Abstract
Varicella zoster virus (VZV) is a neurotropic alphaherpesvirus that, following primary infection (varicella), establishes latency in sensory, autonomic, sympathetic and parasympathetic neurons, where it remains until reactivation (zoster). VZV-specific cell-mediated immune responses maintain VZV latency; thus, immunosuppressed and elderly persons are at risk of reactivation and associated neurological diseases. However, the cytokines produced by the immune system that control VZV in neurons are largely unknown. Therefore, to better understand how the immune system may restrict VZV in neurons, we studied interleukin-6, tumor necrosis factor-alpha and type 1 interferons for their ability to inhibit VZV replication in human neurons in vitro. Our studies revealed that VZV transcription and viral spread were significantly reduced by interleukin-6 and type 1 interferons, and to a lesser extent by tumor necrosis factor-alpha. These findings will help in understanding how the innate immune system limits virus replication in neurons in vivo.
Keywords: VZV, IL-6, TNF, Type 1 interferon, antiviral, neuron
Introduction
Varicella zoster virus (VZV) is an exclusively human neurotropic virus that, upon primary infection, causes varicella (chickenpox). After primary infection with wild-type VZV or vaccination with the live-attenuated varicella vaccine (Varivax, Merck), virus establishes latency in sensory ganglia neurons (Gershon et al., 2012; Gilden et al., 1987; Gilden et al., 1983; Mahalingam et al., 1990). Maintenance of VZV latency requires cell-mediated immunity and production of cytokines. Upon loss or suppression of VZV-specific immunity due to aging, immunosuppressive therapy or HIV/AIDS, VZV can reactivate (Asanuma et al., 2000; Koenig et al., 2013; Levin et al., 2003; Saylor et al., 2015; Weinberg and Levin, 2010; Zhang et al., 1994) to cause zoster (shingles), postherpetic neuralgia, vasculopathies (e.g., stroke and giant cell arteritis) and diseases of the central nervous system (e.g., myelopathy and meningoencephalitis) (Gilden et al., 2016; Minassian et al., 2015; Yawn et al., 2016).
The specific mechanisms of how cell-mediated immunity within VZV-infected human ganglia restricts VZV replication remain largely unknown, due in part to the lack of a suitable animal model; no small-animal model fully recapitulates the human disease when virus reactivates. Human ganglia from patients who died soon after zoster contain neurons expressing VZV antigen as well as large infiltrates of CD8+ cells (Gowrishankar et al., 2010). These immune cells are postulated to help establish and maintain VZV latency via production of cytokines, but the identity of such cytokines remains unknown. Notably, treatment of lupus and rheumatoid arthritis with anti-interleukin-6 (IL-6), antitumor necrosis factor alpha (TNFα) and anti-interferon alpha (IFNα, a type 1 IFN) increases a patient’s likelihood of developing zoster (Cacciapaglia et al., 2015; Furie et al., 2017; Garcia-Doval et al., 2010; Khamashta et al., 2016; Mourgues et al., 2016; Strangfeld et al., 2009), suggesting these cytokines are important in inhibiting VZV replication and maintaining latency. Thus, the aim of this study was to investigate the role of IL-6, TNFα and type 1 IFNs (IFNα and IFNβ) in suppressing VZV in human neurons using our established in vitro model of VZV-infected human neurons (Grose et al., 2013; Yu et al., 2013). In this study, we found that IL-6 significantly inhibits VZV replication and virus production in human neurons, type 1 IFNs were also inhibitory to VZV replication, but less so than IL-6 and TNFα did not inhibit VZV replication in human neurons in vitro.
Results
VZV-infected human neurons express cytokine receptors in vitro.
To ensure human neurons derived from induced pluripotent stem cells (iPSCs) cultured in vitro expressed the appropriate cytokine receptors, and thus would be expected to respond to cytokines, uninfected neurons were tested for the presence of receptors for IL-6 (IL-6R), TNFα (TNFR1 and TNFR2), and type 1 IFNs, IFNα and IFNβ (IFNAR). TNFα can bind to either TNFR1 or TNFR2 (Hohmann et al., 1989), whereas IFNAR is the receptor for both IFNα and IFNβ (Merlin et al., 1985). TNFR1 is expressed on nearly all cells, but TNFR2 is expressed on a limited population of cells including endothelial cells, microglia, oligodendrocytes, hippocampal neurons, cardiac myocytes, thymocytes and mesenchymal stem cells (Arnett et al., 2001; Bocker et al., 2008; Dopp et al., 2002; Grell et al., 1998; Irwin et al., 1999; McCoy and Tansey, 2008; Tartaglia et al., 1991; Yang et al., 2002). Neurons were also stained for β-III tubulin, a neuronal marker, to show neuronal cell morphology. As expected, analysis of uninfected iPSC-derived human neurons using fluorescence microscopy revealed the expression of IL-6R, TNFR1 and IFNAR (Fig. 1, top row) as well as β-III tubulin (Fig. 1, middle row). TNFR2 staining was negative, which was in line with our unpublished RNAseq results that indicated TNFR2 is not expressed at the mRNA level. Uninfected neurons were also negative for the non-neuronal marker glial fibrillary acidic protein (GFAP; data not shown).
