Abstract
The present study focuses on isolation and evaluation of the anti-cancer activity of compounds from the leaves of Abrus precatorius. The bioassay-directed strategy was adopted using chromatographic, gas chromatographic–mass spectrum analysis, nuclear magnetic resonance and X-ray crystallography techniques for purification and characterization of active cytotoxic compounds. Further, MDA-MB-231 breast cancer cell lines and 7,12-dimethylbenz (a) anthracene (DMBA) induced virgin female Sprague Dawley (SD) rats were used for in vitro and in vivo cytotoxicity evaluation. Stigmasterol hemihydrate and 9,12-Octadecadienoic acid (Z,Z)-2-hydroxy-1-(hydroxymethyl)ethyl ester or (β-monolinolein) were the two main cytotoxic constituents of leaf extract of A. precatorius, with an IC50 value of 74.2 and 13.2 µg/ml, respectively, in MDA-MB-231 cells. Additionally, the treatment with the stigmasterol and β-monolinolein as a combinatorial drug therapy in DMBA-induced female SD rats led to recovery of body weight, decreased tumor weight and volume, without any toxic side effects. Immunohistochemical examination showed extensive cell death and low proliferation in the treated tumor tissues that was confirmed by results from H and E staining, TUNEL assay and Ki-67 index as compared to control animal group. The reversion of glycoprotein, lysosomal and tumor marker enzyme levels back to near-normal levels after treatment with the plant compounds clearly demonstrated the reduction of tumor burden in these animals. This is the first report on isolation and characterization of the two active cytotoxic components from leaves of A. precatorius. Additionally, the profound cytotoxic and tumor-suppressive effect of these two compounds as a combinatorial therapy provide an alternative option for breast cancer treatment.
Keywords: Mammary carcinoma; Sprague Dawley rats; 7,12-Dimethylbenz(a)anthracene; Abrus precatorius
Introduction
Human breast cancer is still an extremely complex and dangerous disease with multiple questions (Carlson et al. 2009). The increasing global incidence of breast cancer emphasizes the need to identify novel, safe and efficient anti-cancer drugs for the treatment of breast cancer. Search for natural products represents an area of great interest in which the plant kingdom has been documented the most important source to provide cancer chemopreventive agents with novel structures and unique mechanism of action (Kalt et al. 2001). Considering the safety, efficacy and diversity of phytochemicals, molecular mechanistic research has focused on plants with cancer prevention potential (Shu et al. 2010). With the prevalence and an emergence of multiple drug resistance, nonspecific systemic distribution of anti-tumor agents, inadequate drug concentrations reaching the tumor site, intolerable cytotoxicity and limited ability to monitor therapeutic responses, cancer treatment has become more and more challenging and incurable disease (Misra et al. 2010). To overcome this problem, it is necessary to develop and design new strategies, tools and drugs for treatment of cancer. Hence, for some decades, increased efforts are being made to isolate bioactive products from medicinal plants, for their possible utility in cancer treatment as natural products are known to play a dominant role in the discovery of such new drugs that are known to be effective and less toxic in non-tumor cells as compared to synthetic drugs (Sgariglia et al. 2011).
Abrus precatorius is highly regarded as a universal panacea in the herbal medicine with diverse pharmacological activity spectra. A. precatorius has been reported to contain diverse phytochemicals with numerous pharmacological activities. A. precatorius have been receiving attention as anti-cancer agents, as it has been shown that various phytochemicals from leaves have the property to induce apoptosis on various types of cancers (Hickman 1992; Gul et al. 2013). Different classes of secondary metabolites have been isolated from A. precatorius, including alkaloids (Ghosal and Dutta 1971), phenolic compound, protein, flavonoids (Sujit et al. 2012), isoflavanoquinones, anthocyanins, tannin (Bhardwaj et al. 1980), steroids and other triterpenoids (Chang et al. 1982). There is an enormous need for isolation, characterization and determination of bioactivity of structurally known and unknown compound from A. precatorius for its pharmaceutical applications.
Further animal experimental systems are particularly useful in the study of human mammary carcinogenesis, since the rat mammary gland is highly susceptible to the development of neoplasms, which closely mimic human breast cancer (Samy et al. 2006). The DMBA (7,12-Dimethylbenz(a)anthracene)-induced mammary carcinomas in female Sprague Dawley (SD) rats has become the standard laboratory model of mammary carcinogenesis (Russo and Russo 1996). DMBA is a synthetic polycyclic aromatic hydrocarbon that has been used extensively as a prototype agent in mutation and cancer research as DMBA-mediated biochemical, molecular, genetic and histopathological changes in rats were analogous to those observed in human cancers (Izzotti et al. 1999). Hence, the evaluation in DMBA-induced carcinogenesis may assist in the diagnosis, treatment monitoring and to screen for effective chemopreventive potential of various anti-cancer drugs in cancer patients (Miyata et al. 2001; Minari and Okeke 2014). This model has the potential to examine a single stage of initiation, promotion or progression in mammary carcinogenesis.
The present study was designed to isolate and characterize the cytotoxic components present in the leaves of A. precatorius for their potential anti-cancer activity on MDA-MB-231 cells. Further, the advantages of the fore mentioned attributes of DMBA-induced mammary tumor model were exploited to evaluate the tumor-suppressive effects of the isolated cytotoxic compounds from leaves of A. precatorius for its use as a chemotherapeutic agent. Different biochemical, morphological and histopathological methods were analyzed in tumor-induced female SD rats post several weeks of treatment with the isolated compounds. Biochemical parameters such as glycoprotein content, lysosomal enzymes and other tumor-specific markers were also evaluated in the plasma and liver of control and treated animals.
Materials and methods
Plant material
Fresh, disease-free leaves of A. precatorius were collected from Danvantrivanna, Bangalore University campus, Bangalore, Karnataka, India. The plant material was identified and authenticated by an expert taxonomist. An authenticated voucher specimen of the plant material was deposited in the herbarium of Molecular Diagnostic and Nanobiotechnology Laboratories, Department of Microbiology and Biotechnology, Bangalore University, Bangalore for future reference.
Preparation of leaf extract
Leaves of A. precatorius (1.5 kg) were collected. For solvent extraction, the air-dried and powdered leaves of A. precatorius were loaded to thimble and extracted with ethyl acetate using a soxhlet extractor for 16 h. The filtrate was concentrated under vacuum using rotary evaporator (Heidolph Laborota 4000, Germany) to obtain approximately 109 g of ethyl acetate extract of A. precatorius (EAF-AP). This was later preserved at 4 °C in an airtight bottle until further use.
Isolation of anti-cancer compound
A portion of EAF-AP was subjected to silica gel column chromatography and a stepwise gradient elution was performed with gradients of petroleum ether: ethyl acetate (0–100%) and (0–10%) respectively using different sized columns. The collected column fractions were again tested with TLC chromatogram and the Rf values were determined. The fractions with similar Rf value were combined together and all the fractions and sub-fractions were concentrated under vacuum using rotary evaporator, finally weighed separately and subjected to MTT cytotoxicity assay.
