Introduction
The eosinophil is a leukocyte whose granules stain beautifully and avidly with the acidic red dye eosin (also known as bromoeosin, solvent red 43, 2′,4′,5′,7′-tetrabromofluorescein or C20H8Br4O5). This cell, or a counterpart of this cell, exists in virtually all vertebrates (see Figure 1).1 Even though its existence has only been appreciated for about 150 years, estimates based on evolutionary considerations suggest that the eosinophil lineage is several hundred million years old.2 The fact that this cell type has survived the test of time, including evolutionary pressures, and has been retained as part of innate immunity highlights the important benefits that eosinophils must provide to its host. Yet as clinicians, when elevated numbers show up in a tissue biopsy or on a blood count (typical normal levels <500 per μL, although normal ranges vary from 350 to 600 depending on the lab; eosinophilia defined as an eosinophil count >500 per μL, with the term hypereosinophilia reserved for eosinophil counts >1,500 per μL), distinct subsets of disorders come to mind, so for most of us, this enigmatic cell is instead more often thought of as a troublemaker. Nevertheless, the purpose of this review is to highlight the biology of eosinophils in both sickness and in health. The focus will primarily be restricted to what is known about the useful things eosinophils do, while at the same time providing an update on our current thinking regarding pathogenesis of eosinophil-related disorders, primarily in adults. For those interested in exploring this general topic even further, it has been the subject of one wide-ranging textbook3 and several reviews.4–7 And for those seeking comprehensive reviews on diagnosis and treatment of eosinophil-related diseases, the reader is referred elsewhere.8–13
Figure 1.
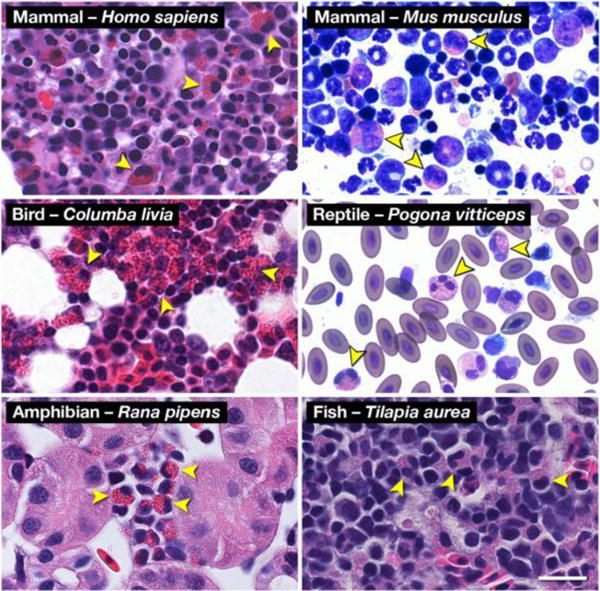
Hematoxylin-eosin (H&E) and Romanowsky-dye (R&D) stained preparations of hematopoietic tissues from representative animals of the five (5) classes of Vertebrata reveal the ubiquitous presence of a uniquely eosinophilic lineage in this sub-phylum. Leukocytes displaying the unique polymorphonucleus and the eosin-binding cytoplasmic granules characteristic of eosinophils are identifiable (arrowheads) in Mammalia (Homo sapiens (human, H&E) and Mus musculus (mouse, R&D)), Aves (Columba livia (rock pigeon, H&E)), Reptilia (Pogona vitticeps (Bearded Dragon, R&D)), Amphibia (Rana pipens (leopard frog, H&E)), and Osteichthyes (Tilapia aurea (Tilapia, H&E)). Scale bar = 20μm. Reproduced from 1.