FIG 1.
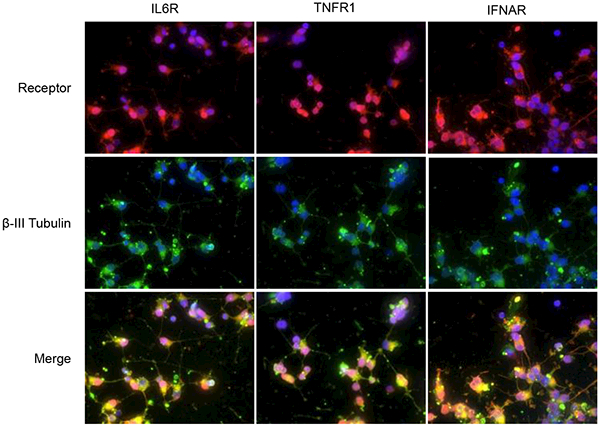
Neurons express cytokine receptors in vitro. Uninfected iPSC-derived human neurons were permeabilized then co-immunostained for indicated cytokine receptor (top row) and β-III tubulin (middle row), followed by fluorescently-conjugated secondary antibodies. IL-6 receptor (IL-6R, left), tumor necrosis factor receptor 1 (TNFR1, middle) and interferon-α/β receptor (IFNAR, right) stainings were all positive, whereas TNFR2 and GFAP stainings were negative (not shown). Merged images (bottom row) indicate all cells are positive for receptor and β-III tubulin. Magnification = 60X; blue, DAPI staining of nuclei.
It was of concern that VZV-infection of iPSC neurons may downregulate cytokine receptors, thereby making the neurons resistant to cytokine treatment. To test this, neuron cultures were infected with VZV for 21 days, after which neurons were fixed and surface protein-stained (non-permeabilized) for the indicated receptors as well as the viral glycoprotein E (gE). At 21 days post-infection (dpi), VZV-infected neurons (Fig. 2, top row) retained surface expression of all cytokine receptors (Fig. 2, middle row). Colocalization of gE and cytokine receptor is visualized in the merge (Fig. 2, bottom row) as a yellow color. As a negative control, infected, non-permeabilized neurons were stained with normal rabbit serum and a mouse isotype Ig, followed by Alexa-conjugated secondary antibodies, as described in Materials and Methods (Fig. 2, bottom row).
FIG 2.
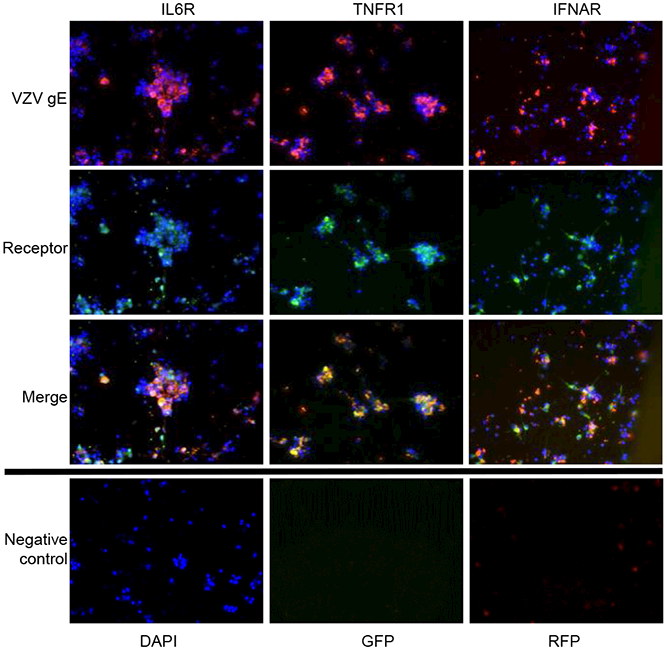
Viral infection does not downregulate cytokine surface receptors. Human neurons were infected with VZV for 21 days, then surface-stained (non-permeabilized) for viral glycoprotein E (row 1) and cytokine receptor (row 2), followed by fluorescently-conjugated secondary antibodies. VZV-infected neurons (red) retain surface expression of cytokine receptor (green), seen as yellow in merge (row 3). Infected neurons (non-permeabilized) were immunostained using control rabbit serum (GFP) and mouse isotype antibody (RFP) (row 4), followed by fluorescently conjugated secondary antibodies. Magnification = 40X; blue, DAPI staining of nuclei.
