Abstract
Chronic heart failure and depressive disorders have a high prevalence and incidence in the elderly. Several studies have shown how depression tends to exacerbate coexisting chronic heart failure and its clinical outcomes and vice versa, especially in the elderly. The negative synergism between chronic heart failure and depression in the elderly may be approached only taking into account the multifaceted pathophysiological characteristics underlying both these conditions, such as behavioural factors, neurohormonal activation, inflammatory mediators, hypercoagulability and vascular damage. Nevertheless, the pathophysiological link between these two conditions is not well established yet. Despite the high prevalence of depression in chronic heart failure elderly patients and its negative prognostic value, it is often unrecognized especially because of shared symptoms. So the screening of mood disorders, using reliable questionnaires, is recommended in elderly patients with chronic heart failure, even if cannot substitute a diagnostic interview by mental health professionals. In this setting, treatment of depression requires a multidisciplinary approach including: psychotherapy, antidepressants, exercise training and electroconvulsive therapy. Pharmacological therapy with selective serotonin reuptake inhibitors, despite conflicting results, improves quality of life but does not guarantee better outcomes. Exercise training is effective in improving quality of life and prognosis but at the same time cardiac rehabilitation services are vastly underutilized.
Keywords: Chronic heart failure, Depression, The elderly
1. Introduction
Chronic heart failure (CHF) is a syndrome caused by a structural and/or functional cardiac abnormality, resulting in a reduced cardiac output and/or elevated intracardiac pressures at rest or during stress.[1] According to the Diagnostic and Statistical of Mental Disorders (DSM)-V criteria, depression is a condition characterized by the presence of depressed mood or loss of pleasure for more than two weeks with at least four additional symptoms among neurovegetative, affective and cognitive ones.[2] The link between cardiovascular diseases (CVDs) and depression has been studied over the past 15 years, because the coexistence of these two conditions represents a frequent observation in clinical practice.[3] Nevertheless, the pathophysiological link between these two conditions is not well established yet. The aim of this review is to analyse the most significant evidences in order to hypothesize possible pathophysiological interplays between these two diseases.
2. Epidemiology
CHF is highly prevalent in the elderly population with an incidence of > 4%, one-year mortality of > 20% and a prevalence of > 20% in individuals ≥ 75 years.[4],[5] Depression is a typical aspect of frail elderly patients with CHF.[6] In particular, in these patients, depression has a prevalence of 20% and it is expected to become the second leading cause of inability and disability after CVD by 2020.[7] Prevalence and incidence of the coexistence of CHF and depression in elderly patients are summarized in Table 1.[8]–[18] While it has been showed an increased prevalence of CHF in elderly patients having major depression,[8] it has been also demonstrated that CHF is an independent risk factor for incident depression in the elderly and that treating the debilitating symptoms of CHF, for example with loop diuretics, may prevent depression development.[18] Moreover, some authors found a stronger relationship between the development of depression and the presence of CHF among elderly women than in elderly men.[12] In this matter the majority of studies described an increased prevalence and incidence of depression among CHF elderly patients,[9]–[11],[13],[14],[16],[17],[19] especially in those with isolated systolic hypertension.[11] In particular, lower social support and hospitalization were identified as worsening factors for the development of psychological distress.[13],[15]
Table 1. Incidence and prevalence of depression in CHF elderly patients and vice versa.
| Citations | Year | Type of study | Age mean | Tools | Outcomes | Results |
| Tresch, et al.[9] | 1985 | Comparative | 73.3 ± 8.7 | DSM-III criteria | Prevalence | CHF is common in patients with major depression |
| Cacciatore, et al.[10] | 1998 | Observational | 73.9 ± 6.2 | GDS | Prevalence | Depression is more prevalent in CHF than in no-CHF elderly patients |
| Koenig HG[11] | 1998 | Prospective | 70.2 | CES-D | Prevalence | Depression has higher prevalence in CHF patients |
| Abramson, et al.[12] | 2001 | Prospective | 72.2 | CES-D | Prevalence | Depression increases the risk of CHF among older persons with isolated systolic hypertension |
| Williams, et al.[13] | 2002 | Prospective | 74.8 | CES-D | Incidence | Depression increases CHF in women but not elderly men |
| Yu, et al.[14] | 2004 | Observational | 77.1 ± 7.9 | HADS | Prevalence | Depression is more prevalent in CHF elderly patients and correlates with lower social support |
| Gottlieb, et al.[15] | 2004 | Descriptive | 64 ± 12 | BDI | Prevalence | Depression is common in patients with CHF |
| Lesman-Leegtel, et al.[16] | 2006 | Observational | 71 ± 12 | CES-D | Prevalence | Depressive symptoms are prominent in hospitalized, elderly CHF patients, especially women |
| Guallar-Castillón, et al.[17] | 2006 | Observational | 77.4 ± 6.8 | GDS | Prevalence | Depression has high prevalence in CHF patients |
| Hägglund, et al.[18] | 2008 | Descriptive | 77.7 ± 8.7 | GDS | Prevalence | Depression is more prevalent in CHF patients |
| Luijendijk, et al.[19] | 2010 | Cohort study | 70.0 ± 8.3 | CES-D | Incidence | CHF increases the incidence of depression |
BDI: beck depression inventory; CES-D: center for epidemiologic studies depression scale; CHF: chronic heart failure; DSM: diagnostic and statistical manual of mental disorders; GDS: geriatric depression scale; HADS: hospital anxiety and depression scale.
