.2014-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Shi Y. Regulation of growth rate and developmental timing by Xenopus thyroid hormone receptor a. Develop Growth Differ. 2016;58:106–115. doi: 10.1111/dgd.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Shibata Y, Su D, Fu L, Luu N, Shi Y. Thyroid Hormone Receptor a Controls Developmental Timing and Regulates the Rate and Coordination of Tissue-Specific Metamorphosis in Xenopus tropicalis. Endocrinology. 2017;158:1985–1998. doi: 10.1210/en.2016-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita Y, Brown DD. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990;4:1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Yen PM. Unliganded TRs regulate growth and developmental timing during early embryogenesis: evidence for a dual function mechanism of TR action. Cell & Bioscience. 2015;5:8. doi: 10.1186/2045-3701-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