Eosinophil hematopoiesis
To make an eosinophil, you need a few key ingredients within its hematopoietic precursor that allows this granulocyte to develop away from a neutrophil or basophil. Studies in mice and/or humans suggest that these include a unique set of transcription factors, including C/EBPε, GATA-1, PU.1, Helios, Aiolos and XBP1 and without them, eosinophils fail to develop (Figure 2).14 Similarly, the eosinophil lineage is dependent on the appearance of a specific receptor on its surface for the cytokine interleukin-5 (IL-5), namely the IL-5 receptor. This heterodimer consists of a specific α chain (CD125, also found on basophils) and a β chain common to the IL-5 receptor (CD131), the latter also part of the receptors for IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF). Surface expression of the IL-5 receptor is one of the earliest eosinophil-specific lineage-commitment events that occurs within the bone marrow, although recent data in mice suggest that another cytokine, IL-33, plays a previously unappreciated role in enhancing eosinophil differentiation at a point upstream of IL-5’s effects.15 Given this pivotal and selective role of the IL-5 receptor, it is not surprising that the recently approved biologic benralizumab, which targets the α chain of the IL-5 receptor for antibody-dependent cellular cytotoxicity and NK cell depletion, is particularly effective at eliminating eosinophils.16 While the source of IL-5 and IL-33 within the bone marrow needed to make eosinophils remains controversial, it is likely that T cells and certain innate lymphoid cells (ILC2) are important sources, at least for IL-5.17, 18
Figure 2.
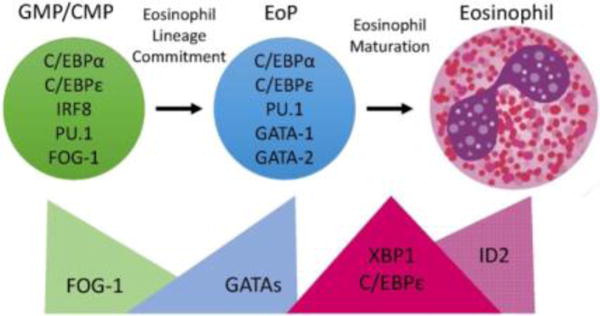
Transcription Factor (TF) expression during eosinophil development. Eosinophils differentiate in the bone marrow from an eosinophil lineage-committed progenitor (EoP) that is derived from the granulocyte/macrophage progenitor (GMP) in mice and the common myeloid progenitor (CMP) in humans. For eosinophil lineage commitment to occur, the myeloid progenitor (GMP or CMP) must express C/EBPα, C/EBPε, interferon regulatory factor 8 (IRF8), and PU.1. Expression of friend of GATA-1 (FOG-1) declines, allowing for increasing expression and activity of GATA TFs, which is necessary for EoP production. Following lineage commitment, eosinophil granule protein gene expression is markedly increased with the collaborative interaction between C/EBPε, PU.1, and GATA-1. To assist with the elevated granule protein synthesis in the EoP and eosinophil precursors, XBP1 expression is increased and promotes survival during the demanding maturation process. Expression of activator isoforms of C/EBPε peaks during eosinophil maturation and then declines during the final stages. Expression of ID2 increases during eosinophil maturation and enhances the rate of maturation. Reproduced from reference 14.
Eosinophil Diaspora and Lifespan
Eosinophils leave the bone marrow as terminally differentiated cells incapable of further division and enter the circulation via steps that include alterations in their adhesive and migratory properties.19 Under normal conditions, once in the circulation, their life-span is about 24 hours in humans, which is about twice as long as it is for neutrophils.20 Some eosinophils appear to traffic to the liver and spleen, but the main site of eosinophil accumulation and residence within the body under homestasis is the gastrointestinal tract from stomach to intestine. Normally, there are very few if any eosinophils in the upper or lower airways or in the esophagus. Extravasation out of the circulation into tissue sites is particularly dependent on the function of β1 and β2 integrin adhesion molecules and their endothelial and tissue counter-receptors vascular cell adhesion molecule-1 (VCAM-1), mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and intercellular cell adhesion molecule-1 (ICAM-1), as well as eosinophil-selective chemoattractants including those that active the CCR3 chemokine receptor such as the eotaxins.21 After entering tissues, it is estimated that eosinophils normally survive only a few days, but at sites of ongoing inflammation, where GM-CSF or other pro-survival cytokines are being produced by tissue-resident cells and perhaps even eosinophils themselves, eosinophils can persist for weeks.22, 23