3. Pathophysiology
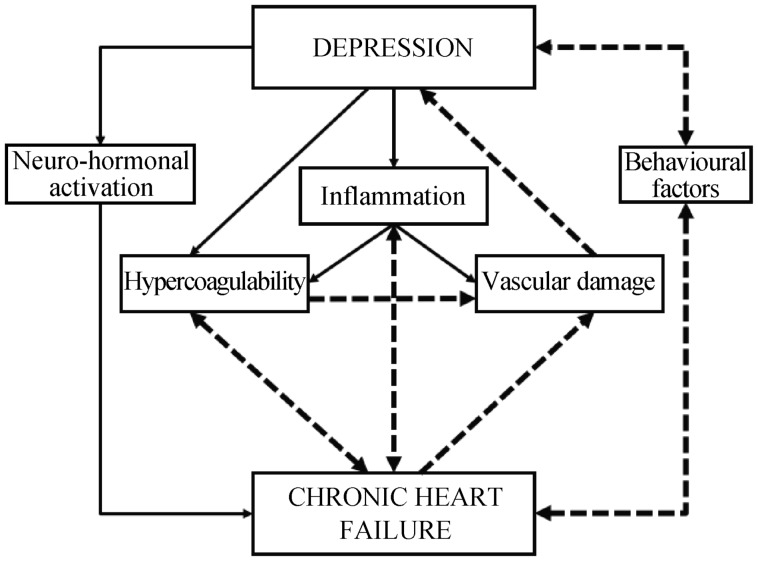
The causal relationship between depression and CHF is not clearly understood. Several mechanisms are involved in the physiopathology of CHF and depression which may represent the common ground for their interaction. We have analysed them to hypothesize possible interplays between the two conditions that involve them all (Figure 1).
Figure 1. Multi-factorial pathophysiology of the relationship between depression and CHF.
Depression and CHF are able to negatively affect one-other, interacting at several different pathophysiological levels. CHF: chronic heart failure.
3.1. Behavioural factors
Depressive symptoms, such as increased fatigue, lack of motivation and inability to concentrate compromise patients' adherence to therapy and healthy lifestyle, with reduced physical activity and increased tobacco and alcohol consumption. This behaviour leads to obesity, atherosclerosis and coronary artery disease, which is the main cause of CHF.[20] Moreover, it is well established that not only CHF symptoms, such as asthenia, fatigue and dyspnoea increase the risk of developing depression, but also drugs commonly used in CHF and their side effects; loop diuretics cause frequent urination and possible social embarrassment, aldosterone antagonists can cause gynecomastia and body dysmorphia in men, β-blockers can cause insomnia, fatigue, sexual dysfunction and depression itself.[21] Furthermore, compliance with CHF treatment regimen is difficult in itself because it frequently involves multiple medications to be taken several times a day.[6] Moreover, cognitive impairment is particularly common in CHF patients (30%–80%) and early dementia could be mistaken for depression because of their shared symptoms, such as apathy, lack of initiative, flat affect, self-neglect, social withdrawal, leading to a misdiagnosis.[22] Finally, the hypothetical drug-induced damage and related neurotoxicity should always be taken into account in elderly patients.
3.2. Neurohormonal activation
Stress factors play a key role in CVDs and depression. In both these conditions, the stress response is mediated by the hypothalamic-pituitary-adrenal (HPA) axis, also known to be more active in the elderly, and by the sympathetic branch of the autonomous nervous system (ANS).[23] The activation of HPA axis leads to high levels of cortisol. Hypercortisolism acts negatively on classic cardiovascular (CV) risk factors such as hypertension, central obesity, insulin resistance and hyperglycaemia. Cortisol also has a mineralocorticoid effect, mediated by its binding to mineralocorticoid receptors (MRs), which is responsible for fluid retention and cardiac remodelling. All these conditions contribute to the progression and exacerbation of CHF.[24] The activation of the sympathetic branch of the ANS causes an imbalance between sympathetic and parasympathetic systems, with a concomitant decrease in vagal tone and an increase in plasma catecholamines. According to the Polyvagal Theory, low vagal tone is associated with reduced social engagement and impaired response to environmental stimuli and challenges.[25] Conversely, high catecholamine levels are associated with negative effects on the CV system, such as vasoconstriction, elevated heart rate and platelet activation. Moreover, the decrease in parasympathetic tone is responsible for a decline in heart rate variability (HRV), which is related to poor prognosis in CHF patients.[26]
3.3. Inflammatory mediators
It is well established that the aging process promotes a proinflammatory state in the elderly. Also depression is associated with elevated levels of inflammatory biomarkers and acute-phase proteins, such as interleukine-1 (IL-1), interleukine-6 (IL-6), C Reactive Protein (CRP) and fibrinogen. This pro-inflammatory state is present even when depression is not associated with other medical conditions.[27] More specifically, IL-6 is one of the most powerful stimulators of the HPA axis leading to chronic elevated levels of cortisol. This latter is responsible for compensatory downregulation of glucocorticoid receptor activity in immune cells resulting in the reduction of the inhibition, usually exerted by cortisol, of several immunoregulatory transcription control pathways, such as nuclear factor-KB, activator-protein 1, and JAK-STAT factors.[28] This process ultimately leads to an excessive production of proinflammatory cytokines, which again feeds the inflammatory process in a vicious circle. An immune activation is also present in CHF. Initially it contributes to cardiac response to physiological stress through myocyte hypertrophy and protection from apoptosis.[21] Subsequently, as CHF progresses, the inflammatory mediators, including cytokines, become part of the trigger to a maladaptive CV response to stressors and play an important role in ventricular remodelling, uncoupling of β-adrenergic receptors, apoptosis, and contractile dysfunction.[29] Finally, inflammation is also associated with increased risk of all-cause dementia,[30] which is responsible for poorer compliance to medications, medical appointments and dietary regimens.[31]
