Abstract
The optimization of the enzymatic hydrolysis of rice protein was determined using an experimental design tool. The semi-purified protease of Bacillus licheniformis LBA 46 and commercial protease Alcalase 2.4 L were used to produce rice hydrolysates using pH values ranging from 6 to 10 and enzyme concentrations varying from 50 to 150 U/mL. The optimized conditions were validated, and using the chosen conditions (pH 10 and 100 U/mL of protease), it was possible to confirm that the model was predictive for oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) responses. The experimental values for the ORAC and FRAP responses were 940 and 18.78 TE µmol/g for the rice protein hydrolysates prepared with LBA protease and 1001.94 and 19.31 TE µmol/g for the rice protein hydrolysates prepared with Alcalase 2.4 L. After optimization of the enzymatic hydrolysis conditions, the antioxidant activity values increased when compared to the values for the intact rice protein: 324.97 TE µmol/g (ORAC) and 6.14 TE µmol/g (FRAP). It was also observed that the LBA protease had an action similar to the commercial protease, showing its potential for application in protein hydrolysis.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1401-1) contains supplementary material, which is available to authorized users.
Keywords: Optimization, Contour curve, Rice, Antioxidant, Protease
Introduction
Plants are an excellent source of proteins and bioactive materials, and thus, their hydrolysates can contribute as alternative sources of protein and health-promoting ingredients (Thamnarathip et al. 2016). According to the United States Department of Agriculture—USDA (2017), the global annual production of rice (Oryza sativa L.) is estimated to be approximately 480 million metric tons between the years 2016 and 2017. This grain is also considered the staple food for 3.5 billion people around the world, mainly in Asia, where rice provides 50% of the dietary caloric supply and a relevant amount of the protein intake for millions of people (Muthayya et al. 2014).
Microbial proteases, especially the alkaline proteases, are used in the preparation of high-value protein hydrolysates. Many species of Bacillus sp. produce high quantities of proteases, which are capable of releasing peptides with beneficial properties (Ward et al. 2009), such as peptides with antioxidant functions, resulting in the enzymatic hydrolysis of food protein (Korhonen and Pihlanto 2006; Ricci et al. 2010). The enzymatic hydrolysis is capable of increasing the exposure of antioxidant amino acids causing an improvement in the antioxidant activity of proteins (Elias et al. 2008).
To improve the antioxidant activities of the hydrolysates, it is important to determine the variables that have an important effect on the hydrolysis process. The traditional univariate method of testing is not efficient or cost-effective to assess the effects of the studied variables, pH and enzyme concentration. The response surface methodology (RSM) is an important tool to evaluate the individual and interaction effects of hydrolysis parameters on the progress of enzymatic hydrolysis (Contreras et al. 2011; Vastag et al. 2010). RSM appears to be a better alternative method to use to optimize the process (Phongthai et al. 2016). Multivariate equations can describe the effects of independent variables (test factors) on the dependent variable (response) studied and also determine the relationships between them (Madamba 2002).
The traditional univariate method of testing is not efficient or cost effective to study the effects of the studied variables, pH and enzyme concentration. The response surface methodology (RSM) appears to be a better alternative method to use to optimize the process (Phongthai et al. 2016). Multivariate equations can describe the effects of the independent variables (test factors) on the dependent variable (response) studied and also determine relationships between them (Madamba 2002).
Antioxidants can be derived from natural sources. The bioactive properties of some protein hydrolysates, such as rice protein, are dependent on the process and enzyme employed. The enzymatic hydrolysis of these proteins may lead to an increase in antioxidant capacity, representing an advantage over intact protein use. The use of different methodologies for measuring antioxidant power, such as 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, ferric reducing antioxidant power (FRAP), and oxygen radical absorbance capacity (ORAC), provides important information about the types of oxidizing processes that the hydrolysates could be used against. DPPH is one of the most stable free radicals and is frequently used in the evaluation of antioxidant activity in foods (Burda and Oleszek 2001). FRAP assay has been widely used to evaluate the total antioxidant activity of several foods and plant extracts. ORAC assay is a method to quantify the antioxidant strength of the substances, since the antioxidant needs to be potent enough to prevent the formation of new free radicals that form during the test (Moniruzzaman et al. 2012).
Process optimization is a powerful tool capable of reducing the time required to obtain the required response and some works have related the benefits of using this tool in obtaining bioactive protein hydrolysates with antioxidant characteristics (Castro and Sato 2014b, 2015; Morales-Medina et al. 2017; Seo et al. 2015).
The objective of this study was to establish the most adequate conditions for the production of rice protein hydrolysates, based on the generation or improvement of the antioxidant activities, and to perform a comparative study between proteases from Bacillus licheniformis (LBA and Alcalase 2.4 L), which were used as reaction catalysts.
Materials and methods
Materials
Rice protein concentrate (RPC), 83.20% protein on a dry weight basis, was obtained from Growth Supplements (Brazil). The chemicals azocasein, trichloroacetic acid (TCA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), iron chloride (FeCl3·6H2O), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), 2,20-azobis(2-methylpropionamidine) dihydrochloride (97%) (AAPH), fluorescein and (7)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (Steinheim, Germany). All other reagents and chemicals used were of analytical grade.