Helpful Eosinophil Functions in Homeostasis and Host Defense
Much of what we know about the role of eosinophils in homeostasis and health is based on data from animal studies. While there are reports of seemingly healthy individuals who appear to lack eosinophils24 and mice genetically engineered to be “eosinophil knockout” animals have no obvious aberrant phenotype,25–27 the current doctrine would suggest that it’s a good thing to have some eosinophils. As summarized in Table 1, eosinophils are felt to contribute a range of beneficial substances that aid in tissue development, remodeling and repair, especially at sites of barriers such as epithelial surfaces.23, 28–30 Their roles in both innate and adaptive immune responses include favorably influencing immune cell development, providing anti-bacterial, anti-fungal and anti-viral responses, and helping to control glucose metabolism, myocyte regeneration, brown fat development and adiposity.1, 4, 5, 7, 23, 31–33 Many of these concepts are mainly rooted in data from animal models, with their translational relevance still uncertain. And despite the seemingly dogmatic, important role of eosinophils during type 2 immune responses to helminths and other parasitic infections, even this concept remains controversial.34 One way to illustrate this point is to examine the safety portion of the package insert (https://www.azpicentral.com/fasenra/fasenra_pi.pdf#page=1, accessed on January 16, 2018) for benralizumab (Fansenra™): “Eosinophils may be involved in the immunological response to some helminth infections. Patients with known helminth infections were excluded from participation in clinical trials. It is unknown if FASENRA will influence a patient’s response against helminth infections. Treat patients with preexisting helminth infections before initiating therapy with FASENRA. If patients become infected while receiving treatment with FASENRA and do not respond to anti-helminth treatment, discontinue treatment with FASENRA until infection resolves.” So while there is a growing interest in what eosinophils actually do to help the host, this area remains ripe for more research.35
Table 1.
Examples of purported roles for eosinophils in health and disease.
| Eosinophils in Health
|
Eosinophils in Disease: complications
|
|---|---|
| Contribute growth factors and matrix metalloproteases for tissue development, remodeling and repair Help maintain mucosal surfaces with high levels of epithelial turnover (e.g., intestine, endometrium) Thymic and plasma cell development Anti-helminth defenses (e.g., via release of granule proteins) Anti-viral responses (e.g., via RNAses in released granule proteins) Anti-bacterial responses (e.g., via release of mitochondrial DNA traps) Anti-fungal immunity (e.g., via recognition of fungal glycans) Control of adiposity (e.g., via release of cytokines that infuence other cells to enhance glucose metabolism and levels of “beneficial” beige fat) |
Tissue injury and inflammation
|
Abbreviations used: EGID, eosinophilic gastrointestinal disorders; IBD, inflammatory bowel disease
Eosinophil-Related Diseases
With the exception of subsets of asthma and atopic dermatitis, eosinophil-related diseases including eosinophilic esophagitis are uncommon, and most would satisfy the definition of a rare disease, namely a prevalence of ≤ 200,000 cases in the United States (Table 2). Eosinophil-related diseases consist of a group of disorders where eosinophils are felt to cause disease, and where there is accompanying evidence of increased blood and/or tissue eosinophils with or without evidence of their activation. While it is common to characterize these as either “primary” (e.g., hypereosinophilic syndromes, HES) or “secondary” or “reactive” (e.g., parasitic diseases), among the shortcomings of this approach to disease categorization is that for many of them, we remain unsure as to what exactly the eosinophil involvement actually represents, except where treating an underlying disease (parasitism, drug reactions) should routinely cause the eosinophilia to resolve. So while this categorization can be useful, it is probably best to think of eosinophil-related diseases as a spectrum of