3.4. Hypercoagulability
CHF, depression and the aging process independently contribute to a hypercoagulable state. Patients with CHF have higher levels of von Willebrand factor (vWf) and fibrinogen and increased plasma viscosity and platelet activity.[32] Similarly, depression and the aging process are associated with an increased expression of Plasminogen Activator Inhibitor-1 (PAI-1), which inhibits tissue- and urokinase-plasminogen activator (tPA and uPA respectively), which are involved in the fibrinolytic process. In both depression and aging, systemic inflammation, the abovementioned hypercortisolism, obesity and sleep disorders lead to high levels of PAI-1 activity with an increased risk of athero-thrombogenesis and CV events.[33] Moreover, PAI-1 directly inhibits tPA mediated cleavage of pro-BDNF (brain-derived neurotrophic factor) to mBDNF (mature BDNF) which is involved in the worsening of depressive symptoms by inducing a volume reduction of specific brain areas involved in mood control, such as hippocampus.[34] In addition, this may further produce a positive feedback loop.[35]
3.5. Vascular damage
In contrast to depressive disorders in younger adults, depression in the elderly, also called late-life depression, is directly associated with ischemic brain lesions. These latter are characterised by white matter hyper-intensities on structural magnetic resonance imaging and frontal and temporal, especially hyppocampal, grey matter changes or atrophy.[36] According to the “vascular depression (VaDep) hypothesis”, CV risk factors, such as hypertension, diabetes, hyperlipidaemia, cause vascular changes, including increased arterial stiffness and endothelial dysfunction. All these modifications ultimately adversely affect cerebral blood flow resulting in ischemic injuries which predispose or worsen geriatric depressive symptoms.[37]
3.6. Depression and CHF relationship: an intriguing hypothesis
Both depression and CHF are able to negatively affect one-other. These two conditions could possibly interact at several different pathophysiological levels, as previously described.
More specifically, starting from a more comprehensive and systemic level, depression and CHF mutually affect themselves through the common pathways related to the induction of a proinflammatory and hypercoagulability state. In this setting, also the neurohormonal dysfunction is involved in both diseases onset and progression. In fact, acting through both hypercortisolism and ANS imbalance, neurohormonal dysfunction not only worsens CHF and other CVDs, but also depression, as hypothesized in the Polyvagal theory. The proinflammatory and hypercoagulability state along with the neurohormonal dysfunction lead to the next level of interaction between depression and CHF, represented by the vascular involvement. This condition is responsible for the onset and worsening of depressive state through ischemic brain lesions but also for the progression of CVDs associated with CHF. Finally, the behavioural component of the interaction between depression and CHF acts when one or both conditions are present contributing either to the rise of the other one or to their mutual worsening (Figure 1), through social issues related to CHF and poor compliance associated to depression.
Nevertheless, in order to argue which condition came first, we suggest that would be necessary a type of study that, starting from healthy subjects, follows them in time to detect the development of depression or CHF and consequently treat the condition raised and verify the effect on the development of the other one. Unfortunately, at the present time, this type of study is not available.
4. Clinical findings
Depression may be unrecognized in cardiac patients for many reasons, the more important being represented by the similarities between the symptoms of depression and CHF (e.g., low energy, fatigue, sleep disturbance, weight loss or gain, decreased attention and concentration, memory impairment).[38] Furthermore, psychological sphere may be disregarded in doctor-patient interaction or a depressive mood might be underestimated considering it a normal reaction to the disease. In this sense, the use of an acronym SIG-E-CAPS [Sleep disorder (increased or decreased); Interest deficit (anhedonia); Guilt (worthlessness, hopelessness, regret); Energy deficit; Concentration deficit; Appetite disorder (increased or decreased); Psychomotor retardation or agitation; Suicidality][39] may be helpful for remembering the main symptoms of major depression.