Enzymes
The alkaline protease was produced by B. licheniformis LBA 46 and belongs to the culture collection of the Laboratory of Food Biochemistry, School of Food Engineering, University of Campinas. The culture medium used in the fermentation process was composed of 32 g/L sugar cane molasses (Fio de Ouro®); 6 g/L corn steep liquor (Corn Products®); 2 g/L yeast extract (Prodex-Lac SD®) and 20 g/L dried whey (Alibra®) adjusted to a pH of 7 as proposed by Contesini (2014) with slight modifications. The cultivation conditions were 30 °C, 300 rpm and 48 h of fermentation. The crude extract was partially purified using precipitation with 80% ammonium sulfate, dialyzed, freeze-dried and stored for enzymatic hydrolysis.
The commercial protease Alcalase 2.4 L obtained from B. licheniformis was purchased from Sigma-Aldrich (Steinheim, Germany).
Experimental design
The parameters of the study, pH (X1) and enzyme concentration (U/mL) (X2), were evaluated using a central composite rotatable design (CCRD) with four factorial points, three replicates at the central point and four axial points (11 runs). The responses were based on the antioxidant analysis using the following methods: DPPH radical scavenging, ORAC and FRAP. A second-order model equation was used to explain the performance of the system as follows:
| 1 |
where Y is the dependent variable or response; i and j have values varying from 1 to the number of variables (n), β0 is the intercept, βi is the linear coefficient, βij is the quadratic coefficient, and Xi and Xj are the coded values of the independent variables. ANOVA, the coefficient of determination R2 and F test at p ≤ 0.10 were used to measure the goodness of fit of the model. Table 1 provides the coded and uncoded levels for the CCRD.
Table 1.
Coded and uncoded levels for the CCRD
| Variables | Levels | ||||
|---|---|---|---|---|---|
| − 1.41 (−α) | − 1 | 0 | + 1 | + 1.41 (+α) | |
| X1: pH | 6 | 6.58 | 8 | 9.42 | 10 |
| X2: E (U/mL) | 50 | 64.54 | 100 | 135.46 | 150 |
Preparation of protein hydrolysates
The rice protein hydrolysates were prepared according to the method described by Castro and Sato (2014a). The substrate utilized for enzymatic hydrolysis was RPC. A concentration of 10% RPC was suspended in 50 mL of the appropriate buffer according to the values described in the CCRD in 125 mL Erlenmeyer flasks. The concentrations of both enzymes were adjusted according to their activities at each pH using 0.1 M sodium phosphate buffer (pH 6, 6.58 and 8) and 0.1 M sodium bicarbonate–NaOH buffer (pH 9.42 and 10). The enzymatic hydrolysis was performed at 60 °C for 120 min. After hydrolysis, the proteases were inactivated at 100 °C for 20 min. The mixtures were centrifuged at 17,000×g and 5 °C for 20 min, and the supernatants containing the peptides were collected, freeze-dried and used for the posterior analysis.
Determination of protease activity
The protease activity was determined according to the method described by Charney and Tomarelli (1947) and modified by Castro and Sato (2014a) using azocasein as a substrate. Azocasein and enzyme solutions were prepared in different buffers, 0.1 M sodium phosphate (pH 6, 6.58 and 8) and 0.1 M sodium bicarbonate-NaOH (pH 9.42 and 10). An aliquot of 0.5 mL of 0.5% azocasein and 0.5 mL of the enzyme solution was incubated for 40 min at 60 °C. Then, the reaction was paralyzed by adding 0.5 mL of 10% trichloroacetic acid (TCA). The mixture was centrifuged at 17,000×g for 15 min at 15 °C. An aliquot of 1 mL of the supernatant was added to 1 mL of 5 M KOH, and the absorbance was measured at 428 nm. One unit of protease activity (U) was defined as the amount of enzyme that causes an increase of 0.01 in absorbance under the assay conditions.
Determination of the TCA soluble protein content
The TCA soluble protein content was applied as the indirect way to measure the degree of hydrolysis (DH) using the method proposed by Peričin et al. (2009). An aliquot of 0.5 mL of the hydrolysate (0.8 mg/mL) was added to 0.5 mL of 0.44 mol/L TCA. The mixture was incubated for 30 min at room temperature and centrifuged at 17,000×g and 15 °C for 15 min. Then, the supernatant was utilized to measure the protein content. Lowry’s method (with modifications) was utilized to measure the protein, and bovine serum albumin was the protein standard (Hartree 1972). The results were calculated as the ratio of the TCA soluble protein content and the total protein content in the supernatant of the hydrolysate mixture expressed as a percentage.