MTT assay for cytotoxicity
MDA-MB-231 breast cancer cell lines (5 × 103 cells/well) were seeded to 96-well micro-titer plates. After 24 h, cells were treated, respectively, with all isolated fractions and sub-fractions. Again after 24 h, 20 µl of 5 mg/ml MTT was added to each wells and kept in CO2 incubator for 2 h. After 2 h, medium was removed and 150 µl of DMSO was added and mixed in each well. Absorbance was recorded at 595 nm with multiwell plate reader. Percentage of cell viability = (OD of treated cells/OD of control cells) × 100. The concentration of drug that inhibits 50% of the cells (IC50 values) for these samples were obtained from dose–response curves.
Crystallization and data collection
Single-crystal X-ray data for the compound was collected on Bruker Kappa Apex II CCD diffractometer using Mo-KΣ (λ = 0.71073 Å). The crystal was maintained at the desired temperature during data collection using Oxford cryo-systems N2 open flow cryostat. Structure solution and refinement were performed using SHELX-2013 embedded in the WinGX Suite. Refinement of coordinates and anisotropic thermal parameters of nonhydrogen atoms were carried out by the full-matrix least-squares method. The hydrogen atoms for all structures were located on the difference Fourier map. Mercury version 3.0 was used for molecular representations and packing diagrams.
Nuclear magnetic resonance spectroscopy (NMR)
Approximately, 15 mg of bioactive pure fraction was dissolved in 3 ml of deuterated chloroform (CD2Cl3) and filtered through a 0.2 µm PTFE membrane. The deuteratedchloroform containing the bioactive pure compound was transferred into a round-bottom NMR tube 178 mm long and 4.2 mm diameter (Norell, USA). The sample was subjected to analysis on a 400 MHz NMR (Bruker Advance-400 MHz FT-NMR) to obtain 1H NMR and 13C NMR spectrums.
Gas chromatography–mass spectrum analysis (GC–MS)
GC–MS analysis was performed according to the procedure using a Shimadzu Japan GC-2010 plus series. Interpretation on GC–MS profile was conducted using the database of national institute of standard and technology (NIST). The retention time, name, molecular weight and structure were recorded for test material. The spectrum of unknown component was compared with NIST library and the name, molecular weight and structure of components of test materials were ascertained.
Animals and experimental design
Inbred healthy female 6-week-old Sprague Dawley rats (virgin) weighing between 122 and 135 g were used for the study. The animals were maintained under standard conditions of temperature (25 ± 2 °C), humidity (30–70%) and light (12 h light/dark). Standard pellet diet was provided along with tap water ad libitum. The study has got an approval from the Institutional Animal Ethical Committee (IAEC), regulated by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India Approval no: (AACP/IAEC/Dec-2012/19). Thirty female Sprague Dawley rats were divided into five groups (Fig. 1):
Fig. 1.
Experimental design for the evaluation of anti-tumor effect of stigmasterol and β-monolinolein in female Sprague Dawley rats
Group A (normal rats; n = 6): provided only with diet and water.
Group B (plant compound control; n = 6): Only stigmasterol and β-monolinolein treated.
Group C (tumor control; n = 6): DMBA alone; 20 mg/Kg body weight in 0.5 ml sesame oil administered one time orally at 7 weeks of age.
Group D (DMBA + plant compound treated; n = 6): Treated every fourth day with 10 mg/kg of stigmasterol and β-monolinolein dissolved in sesame oil for 5 weeks.
Group E (DMBA + doxorubicin-treated positive control; n = 6): Treated every fourth day with 10 mg/kg of doxorubicin dissolved in water for 5 weeks.
The animals were checked once per week for mammary tumor development by palpation. Initial body weight and final body weight (g) were recorded (Barros et al. 2004). The tumor mass was measured horizontally and vertically in group C, D and E animals using a vernier calliper. Tumor volume (mm in diameter) was calculated using the formula V = 0.5 ab2, where ‘a’ and ‘b’ indicates the major and minor diameter, respectively (Sharma et al. 2013). At the end of an experimental period (17 weeks of DMBA induction + 5 weeks of plant compound treatment), all the animal groups were anesthetized with diethyl ether and sacrificed by decapitation. Animals were starved overnight before sacrificing them. Blood was collected and the serum was separated by centrifugation. The tumors formed were collected and washed 2–3 times with saline. The tissue (5 g) was then homogenized in 0.1 M Tris–HCl buffer (pH 7.4) and stored under appropriate conditions.
Immunohistochemistry
Paraffin-embedded blocks of tumors were prepared from the formalin-fixed tumors of all the groups. The 5-µm-thick sections were cut from the paraffin-embedded tumor blocks and collected on silane-coated glass slides. Hematoxylin and eosin (H and E) staining was done to the sections to visually analyze cell death in the tumors. The apoptosis was analyzed by performing TUNEL assay using TACS® 2 TdT DAB-in situ apoptosis detection kit according to the manufacturer’s protocol (Trevigen Inc., Gaithersburg, USA). For ki-67 staining (cell proliferation marker), sections were first deparaffinized in xylene for 15 min followed by gradual rehydration in ethanol. Heat-mediated antigen retrieval in Tris–EDTA (pH 9) or 10 mM citrate buffer (pH 6) was performed. All the sections were further treated with 3% hydrogen peroxide in methanol to block endogenous peroxidase activity and then washed with PBS. It was followed by blocking with 5% fat-free milk powder for 30 min. Anti-ki-67 Antibody (Biogenex, Fremont, USA) incubation was done at room temperature for 2 h followed by incubation with an appropriate horseradish peroxidase (HRP) and conjugated secondary antibody for 30 min. Detection of antibody was carried out using supersensitive polymer-HRP IHC/DAB detection system (Biogenex, Fremont, USA). Hematoxylin was used for counter staining the sections. Appropriate antibody controls (IgG) were used during the procedure to rule out the possibility of nonspecific binding. Percentage of cells showing positive staining for each sample was counted (three independent samples were stained from each group) and plotted as relative difference with respect to the control tumors.
Biochemical estimations in plasma and liver of control and treated animals
The levels of glycoprotein components, namely hexose, hexosamine and sialic acid in plasma and liver were estimated by various methods (Glossman and Neville 1971; Niebes 1972; Warren 1959) with minor modifications. The values were expressed as mg/g of defatted tissues and mg/dl in plasma. Lysosomal enzymes including acid phosphatases, β-d-glucuronidase and cathepsin proteases were assayed by various method (Rosenblit et al. 1974; Kawai and Anno 1971; Sapolsky et al. 1973). The activity of cathepsin-D was expressed as µmoles of tyrosine-released/minute/mg of protein.The activity of the tumor marker enzymes including gamma-glutamyltransferase, lactate dehydrogenase (LDH) and alkaline phosphatases (ALP’s)in the plasma and liver were estimated according to (Rosalki and Rau 1972; King 1965a, b) with minor modifications.
Statistical analysis
The data represent the mean ± SD of three independent experiments each in a triplicate. The significance between control and treated groups was analyzed by Student’s t test and p values less than 0.05 were taken as significant by GraphPad Prism 5.0 software (GraphPad Software Inc., CA, USA).