These symptoms, worsen quality of life (QoL), are independent risk factors for adverse clinical outcomes among CHF patients and are associated with an increased risk of adverse cardiac events thus making their recognition and treatment crucial and time sensitive issues.[30],[40] Recurring symptoms in CHF such as dyspnea, fatigue, and edema and sleep disturbance combined with recurrent hospitalizations and poor functional status put these patients at risk to develop or worsen a coexistent depression.[41]
5. Diagnosis
In a meta-analysis of 27 studies, the mean prevalence rate of clinically significant depression among CHF patients was 21.5%, even if this result is influenced by the use of either screening tools or diagnostic interview (33.6% and 19.3%, respectively) and by NYHA class (11% in class I, 42% in class IV).[41] Nevertheless, the overlapping signs and symptoms make the diagnosis of depression a challenge in patients with heart failure.[14] Indeed, fewer than 25% of cardiac patients with depressive disorders are identified of which only about one-half receive any treatment.[3] This is a well-defined clinical issue which prompted the American Heart Association (AHA) to screen for depression all patients with coronary heart disease (CHD), the most common cause of CHF, using Patient Health Questionnaire (PHQ-9 & PHQ-2).[42]
Similarly, the European Society of Cardiology (ESC), in the recently released CHF guidelines, defined as good practice the routine screening for depression in these patients using validated questionnaires.[1] For this purpose, the Beck Depression Inventory (BDI)[43] and Cardiac Depression Scale (CDS)[44] are reliable tools even if other questionnaires, such as the Geriatric Depression Scale (GDS),[45] have been widely used.
It is important to underline that screening questionnaires facilitate the assessment of depression but cannot stand for a diagnostic interview by mental health professionals and that the diagnosis of major depression is clinical and based on DSM-V criteria.[2]
6. Prognosis
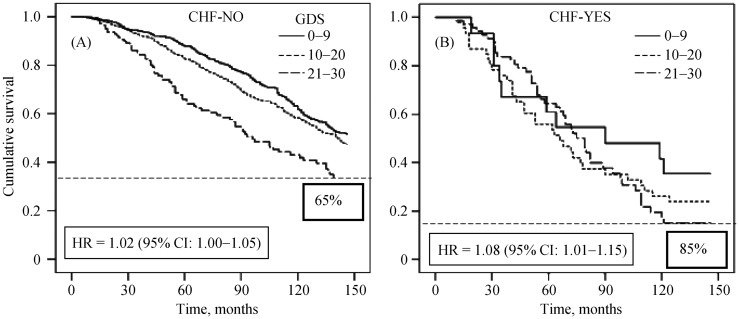
In Table 2[4],[16],[38],[46]–[51] are listed the studies that show how depression worsens clinical outcomes in elderly patients with CHF. As shown in Figure 2, it has been demonstrated that depression is predictive of long-term mortality in the absence and even more in the presence of CHF,[4],[16],[48],[49] being the increasing GDS score associated with a decreased survival, significantly more marked in CHF patients.[52] Some authors assessed that depressive symptoms among CHF patients tend to worsen physical functioning[38],[46] heart failure symptoms[46] and QoL[46],[51] especially in elderly women.[50] These negative effects of depression on CHF elderly patients are, as a consequence, responsible of increased health care utilization and costs in this population[46] and risk of nursing home (NH) admission, which is considered a marker of loss of independent living and poor outcomes.[47] Other recent studies identified depression also as an independent risk factor for hospital re-admission in patients with CHF, in particular in geriatric population.[53]
Table 2. Depression worsens clinical outcomes in elderly patients with CHF.
| Citations | Year | Type of study | Mean age, yrs | Tools | Outcomes | Results |
| Skotzko, et al.[37] | 2000 | Comparative | 64 ± 11 | CES-D | Physical functioning and functional ability | Depression worsens physical functioning and reduces the perception of functional ability |
| Sullivan, et al.[46] | 2004 | Prospective | 75.0 ± 9.7 | HDRS | Health status | Depression worsens health status of elderly CHF patients |
| Guallar-Castillón, et al.[17] | 2006 | Observational | 77.4 ± 6.8 | GDS | Prognosis | Depression worsens prognosis in CHF patients |
| Ahmed, et al.[47] | 2006 | Observational | 78.9 ± 7.6 | ICD-9 | Nursing home admission | Depression increases the risk of nursing home admission in HF elderly patients |
| Johansson, et al.[48] | 2007 | Prospective | 73 ± 5.6 | MHI-5 | Mortality | Depression increases mortality in HF elderly patients |
| Macchia, et al.[49] | 2008 | Prospective | 79.02 | Exposure to antidepressants | Mortality | Depression impairs survival in elderly patients with HF |
| Lesman-LeegteI, et al.[50] | 2009 | Observational | 72 ± 9 | CES-D | QoL | HF worsens HRQoL and is associated to depressive symptoms, especially in elderly women |
| Testa, et al.[4] | 2011 | Observational | 74.2 ± 6.3 | GDS | Mortality | Depression increases mortality in HF patients |
| Uchmanowicz, et al.[51] | 2015 | Observational | 67.9 ± 10.7 | HADS | HRQoL | Depression worsens HRQoL in HF patients |
CES-D: center for epidemiologic studies depression scale; HDRS: Hamilton depression rating scale; HF: heart failure; HRQoL: health related quality of life; GDS: geriatric depression scale; ICD-9: international classification of diseases 9th version; MHI-5: mental health inventory 5.