Determination of the antioxidant activities
DPPH radical-scavenging
The DPPH radical-scavenging was determined using the method described by Bougatef et al. (2009). An aliquot of 0.5 mL of each sample (5 mg/mL) was mixed with 0.5 mL of 99.5% ethanol and 125 µL of 0.02% DPPH solution in 99.5% ethanol. The mixture was incubated for 60 min at room temperature in the dark. The measurements were performed at 517 nm using a UV–visible spectrophotometer. A calibration curve of standard Trolox (1.5–37.5 µM) was used to calculate the DPPH radical-scavenging (Trolox equivalents/g of protein hydrolysates, TE µmol/g). The results were also represented as a DPPH inhibition (%) as in Eq. 2:
| 2 |
Distilled water was used instead of sample in the control. The control sample was performed with no DPPH solution.
Oxygen radical absorbance capacity (ORAC)
The ORAC assay was performed according to the method of Dávalos et al. (2004) as described by Castro and Sato (2014a). The automated ORAC assay was carried out on a Novo Star Microplate reader (BMG LABTECH, Germany) with fluorescence filters (excitation wavelength of 485 nm and an emission wavelength of 520 nm) over 120 min at 37 °C. The reaction mixture contained 20 µL of sample (1 mg/mL), blank (distilled water) or standard (Trolox solutions); 120 µL of 0.4 mg/mL fluorescein; and 60 µL of 108 mg/mL AAPH in 75 mM sodium phosphate buffer (pH 7.4). The area under the fluorescence decay curve (AUC) was calculated according to Eq. (3):
| 3 |
where fi is the fluorescence reading at time i and f0 is the fluorescence obtained after the addition of AAPH.
The net AUC was calculated according to Eq. (4):
| 4 |
The ORAC values were calculated using the calibration curve, which was constructed with Trolox standards (1.5–1500 µM) and a blank without antioxidants. The results were expressed as TE µmol/g of protein hydrolysates. All assays were performed in three independent replicates.
Ferric reducing antioxidant power assay (FRAP)
The FRAP assay was performed according to the method described by Benzie and Strain (1996) and modified by Wiriyaphan et al. (2012). An aliquot of 100 µL of each sample (5 mg/mL) was mixed with 1 mL of fresh FRAP reagent (25 mL of 300 mM acetate buffer, pH 3.6; 2.5 mL of 10 mM TPTZ in 40 mM HCl; and 2.5 mL of 20 mM FeCl3·6H2O). The mixture was incubated for 15 min at 37 °C. The absorbance was measured at 593 nm. The results were calculated based on a standard curve of Trolox (1.5–300 µM) and expressed in TE µmol/g of protein hydrolysates.
Statistical analysis
The Statistica 7.0 program (Statsoft®/Dell, USA) was used for the experimental design, matrix and statistical analysis. The differences were considered significant at p ≤ 0.10. All assays were performed in three independent replicates.
Results and discussion
To optimize the hydrolysis process, it is necessary to determine the conditions and variables that significantly affect the responses evaluated (Castro and Sato 2015). The RSM statistical evaluation provides this kind of information indicating the best condition that could be used in obtaining hydrolysates with increased antioxidant activities (Castro et al. 2017).
The limited variability of the central points indicated the good reproducibility of the experimental data. The highest values for the DPPH, ORAC and FRAP of rice protein hydrolysates using the LBA enzyme were found in assays 1 (6.22 TE µmol/g), 4 (1019.94 TE µmol/g) and 2 (18.72 TE µmol/g), respectively (Table S1). These differed from the results for Alcalase 2.4 L, which showed better results for DPPH in assay 1 (6.74 TE µmol/g) and for ORAC and FRAP in assay 6 (1012.57 and 18.45 TE µmol/g). Slightly higher values can be observed for the hydrolysis responses with Alcalase 2.4 L (Table S2).
The lowest values obtained using the protease produced by B. licheniformis LBA 46 were found in assays 6 (37.60 TE µmol/g), 7 (658.10 TE µmol/g) and 5 (8.08 TE µmol/g) for DPPH, ORAC and FRAP, respectively (Table S1). For the hydrolysis with Alcalase 2.4 L, the assays with the lowest values were 7 for DPPH (43.21 TE µmol/g), 11 for ORAC (722.14 TE µmol/g) and 5 for FRAP (8.54 TE µmol/g) (Table S2).
The evaluation of the DH is used to verify how much of the protein was broken down by the enzyme used during the hydrolysis, which is related to the catalytic action of the enzyme, and it is used to monitor the proteolysis and compare the different protein hydrolysates produced (Hsu 2010). The hydrolysates presented similar DH values independent of the enzyme employed in the process. For the LBA enzyme, the lowest value was found for the hydrolysates obtained in assay 2 (49%). The other tests presented median values between 52.45–55.83%. For Alcalase 2.4 L, the degree of hydrolysis ranged from 50.83 to 54.69%.
The DH of bovine plasma protein increased after hydrolysis with Alcalase, ranging from 18.70 to 20.42%. The highest antioxidant activity was obtained when DH values were lower (Seo et al. 2015). Kong and Xiong (2006) also observed higher DH after the use of Alcalase in zein hydrolysis. It was also reported that the kind of protease used during the process, as well as the DH obtained, can affect the antioxidant activity.