Results
Isolation of anti-cancer compound
Thin-layer chromatographic analysis of EAF-AP result showed that petroleum ether: ethyl acetate (5:5) solvent combination was the most appropriate solvent system and has shown clear separate six bands with good resolution at distinct Rf values. Hence, this solvent system was used for further isolation process. Gradient column chromatography of the EAF-AP produced 93 fractions of 100 ml each using solvent system petroleum ether : ethyl acetate (0–100%) as an eluent. These fractions were combined on the basis of thin-layer chromatographic (TLC) analysis to afford eight fractions (A to H) with their respective Rf values (Table 1).
Table 1.
Column-chromatographic fractions isolated from A. precatorius extract
| Column fractions | Solvent system | Weight of fraction (g) | No. of bands | R f value |
|---|---|---|---|---|
| Fraction 0 | Petroleum ether [100%] | – | No band | – |
| Fraction A | 10% ethyl acetate : petroleum ether [10 ml E.A + 90 ml P.E] | 2.62 | 1 | 0.38 |
| Fraction B | 20% ethyl acetate : petroleum ether [20 ml E.A + 80 ml P.E] | 1.33 | 1 | 0.46 |
| Fraction C | 30% ethyl acetate : petroleum ether [30 ml E.A + 70 ml P.E] | 3.44 | 1 | 0.57 |
| Fraction D | 40% ethyl acetate : petroleum ether [40 ml E.A + 60 ml P.E] | 2.43 | 1 | 0.60 |
| Fraction E | 50% ethyl acetate : petroleum ether [50 ml E.A + 50 ml P.E] | 1.96 | 1 | 0.63 |
| Fraction F | 60% ethyl acetate : petroleum ether [60 ml E.A + 40 ml P.E] | 2.32 | 1 | 0.66 |
| Fraction G | 70% ethyl acetate : petroleum ether [70 ml E.A + 30 ml P.E] | 2.11 | 1 | 0.68 |
| Fraction H | 80% ethyl acetate : petroleum ether [80 ml E.A + 20 ml P.E] | 4.21 | 2 | 0.72, 0.74 |
MTT assay for cytotoxicity
Among all the eight column-chromatographic fractions isolated from EAF-AP, only fraction C showed significant cytotoxic activity on MDA-MB-231 cells at concentrations ranging from 50 to 150 µg/ml after 48 h of treatment (Fig. 2). Hence, fraction C was selected for further characterization.
Fig. 2.
Effect of fraction A to H on MDA-MB-231 cells at concentrations ranging from 50 to 150 µg/ml. Values are means ± SE of at least three independent experiments, each in triplicates
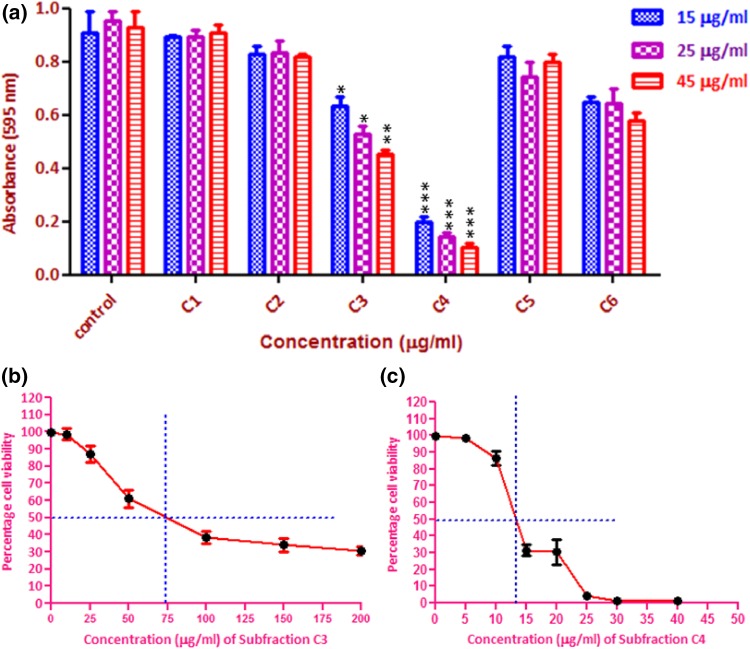
Gradient column chromatography of the cytotoxic fraction C produced seventy fractions of 10 ml each, using petroleum ether–ethyl acetate (0–10%) as an eluent. These fractions were further combined on the basis of thin-layer chromatographic (TLC) analysis to afford six sub-fractions as C1–C6. Among all the six sub-fractions isolated from fraction C, only sub-fractions C3 and C4 were found to be significantly active at concentrations ranging from 15 to 45 µg/ml (Fig. 3a). To calculate the IC50 value of these sub-fractions, MDA-MB-231 cells were treated for 48 h in a dose-dependent manner. The sub-fraction C3 and C4 showed a very significant cytotoxic effect with an IC50 value of 74.2 and 13.2 µg/ml, respectively (Fig. 3b, c).
Fig. 3.
Cytotoxic activity of sub-fractions. a C1 to C6 on MDA-MB-231 cells at different concentrations, b IC50 values of fractions C3 and c fractions C4
Morphological studies
Microscopic examination of MDA-MB-231 cells treated with fractions C3 and C4 for 48 h showed distinct changes such as shrinkage and detachment of MDA-MB-231 cells from the surface of the dish (Fig. 4). These results indicate that fractions C3 and C4 have potent cytotoxic activity on MDA-MB-231 breast cancer cells. However, the fraction C4 showed much higher cytotoxic activity than the fraction C3.
Fig. 4.
Microscopic images of human breast cancer MDA-MB-231 cells. After treatment with fractions C3 (74.2 µg/ml) and C4 (13.2 µg/ml) for 48 h (×10 magnification)
Identification and structure elucidation
Fractions C3 was recrystallized in dichloromethane and colorless needle-shaped crystals suitable for single-crystal diffraction was harvested after 3 days. Finally, the crystals obtained from fraction C3 were submitted for X-ray crystallographic analysis.
Crystal data of cytotoxic fractions C3
Compound name = stigmasterol hemihydrate; sum formula = 2C29H48O·0.5H2O; Molecular weight = 412.6908 g/mol; crystal description and color = needle, colorless; Dx = 1.019 g/cm3; Mr = 825.36; space group = monoclinic, P21; a = 9.5607 [12] Å; b = 7.5768 [10] Å; c = 37.162 [5] Å; β = 94.141 [3]; V = 2685.0 [6] Å3; Z = 2; µ = 0.060 [m/m]; T = 150 K; S = 0.968; Mo Kα radiation; R [reflections] = 0.0898 [3559]; wR2 [reflections] = 0.2720 [9595] (Fig. 5a–d).
Fig. 5.