Figure 2. Cox regression survival curve in the absence (A) and presence (B) of chronic heart failure stratified in tertiles of geriatric depression scale.
Depression is predictive of long-term mortality in the absence and even more in the presence of CHF, being the increasing GDS score associated with a decreased survival, significantly more marked in CHF patients. CHF: chronic heart failure; CI: confidence interval; GDS: geriatric depression scale; HR: hazard ratio. Modified with permission.[4]
7. Therapy
Therapeutic approach in elderly patients with CHF and depression is complex. Of course, CHF therapy should be optimized according to the more recent guidelines.[1] Moreover, treatment of depression requires an additional approach including psychotherapy, antidepressants, exercise training and electroconvulsive therapy (ECT).
7.1. Antidepressants
Several studies have been conducted on the use of antidepressants in elderly CHF patients and the results have been conflicting. Fosbol, et al.[54] in their large study have observed 99,335 patients with CHF between 1997 and 2005; among these patients 19,411 were treated both with CHF therapy and antidepressants [tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitor (SSRIs)] showing that the use of antidepressants was associated with an increased risk of CV mortality. Similar findings were also reported in a Danish study, in which in patients treated with at least one antidepressant an increased risk of mortality was reported.[55] In the MOOD-HF (Mortality, Morbidity and Mood in Depressed Heart Failure Patients) study major depression was treated with escitalopram (a SSRI) or placebo for 12–24 months. Also in this trial, the antidepressive treatment failed to reduce hospitalization and all-cause mortality in patients with CHF and reduced left ventricular ejection fraction (LVEF 45%) but above all it failed in improve symptoms of depression.[56] However, in the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial, in patients with CHF, major depression was treated with sertraline (a SSRI) or placebo for 12 weeks. In this study CHF patients, who achieved remission of depressive symptoms, had a better QoL although it did not guarantee an improvement in overall outcome.[57],[58] In fact, the two main classes of antidepressant medications used in clinical practice are SSRIs and the TCAs.[59] TCAs are known to have several CV side effects: fatal arrhythmias, increased heart rate, orthostatic hypotension and prolong QT-interval, especially in association with β-blockers that are a cornerstone of CHF therapy. In fact, the association of these two classes of drugs causes an abrupt reduction in the survival curve.[53] Moreover, there are few evidences of an increased risk for CVD, ischemic heart disease or myocardial infarction in population-based samples, or mortality in patients with pre-existing CHF.[54],[60],[61] Despite several studies have shown an association between SSRIs use and an increased CV risk, especially of myocardial infarction, SSRIs are preferable over TCAs because of their safety in cardiac patients.[60]–[64] Two studies confirmed the safety of SSRIs showing reduced incidence of myocardial infarction or mortality in CHF patients using SSRIs.[65],[66] β-blockers are associated with lower CV mortality in patients with end-stage, severe heart failure and depression only when they are in association with SSRIs, instead of as observed for the TCA. In fact, this combination is responsible for an increase in survival.[53] SSRIs have also shown an anti-inflammatory effect, based on the ability to reduce IL-6, IL-1 and TNFα levels, which are involved in the pathogenesis of both depression and CHF.[67] Moreover, in some cases SSRIs have been reported to have also a protective effect on CV system.[68]
7.2. Non-pharmacological interventions
Psychotherapy is the application of clinical methods and interpersonal stances derived from established psychological principles with the aim to take care of individual's mental health problems. This can be reached through several methods, among these the most useful for treating depression is cognitive behavioral therapy (CBT).[20] It consists in educate patients to formulate alternative ways to view and react to troubles that usually lead to a depressive mood. In this way CBT may achieve an improvement of patient self-efficacy, a better adherence and a more positive approach to health and life.[69]
Several studies show how exercise training is an effective non-pharmacologic therapeutic choice able to reduce depressive symptoms among CHF patients.[69] There are several mechanisms through which exercise can help CHF patients with coexisting depression. According to the pathophysiology mechanisms that subtend depression, exercise increases the release of neurotransmitters (serotonin, dopamine, and norepinephrine), which are known to be reduced in depressive disorders.[39] The outcome is variable because of the involvement of several variables such as intensity of exercise, severity of underlying disease, and concomitant use of beta-blockers or ACE inhibitors. A recent meta-analysis of randomized clinical trials (19 trials with 3447 patients enrolled) looked into the effects of exercise training on symptoms of depression in CHF patients. Physical activities (walking, bicycle, treadmill, etc.) widely varied across trials and were center-based or home-based or combined. However, the interventions were able to reduce the depressive symptoms more consistently when aerobic exercise was performed by patients with reduced ejection fraction.[70] Although 2016 ESC Guidelines[1] strongly recommend cardiac rehabilitation to improve the prognosis of all CHF patients, this therapeutic approach is still underutilized and, when prescribed, rarely performed.