Tables 2 and 3 show the regression coefficients of the variables calculated for the responses studied. According to Solouk et al. (2011), the importance of variables and their effects within experimental planning can be explained by the magnitude and sign of the coefficients. The significance of the regression coefficients was determined based on their p value. Statistical analysis showed that the variations in pH (6–10) and enzyme concentration (50–150 U/mL) studied had no effect on the antioxidant activity of the hydrolysates measured by the DPPH assay. Thus, it was not possible to predict a mathematical model for this response.
Table 2.
Regression coefficients, standard error, tcalc and p value of rice hydrolysis using the LBA protease
| Factors | Coefficients | Standard error | t calc | p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | ORAC | FRAP | DPPH | ORAC | FRAP | DPPH | ORAC | FRAP | DPPH | ORAC | FRAP | |
| Intercept | 5.21 | 699.87 | 10.03 | 0.23 | 39.45 | 1.15 | 22.22 | 17.74 | 8.71 | 0.000 | 0.000 | 0.000 |
| pH | − 0.39 | 67.68 | 3.48 | 0.14 | 24.20 | 0.71 | − 2.68 | 2.80 | 4.92 | 0.044 | 0.038 | 0.004 |
| pH2 | 0.12 | 66.22 | 1.75 | 0.17 | 28.87 | 0.84 | 0.73 | 2.29 | 2.08 | 0.500 | 0.070 | 0.093 |
| Protease | 0.20 | 69.12 | 0.20 | 0.14 | 24.20 | 0.71 | 1.39 | 2.86 | 0.28 | 0.224 | 0.036 | 0.790 |
| Protease2 | 0.12 | 43.01 | 0.83 | 0.17 | 28.87 | 0.84 | 0.71 | 1.49 | 0.99 | 0.510 | 0.197 | 0.370 |
| pH x protease | 0.29 | 29.42 | − 0.68 | 0.20 | 34.17 | 1.00 | 1.44 | 0.86 | − 0.68 | 0.211 | 0.429 | 0.527 |
tcalc calculated with five degrees of freedom
Table 3.
Regression coefficients, standard error, tcalc and p value of pea hydrolys using the Alcalase 2.4 L
| Factors | Coefficients | Standard error | t calc | p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | ORAC | FRAP | DPPH | ORAC | FRAP | DPPH | ORAC | FRAP | DPPH | ORAC | FRAP | |
| Intercept | 5.74 | 729.24 | 11.41 | 0.24 | 15.37 | 0.36 | 24.32 | 47.45 | 31.80 | 0.000 | 0.000 | 0.000 |
| pH | − 0.26 | 63.77 | 3.66 | 0.14 | 9.43 | 0.22 | − 1.77 | 6.77 | 16.62 | 0.137 | 0.001 | 0.000 |
| pH2 | 0.20 | 94.22 | 1.26 | 0.17 | 11.25 | 0.26 | 1.18 | 8.38 | 4.80 | 0.291 | 0.000 | 0.005 |
| Protease | 0.18 | 21.09 | 0.22 | 0.14 | 9.43 | 0.22 | 1.25 | 2.24 | 0.99 | 0.266 | 0.075 | 0.370 |
| Protease2 | 0.10 | 40.42 | 0.06 | 0.17 | 11.25 | 0.26 | 0.58 | 3.59 | 0.24 | 0.589 | 0.016 | 0.821 |
| pH x protease | 0.24 | − 25.15 | − 0.31 | 0.20 | 13.31 | 0.31 | 1.16 | − 1.89 | − 0.98 | 0.297 | 0.117 | 0.371 |
tcalc calculated with five degrees of freedom
The regression coefficients for LBA protease hydrolysates indicated that the pH of hydrolysis had linear and quadratic effects (p < 0.10) on the ORAC and FRAP responses. The linear term of protease hydrolysates had a significant effect (p < 0.05) only on the ORAC response. With regards to Alcalase 2.4 L, the regression coefficients indicated that the pH of hydrolysis had strong linear and quadratic effects (p < 0.01) on the ORAC and FRAP responses. The linear and quadratic terms of protease only affected the ORAC response (p < 0.10).
Analysis of variance (ANOVA) for the regression model was performed (Tables S3 and S4). The significance of each coefficient was verified using the F test, p value (associated probability) and coefficient of determination, R2. The F values calculated for the regressions were greater than the tabulated F values and are presented as F ratios, demonstrating the statistical significance of the models. The R2 value of the regression model can be used to determine the variability in the experimental data. The statistical parameter R2 ranged from 0.71 to 0.98, meaning that the models are able to explain from 71 to 98% of the variability of the experimental data. The p values for the ORAC and FRAP responses were less than 0.05, showing that the proposed models presented high significance at a 95% confidence level. The models with significant factors for the experimental data representing each of the responses are presented in Eq. (5) (ORAC response using the LBA protease); Eq. (6) (FRAP response using the LBA protease); Eq. (7) (ORAC response using Alcalase 2.4 L) and Eq. 8 (FRAP response using Alcalase 2.4 L).
| 5 |
| 6 |
| 7 |
| 8 |
where Y is the response and X1 and X2 are the coded values of the independent variables, the pH and protease concentration, respectively.