IL-6 and type 1 IFNs, but not TNFα, inhibit viral replication in vitro.
To demonstrate that cytokine treatment over the course of the experiments would not be toxic to neurons, both uninfected and VZV-infected neuronal cultures were maintained without cytokine (untreated control, UT) or with either IL-6, TNFα, IFNα or IFNβ for 21 days. After 21 days, cytotoxicity was measured by lactate dehydrogenase (LDH) release into the culture supernatants. In uninfected cultures, IL-6 induced an 8.17 ± 4.01% cytotoxicity relative to the maximum release control, and no other cytokine induced any cytotoxicity. Also, acyclovir (ACV) toxicity was measured since ACV was used as a positive control to inhibit VZV replication (used in virus replication experiments, below); this treatment induced no cytotoxicity in uninfected cultures. Similarly, after 21 days of infection, neurons cultured in the presence of cytokine did not release any additional LDH compared to infection alone, indicating cytokine treatment did not accelerate death of infected neurons (LDH release, relative to uninfected, untreated maximum release control: VZV only, 4.70 ± 0.28%; VZV+IL-6, 5.13 ± 1.30%; VZV+TNFα, 21.15 ± 19.55%; VZV + IFNα, 2.99 ± 1.30%; IFNβ, 6.52 ± 3.64%). To test the hypothesis that IL-6, TNFα or either type 1 IFN would inhibit VZV replication in human neurons, neuronal cultures were either pretreated with cytokine 24 h prior to VZV infection or with medium lacking any cytokine (UT control). Pretreatment was done to recapitulate what may occur in vivo, where neurons experience cytokine exposure prior to retrograde transport of alphaherpesviruses from peripheral tissue and deposition of viral DNA into the neuron nucleus (Rosato and Leib, 2015; Song et al., 2016). Following infection, neurons were continuously cultured in the presence or absence of cytokine as described above for the toxicity assay. At 21 dpi, UT and cytokine-treated cultures were harvested and mRNA levels measured. Treatment with IL-6 resulted in a significant reduction of all viral mRNAs measured, irrespective of gene class, relative to UT cultures (ORF62 and ORF63, immediate early; ORF29, early; ORF68, late; p<0.05) (Fig. 3A). However, treatment of VZV-infected neurons with either type 1 IFN had no significant effect on levels of ORF63 transcript (p>0.05), but did significantly reduce all other viral genes examined. TNFα followed the trend of inhibiting viral transcription relative to UT cells, but to a lesser extent than other cytokines studied and was not statistically significant. As a positive control for inhibition of viral replication, a parallel culture of VZV-infected neurons was maintained in the presence of ACV; at 21 dpi, VZV transcripts were either not detectable or not quantifiable (Ct > 35) (Fig. 3A). From the same cultures that were used to measure mRNA abundance, DNA was also extracted and abundance of viral genomes measured (Fig. 3B). None of the treatments, including acyclovir, caused a significant decrease in viral DNA abundance compared to UT. Next, the ability of the cytokines to limit virus spread was measured. Neurons were pretreated or remained UT, infected, then maintained as described in the methods. The ability of IL-6, TNFα or either type 1 IFN to limit VZV spread within neuronal cultures was examined by measuring viral plaque area at 21 dpi. At 21 dpi, VZV-infected neuron cultures without cytokine treatment had viral antigen covering large areas (0.317 ± 0.121 mm2). Conversely, VZV-infected neurons cultured in the presence of either IL-6, TNFα, IFNα or IFNβ had much reduced spread (virus area: IL-6, 0.059 ± 0.022 mm2; TNFα, 0.092 ± 0.032 mm2; IFNα, 0.017 ± 0.003 mm2; IFNβ, 0.008 ± 0.01 mm2). Compared to UT, cytokine-treated cultures contained plaques that were reduced by: IL-6, 83 ± 8%; TNFα, 50 ± 22%; IFNα, 93 ± 2%; and IFNβ, 96 ± 3% (Fig. 3C).