disorders ranging from those that are more systemic versus those that are distinguished by a more predictable pattern of organ specificity (see Table 2).35–37 And even within the realm of eosinophil-related diseases, the location, type of tissue injury and magnitude of eosinophilia varies widely. Under inflammatory conditions, eosinophil numbers in the circulation or in tissues can expand greatly, and the development of eosinophilia can be rapid. For example, allergen provocation of the airway can result in blood eosinophilia within hours of the challenge. Often, the total eosinophil count in the blood can provide useful information when initially considering the differential diagnosis and subsequent workup for patients presenting with eosinophilia or hypereosinophilia (Figure 3). Extremely high total eosinophil counts (e.g., >5,000/μL) have a much narrower list of possible causes, while many eosinophil-related diseases can present with a normal blood total eosinophil count (e.g., eosinophilic esophagitis). Therefore, the general approach to a patient suspected of having an eosinophil-related disease should include consideration of a customized diagnostic investigation based on signs and symptoms of organ involvement, and determining what tissue, if any, to biopsy. When faced with a patient with persistent hypereosinophilia, the differential diagnosis can be narrowed based on the clinical presentation (Figure 3).8 For example, suspicion for eosinophilic granulomatosis with polyangiitis (EGPA, formerly known as Churg Strauss syndrome, one of the “overlap” forms of HES) should arise if there is accompanying asthma, often with sinus disease, and evidence of involvement of other organs such as nerves, skin or the gastrointestinal system. Primary skin involvement in the setting of hypereosinophilia, such as a pruritic macular rash with or without angioedema, or even severe pruritus without rash, should result in the consideration of HES, including the lymphocytic variant of HES. The finding of lymphadenopathy and/or splenomegaly with hypereosinophilia would suggest the myeloid variant of HES. Defining the type of HES, using specific blood testing and biopsies of involved organs including the bone marrow, then dictates both prognosis and specific treatments.9, 13 Thus, treatments range from the use of systemic corticosteroids to tyrosine kinase inhibitors for specific variants of myeloid HES to biologics (e.g., mepolizumab for EGPA38 or idiopathic HES39) to interferon-α, mepolizumab or immunosuppressive agents (for idiopathic and lymphocytic forms of HES40, 41) to hydroxyurea (for idiopathic and myeloid HES42), and most recently JAK inhibitors (for myeloid and lymphocytic forms of HES43).
Table 2.
Examples of some eosinophil-associated diseases
| Mainly organ-specific involvement or cause |
| Skin: atopic dermatitis, bullous pemphigoid |
| Lung: asthma, allergic bronchopulmonary aspergillosis |
| Eye: atopic keratoconjunctivitis |
| Sinuses: nasal polyposis |
| Adrenal glands: hypoadrenalism |
| Gastrointestinal tract: eosinophilic esophagitis, gastritis and colitis; inflammatory bowel disease |
| Multi-organ involvement |
| Helminth, protozoan, ectoparasite and fungal infections |
| Drug reactions |
| Leukemia, lymphoma |
| Immune deficiencies such as those associated with Omenn syndrome and various forms of Hyper-IgE syndrome |
| Autoimmune diseases including eosinophilic granulomatosis with polyangiitis, sarcoidosis and IgG4 disease |
| Hypereosinophilic syndromes |
Figure 3.
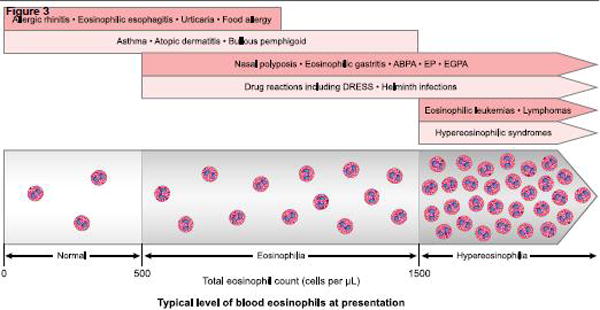
Potential diagnosis based on the total blood eosinophil count at presentation. This general approach is not designed to provide absolute cutoffs, but instead is meant to help narrow down the list of likely diagnoses, which can then be further delineated based on history, physical exam and additional testing.
Abbreviations used: ABPA, allergic bronchopulmonary aspegillosis; EP, eosinophilic pneumonia; EGPA, eosinophilic granulomatosis with polyangiitis, formerly known as Churg-Strauss syndrome; DRESS, drug reaction with eosinophilia and systemic symptoms. Artwork by Jacqueline Schaffer.
Pharmacologic Manipulation of Eosinophils: What has this taught us about the role of eosinophils in health and disease?