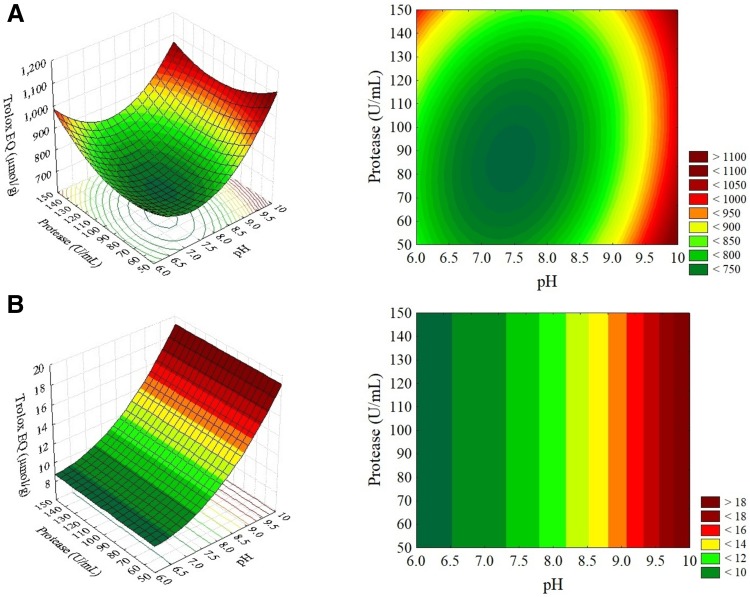
RSM is a statistical procedure frequently used for the optimization of studies that use quantitative data from an experimental design to determine and solve problems. The equations describe the effect and determine the interrelationships representing the combined effects of the independent variables on the responses, allowing the researcher to efficiently explore the studied process (Madamba 2002). The effects of the independent variables (pH and enzyme concentration) are presented as response surface and contour curves, as shown in Figs. 1 and 2. In general, these graphs show the optimal conditions (Cao et al. 2012), which expose the values for the variables to find the maximum or minimum response according to the needs of the study.
Fig. 1.
Response surfaces and contour diagrams for the ORAC (a) and FRAP (b) assays as a function of the pH value and protease (U/mL) concentration for rice protein hydrolysates prepared with LBA protease
Fig. 2.
Response surfaces and contour diagrams for the ORAC (a) and FRAP (b) assays as a function of the pH value and protease (U/mL) concentration for rice protein hydrolysates prepared with Alcalase 2.4 L
For rice protein hydrolysates prepared with the two enzymes evaluated, a similar behavior can be observed, where the ORAC and FRAP responses have maximal (red region) and minimal (green region) values in similar intervals. The pH values that give the maximum responses for both antioxidant activities are in the range of 9–10, and the enzyme concentration is in the entire studied range, from 50 to 150 U/mL, representing that both enzymes evaluated have the catalytic power to produce rice protein hydrolysates with antioxidant activity even at the lowest evaluated concentration (50 U/mL). The enzyme produced in the laboratory by the microorganism B. licheniformis LBA 46, although producing peptides with values of antioxidant activity lower than the values obtained when the hydrolysis was performed with Alcalase 2.4 L, acts in a similar way to this enzyme since both are enzymes with a pH range in the alkaline region.
Asp and Glu are amino acids that exhibit strong antioxidant activity and are important in the composition of protein hydrolysates (Saiga et al. 2003). A study developed by Zhao et al. (2012) using Alcalase 2.4 L for the hydrolysis of rice dreg protein (pH 8.5 and temperature of 55 °C) showed that Asp and Glu were the amino acids found in higher concentrations in the hydrolysates produced. The authors also observed a high amount of low molecular weight peptides, 41.75% (1–3 kDa) and 26.75% (< 1 kDa), which is related to higher antioxidant activities.
Zhou et al. (2013) described rice protein hydrolysates prepared by different microbial proteases (validase protease from Aspergillus oryzae, pH 7; alkaline protease from B. licheniformis, pH 10; and neutral protease from Bacillus subtillis, pH 7) incubated at 55 °C and ultrafiltered. The authors reported ORAC values lower than those found in this study, ranging between 34.20 and 87.30 TE µmol/g. The hydrolysate fractions at 100 mg/mL registered 31.2–49.7% of DPPH reduction. In this study, the hydrolysates at 5 mg/mL showed 37.95–49.50 and 43.20–53.91% for hydrolyses performed by the LBA enzyme and Alcalase 2.4 L, respectively.