FIG 3.
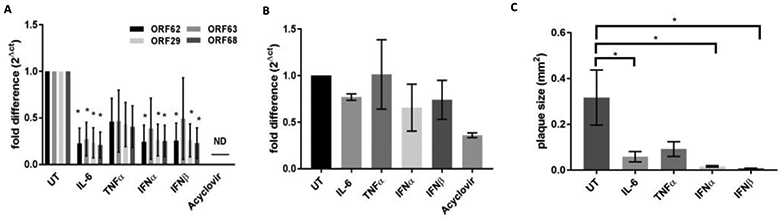
Inhibition of viral replication. VZV-infected neurons were either untreated (UT) or treated with the indicated cytokine (IL-6, TNFα, IFNα, IFNβ) or acyclovir for 21 days, when abundance of either viral mRNA (A) or viral DNA (B) was assessed using qPCR, or virus spread was assessed by measuring viral plaque area (C). Viral transcripts corresponding to VZV ORFs 62, 63, 29 and 68 were assayed. Values are fold-difference of abundance in treated culture relative to UT culture, calculated using the 2ΔCt method (2(Ct,UT)-(Ct, treated)) (a and B). Data are mean ± SEM from 3 experiments; ND, not detected; * indicates a p value <0.05.
IL-6 inhibits production of VZV in human neurons in vitro.
To analyze the quantity of infectious virus produced, cultures were pretreated or remained UT, infected, and cultured as above. At 21 dpi, neuron cultures were harvested, dispersed into single cell suspension then co-cultured on a monolayer of uninfected fibroblasts and plaques were allowed to form. Compared to UT, only IL-6-treated VZV-infected neurons had a significantly reduced (p < 0.05) number of plaques form (reduced 61 ± 15%; Fig. 4); all other treatments reduced the average number of plaques, compared to UT, but did not reach statistical significance.
FIG 4.
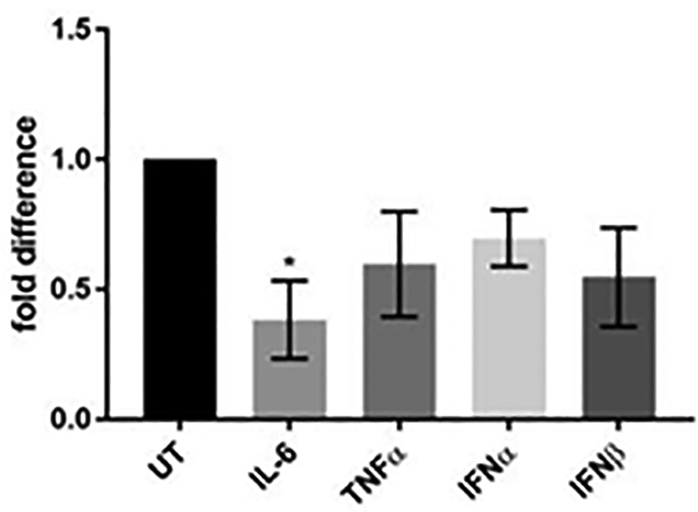
Production of infectious virus. VZV-infected neurons were either untreated (UT) or treated with the indicated cytokine (IL-6, TNFα, IFNα or IFNβ) for 21 days. Neurons were harvested and co-cultured on uninfected HFL cells in the absence of cytokine. Plaques in fibroblast monolayers were detected and counted following crystal violet staining and represented and fold difference relative to UT culture. Data are mean ± SEM from 3 experiments; * indicates a p value <0.05.
Discussion
How cell-mediated immunity and/or cytokines restrict VZV replication in ganglia in vivo is unknown. To better understand this process, we have examined effects of IL-6, TNFα and type 1 IFNs on VZV replication in human neurons. IL-6 exhibited the most consistent and strongest antiviral characteristics of the studied cytokines, significantly limiting transcription of all viral kinetic classes (Fig. 3A), as well as virus spread (Fig. 3C), and production of infectious particles (Fig. 4), compared to UT controls. The strong anti-viral nature of IL-6 in human neurons in vitro is supported by the high levels of IL-6 present during VZV reactivation in vivo causing VZV vasculopathy (Jones et al., 2016) and giant cell arteritis (Gilden et al., 2016; Martinez-Taboada et al., 2008).