ECT is considered the “gold standard” in the treatment of geriatric patients with major depression resistant to pharmacotherapy and psychotherapy and who require a rapid response because of the severity of their psychiatric or medical condition.[71] ECT has been used safely in patients with multiple medical comorbidities, but it may be conducted with caution in cardiac patients. Although there are no absolute medical contraindication to ECT, several CVDs may increase the mortality risk associated with ECT (i.e. recent myocardial infarction, poorly compensated congestive heart failure, severe cardiac valvular disease). Older age per se is not a risk factor for mortality associated with ECT, although older adults may be at a greater risk because of a higher prevalence of medical comorbidity, especially CVDs.[72] Because of a brief parasympathetic discharge during ECT, the main CV adverse effects of ECT are arrhythmias (ventricular tachycardia, ventricular fibrillation, bradycardia with second degree heart block, asystole), ischemic changes on ECG and hypotension.[73] So, ECT should be used with caution in elderly depressed CHF patients.
8. Conclusions
CHF and depressive disorders have a high prevalence and incidence in the elderly. Depression tends to exacerbate CHF and this latter worsens QoL and leads to the development of depressive symptoms. Depression is a well-established independent negative prognostic factor in elderly patients with CHF but is often unrecognized in cardiac patients. Indeed, the negative synergism between CHF and depression in the elderly may be approached only taking into account the multifaceted pathophysiological characteristics underlying both these conditions. Accordingly, a multidisciplinary therapeutical approach represents the preferable way to manage this harmful condition, particularly frequent in the elderly.
Acknowledgments
The authors declare that they have no conflict of interest.
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th Edition. 2013. [Google Scholar]
- 3.Rustad JK, Stern TA, Hebert KA, et al. Diagnosis and treatment of depression in patients with congestive heart failure: a review of the literature. Prim Care Companion CNS Disord. 2013;15:PCC.13r01511. doi: 10.4088/PCC.13r01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Testa G, Cacciatore F, Galizia G, et al. Depressive symptoms predict mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2011;41:1310–1317. doi: 10.1111/j.1365-2362.2011.02544.x. [DOI] [PubMed] [Google Scholar]
- 5.Abete P, Testa G, Della-Morte D, et al. Treatment for chronic heart failure in the elderly: current practice and problems. Heart Fail Rev. 2013;18:529–551. doi: 10.1007/s10741-012-9363-6. [DOI] [PubMed] [Google Scholar]
- 6.Cacciatore F, Abete P, Mazzella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 7.Stranieri G, Carabetta C. Socio-economic cultural transformations and depression in elderly people. Psychiatr Danub. 2015;27:S212–S215. [PubMed] [Google Scholar]
- 8.Ghosh RK, Ball S, Prasad V, et al. Depression in heart failure: Intricate relationship, pathophysiology and most updated evidence of interventions from recent clinical studies. Int J Cardiol. 2015;224:170–177. doi: 10.1016/j.ijcard.2016.09.063. [DOI] [PubMed] [Google Scholar]
- 9.Tresch DD, Folstein MF, Rabins PV, et al. Prevalence and significance of cardiovascular disease and hypertension in elderly patients with dementia and depression. J Am Geriatr Soc. 1985;33:530–537. doi: 10.1111/j.1532-5415.1985.tb04616.x. [DOI] [PubMed] [Google Scholar]
- 10.Cacciatore F, Testa G, Galizia G, et al. Clinical frailty and long-term mortality in elderly subjects with diabetes. Acta Diabetol. 2013;50:251–260. doi: 10.1007/s00592-012-0413-2. [DOI] [PubMed] [Google Scholar]
- 11.Koenig HG. Depression in hospitalized older patients with congestive heart failure. Gen Hosp Psychiatry. 1998;20:29–43. doi: 10.1016/s0163-8343(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 12.Abramson J, Berger A, Krumholz HM, et al. Depression and risk of heart failure among older persons with isolated systolic hypertension. Arch Intern Med. 2001;161:1725–1730. doi: 10.1001/archinte.161.14.1725. [DOI] [PubMed] [Google Scholar]
- 13.Williams SA, Kasl SV, Heiat A, et al. Depression and risk of heart failure among the elderly: a prospective community-based study. Psychosom Med. 2002;64:6–12. doi: 10.1097/00006842-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Yu DS, Lee DT, Woo J, et al. Correlates of psychological distress in elderly patients with congestive heart failure. J Psychosom Res. 2004;57:573–581. doi: 10.1016/j.jpsychores.2004.04.368. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb SS, Khatta M, Friedmann E, et al. The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol. 2004;43:1542–1549. doi: 10.1016/j.jacc.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 16.Lesman-Leegte I, Jaarsma T, Sanderman R, et al. Depressive symptoms are prominent among elderly hospitalised heart failure patients. Eur J Heart Fail. 2006;8:634–640. doi: 10.1016/j.ejheart.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Guallar-Castillón P, Magariños-Losada MM, Montoto-Otero C, et al. [Prevalence of depression and associated medical and psychosocial factors in elderly hospitalized patients with heart failure in Spain] Rev Esp Cardiol. 2006;59:770–778. [Article in Spanish] [PubMed] [Google Scholar]