On the other hand, Wattanasiritham et al. (2016) studied the native and denatured fractions of rice bran after hydrolysis with trypsin (pH 7.8) and papain (pH 7) at 37 °C. The denatured fraction (albumin) hydrolyzed by trypsin exhibited the highest antioxidant activity with an ORAC value of 4067 TE µmol/mg protein when compared to the native protein. In this case, after the denaturation, the enzyme could be penetrating, thus cleaving a greater number of peptide bonds producing low molecular weight peptides, which have high antioxidant activity. The effect of the protease type on the hydrolysis of rice bran was evaluated by Thamnarathip et al. (2016). It was observed that Alcalase was the most efficient enzyme in the production of hydrolysates with higher antioxidant activity.
Based on the range of values already studied and taking into account that antioxidant activity analyses are susceptible to variation, to optimize the responses obtained for the antioxidant activity, the chosen pH was the highest (10) and the protease concentration was an intermediate value (100 U/mL) since this variable had practically the same effect throughout the CCRD. Due to the fact that the responses to the hydrolysis of the rice protein were similar, the optimized parameters were the same for the two enzymes used. According to the regression models (Eqs. 5–8), the predicted values for the ORAC and FRAP under these conditions were 942.30 and 18.70 TE µmol/g for the rice protein hydrolysates prepared with LBA protease and 1006.46 and 19.10 TE µmol/g for the rice protein hydrolysates prepared with Alcalase 2.4 L. The hydrolysis assays, in the conditions defined as optimal according to the interval presented in the contour curves, were performed in triplicate to confirm the validity of the model. The experimental values obtained are in agreement with the expected values (p ≤ 0.10), confirming once again the validity of the model (Table 4).
Table 4.
Validation tests to determine the adequacy of the models obtained for maximum antioxidant activities of rice protein hydrolysates prepared with LBA protease and Alcalase 2.4 L
| Validation LBA 46 hydrolysates | ||||
|---|---|---|---|---|
| Conditions | ||||
| ORAC (TE µmol/g) | FRAP (TE µmol/g) | |||
| Independent variables | pH | Protease (U/mL) | pH | Protease (U/mL) |
| Experimental coded value | 1.41 | 0 | 1.41 | 0 |
| Experimental real value | 10 | 100 | 10 | 100 |
| Predicted response | 942.30a | 18.70b | ||
| Experimental response | 940.05 ± 20.18a | 18.78 ± 0.15b | ||
| Validation Alcalase 2.4 L hydrolysates | ||||
|---|---|---|---|---|
| Conditions | ||||
| ORAC (TE µmol/g) | FRAP (TE µmol/g) | |||
| Independent variables | pH | Protease (U/mL) | pH | Protease (U/mL) |
| Experimental coded value | 1.41 | 0 | 1.41 | 0 |
| Experimental real value | 10 | 100 | 10 | 100 |
| Predicted response | 1006.46a | 19.10b | ||
| Experimental response | 1001.94 ± 24.58a | 19.31 ± 0.19b | ||
Results presented as mean (n = 3) ± standard deviation. Values with different letters are significantly different (p ≤ 0.05)
The unhydrolyzed rice protein sample (control) presented values of 324.97 ± 13.25 (ORAC) and 6.14 ± 0.11 (FRAP) all in TE µmol/g. As seen in Table 4, after optimization of the enzymatic hydrolysis conditions, the antioxidant activity values increased regardless of the enzyme used in the hydrolysis. For the hydrolysis performed with the LBA enzyme, there were increases of 2.9-fold for ORAC assay and 3.05-fold for the FRAP assay. For the hydrolysis performed with Alcalase 2.4 L, the increases were slightly higher, 3.08-fold for the ORAC assay and 3.14-fold for the FRAP assay.
Each method of determining the antioxidant capacity is based on a different mechanism. Therefore, it is important that the efficiency of peptides as antioxidants be evaluated by different methodologies. The FRAP assay is a method that relies on oxide-reduction reactions. At low pH values, the complex Fe3-TPTZ is reduced to Fe2 producing an intense blue color showing a maximum absorbance at 593 nm. The presence of a reducing agent, i.e., antioxidant, favors the reduction of the complex, and the blue color becomes stronger (Benzie and Strain 1996). The ORAC method is based on the capture of the peroxyl radical. The radical reacts with a fluorescent probe to produce a non-fluorescent product. The antioxidant activity is measured by the maintenance of the fluorescence (Dávalos et al. 2004). In this work the rice protein hydrolysates showed higher efficiency against the oxidation reaction evaluated by the FRAP assay and were also efficient in capturing the peroxyl radical (measured by the ORAC assay). It would be coherent to propose that the hydrolysates produced had peptides and/or amino acids capable of donating electrons that could stabilize the radicals and disrupt the radical chain reaction.
Other authors reported the use of rice and its byproducts and derivatives in the production of peptides with bioactivity (Phongthai et al. 2016; Selamassakul et al. 2016; Wang et al. 2016, 2017), demonstrating that the antioxidant activity was improved after the hydrolysis of the intact protein. However, few studies that use both the RSM tool as well as the comparison of two enzymes used in the enzymatic hydrolysis of rice and its derivatives are found in the literature. Since the cost of enzymes is a rate-limiting step in the production of bioactive protein hydrolysates, the discovery and study of new enzymes is needed. One of these enzymes, LBA protease, a semi-purified enzyme with similar function to that of the pure commercial enzyme Alcalase 2.4 L, represents an advantage due to its potential use in the preparation of bioactive rice hydrolysates in optimized conditions.