While IL-6 demonstrated the most consistent antiviral effect, type 1 IFNs also exhibited antiviral properties. Type 1 IFNs significantly reduced the levels of VZV transcripts compared to untreated, with the exception of VZV ORF63 transcript (Fig. 3A), suggesting there may be a differential block of virus transcription by type 1 IFNs compared to IL-6. This may be explained by the fact that type 1 IFNs primarily signal through signal transducer and activator of transcription 1 and 2 (STAT-1 and −2), whereas IL-6 primarily signals through STAT-3.
TNFα has been shown to be strongly antiviral in non-neuronal cells (Ito et al., 1991; Torigoe et al., 2000); however, these studies indicate TNFα may be less antiviral in neurons as it did not significantly inhibit VZV replication in any of our assays. Notably, TNFα signals through several different pathways following binding to either TNFR1 or TNFR2 surface receptors (Leong and Karsan, 2000); future studies will examine if differential downstream pathways are used in neuronal versus non-neuronal cell types in the context of VZV-infection.
Zostavax has a high ratio of non-infectious DNA to plaque forming units (PFU) and VZV replicates slowly in neurons compared to non-neuronal cells; therefore, differentiation between newly replicated DNA and adhered inoculum is difficult. As such, viral DNA abundance does not increase compared to input by 14 dpi (Baird et al., 2014b). This is likely the reason that, after 21 days of infection, the abundance of viral DNA in ACV- or cytokine-treated neurons, although reduced, is statistically the same as UT neurons (Fig. 3B). It is likely that, if cultures were permitted to incubate longer, differences in DNA abundance would be observed. Despite the lack of statistical significance, IL-6-and type 1 IFN-treated, as well as ACV-treated, cultures had reduced average DNA abundance compared to UT cultures at 21 dpi.
Overall, IL-6 and type 1 IFNs had an inhibitory effect on VZV in human neurons in vitro, where TNFα did not. Whereas this study utilized Zostavax as inoculum, it is possible a wild-type strain of VZV may respond differently to cytokine treatment. Future studies will examine how each cytokine specifically modulates virus replication, as well as additive effects when cytokines are combined.
Materials and Methods
Cells and viruses
Human induced pluripotent stem cell (iPSC; Cellular Dynamics International, Madison, WI)-derived neurons were cultured and infected at a low multiplicity of infection (MOI; approx. 0.001 to 0.0001) with vaccine-strain VZV (Zostavax; Merck, Kenilworth, NJ) as previously described (Baird et al., 2014a; Baird et al., 2015; Baird et al., 2014b). After 3 h of incubation, neurons were washed twice with medium to remove inoculum and cultured for 21 days. Neurons received a 50% medium change with fresh medium twice weekly. Human fetal lung fibroblasts (HFL; ATCC, Manassas, VA) were cultured in Dulbecco’s modified eagle medium supplemented with 10% fetal bovine serum. All cultures were incubated at 37°C and 5% CO2.
Cytokine treatment
Cytokines were prepared in water per the manufacturer’s instructions (IL-6 and TNFα, Millipore, Temecula, CA; IFNα, PBL Assay Science, Piscataway, NJ; IFNβ, Peprotech, Rocky Hill, NJ). Cytokines were used at concentrations observed in the literature: IL-6 and TNFα were each used at 100 ng/ml while type 1 IFNs were used at 10 U/ml, acyclovir was used at 50 μM. For cytokine treatment of VZV-infected cultures, cultures were pretreated for 24 h prior to infection or remained untreated without any cytokine. During the 3 h infection period, cytokine was removed, after which neurons were washed twice with cell medium and cytokine was reintroduced at concentration indicated above. Cytokine-treated cultures, whether infected and uninfected, received 50% medium changes twice weekly with fresh medium containing fresh cytokine at concentration indicated above.
Cytotoxicity
To measure cytotoxicity, parallel cultures of both infected and uninfected neurons were prepared and were either untreated or treated with indicated cytokine as detailed above. After 21 days, cytotoxicity was measured using the Pierce LDH cytotoxicity assay kit (Thermo Fisher Scientific, Waltham, MA) following manufacturer’s instructions. All percent cytotoxicity measurements were calculated relative to maximum release control of uninfected, untreated cultures.