Separate from the discussion of the normal lifespan of the eosinophil, it is important to briefly review ways to actively eliminate eosinophils via pharmacology, and how this influences our knowledge of the role of eosinophils in sickness and in health. Best known and most used among these treatment approaches are glucocorticosteroids, which reduce circulating and tissue eosinophils within hours via mechanisms that include direct activation of eosinophil death.44 Under certain circumstances, however, eosinophils can become resistant to these drugs, such as in myeloproliferative forms of hypereosinophilic syndrome45 or in the presence of high levels of eosinophil-survival cytokines like IL-5, at least in vitro. With the administration of anti-IL-5 antibodies like mepolizumab or reslizumab, blood eosinophils typically decline by ≥80% within days, but do not necessarily decline as much in tissues, such as the lower airway in asthma, presumably because other eosinophil pro-survival cytokines like GM-CSF might still be active at sites of tissue eosinophilia.46–48 By comparison, administration of the anti-IL-5 receptor antibody benralizumab causes a more profound and prolonged active depletion of eosinophils due to antibody-dependent cellular cytotoxicity.16, 49 These anti-eosinophil biological agents are steroid-sparing in asthma and eosinophilic granulomatosis with polyangiitis.38, 50, 51 And it is worth noting that the targeting of eosinophils in eosinophilic asthma reduces exacerbations52 and may improve airway remodeling through mechanisms that remain elusive,53 even though its effects on lung function are variable and generally fail to alter airways hyperreactivity.48, 52, 54 So far, data from multi-year use suggest they are safe in that chronic reductions of eosinophils in asthma and hypereosinophilc syndromes appears well tolerated,41, 55 yet unexpected issues such as herpes zoster have appeared in very small numbers during asthma trials with some anti-eosinophil agents such as mepolizumab but not others, so whether rare side effects will emerge with chronic eosinopenia remains to be determined.
Studies of hypereosinophilic syndrome and eosinophilic esophagitis provide us with additional opportunities to gain insight into the role of eosinophils in these conditions. While mepolizumab was shown to have corticosteroid-sparing properties in certain hypereosinophilic syndromes,39, 56 it was the paradigm-changing discovery that some forms of hypereosinophilic syndrome are “cured” with imatinib, a tyrosine kinase inhibitor, because of gain-of-function molecular rearrangements such as the FIP1L1-PDGFRA fusion gene resulting from an interstitial deletion of a portion of chromosome 4. This is a wonderful example of how pharmacology completely and definitively redefined one subset of hypereosinophilic syndrome into that of a form of eosinophilic leukemia.57–59 In contrast, the clinical effects of mepolizumab and reslizumab in eosinophilic esophagitis have so far been inconsistent and mostly disappointing despite reducing esophageal mucosal eosinophil counts (but not homeostatic numbers of eosinophils in the duodenal mucosa).60, 61 In nasal polyposis, the role of eosinophils is still evolving. For instance, in asthma trials, the subgroup with nasal polyposis was more likely to show a greater benefit from reslizumab.62 When specifically studied for effects on nasal polyposis per se, mepolizumab had some clinical benefit, but it was neither profound nor consistent across all subjects.63 The same can be said for dexpramipexole, an oral small molecule that was fortuitously discovered to selectively reduce blood eosinophil counts.64 When tested in chronic rhinosinusitis with nasal polyposis, >90% reductions were seen in blood and nasal tissue eosinophils, but clinical outcome measures were disappointing.65 It will nonetheless be interesting to see what impact benralizumab and other anti-eosinophil drugs will have on these and other eosinophil-related diseases over time.
Other pharmacologic agents are teaching us more about eosinophils in sickness too. Small molecules targeting CCR3, a chemokine receptor selectively expressed on eosinophils, have so far proved ineffective in asthma,66 bringing into question the role of eotaxins in this disease. A phase 2 clinical trial in subjects with inadequately controlled asthma using a biologic targeting GM-CSF failed to show efficacy.67 In contrast, given their involvement in type 2 inflammation and in eosinophil recruitment, excitement is building for drugs targeting cytokines like IL-4, IL-13 and their shared IL-4 receptor α subunit. For example, dupilumab has shown efficacy in eosinophil-related diseases such as atopic dermatitis, asthma, nasal polyposis and eosinophilic esophagitis.68–71 Whether its benefits in these conditions are teaching us anything definitive about the role of eosinophils in each condition remains uncertain. Interestingly, a worsening of blood eosinophilia can be seen with dupilumab, reminiscent of what is observed during treatment with the anti α4 integrin biologic natalizumab72 but not the anti α4β7 integrin biologic vedolizumab,73 suggesting that IL-4 receptor α and α4β1 integrins, but not α4β7 integrins, are involved in regulating eosinophil trafficking under homeostatic and inflammatory conditions.19, 21 As more drugs are developed that either directly74, 75 or indirectly target eosinophils,76–78 we should learn even more about their role in specific diseases.