Conclusion
The hydrolysis of rice protein for obtaining peptides with antioxidant activity was optimized with a pH of 10 and 100 U/mL protease. The protease from B. licheniformis LBA 46 was shown to be as efficient in the production of bioactive peptides with antioxidant activity as the commercial protease Alcalase 2.4 L, presenting similar responses after the validation. This means that the LBA protease presents potential to be used as a catalyst in the production of rice protein hydrolysates. For both protease preparations used in this study, there was an increase in the antioxidant activity of rice protein hydrolysates when compared to the control sample (non-hydrolyzed sample). This work amplifies the existing knowledge about the use of microbial proteases in the production of rice protein peptides with antioxidant activity and suggests, in view of the results found, that these hydrolysates have the potential to be used as a natural source of antioxidants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Jessika Gonçalves dos Santos Aguilar, Phone: +55 19 3521-2175, Email: jessgsantos@gmail.com.
Ruann Janser Soares de Castro, Email: ruannjanser@hotmail.com.
Helia Harumi Sato, Email: heliah@unicamp.br.
References
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal Biochem. 1996;239:1 70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bougatef A, Hajji M, Balti R, Lassoued I, Triki-Ellouz Y, Nasri M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009;114:4 1198–1205. doi: 10.1016/j.foodchem.2008.10.075. [DOI] [Google Scholar]
- Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. J Agric Food Chem. 2001;49:2774–2779. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- Cao W, Zhang C, Ji H, Hao J. Optimization of peptic hydrolysis parameters for the production of angiotensin I-convertingenzyme inhibitory hydrolysate from Aceteschinensis through Plackett–Burman and response surface methodological approaches. J Sci Food Agric. 2012;92:42–48. doi: 10.1002/jsfa.4538. [DOI] [PubMed] [Google Scholar]
- Castro RJS, Sato HH. Advantages of an acid protease from Aspergillus oryzae over commercial preparations for production of whey protein hydrolysates with antioxidant activities. J Food Process. 2014;3:58–65. [Google Scholar]
- Castro RJS, Sato HH. Comparison and synergistic effects of intact proteins and their hydrolysates on the functional properties and antioxidant activities in a simultaneous process of enzymatic hydrolysis. Food Bioprod Process. 2014;92:1 80–88. doi: 10.1016/j.fbp.2013.07.004. [DOI] [Google Scholar]
- Castro RJS, Sato HH. A response surface approach on optimization of hydrolysis parameters for the production of egg white protein hydrolysates with antioxidant activities. Biocatal Agric Biotechnol. 2015;4:1 55–62. [Google Scholar]
- Castro RJS, Cason VG, Sato HH. Binary mixture of proteases increases the antioxidant properties of white bean (Phaseolus vulgaris L.) protein-derived peptides obtained by enzymatic hydrolysis. Biocatal Agric Biotechnol. 2017;10:291–297. [Google Scholar]
- Charney J, Tomarelli RM. A colorimetric method for the determination the proteolytic activity of duodenal juice. J Biol Chem. 1947;170:23 501–505. [PubMed] [Google Scholar]
- Contesini FJ (2014) Production, characterization and application of proteases from Bacillus sp. Ph.D. thesis, University of Campinas
- Contreras MM, Hernández-Ledesma B, Amigo L, Martín-Álvarez PJ, Recio I. Production of antioxidant hydrolyzates from a whey protein concentrate with thermolysin: optimization by response surface methodology. LWT Food Sci Technol. 2011;44:9–15. doi: 10.1016/j.lwt.2010.06.017. [DOI] [Google Scholar]
- Dávalos A, Gómez-Cordovés C, Bartolomé B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem. 2004;52:1 48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- Elias RJ, Kellerby SS, Decker EA. Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr. 2008;48:430–441. doi: 10.1080/10408390701425615. [DOI] [PubMed] [Google Scholar]
- Hartree EF. Determination of protein: a modification of the Lowry methods that gives a linear photometric response. Anal Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hsu KC. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem. 2010;122:42–48. doi: 10.1016/j.foodchem.2010.02.013. [DOI] [Google Scholar]
- Kong BH, Xiong YL. Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J Agric Food Chem. 2006;54:6059–6068. doi: 10.1021/jf060632q. [DOI] [PubMed] [Google Scholar]
- Korhonen H, Pihlanto A. Bioactive peptides: production and functionality. Int Dairy J. 2006;16:9 945–960. [Google Scholar]