Elucidated structure of stigmasterol. a Chemical structure of stigmasterol hemihydrate. b Ellipsoid plot indicating magnitudes and directions of the thermal vibration of atoms in crystal structures. c, d Oak Ridge Thermal Ellipsoid Plot (ORTEP) diagrams for the asymmetric unit of the crystal structures (ellipsoids were drawn at 50% probability)
| Hydrogen bonds with H.A < r [A] + 2.000 Angstroms and <DHA>110° | ||||||
|---|---|---|---|---|---|---|
| D–H | d[D–H] | d[H·A] < DHA | d[D⋯A] | A | Symmetry code | |
| O1–H1O | 0.820 | 2.280 | 165.84 | 3.081 | O3 | [− x + 1, y − 1/2, − z] |
| O2–H2O | 0.820 | 2.408 | 144.13 | 3.109 | O3 | [− x + 1, y + 1/2, − z] |
Structure elucidation of cytotoxic fraction C4
The compound was obtained as dark green powder (0.9 g) and its detailed analysis of 1H, 13C NMR spectra is as follows:
1H NMR [400 MHz, CDCl3]:
9.52 [s, 1H–OH], 9.39 [s, 1H–OH], 8.02 [d, J = 10 Hz, 1H–CH=CH], 7.97 [d, J = 10 Hz, 1H–CH=CH], 6.17 [m, 2H–CH=CH], 4.46 [m, 1H–COOCH[R]2], 3.67 [d, J = 8.4 Hz, 2H–CH2[CH=CH]2], 3.4 [s, 2H–CH2OH], 3.2 [s, 2H–CH2OH], 2.3 [m, 2H–CH2COOR], 1.6 [m, 4H–CH2CH=CH], 1.25–0.7 [m, 19H-alkyl].
13C NMR [100 MHz, CDCl3]:
δ 172.3, 129.1, 122.8, 117.7, 104.4, 77.2, 64.7, 31.9, 24.9, 24.7, 24.4, 23.0, 22.7, 19.6, 17.4, 16.2,14.13, 12.1,11.2.
GCMS:
m/z 355 [calcd for C21H38O4, 354.5310].
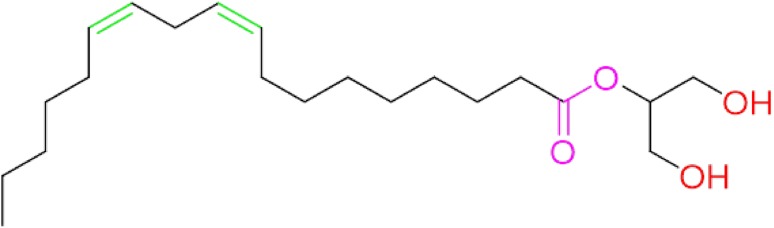
Molecular formula was deduced as C21H38O4 (9, 12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl) ethylester) or (β-Monolinolein) (Fig. 6).
Fig. 6.
Chemical structure of fraction C4 from the spectroscopic data. Fraction C4 was identified as C21H38O4(9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl) ethylester)
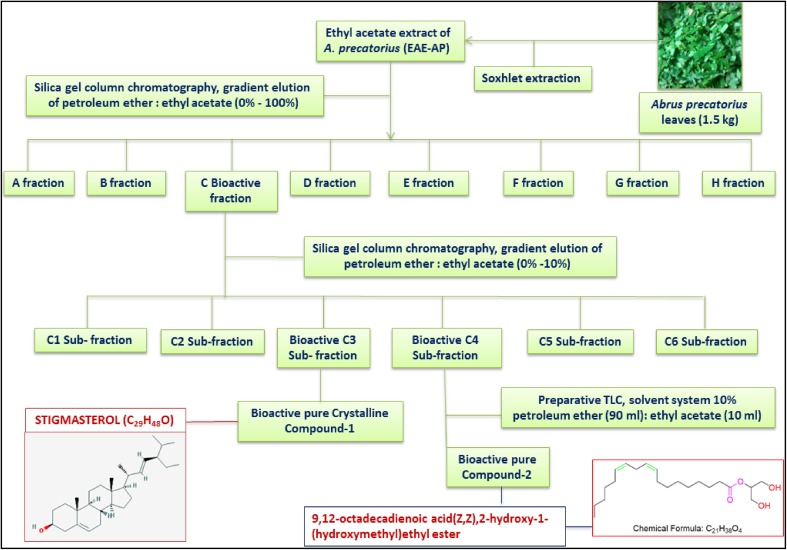
Further, the overall representation of how the two cytotoxic compounds were isolated and characterized from the leaves of A. precatorius is shown in (Fig. 7).
Fig. 7.
Flow chart showing bioactive guided isolation and identification of anti-cancer compounds from leaves of Abrus precatorius
The anti-tumor activity of stigmasterol and β-monolinolein in DMBA-induced Sprague Dawley rats
DMBA-induced tumors bearing female Sprague Dawley rats were given stigmasterol and β-monolinolein as a combinatorial therapy to assess its effect on tumor growth. Tumor-bearing animals show a reduced body weight in comparison to body weight of control animals (Table 2). Upon the oral administration of the stigmasterol and β-monolinolein combination (20 mg/kg body weight), the animals show a recovery in their body weight from 152 ± 6.7 g (control mice) to 187 ± 5.8 g (treated mice), suggesting reduction in the tumor burden of these animals. A significant difference between the body weight of control and stigmasterol and β-monolinolein-treated animals suggesting that stigmasterol and β-monolinolein has a tumor-suppressing effect in vivo.
Table 2.
Effect of stigmasterol and β-monolinolein on body weight in control (without tumor), DMBA-treated (with tumor) and plant compound-treated SD rats
| Body weight (g) | Group A (normal rats) | Group B (stigmasterol and β-monolinolein) | Group C (DMBA alone) | Group D* (DMBA + Stigm-asterol and β-monolinolein) | Group E* (DMBA and doxorubicin) |
|---|---|---|---|---|---|
| Initial (7 weeks old) | 126 ± 3.4 | 131 ± 3.5 | 125 ± 3.1 | 130 ± 4.7 | 127 ± 3.4 |
| Final (22 weeks old) | 191 ± 4.8 | 188 ± 4.3 | 152 ± 6.7 | 187 ± 5.8 | 171 ± 7.5 |
(n = 6), values are expressed as mean ± SD
*Significant difference in body weight at p < 0.05 level
After 17 weeks of DMBA treatment, all the treated groups C, D and E (Fig. 8a) were assessed for tumor formation and growth. Following mammary tumor development, the tumor weight and volume was also analyzed in these different groups (DMBA treated). The average tumor volume recorded for DMBA (control) and stigmasterol and β-monolinolein-treated animals, respectively, is 4896.2 and 2558 mm3. Similarly, the average tumor weight recorded for control and stigmasterol and β-monolinolein-treated animals, respectively, was 19.4 and 8.16 g. The overall difference in tumor volume and weight are represented graphically in Fig. 8b, c. As the results show, plant compound-treated animals show a very significant reduction in both volume and weight of tumors in comparison to control animals in vivo. Stigmasterol and β-monolinolein treatment can be used as an effective therapeutic combination in controlling tumor growth in vivo. This data indicates that this combination is as efficient as approved drug (doxorubicin) in tumor regression.
Fig. 8.