Conclusion
Recent advances have both highlighted and expanded our knowledge of the role of eosinophils in health and disease. Many intriguing findings from mouse models remain to be verified in humans. The availability of new drugs, especially highly specific biologics targeting eosinophils or IL-5, are providing us with the precise tools needed to do pharmacologic hypothesis testing in humans regarding the benefits and risks of reducing eosinophils on a chronic basis. Such opportunities will undoubtedly inform and shape our evolving understanding of the contribution of the enigmatic eosinophil in sickness and in health.
Key messages.
Eosinophils are ancient cells, having survived evolutionary pressures among virtually all vertebrates, which suggests that they provide important functions to their hosts.
Eosinophils likely contribute beneficially to most forms of immunity including viral, bacterial, fungal and parasitic diseases.
The degree of blood eosinophilia or hypereosinophilia can be helpful during initial considerations of a differential diagnosis.
Newly approved biologic agents for asthma that reduce or even eliminate eosinophils have generally been well tolerated, not just in in asthma but in other chronic eosinophil-associated diseases, suggesting that reductions in eosinophil numbers achieved by such agents is relatively innocuous.
Acknowledgments
The author thanks Dr. Sergejs Bernikovs for helpful input, and Jacqueline Schaffer for artwork in Figure 2.
This work was supported by grants AI72265 and HL107151 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Sanofi-Aventis, GSK, TEV: consultant
Allakos, Inc.: SAB, stockholder
Elsevier, UpToDate: editor
NCT – not applicable
References
- 1.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGarry MP. The evolutionary origins and presence of eosinophils in extant species. In: Lee JJ, Rosenberg HF, editors. Eosinophils in Health and Disease. Amsterdam: Elsevier; 2012. pp. 13–18. [Google Scholar]
- 3.Lee JJ, Rosenberg HF, editors. Eosinophils in Health and Disease. Amsterdam: Elsevier; 2012. [Google Scholar]
- 4.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120:3882–3890. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klion A. Recent advances in understanding eosinophil biology. F1000Res. 2017;6:1084. doi: 10.12688/f1000research.11133.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Sullivan JA, Bochner BS. Eosinophils and eosinophil-associated diseases: An update. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.09.022. doi: 101016/jjaci201709022 [Epub ahead of print[ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klion AD. Eosinophilia: a pragmatic approach to diagnosis and treatment. Hematology Am Soc Hematol Educ Program. 2015;2015:92–97. doi: 10.1182/asheducation-2015.1.92. [DOI] [PubMed] [Google Scholar]
- 9.Klion AD. How I treat hypereosinophilic syndromes. Blood. 2015;126:1069–1077. doi: 10.1182/blood-2014-11-551614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legrand F, Klion AD. Biologic therapies targeting eosinophils: current status and future prospects. J Allergy Clin Immunol Pract. 2015;3:167–174. doi: 10.1016/j.jaip.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radonjic-Hoesli S, Valent P, Klion AD, Wechsler ME, Simon HU. Novel targeted therapies for eosinophil-associated diseases and allergy. Annu Rev Pharmacol Toxicol. 2015;55:633–656. doi: 10.1146/annurev-pharmtox-010814-124407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochner BS. Novel therapies for eosinophilic disorders. Immunol Allergy Clin North Am. 2015;35:577–598. doi: 10.1016/j.iac.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuang FL, Klion AD. Biologic agents for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol Pract. 2017;5:1502–1509. doi: 10.1016/j.jaip.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulkerson PC. Transcription factors in eosinophil development and as therapeutic targets. Front Med (Lausanne) 2017;4:115. doi: 10.3389/fmed.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston LK, Hsu CL, Krier-Burris RA, et al. IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol. 2016;197:3445–3453. doi: 10.4049/jimmunol.1600611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125:1237–1244. doi: 10.1016/j.jaci.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Sehmi R, Wood LJ, Watson R, et al. Allergen-induced increases in IL-5 receptor alpha-subunit expression on bone marrow-derived CD34+ cells from asthmatic subjects. J Clin Invest. 1997;100:2466–2475. doi: 10.1172/JCI119789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson MW. Eosinophil activation status in separate compartments and association with asthma. Front Med (Lausanne) 2017;4:75. doi: 10.3389/fmed.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 20.Farahi N, Singh NR, Heard S, et al. Use of 111-Indium-labeled autologous eosinophils to establish the in vivo kinetics of human eosinophils in healthy subjects. Blood. 2012;120:4068–4071. doi: 10.1182/blood-2012-07-443424. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Bochner BS. 6.3 Eosinophil trafficking. In: Rosenberg HF, Lee JJ, editors. Eosinophils in Health and Disease. Amsterdam: Elsevier; 2012. pp. 131–139. [Google Scholar]