- 18.Hägglund L, Boman K, Lundman B, et al. Depression among elderly people with and without heart failure, managed in a primary healthcare setting. Scand J Caring Sci. 2008;22:376–382. doi: 10.1111/j.1471-6712.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- 19.Luijendijk HJ, Tiemeier H, van den Berg JF, et al. Heart failure and incident late-life depression. J Am Geriatr Soc. 2010;58:1441–1448. doi: 10.1111/j.1532-5415.2010.02921.x. [DOI] [PubMed] [Google Scholar]
- 20.Abete P, Cacciatore F, Ferrara N, et al. Body mass index and pre-infarction angina in elderly patients with acute myocardial infarction. Am J Clin Nutr. 2003;78:796–801. doi: 10.1093/ajcn/78.4.796. [DOI] [PubMed] [Google Scholar]
- 21.Nair N, Farmer C, Gongora E, et al. Commonality between depression and heart failure. Am J Cardiol. 2012;109:768–772. doi: 10.1016/j.amjcard.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara N, Abete P, Giordano M, et al. Neurotoxicity induced by Cefepime in a very old hemodialysis patient. Clin Nephrol. 2003;59:388–390. doi: 10.5414/cnp59388. [DOI] [PubMed] [Google Scholar]
- 23.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Guder G, Bauersachs J, Frantz S, et al. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964. [DOI] [PubMed] [Google Scholar]
- 25.Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 26.Otte C, Neylan TC, Pipkin SS, et al. Depressive symptoms and 24-hour urinary norepinephrine excretion levels in patients with coronary disease: findings from the Heart and Soul Study. Am J Psychiatry. 2005;162:2139–2145. doi: 10.1176/appi.ajp.162.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin. 2006;24:521–538. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blum A, Miller H. Pathophysiological role of cytokines in congestive heart failure. Annu Rev Med. 2001;52:15–27. doi: 10.1146/annurev.med.52.1.15. [DOI] [PubMed] [Google Scholar]
- 30.Koyama A, O'Brien J, Weuve J, et al. The role of peripheral inflammatory markers in dementia and Alzheimer's disease: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2013;68:433–440. doi: 10.1093/gerona/gls187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Lorenzo F, Saba N, Kakkar VV. Blood coagulation in patients with chronic heart failure: evidence for hypercoagulable state and potential for pharmacological intervention. Drugs. 2003;63:565–576. doi: 10.2165/00003495-200363060-00004. [DOI] [PubMed] [Google Scholar]
- 32.Savoy C, Van Lieshout RJ, Steiner M. Is plasminogen activator inhibitor-1 a physiological bottleneck bridging major depressive disorder and cardiovascular disease? Acta Physiol (Oxf) 2017;219:715–727. doi: 10.1111/apha.12726. [DOI] [PubMed] [Google Scholar]
- 33.Rodier M, Prigent-Tessier A, Béjot Y, et al. Exogenous t-PA administration increases hippocampal mature BDNF levels. plasmin- or NMDA-dependent mechanism? PLoS One. 2014;9:e92416. doi: 10.1371/journal.pone.0092416. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Aizenstein HJ, Baskys A, Boldrini M, et al. Vascular depression consensus report—a critical update. BMC Med. 2016;14:161. doi: 10.1186/s12916-016-0720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skotzko CE, Krichten C, Zietowski G, et al. Depression is common and precludes accurate assessment of functional status in elderly patients with congestive heart failure. J Card Fail. 2000;6:300–305. doi: 10.1054/jcaf.2000.19222. [DOI] [PubMed] [Google Scholar]
- 38.Guck TP, Elsasser GN, Kavan MG, et al. Depression and congestive heart failure. Congest Heart Fail. 2003;9:163–169. doi: 10.1111/j.1527-5299.2003.01356.x. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W, Kuchibhatla M, Clary GL, et al. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154:102–108. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 40.Kato N, Kinugawa K, Shiga T, et al. Depressive symptoms are common and associated with adverse clinical outcomes in heart failure with reduced and preserved ejection fraction. J Cardiol. 2012;60:23–30. doi: 10.1016/j.jjcc.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 42.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 43.Lahlou-Laforet K, Ledru F, Niarra R, et al. Validity of Beck Depression Inventory for the assessment of depressive mood in chronic heart failure patients. J Affect Disord. 2015;184:256–260. doi: 10.1016/j.jad.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 44.Ski CF, Thompson DR, Hare DL, et al. Cardiac Depression Scale: Mokken scaling in heart failure patients. Health Qual Life Outcomes. 2012;10:141. doi: 10.1186/1477-7525-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan MD, Newton K, Hecht J, et al. Depression and health status in elderly patients with heart failure: a 6-month prospective study in primary care. Am J Geriatr Cardiol. 2004;13:252–260. doi: 10.1111/j.1076-7460.2004.03072.x. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed A, Ali M, Lefante CM, et al. Geriatric heart failure, depression, and nursing home admission: an observational study using propensity score analysis. Am J Geriatr Psychiatry. 2006;14:867–875. doi: 10.1097/01.JGP.0000209639.30899.72. [DOI] [PubMed] [Google Scholar]
- 48.Johansson P, Dahlström U, Alehagen U. Depressive symptoms and six-year cardiovascular mortality in elderly patients with and without heart failure. Scand Cardiovasc J. 2007;41:299–307. doi: 10.1080/14017430701534829. [DOI] [PubMed] [Google Scholar]