Effect of plant compounds on growth of mammary tumors. a Images show the appearance of tumors (encircled) after DMBA treatment in female SD rats. b Representative images show dissected mammary tumors from different treatment groups. c The graphs show effect of stigmasterol and β-monolinolein on tumor weight and volume in treated animals compared to control and doxorubicin-treated animals
Immunohistochemistry
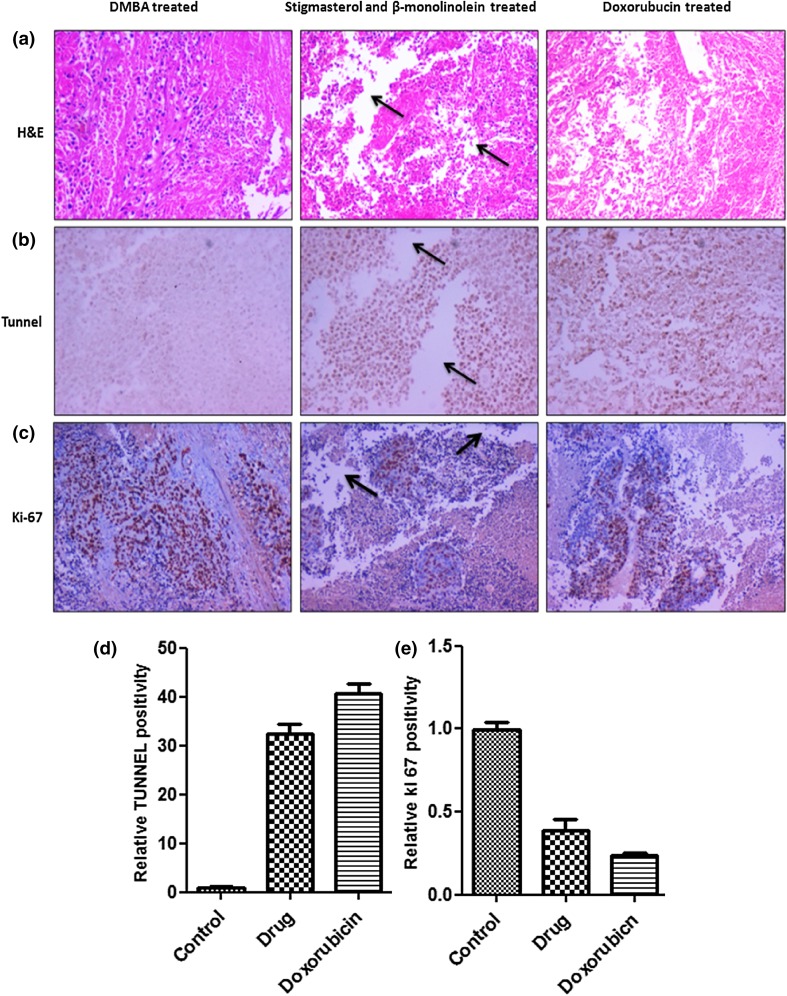
As stigmasterol and β-monolinolein has a potent effect on tumor growth in vivo, it is important to analyze these results at the molecular level. In this regard, immunohistochemistry was performed on the tumor sections from different groups. To analyze the effects quantitatively, apoptotic and proliferation index was also calculated on different tumor sections from each group.As shown in Fig. 9a, H and E staining of tumors revealed extensive tissue damage in tumors treated with stigmasterol and β-monolinolein in comparison to control animals. To evaluate extent of apoptosis, TUNEL assay, which is a measure of DNA damage, was performed. As shown in Fig. 9b and represented graphically in Fig. 9d, the results show that tumors treated with stigmasterol and β-monolinolein have approximately 35-fold high-apoptotic index in comparison to control mice. Conversely, Ki-67 staining was performed to measure extent of proliferation in these different groups. As shown in Fig. 9c and represented graphically in Fig. 9e, the results show that tumors treated with stigmasterol and β-monolinolein have a significantly low proliferative index in comparison to control animals. This is indicated by percentage of nuclei staining positive (intense brown staining) for the assay. The results indicate an approximately 2.5-fold decrease in proliferation of tumor cells in stigmasterol and β-monolinolein-treated animals. These results were arrived at by calculating percentage of positively stained nuclei in control versus stigmasterol and β-monolinolein-treated and doxorubicin-treated animals. These results demonstrated that stigmasterol and β-monolinolein treatment was associated with a very significant decreased in proliferation and increased apoptosis. Analysis of data suggests that stigmasterol and β-monolinolein has a potent anti-tumor activity in DMBA-induced female Sprague Dawley rats.
Fig. 9.
Immunohistochemistry analysis for detection of apoptosis and cell proliferation in tumors. a Histological features of tumors with H and E staining extensive tissue damage in tumors treated animals (arrow). b TUNEL staining on paraffin sections from solid tumor tissues and c Ki-67 staining of tumor tissue sections (×10 magnification). d, e The graph represents the quantitative estimation of increase in number of TUNEL-positive and decrease in number of ki-67-positive cells upon treatment with stigmasterol and β-monolinolein, respectively
Estimation of glycoprotein components in plasma and liver
The results indicated the elevated levels of hexoses from 122 ± 9.2 and 167 ± 11.4 mg/dl in plasma, 3.11 ± 0.56 and 7.56 ± 0.82 mg/g in liver of normal and tumor-bearing animals, respectively (Table 3). After stigmasterol and β-monolinolein administration, the levels of hexoses were reduced 135 ± 7.8 mg/dl in the plasma compared to 167 ± 11.4 mg/dl in the tumor control (Group C). Similarly, the levels of hexoses were reduced to 4.33 ± 0.86 mg/g in the liver of treated animals compared to 7.56 ± 0.82 mg/g in the tumor control. The results also showed that the levels of hexosamine and sialic acid showed similar tendency (Table 3). The reduction in the level of glycoprotein activity in stigmasterol and β-monolinolein treated animals as compared to the tumor-bearing confirm its anti-tumor activity.
Table 3.
Effect of stigmasterol and β-monolinolein on glycoprotein components in plasma and liver in control and experimental animals
| Parameters | Group A (normal rats) | Group B (stigmasterol and β-monolinolein) | Group C (DMBA alone) | Group Da (DMBA and stigmasterol and β-monolinolein) | Group Ea (doxorubicin) |
|---|---|---|---|---|---|
| Plasma (mg/dl) | |||||
| Hexose | 122 ± 9.2 | 119 ± 8.3 | 167 ± 11.4 | 135 ± 7.8 | 129 ± 7.7 |
| Hexosamine | 30.6 ± 3.14 | 30 ± 3.0 | 46 ± 4.6 | 37 ± 3.5 | 34 ± 3.6 |
| Sialic acid | 8.9 ± 4.2 | 44.7 ± 3.7 | 112 ± 8.3 | 78 ± 4.7 | 69 ± 4.8 |
| Liver (mg/g of defatted tissue) | |||||
| Hexose | 3.11 ± 0.56 | 3.16 ± 0.76 | 7.56 ± 0.82 | 4.33 ± 0.86 | 3.87 ± 0.64 |
| Hexosamine | 2.98 ± 0.43 | 3.05 ± 0.79 | 9.12 ± 0.47 | 4.07 ± 0.44 | 3.31 ± 0.90 |
| Sialic acid | 2.47 ± 0.38 | 3.51 ± 0.6 | 5.23 ± 0.66 | 3.17 ± 0.54 | 2.83 ± 0.73 |
Values are expressed as mean ± SD
aWhen compared with Group C, Group D and E showed significant difference
Estimation of lysosomal enzymes
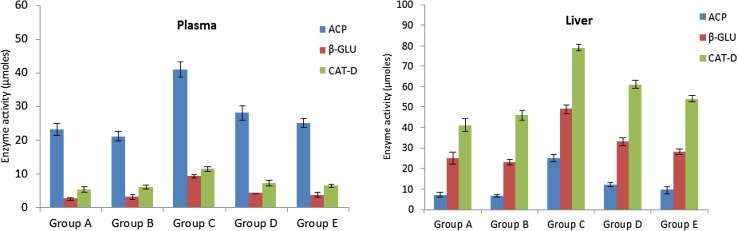
During the tumor progression, high levels of lysosomal enzymes are released from the tumor cells which serve as tumor biomarkers. In this regard, activities of various lysosomal enzymes such as acid phosphatase, β-d-glucuronidase and cathepsin-d were analyzed in plasma and liver of control and experimental animals to assess the rate of tumor progression (Fig. 10). The activities of lysosomal enzymes were found to be significantly increased in DMBA-treated animals compared to animals without tumors. The enzyme activities were significantly reverted to near-normal levels upon treatment with stigmasterol and β-monolinolein, indicating a reduction of tumor burden in these animals.