- Madamba PS. The response surface methodology: an application to optimize dehydration operations of selected agricultural crops. LWT Food Sci Technol. 2002;35:7 584–592. doi: 10.1016/S0023-6438(02)90914-X. [DOI] [Google Scholar]
- Moniruzzaman M, Khalil MI, Gan SH. Advances in the analytical methods for determining the antioxidant properties of honey: a review. Afr J Tradit Complement Altern Med. 2012;9(1):36–42. doi: 10.4314/ajtcam.v9i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Medina R, Pérez-Gálvez R, Guadix A, Guadix EM. Multiobjective optimization of the antioxidant activities of horse mackerel hydrolysates produced with protease mixtures. Process Biochem. 2017;52:149–158. doi: 10.1016/j.procbio.2016.11.001. [DOI] [Google Scholar]
- Muthayya S, Sugimoto JD, Montgomery S, Maberly GF. An overview of global rice production, supply, trade, and consumption. Ann N Y Acad Sci. 2014;1324:1 7–14. doi: 10.1111/nyas.12540. [DOI] [PubMed] [Google Scholar]
- Peričin D, Radulović-Popović L, Vaštag Ž, Madarev-Popović S, Trivić S. Enzymatic hydrolysis of protein isolate from hull-less pumpkin oil cake: application of response surface methodology. Food Chem. 2009;115:2 753–757. [Google Scholar]
- Phongthai S, Lim ST, Rawdkuen S. Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. J Cereal Sci. 2016;70:146–154. doi: 10.1016/j.jcs.2016.06.001. [DOI] [Google Scholar]
- Ricci I, Artacho R, Olalla M. Milk protein peptides with angiotensin I-converting enzyme inhibitory (ACEI) activity. Crit Rev Food Sci Nutr. 2010;50:5 390–402. doi: 10.1080/10408390802304198. [DOI] [PubMed] [Google Scholar]
- Saiga A, Tanabe S, Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agric Food Chem. 2003;51:12 3661–3667. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- Selamassakul O, Laohakunjit N, Kerdchoechuen O, Ratanakhanokchai K. A novel multi-biofunctional protein from brown rice hydrolysed by endo/endo-exoproteases. Food Funct. 2016;7:6 2635–2644. doi: 10.1039/C5FO01344E. [DOI] [PubMed] [Google Scholar]
- Seo H-W, Jung E-Y, Go G, Kim G-D, Joo S-T, Yang H-S. Optimization of hydrolysis conditions for bovine plasma protein using response surface methodology. Food Chem. 2015;185:106–111. doi: 10.1016/j.foodchem.2015.03.133. [DOI] [PubMed] [Google Scholar]
- Solouk A, Solati-Hashjin M, Najarian S, Mirzadeh H, Seifalian AM. Optimization of acrylic acid grafting onto POSS-PCU nanocomposite using response surface methodology. Iran Polym J. 2011;20:2 91–107. [Google Scholar]
- Thamnarathip P, Jangchud K, Nitisinprasert S, Vardhanabhuti B. Identification of peptide molecular weight from rice bran protein hydrolysate with high antioxidant activity. J Cereal Sci. 2016;69:329–335. doi: 10.1016/j.jcs.2016.04.011. [DOI] [Google Scholar]
- Vastag Z, Popovic L, Popovic S, Krimer V, Peričin D. Hydrolysis of pumpkin oil cake protein isolate and free radical scavenging activity of hydrolysates: influence of temperature, enzyme/substrate ratio and time. Food Bioprod Process. 2010;88:277–282. doi: 10.1016/j.fbp.2009.12.003. [DOI] [Google Scholar]
- Wang Z, Li H, Liang M, Yang L. Glutelin and prolamin, different components of rice protein, exert differently in vitro antioxidant activities. J Cereal Sci. 2016;72:108–116. doi: 10.1016/j.jcs.2016.10.006. [DOI] [Google Scholar]
- Wang X, Chen H, Fu X, Li S, Wei J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: biochemical characterization and molecular docking study. LWT Food Sci Technol. 2017;75:93–99. doi: 10.1016/j.lwt.2016.08.047. [DOI] [Google Scholar]
- Ward OP, Rao MB, Kulkarni A. Proteases. In: Schaechter M, editor. Encyclopedia of microbiology. Amsterdam: Elsevier; 2009. pp. 495–511. [Google Scholar]
- Wattanasiritham L, Theerakulkait C, Wickramasekara S, Maier CS, Stevens JF. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016;192:156–162. doi: 10.1016/j.foodchem.2015.06.057. [DOI] [PubMed] [Google Scholar]
- Wiriyaphan C, Chitsomboon B, Yongsawadigul J. Antioxidant activity of protein hydrolysates derived from threadfin bream surimi byproducts. Food Chem. 2012;132:1 104–111. doi: 10.1016/j.foodchem.2011.10.040. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Xiong H, Selomulya C, Chen XD, Zhong H, Wang S, Sun W, Zhou Q. Enzymatic hydrolysis of rice dreg protein: effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012;134:3 1360–1367. doi: 10.1016/j.foodchem.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Zhou K, Canning C, Sun S. Effects of rice protein hydrolysates prepared by microbial proteases and ultrafiltration on free radicals and meat lipid oxidation. LWT Food Sci Technol. 2013;50:1 331–335. doi: 10.1016/j.lwt.2012.05.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.