- 49.Macchia A, Monte S, Pellegrini F, et al. Depression worsens outcomes in elderly patients with heart failure: an analysis of 48,117 patients in a community setting. Eur J Heart Fail. 2008;10:714–721. doi: 10.1016/j.ejheart.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Lesman-Leegtel I, Jaarsma T, Coyne JC. Quality of life and depressive symptoms in the elderly: a comparison between patients with heart failure and age- and gender-matched community controls. J Card Fail. 2009;15:17–23. doi: 10.1016/j.cardfail.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Uchmanowicz I, Gobbens RJ. The relationship between frailty, anxiety and depression, and health-related quality of life in elderly patients with heart failure. Clin Interv Aging. 2015;10:1595–1600. doi: 10.2147/CIA.S90077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaccarino V, Kasl SV, Abramson J, et al. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol. 2001;38:199–205. doi: 10.1016/s0735-1097(01)01334-1. [DOI] [PubMed] [Google Scholar]
- 53.Jünger J, Schellberg D, Müller-Tasch T, et al. Depression increasingly predicts mortality in the course of congestive heart failure. Eur J Heart Fail. 2005;7:261–267. doi: 10.1016/j.ejheart.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Fosbøl EL, Gislason GH, Poulsen HE, et al. Prognosis in heart failure and the value of β-blockers are altered by the use of antidepressants and depend on the type of antidepressants used. Circ Heart Fail. 2009;2:582–590. doi: 10.1161/CIRCHEARTFAILURE.109.851246. [DOI] [PubMed] [Google Scholar]
- 55.Veien KT, Videbæk L, Schou M, et al. High mortality among heart failure patients treated with antidepressants. Int J Cardiol. 2011;146:64–67. doi: 10.1016/j.ijcard.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Angermann CE, Gelbrich G, Störk S, et al. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: The MOOD-HF Randomized Clinical Trial. JAMA. 2016;315:2683–2693. doi: 10.1001/jama.2016.7635. [DOI] [PubMed] [Google Scholar]
- 57.Xiong GL, Fiuzat M, Kuchibhatla M, et al. Health status and depression remission in patients with chronic heart failure: patient-reported outcomes from the SADHART-CHF trial. Circ Heart Fail. 2012;5:688–692. doi: 10.1161/CIRCHEARTFAILURE.112.967620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692–699. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang W, Glassman A, Krishnan R, et al. Depression and ischemic heart disease: what have we learned so far and what must we do in the future? Am Heart J. 2005;150:54–78. doi: 10.1016/j.ahj.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Hippisley-Cox J, Pringle M, Hammersley V, et al. Antidepressants as risk factor for ischaemic heart disease: case-control study in primary care. BMJ. 2001;323:666–669. doi: 10.1136/bmj.323.7314.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med. 2000;108:2–8. doi: 10.1016/s0002-9343(99)00301-0. [DOI] [PubMed] [Google Scholar]
- 62.Hamer M, Batty GD, Seldenrijk A, et al. Antidepressant medication use and future risk of cardiovascular disease: the Scottish Health Survey. Eur Heart J. 2011;32:437–442. doi: 10.1093/eurheartj/ehq438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monster TB, Johnsen SP, Olsen ML, et al. Antidepressants and risk of first-time hospitalization for myocardial infarction: a population-based case-control study. Am J Med. 2004;117:732–737. doi: 10.1016/j.amjmed.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 64.Brouwers C, Christensen SB, Damen NL, et al. Antidepressant use and risk for mortality in 121,252 heart failure patients with or without a diagnosis of clinical depression. Int J Cardiol. 2016;203:867–873. doi: 10.1016/j.ijcard.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 65.Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation. 2001;104:1894–1898. doi: 10.1161/hc4101.097519. [DOI] [PubMed] [Google Scholar]
- 66.Schlienger R, Fischer LM, Jick H, et al. Current use of selective serotonin reuptake inhibitors and risk of acute myocardial infarction. Drug Saf. 2004;27:1157–1165. doi: 10.2165/00002018-200427140-00006. [DOI] [PubMed] [Google Scholar]
- 67.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nezafati MH, Vojdanparast M, Nezafati P. Antidepressants and cardiovascular adverse events: a narrative review. ARYA Atheroscler. 2015;11:295–304. [PMC free article] [PubMed] [Google Scholar]
- 69.Herring MP, Puetz TW, O'Connor PJ, et al. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:101–111. doi: 10.1001/archinternmed.2011.696. [DOI] [PubMed] [Google Scholar]
- 70.Tu RH, Zeng ZY, Zhong GQ, et al. Effects of exercise training on depression in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Eur J Heart Fail. 2014;16:749–757. doi: 10.1002/ejhf.101. [DOI] [PubMed] [Google Scholar]
- 71.McDonald WM. Neuromodulation treatments for geriatric mood and cognitive disorders. Am J Geriatr Psychiatry. 2016;24:1130–1141. doi: 10.1016/j.jagp.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 72.Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London) 2014;4:33–54. doi: 10.2217/npy.14.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zielinski RJ, Roose SP, Devanand DP, et al. Cardiovascular complications of ECT in depressed patients with cardiac disease. Am J Psychiatry. 1993;150:904–909. doi: 10.1176/ajp.150.6.904. [DOI] [PubMed] [Google Scholar]