Immunofluorescent microscopy
To determine expression of cytokine receptors, neurons were cultured on either standard tissue culture plastic (12-well plates) or poly-D/laminin-coated glass coverslips (Corning, Corning, NY). Medium was removed and cells were washed once with 1X PBS, fixed in 4% paraformaldehyde for 20 min at 4°C, then either permeabilized with 0.1% Triton-X for 20 min at room temperature (internal staining, Fig. 1) or remained non-permeabilized (surface staining, Fig. 2), and blocked with 5% normal goat serum (Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. Cytokine receptors, β-III tubulin or VZV glycoprotein E (gE) were identified by overnight incubation at 4°C with a primary antibody [anti-IL6R (cat #: sc-13947), -TNFR1 (cat #: sc-7895) and -TNFR2 (cat #: sc-8041), -IFNAR (cat #: sc-704), and -VZV gE (cat #: sc-56995), Santa Cruz Biotechnology, Dallas, TX; β-III tubulin (cat#: 60052), Stem cell Technologies, Cambridge, MA], followed by Alexa Fluor 594- or 488-conjugated secondary antibodies (Thermo Fisher Scientific) for 1 h at 4°C. As negative controls, non-permeabilized, infected neurons were surface stained with normal rabbit serum and mouse isotype Ig antibody followed by Alexa Fluor 594- or 488-conjugated secondary antibodies, respectively (Fig. 2). Cell nuclei were stained with DAPI for 5 min at room temperature. Images were captured using an Olympus (Olympus, Center Valley, PA) IX73 inverted fluorescent microscope fitted with an XM10 cooled CCD camera and processed using Cellsense (Olympus) software.
Nucleic acid isolation and qPCR/RT-qPCR
VZV-infected human neurons harvested at 21 dpi were analyzed for abundance of viral DNA and RNA. DNA and RNA were isolated using ZR Duet DNA/RNA Kit (Zymo Research, Irvine, CA), after which mRNA was isolated by binding to oligo(dT) beads (uMACS; Miltenyi, Bergisch Gladback, Germany) and reverse transcribed using the Superscript IV cDNA Synthesis Kit (LifeTechnologies). Quantitative TaqMan PCR (FastStart Universal MasterMix, Roche, Basel, Switzerland) was performed on an ABI 7500 FAST to quantify viral DNA using primers targeted to a locus within ORF68 (F: GTACATTTGGAACATGCGCG; R: TCCACATATGAAACTCAGCCC) and cDNA with primers targeted to four different viral loci: ORF62 (F: CCTTGGAAACCACATGATCGT; R: AGCAGAAGCCTCCTCGACAA), ORF63 (F: TAGCGACGATGATGGGTCTA; R: GTGCTCTCCTCTGATTCTTCTTC), ORF29 (F: GGCGGAACTTTCGTAACCAA; R: CCCCATTAAACAGGTCAACAAAA), and ORF68 (same as DNA primers, above).
Plaque area measurement
VZV-infected cultures were cultured in the presence or absence of indicated cytokine as detailed above, then fixed at 21 dpi and stained for viral gE. Images of infected neurons were captured as above and area of infection was measured by manually outlining VZV antigen-positive neurons using Cellsense software.
VZV titration
To determine production of infectious virus, a single well of a 12-well plate containing VZV-infected neurons (5×105 cells; with or without cytokine treatment) was harvested at 21 dpi by brief trypsinization and pipetted vigorously to disperse neurons into a single cell suspension. The entire neuron suspension was added to 40% confluent monolayers of HFL cells and co-cultured for 7 days, at which time cells were fixed and stained using 0.1% crystal violet in 70% ethanol/1X PBS and plaques counted.
Statistical analysis
Statistical significance of mean plaque size and DNA inhibition were determined using one-way analysis of variance (ANOVA) between untreated and treated groups. Statistical significance of mRNA transcription inhibition was determined using a 2-way ANOVA (treatment x ORF) to compare treatments between different ORFs. The subsequent significant effect of treatments was followed by Fisher’s LSD tests to compare untreated groups with all subsequent treatments. All statistical tests were set at a 0.05-α level and conducted using Prism 7 (GraphPad software, La Jolla, CA).
Acknowledgments
We thank Dr. Andrew Bubak for assistance with figure preparation and statistical analysis, Marina Hoffman for editorial review, and Cathy Allen for manuscript preparation.