Fig. 10.
The activity of acid phosphatases (ACP), β-glucuronidase (β-GLU) and cathepsin-D (CAT-D) in the plasma and liver of various experimental groups. Compared to Group C, Group D and E show prominent decrease in the levels of these lysosomal enzymes that was similar to normal rats
Estimation of tumor marker enzymes
The analysis of tumor marker enzymes can be used as an indication of neoplastic condition and therapy. The effect of stigmasterol and β-monolinolein on the levels of gamma-glutamyltransferase (γ-GT), lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) in plasma and liver showed similar values with normal animals (Table 4) kept as group A. DMBA-treated animals have shown an increase in levels of these tumor marker enzymes compared to normal (without DMBA) rats. Stigmasterol and β-monolinolein (group B) did not show any toxic side effects on rats and this was indicated by similar type of enzyme response seen in the normal rats. Hence due to the treatment of stigmasterol and β-monolinolein in tumor bearing rats, the levels of these marker enzymes were significantly reduced, suggesting the suppression of mammary tumors in stigmasterol and β-monolinolein treated animals.
Table 4.
Effect of stigmasterol and β-monolinolein on tumor marker enzymes in plasma and liver tissue of control and treated animals
| Parameters | Group A (normal rats) | Group B (stigmasterol + β-monolinolein) | Group C (DMBA alone) | Group Da (DMBA + stigmasterol and β-monolinolein) | Group Ea (doxorubicin) |
|---|---|---|---|---|---|
| Plasma | |||||
| Gamma-glutamyltransferase (IU/dl) | 1.13 ± 0.16 | 1.07 ± 0.11 | 2.72 ± 0.19 | 1.69 ± 0.15 | 1.39 ± 0.18 |
| Lactate dehydrogenase (IU/dl) | 0.29 ± 0.04 | 0.35 ± 0.07 | 1.27 ± 0.09 | 0.59 ± 0.08 | 0.46 ± 0.10 |
| Alkaline phosphatase µmol/min/mg protein | 1.21 ± 0.07 | 1.32 ± 0.13 | 2.69 ± 0.09 | 1.93 ± 0.16 | 1.61 ± 0.12 |
| Liver | |||||
| Gamma glutamyl transferase (µmol/min/mg protein) | 5.16 ± 0.31 | 5.31 ± 0.27 | 8.93 ± 0.16 | 6.25 ± 0.17 | 5.80 ± 0.19 |
| Lactate dehydrogenase (µmol/min/mg protein) | 2.95 ± 0.21 | 3.15 ± 0.27 | 6.35 ± 0.15 | 4.11 ± 0.19 | 3.65 ± 0.11 |
| Alkaline phosphatase µmol/min/mg protein | 1.19 ± 0.11 | 1.26 ± 0.19 | 3.07 ± 0.27 | 2.09 ± 0.21 | 1.63 ± 0.23 |
Values are expressed as mean ± SD
aWhen compared with Group C, Group D and E show a significant difference; statistical significance
Discussion
Breast cancer is known to be one of the primary causes of death among women worldwide. The breast cancer mortality was recorded to be around 23% (1.38 million) cases worldwide and hence poses a major challenge to human health (Jemal et al. 2011). The high mortality rate among cancer patients is due to lack of efficient cancer diagnostics and therapeutics. This necessitates search for novel methods and molecules for treatment and prevention of cancer. In this regard, plants offer a diverse source of compounds with varied biological activities for development of novel therapeutic agents (Kellof 2000). A large number of drugs that are currently in clinical use are of plant origin. Some of these molecules are either directly obtained from plants or exist as a modified form of plant compounds. Hence, the present investigations were undertaken for bioassay-guided isolation, identification and characterization of cytotoxic molecules from the leaves of A. precatorius for their potential use in cancer treatment.
The bioactivity-directed strategy was adopted for purification and characterization of active compounds in EAF-AP. Column-chromatographic fractionation using petroleum ether: ethyl acetate as a solvent system yielded eight fractions in the EAF-AP (fraction A to H). Among these, only fraction C showed significant cytotoxic activity on MDA-MB-231 cells in a concentration-dependent manner. This fraction was further subjected to column-chromatographic fractionation to isolate specific cytotoxic compounds in pure form. Gradient column chromatography of the fraction C produced six sub-fractions. Among these, only sub-fractions C3 and C4 were found to be significantly active with an IC50 value of 74.2 and 13.2 µg/ml, respectively. Both fractions C3 and C4 were subjected for further spectroscopic analyses. Fractions C3 was recrystallized in dichloromethane and colorless needle-shaped crystals were harvested and used for X-ray crystallographic analysis. This identifies C3 fraction as stigmasterol hemihydrate with molecular formula as C29H48O.0.5H2O with molecular weight as 412.6908 g/mol. The fraction C4 was elucidated as 9,12-Octadecadienoic acid (Z, Z)-2-hydroxy-1-(hydroxymethyl) ethylester or (β-monolinolein) with molecular formula was deduced as C21H38O4 with molecular weight as 354.5310 g/mol. Stigmasterol has been isolated from various medicinal plants such as Croton sublyratus (De-Eknamkul and Potduang 2003), Akebiaquinata (Liu et al. 2005), etc. Stigmasterol is known to have anti-cancer activity against various types of cancer cells. Gomez et al. stated that stigmasterol isolated from chloroform extract of Achillea ageratum has shown significant cytostatic activity against Hep-2 and McCoy cells (Gomez et al. 2001). These results are in concordance with Rashed and Fouche 2013; Sudha et al. 2013, that stigmasterol is known to contain anti-cancer activity. The β-monolinolein is a long-chain unsaturated fatty acids. It was previously reported that fatty acid esters are known to exhibit cytotoxicity against HeLa, HepG2 and MCF-7 cells (Aziz et al. 2009; Lee et al. 2009).