- 22.Zimmermann N, Rothenberg ME. Mechanism of enhanced eosinophil survival in inflammation. Blood. 2015;125:3831–3832. doi: 10.1182/blood-2015-04-640623. [DOI] [PubMed] [Google Scholar]
- 23.Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017;17:746–760. doi: 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gleich GJ, Klion AD, Lee JJ, Weller PF. The consequences of not having eosinophils. Allergy. 2013;68:829–835. doi: 10.1111/all.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu C, Cantor AB, Yang H, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 27.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg HF, Dyer KD, Domachowske JB. Eosinophils and their interactions with respiratory virus pathogens. Immunol Res. 2009;43:128–137. doi: 10.1007/s12026-008-8058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marichal T, Mesnil C, Bureau F. Homeostatic eosinophils: characteristics and functions. Front Med (Lausanne) 2017;4:101. doi: 10.3389/fmed.2017.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gieseck RL, 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 31.Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242:161–177. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343:57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heredia JE, Mukundan L, Chen FM, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strandmark J, Rausch S, Hartmann S. Eosinophils in homeostasis and their contrasting roles during inflammation and helminth infections. Crit Rev Immunol. 2016;36:193–238. doi: 10.1615/CritRevImmunol.2016018726. [DOI] [PubMed] [Google Scholar]
- 35.Bochner BS, Book W, Busse WW, et al. Workshop report from the National Institutes of Health Taskforce on the Research Needs of Eosinophil-Associated Diseases (TREAD) J Allergy Clin Immunol. 2012;130:587–596. doi: 10.1016/j.jaci.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valent P, Gleich GJ, Reiter A, et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev Hematol. 2012;5:157–176. doi: 10.1586/ehm.11.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607–612 e609. doi: 10.1016/j.jaci.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376:1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–1228. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 40.Butterfield JH. Treatment of hypereosinophilic syndromes with prednisone, hydroxyurea, and interferon. Immunol Allergy Clin North Am. 2007;27:493–518. doi: 10.1016/j.iac.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Roufosse FE, Kahn JE, Gleich GJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. 2013;131:461–467. doi: 10.1016/j.jaci.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: A multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124:1319–1325. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King B, Lee AI, Choi J. Treatment of hypereosinophilic syndrome with cutaneous involvement with the jak inhibitors tofacitinib and ruxolitinib. J Invest Dermatol. 2017;137:951–954. doi: 10.1016/j.jid.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends in immunology. 2013;34:398–409. doi: 10.1016/j.it.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Khoury P, Abiodun AO, Holland-Thomas N, Fay MP, Klion AD. Hypereosinophilic syndrome subtype predicts responsiveness to glucocorticoids. The journal of allergy and clinical immunology In practice. 2018;6:190–195. doi: 10.1016/j.jaip.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menzies-Gow A, Flood-Page P, Sehmi R, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111:714–719. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 47.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 48.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol. 2015;135:299–310. doi: 10.1016/j.jaci.2014.12.1871. [DOI] [PubMed] [Google Scholar]