Funding Sources
This work was supported by Public Health Service grants NS093716 (N.L.B.), AG032958 (R.J.C.) and NS092228 (R.J.C.) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP, 2001. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci 4, 1116–1122. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Sharp M, Maecker HT, Maino VC, Arvin AM, 2000. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J. Infect. Dis 181, 859–866. [DOI] [PubMed] [Google Scholar]
- Baird NL, Bowlin JL, Cohrs RJ, Gilden D, Jones KL, 2014a. Comparison of varicella-zoster virus RNA sequences in human neurons and fibroblasts. J. Virol 88, 5877–5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NL, Bowlin JL, Hotz TJ, Cohrs RJ, Gilden D, 2015. Interferon Gamma Prolongs Survival of Varicella-Zoster Virus-Infected Human Neurons In Vitro. J. Virol 89, 7425–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NL, Bowlin JL, Yu X, Jonjic S, Haas J, Cohrs RJ, Gilden D, 2014b. Varicella zoster virus DNA does not accumulate in infected human neurons. Virology 458–459, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocker W, Docheva D, Prall WC, Egea V, Pappou E, Rossmann O, Popov C, Mutschler W, Ries C, Schieker M, 2008. IKK-2 is required for TNF-alpha-induced invasion and proliferation of human mesenchymal stem cells. J. Mol. Med. (Berl.) 86, 1183–1192. [DOI] [PubMed] [Google Scholar]
- Cacciapaglia F, Zuccaro C, Iannone F, 2015. Varicella-zoster virus infection in rheumatoid arthritis patients in the anti-tumour necrosis factor era. Clin. Exp. Rheumatol 33, 917–923. [PubMed] [Google Scholar]
- Dopp JM, Sarafian TA, Spinella FM, Kahn MA, Shau H, de Vellis J, 2002. Expression of the p75 TNF receptor is linked to TNF-induced NFkappaB translocation and oxyradical neutralization in glial cells. Neurochem. Res 27, 1535–1542. [DOI] [PubMed] [Google Scholar]
- Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, Illei GG, Drappa J, Wang L, Yoo S, 2017. Anifrolumab, an Anti-Interferon-alpha Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol 69, 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Doval I, Perez-Zafrilla B, Descalzo MA, Rosello R, Hernandez MV, Gomez-Reino JJ, Carmona L, Group BS, 2010. Incidence and risk of hospitalisation due to shingles and chickenpox in patients with rheumatic diseases treated with TNF antagonists. Ann. Rheum. Dis 69, 1751–1755. [DOI] [PubMed] [Google Scholar]
- Gershon AA, Chen J, Davis L, Krinsky C, Cowles R, Reichard R, Gershon M, 2012. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans. Am. Clin. Climatol. Assoc 123, 17–33; discussion 33–15. [PMC free article] [PubMed] [Google Scholar]
- Gilden D, White T, Khmeleva N, Boyer PJ, Nagel MA, 2016. VZV in biopsy-positive and -negative giant cell arteritis: Analysis of 100+ temporal arteries. Neurol Neuroimmunol Neuroinflamm 3, e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden DH, Rozenman Y, Murray R, Devlin M, Vafai A, 1987. Detection of varicella-zoster virus nucleic acid in neurons of normal human thoracic ganglia. Ann. Neurol 22, 377–380. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Vafai A, Shtram Y, Becker Y, Devlin M, Wellish M, 1983. Varicella-zoster virus DNA in human sensory ganglia. Nature 306, 478–480. [DOI] [PubMed] [Google Scholar]
- Gowrishankar K, Steain M, Cunningham AL, Rodriguez M, Blumbergs P, Slobedman B, Abendroth A, 2010. Characterization of the host immune response in human Ganglia after herpes zoster. J. Virol 84, 8861–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell M, Becke FM, Wajant H, Mannel DN, Scheurich P, 1998. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur. J. Immunol 28, 257–263. [DOI] [PubMed] [Google Scholar]
- Grose C, Yu X, Cohrs RJ, Carpenter JE, Bowlin JL, Gilden D, 2013. Aberrant virion assembly and limited glycoprotein C production in varicella-zoster virus-infected neurons. J. Virol 87, 9643–9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann HP, Remy R, Brockhaus M, van Loon AP, 1989. Two different cell types have different major receptors for human tumor necrosis factor (TNF alpha). J. Biol. Chem 264, 14927–14934. [PubMed] [Google Scholar]
- Irwin MW, Mak S, Mann DL, Qu R, Penninger JM, Yan A, Dawood F, Wen WH, Shou Z, Liu P, 1999. Tissue expression and immunolocalization of tumor necrosis factor-alpha in postinfarction dysfunctional myocardium. Circulation 99, 1492–1498. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakano T, Kamiya T, Kitamura K, Ihara T, Kamiya H, Sakurai M, 1991. Effects of tumor necrosis factor alpha on replication of varicella-zoster virus. Antiviral Res. 15, 183–192. [DOI] [PubMed] [Google Scholar]