Animal experimental systems are particularly useful in the study of human mammary carcinogenesis, since the rat mammary gland is highly susceptible to the development of neoplasms, which closely mimic human breast cancer (Samy et al. 2006). In the present study, 30 female Sprague Dawley rats were used for the evaluation of anti-cancer activity of stigmasterol and β-monolinolein, as a combinatorial drug therapy for the treatment of breast cancer. It has been demonstrated that oral administration of various phytochemicals in combination significantly protected tumor formation in DMBA-induced mammary carcinogenesis in SD rats (Pugalendhi and Manoharan 2010). This is because the tumor cells develop resistance to single drug molecules more easily than multiple drugs given simultaneously. Hence, combinations of drugs are tested for better tumor regression. In this direction, combined use of stigmasterol and β-monolinolein derived from A. precatorius leaves were tested in DMBA-treated female SD rats for its use as a chemotherapeutic agent. This was determined by checking various parameters such as the body weight, levels of various tumor biomarkers, tumor weight/volume and histopathological examination of paraffin-embedded tumor sections. A severe loss in body weight was observed in cancer-bearing SD rats compared to normal rats due to the carcinogenic effects of DMBA. The loss in the body weight of the plant compound treated animals was recovered significantly after oral administration of stigmasterol and β-monolinolein. Significant reduction was observed in the tumor weight and volume of stigmasterol and β-monolinolein-treated animals compared to DMBA tumor-bearing animals. Further, stigmasterol and β-monolinolein-treated animals does not show any toxic side effects. This showed that stigmasterol and β-monolinolein treatment was effective in controlling the tumor growth in vivo. The data also suggested that in comparison to doxorubicin (standard drug), stigmasterol and β-monolinolein treatment was also efficient in inducing tumor regression.
The histopathological examination (H and E staining) revealed extensive tumor cellular damage in rats treated with stigmasterol and β-monolinolein. To evaluate extent of apoptosis, TUNEL assay was performed on stigmasterol and β-monolinolein-treated tumor sections. The results indicated relatively high-apoptotic index of the treated tumors (indicated by high-nuclear staining intensity) in comparison to control (DMBA-treated) SD rats. Cell proliferation, which was reflected by immunohistochemical staining in Ki-67, was compared with the control, doxorubicin alone and stigmasterol and β-monolinolein-treated SD rats. Mammary tumors in the tumor control (group C) showed an intense staining for Ki-67 in the nucleus, suggesting a high rate of proliferation in these cells. In contrast, the expression of Ki-67 from the stigmasterol and β-monolinolein treated SD rats (group D) exhibited lower staining indicating reduced number of proliferating cells in these tumors. Collectively, immunohistochemical data suggests chemopreventive efficacy of stigmasterol and β-monolinolein treatment in mammary carcinoma-induced animal models.
Glycoproteins are the potential tumor markers (Dacremont 1972), when the cells undergo neoplastic transformations, the activities of glycosidases increase two to three times as compared to normal tissue (Bossmann and Hall 1974). Thus, the combined evaluation of hexose, hexosamine and sialic acid residues of glycoproteins might help to establish a useful aid in strengthening the diagnosis and treatment of mammary cancer patients (Dube and Bertozzi 2005). The expression of glycoprotein components in DMBA-generated mammary tumors was significantly increased compared to normal rats. On stigmasterol and β-monolinolein treatment, glycoprotein component levels were reverted back to near-normal levels. Similarly, high levels of lysosomal enzymes were released from the tumor sites (Beem et al. 1987). The expression of lysosomal enzymes in DMBA-generated mammary tumors in group was significantly increased when compared to normal rats. On stigmasterol and β-monolinolein treatment, expression of these lysosomal enzymes were reverted back to near normal. In addition, increase in the activities of marker enzymes such as gamma-glutamyltransferase, lactate dehydrogenase and alkaline phosphatase can be attributed to over expression of enzymes and higher rate of glycolysis by proliferated cells in various types of cancers (Lippert et al. 1981; Perumal et al. 2015). On stigmasterol and β-monolinolein treatment, expression of these tumor marker enzymes were reverted back to near-normal levels suggesting the tumor-suppressive effect of stigmasterol and β-monolinolein. This showed that the stigmasterol and β-monolinolein acts as a safe-positive pharmacological agent in treating breast tumors in female SD rats. In conclusion, the combinatorial drug therapy with stigmasterol and β-monolinolein can be very useful for the treatment of breast cancer.
Acknowledgements
The authors are grateful to the Department of Microbiology and Biotechnology, Bangalore University, Bangalore, Karnataka, India for support and facilities to carry out our research. Mohammad Shafi Sofi conveys sincere thanks to UGC, New Delhi for providing UGC-BSR Meritorious Fellowship (no. F.7-322/2011-BSR).
Compliance with ethical standards
Conflict of interest
No conflict of interest was declared.
References
- Aziz AA, Rady HM, Amer MA, Kiwan HS. Effect of some honey bee extracts on the proliferation, proteolytic and gelatinolytic activities of the hepatocellular carcinoma Hep-G2 cell line. Aust J Basic Appl Sci. 2009;3:2754–2769. [Google Scholar]
- Barros AC, Muranaka ENK, Mori LJ, Pelizon HTI, Kyoshi G, Giovana P, Jose A. Induction of experimental mammary carcinogenesis in rats with 7, 12- dimethylbenz (a) anthracene. J Hosp Clin. 2004;59:257–261. doi: 10.1590/S0041-87812004000500006. [DOI] [PubMed] [Google Scholar]
- Beem EP, Hillebrand MJ, Benckhuijsen C, Overdijk B. Origin of the increased activity of beta-glucuronidase in the soluble fraction of rat mammary tumors during ovariectomy-induced regression. Can Res. 1987;47:3980–3987. [PubMed] [Google Scholar]
- Bhardwaj DK, Bisht MS, Mehta CK. Flavonoids from Abrus precatorius. Phytochemistry. 1980;19:2040–2041. doi: 10.1016/0031-9422(80)83038-X. [DOI] [Google Scholar]
- Bossmann HB, Hall TC. Enzyme activity in invasive tumors of human breast and colon. Proc Natl Acad Sci USA. 1974;71:18331837. doi: 10.1073/pnas.71.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB. Breast cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- Chang HM, Chiang TC, Thomas CWM. Isolation and structure elucidation of abruslactone A: a new oleanene-type triterpene from the roots and vines of Abrus precatorius L. J Chem Soc Chem Commun. 1982;20:1197–1198. doi: 10.1039/c39820001197. [DOI] [Google Scholar]
- Dacremont G. Ganglioside concentration in human plasma. Clin Chim Acta. 1972;37:449–454. doi: 10.1016/0009-8981(72)90468-8. [DOI] [PubMed] [Google Scholar]