- 49.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 50.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 51.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 52.Katial RK, Bensch GW, Busse WW, et al. Changing paradigms in the treatment of severe asthma: the role of biologic therapies. J Allergy Clin Immunol Pract. 2017;5:S1–S14. doi: 10.1016/j.jaip.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 53.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of extracellular matrix proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters SP, Busse WW. New and anticipated therapies for severe asthma. The journal of allergy and clinical immunology In practice. 2017;5:S15–S24. doi: 10.1016/j.jaip.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Murphy K, Jacobs J, Bjermer L, et al. Long-term safety and efficacy of reslizumab in patients with eosinophilic asthma. The journal of allergy and clinical immunology In practice. 2017;5:1572–1581. doi: 10.1016/j.jaip.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 56.Roufosse F, de Lavareille A, Schandene L, et al. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126:828–835 e823. doi: 10.1016/j.jaci.2010.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 58.Klion AD, Noel P, Akin C, et al. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood. 2003;101:4660–4666. doi: 10.1182/blood-2003-01-0006. [DOI] [PubMed] [Google Scholar]
- 59.Khoury P, Desmond R, Pabon A, et al. Clinical features predict responsiveness to imatinib in platelet-derived growth factor receptor-alpha-negative hypereosinophilic syndrome. Allergy. 2016;71:803–810. doi: 10.1111/all.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conus S, Straumann A, Bettler E, Simon HU. Mepolizumab does not alter levels of eosinophils, T cells, and mast cells in the duodenal mucosa in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:175–177. doi: 10.1016/j.jaci.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 61.Schoepfer AM, Straumann A, Safroneeva E. Pharmacologic treatment of eosinophilic esophagitis: an update. Gastrointest Endosc Clin N Am. 2018;28:77–88. doi: 10.1016/j.giec.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 63.Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–995. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 64.Dworetzky SI, Hebrank GT, Archibald DG, Reynolds IJ, Farwell W, Bozik ME. The targeted eosinophil-lowering effects of dexpramipexole in clinical studies. Blood Cells Mol Dis. 2017;63:62–65. doi: 10.1016/j.bcmd.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Prussin C, Laidlaw TM, Panettieri RA, et al. Dexpramipexole effectively lowers blood and tissue eosinophils in subjects with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2017;139:AB64. abstr. [Google Scholar]
- 66.Neighbour H, Boulet LP, Lemiere C, et al. Safety and efficacy of an oral CCR3 antagonist in patients with asthma and eosinophilic bronchitis: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2014;44:508–516. doi: 10.1111/cea.12244. [DOI] [PubMed] [Google Scholar]
- 67.Molfino NA, Kuna P, Leff JA, et al. Phase 2, randomised placebo-controlled trial to evaluate the efficacy and safety of an anti-GM-CSF antibody (KB003) in patients with inadequately controlled asthma. BMJ Open. 2016;6:e007709. doi: 10.1136/bmjopen-2015-007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469–479. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 69.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 70.Beck LA, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 71.Hirano I, Dellon ES, Hamilton JD, et al. Dupilumab efficacy and safety in adult patients with active eosinophilic esophagitis: a randomized double-blind placebo-controlled phase 2 trial. United Eur Gastro J. 2017;5(Suppl 1) [Google Scholar]
- 72.Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 73.Fedyk ER, Wyant T, Yang LL, et al. Exclusive antagonism of the α4β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflammatory bowel diseases. 2012;18:2107–2119. doi: 10.1002/ibd.22940. [DOI] [PubMed] [Google Scholar]
- 74.Legrand F, Tomasevic N, Simakova O, et al. The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J Allergy Clin Immunol. 2014;133:1439–1447. doi: 10.1016/j.jaci.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bochner BS. “Siglec”ting the allergic response for therapeutic targeting. Glycobiology. 2016;26:546–552. doi: 10.1093/glycob/cww024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barnes N, Pavord I, Chuchalin A, et al. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin Exp Allergy. 2012;42:38–48. doi: 10.1111/j.1365-2222.2011.03813.x. [DOI] [PubMed] [Google Scholar]
- 77.Straumann A, Hoesli S, Bussmann C, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68:375–385. doi: 10.1111/all.12096. [DOI] [PubMed] [Google Scholar]
- 78.Hanania NA, Noonan M, Corren J, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70:748–756. doi: 10.1136/thoraxjnl-2014-206719. [DOI] [PMC free article] [PubMed] [Google Scholar]