- Jones D, Alvarez E, Selva S, Gilden D, Nagel MA, 2016. Proinflammatory cytokines and matrix metalloproteinases in CSF of patients with VZV vasculopathy. Neurol Neuroimmunol Neuroinflamm 3, e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamashta M, Merrill JT, Werth VP, Furie R, Kalunian K, Illei GG, Drappa J, Wang L, Greth W, 2016. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis 75, 1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig HC, Garland JM, Weissman D, Mounzer K, 2013. Vaccinating HIV patients: focus on human papillomavirus and herpes zoster vaccines. AIDS Rev 15, 77–86. [PubMed] [Google Scholar]
- Leong KG, Karsan A, 2000. Signaling pathways mediated by tumor necrosis factor alpha. Histol. Histopathol 15, 1303–1325. [DOI] [PubMed] [Google Scholar]
- Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, Chan IS, Li DJ, Wang W, Keller PM, Shaw A, Silber JL, Schlienger K, Chalikonda I, Vessey SJ, Caulfield MJ, 2003. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J. Infect. Dis 188, 1336–1344. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Wolf W, Dueland AN, Cohrs R, Vafai A, Gilden D, 1990. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N. Engl. J. Med 323, 627–631. [DOI] [PubMed] [Google Scholar]
- Martinez-Taboada VM, Alvarez L, RuizSoto M, Marin-Vidalled MJ, Lopez-Hoyos M, 2008. Giant cell arteritis and polymyalgia rheumatica: role of cytokines in the pathogenesis and implications for treatment. Cytokine 44, 207–220. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG, 2008. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J. Neuroinflammation 5, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin G, Falcoff E, Aguet M, 1985. 125I-labelled human interferons alpha, beta and gamma: comparative receptor-binding data. J. Gen. Virol 66 ( Pt 5), 1149–1152. [DOI] [PubMed] [Google Scholar]
- Minassian C, Thomas SL, Smeeth L, Douglas I, Brauer R, Langan SM, 2015. Acute Cardiovascular Events after Herpes Zoster: A Self-Controlled Case Series Analysis in Vaccinated and Unvaccinated Older Residents of the United States. PLoS Med. 12, e1001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourgues C, Henquell C, Tatar Z, Pereira B, Nourisson C, Tournadre A, Soubrier M, Couderc M, 2016. Monitoring of Epstein-Barr virus (EBV)/cytomegalovirus (CMV)/varicella-zoster virus (VZV) load in patients receiving tocilizumab for rheumatoid arthritis. Joint Bone Spine 83, 412–415. [DOI] [PubMed] [Google Scholar]
- Rosato PC, Leib DA, 2015. Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis. PLoS Pathog. 11, e1005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Thakur K, Venkatesan A, 2015. Acute encephalitis in the immunocompromised individual. Curr. Opin. Infect. Dis 28, 330–336. [DOI] [PubMed] [Google Scholar]
- Song R, Koyuncu OO, Greco TM, Diner BA, Cristea IM, Enquist LW, 2016. Two Modes of the Axonal Interferon Response Limit Alphaherpesvirus Neuroinvasion. MBio 7, e02145–02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, Zink A, 2009. Risk of Herpes Zoster in Patients With Rheumatoid Arthritis Treated With Anti-TNF-alpha Agents. Jama-Journal of the American Medical Association 301, 737–744. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA Jr., Goeddel DV, 1991. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc. Natl. Acad. Sci. U. S. A 88, 9292–9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe S, Ihara T, Kamiya H, 2000. IL-12, IFN-gamma, and TNF-alpha released from mononuclear cells inhibit the spread of varicella-zoster virus at an early stage of varicella. Microbiol. Immunol 44, 1027–1031. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Levin MJ, 2010. VZV T cell-mediated immunity. Curr. Top. Microbiol. Immunol 342, 341–357. [DOI] [PubMed] [Google Scholar]
- Yang L, Lindholm K, Konishi Y, Li R, Shen Y, 2002. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J. Neurosci 22, 3025–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn BP, Wollan PC, Nagel MA, Gilden D, 2016. Risk of Stroke and Myocardial Infarction After Herpes Zoster in Older Adults in a US Community Population. Mayo Clin. Proc 91, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Seitz S, Pointon T, Bowlin JL, Cohrs RJ, Jonjic S, Haas J, Wellish M, Gilden D, 2013. Varicella zoster virus infection of highly pure terminally differentiated human neurons. J. Neurovirol 19, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cosyns M, Levin MJ, Hayward AR, 1994. Cytokine production in varicella zoster virus-stimulated limiting dilution lymphocyte cultures. Clin. Exp. Immunol 98, 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]