- De-Eknamkul W, Potduang B. Biosynthesis of β-sitosterol and stigmasterol in Croton sublyratus proceeds via a mixed origin of isoprene units. Phytochemistry. 2003;623:389–398. doi: 10.1016/S0031-9422(02)00555-1. [DOI] [PubMed] [Google Scholar]
- Dube DH, Bertozzi CR. Glycans in cancer and inflammation potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- Ghosal S, Dutta SK. Alkaloids of Abrus precatorius. Phytochemistry. 1971;10:195–198. doi: 10.1016/S0031-9422(00)90270-X. [DOI] [Google Scholar]
- Glossman H, Neville DM. Glycoproteins of cell surfaces: a comparative study of three different cell surfaces of the rat. J Biol Chem. 1971;246:6339–6346. [PubMed] [Google Scholar]
- Gomez MA, García MD, Saenz MT. Cytostatic activity of Achillea ageratum L. Phytother Res. 2001;15:633–634. doi: 10.1002/ptr.837. [DOI] [PubMed] [Google Scholar]
- Gul MZ, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA. Antioxidant and antiproliferative activities of Abrus precatorius leaf extracts-an in vitro study. BMC Complement Altern Med. 2013;13:53–65. doi: 10.1186/1472-6882-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11:121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Camoirano A, Cartiglia C, Grubbs CJ, Lubet RA, Kelloff GJ, Flora SD. Patterns of DNA adduct formation in liver and mammary epithelial cells of rats treated with 7, 12-dimethylbenz(a)anthracene, and selective effects of chemopreventive agents. Can Res. 1999;59:4285–4290. [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;2:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kalt W, Ryan DAJ, Duy JC, Prior RL, Ehlenfeldt MK, Kloet SPV. Interspecific variation in anthocyanins, phenolics, and antioxidant capacity among genotypes of high bush and low bush blueberries (Vaccinium section Cyanococcus spp.) J Agric Food Chem. 2001;49:4761–4767. doi: 10.1021/jf010653e. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Anno K. Mucopolysaccharide degrading enzymes from the liver of squid Omma strephessolani pacificus I Hyaluronidase. Biochimica et Biophyicas Acta. 1971;242:428–436. doi: 10.1016/0005-2744(71)90234-8. [DOI] [PubMed] [Google Scholar]
- Kellof GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2000;78:199–334. doi: 10.1016/S0065-230X(08)61026-X. [DOI] [PubMed] [Google Scholar]
- King J. Practical clinical enzymology. London: Nostrand Company Pvt. Ltd.; 1965. The hydrolases-acid and alkaline phosphatises. [Google Scholar]
- King J. Practical clinical enzymology. London: Nostrand Company Pvt. Ltd.; 1965. The dehydrogenase of oxidoreductase-lactate dehydrogenase. [Google Scholar]
- Lee SD, Guijae YE, Hee JCE, Man-Jin IE, Nam-Soon OE, Yoon KHE, Woo IHE, Dong CK. Lipid soluble extracts as the main source of anticancer activity in ginseng and ginseng marc. J Am Oil Chem Soc. 2009;86:1065–1071. doi: 10.1007/s11746-009-1460-x. [DOI] [Google Scholar]
- Lippert M, Papadopoulos N, Javadpour NR. Role of lactate dehydrogenase isoenzymes in testicular cancer. Urology. 1981;18:50–53. doi: 10.1016/0090-4295(81)90495-7. [DOI] [PubMed] [Google Scholar]
- Liu G, Zheng J, Yu Z, Zhang J, Lin R. Study on sterols and triterpenes from the stems of Akebia quinata. J Chin Med Mater. 2005;28:1060–1062. [PubMed] [Google Scholar]
- Minari JB, Okeke U. Chemopreventive effect of Annona muricata on DMBA induced cell proliferation in the breast tissues of female albino mice. Egypt J Med Hum Genet. 2014;15:327–334. doi: 10.1016/j.ejmhg.2014.05.001. [DOI] [Google Scholar]
- Misra R, Acharya S, Sahoo SK. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov Today. 2010;15:842–850. doi: 10.1016/j.drudis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Miyata M, Furukawa M, Takahashi K, Gonzalez FJ, Yamazoe Y. Mechanism of 7, 12-dimethylbenz[a]anthracene induced immunotoxicity: role of metabolic activation at the target organ. Jpn J Pharmacol. 2001;86:302–309. doi: 10.1254/jjp.86.302. [DOI] [PubMed] [Google Scholar]
- Niebes P. Determination of enzyme and degradation products of GAG metabolism in the serum of healthy and various subjects. Clin Chim Acta. 1972;42:399–408. doi: 10.1016/0009-8981(72)90105-2. [DOI] [Google Scholar]
- Perumal S, Babu LH, Langeswaran K, Kumar SG, Balasubramanian MP. Pharmacological potential of natural flavonoid diosmin against n-nitrosodiethylamine induced hepatocellular carcinogenesis in wistar albino rats. Int J Res Biosci. 2015;4:25–36. [Google Scholar]
- Pugalendhi P, Manoharan S. Chemopreventive potential of genistein and daidzein in combination during 7, 12-dimethylbenz(a)anthracene (DMBA) induced mammary carcinogenesis in Sprague–Dawley rats. Pak J Biol Sci. 2010;13:279–286. doi: 10.3923/pjbs.2010.279.286. [DOI] [PubMed] [Google Scholar]
- Rashed KN, Fouche G. Chemical constituents, phytochemical analysis and in vitro anticancer activity of Hedera helix L. Topclass. J Herb Med. 2013;2:223–227. [Google Scholar]
- Rosalki SB, Rau D. Serum gamma glutamyl transpeptidase activity in alcoholism. Int J Clin Chem. 1972;39:41–47. doi: 10.1016/0009-8981(72)90297-5. [DOI] [PubMed] [Google Scholar]
- Rosenblit PD, Metzger RP, Wick AN. Effect of streptozotocin diabetes on acid phosphatase and selected glycosidase activities of serum and various rat organs. Proc Soc Exp Biol Med. 1974;145:244248. doi: 10.3181/00379727-145-37786. [DOI] [PubMed] [Google Scholar]
- Russo J, Russo IH. Experimentally induced mammary tumors in rats. Breast Cancer Res Treat. 1996;39:7–20. doi: 10.1007/BF01806074. [DOI] [PubMed] [Google Scholar]
- Samy RP, Gopalakrishnakone P, Ignacimuthu S. Antitumor promoting potential of luteolin against 7, 12-dimethylbenz(a)anthracene induced mammary tumors in rats. Chem Biol Interact. 2006;164:1–14. doi: 10.1016/j.cbi.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Sapolsky AI, Atlman RD, Howell DS. Cathepsin D activity in normal and osteoarthritic human cartilage. Fed Proc. 1973;32:14891493. [PubMed] [Google Scholar]
- Sgariglia MA, Soberon JR, Sampietro DA, Quiroga EN, Vattuone MA. Isolation of antibacterial components from infusion of Caesalpinia paraguariensis bark—a bioguided phytochemical study. Food Chem. 2011;126:395–404. doi: 10.1016/j.foodchem.2010.10.104. [DOI] [Google Scholar]
- Sharma S, Panjamurthy K, Choudhary B, Srivastava M, Shahabuddin M, Giri R, Advirao GM, Raghavan SC. A novel DNA intercalator, 8-methoxy pyrimido[4′,5′:4,5] thieno (2,3-b)quinoline-4(3H)-one induces apoptosis in cancer cells, inhibits the tumor progression and enhances lifespan in mice with tumor. Mol Carcinog. 2013;52:413–425. doi: 10.1002/mc.21867. [DOI] [PubMed] [Google Scholar]
- Shu LM, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010;29:483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- Sudha T, Chidambarampillai S, Mohan VR. GC–MS analysis of bioactive components of aerial parts of Fluggea leucopyrus Willd. (Euphorbiaceae) J Appl Pharm Sci. 2013;3:126–130. [Google Scholar]
- Sujit K, Tanusri B, Sourav P, Jadupati M, Amites G, Amitava G. Pharmacognostical studies and chromatographic evaluation of the different extracts of Abrus precatorius Linn. Int J Pharm Res Dev. 2012;4:225–233. [Google Scholar]
- Warren L. The thiobarbituric acid assay of sialic acid. J Biol Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]