Abstract
Objective
To evaluate the predictive value of red cell distribution width (RDW) on left atrial thrombus (LAT) or left atrial spontaneous echo contrast (LASEC) in patients with non-valvular atrial fibrillation (AF).
Methods
We reviewed 692 patients who were diagnosed as non-valvular AF and underwent transesophageal echocardiography (TEE) in Guangdong Cardiovascular Institute from April 2014 to December 2015. The baseline clinical characteristics, laboratory test of blood routine, electrocardiograph measurements were analyzed.
Results
Eighty-four patients were examined with LAT/LASEC under TEE. The mean RDW level was significantly higher in LAT/LASEC patients compared with the non-LAT/LASEC patients (13.59% ± 1.07% vs. 14.34% ± 1.34%; P < 0.001). Receiver–operating characteristic curve analysis was performed and indicated the best RDW cut point was 13.16%. Furthermore, multivariate logistic regression analysis indicated that RDW level > 13.16% could be an independent risk factor for LAT/LASEC in patients with AF.
Conclusion
Elevated RDW level is associated with the presence of LAT/LASEC and could be with moderate predictive value for LAT/LASEC in patients with non-valvular AF.
Keywords: Atrial fibrillation, Left atrial spontaneous echo contrast, Left atrial thrombus, Red cell distribution width
1. Introduction
Atrial fibrillation (AF), as one of the most common cardiac arrhythmia, is associated with significant morbidity and mortality and essential risk factor for thromboembolic events.[1] The development of thrombi within the left atrium is supposed to be the pathogenesis of thrombo-embolism.[2] Nowadays transesophageal electrocardiograph (TEE) is the gold-standard for identifying left atrium thrombus (LAT) or left atrial spontaneous echo contrast (LASEC) which has been regarded as a precursor of LAT. However, the mechanisms and pathways underlying LAT or LASEC have not yet been fully clarified.
Red cell distribution width (RDW) is a measure of the variability in the size of circulating erythrocytes. Its clinical value has been confined to the differential diagnosis among several causes of anemia.[3] Several studies have shown that RDW is associated with the presence and outcomes of several cardiovascular diseases.[4]–[6] But very limited data were found regarding the predictive value of RDW for the development of LAT or LASEC in patients with non-valvular AF.[6] Therefore, we would aim to reveal the relationship between RDW and the development of LAT/LASEC in patients with non-valvular AF.
2. Methods
2.1. Study population
We retrospectively reviewed the clinical, laboratory, and electrocardiograph data of patients with AF who underwent TEE prior to radiofrequency catheter ablation or cardioversion in our institution from April 2014 to December 2015. Patients with valvular heart disease or severe anemia (hemoglobin level < 10 g/dL) were excluded from the present study. Our study was approved by the local ethics committee.
2.2. Laboratory data
The blood routine tests were performed on all patients prior to radiofrequency catheter ablation or cardioversion. Hemoglobin (HGB), hematocrit (HCT), red blood cell count (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet (PLT), white blood cell counts (WBC), and RDW were measured by an automated complete blood count. The reference range of RDW is from 11.0% to 16.0% in our laboratory.
2.3. Electrocardiograph data
Transthoracic echocardiography was performed using a Vivid S5 (2–4 MHz phased array transducer; GE, Horten, Norway) system. Standard parasternal long-axis and short-axis views and apical 2- and 4-chamber views were obtained in all patients. Left atrial diameters were measured in parasternal long axis view. LV ejection fraction (LVEF) was calculated by using the modified Simpson method. TEE was performed without anesthesia or sedation in all the patients by using a 5 MHz biplane phased array transducer (Vivid S5, GE, Horten, Norway). The left atrium and left atrial appendage were imaged in different tomographic planes to detect the presence of LAT or LASEC. SEC was classified according to previously described criteria.[7] LASEC was defined as minimal echogenicity located in the left atrial appendage (LAA) or rarely in the main cavity of the left atrium. LAT was defined as dense swirling pattern in the LAA, less intense in the main atrium cavity, may fluctuate in intensity but constantly detectable throughout the cardiac cycle or intense echo density and very slow swirling pattern in the LAA and with similar intensity in the main atrium cavity. All patients were divided into LAT/LASEC group or non-LAT/LASEC group basing on the result of TEE.
2.4. Statistical analysis
Numerical variables were expressed as mean ± SD and categorical variables were expressed as percentage. Comparisons between the two groups were made using the Student t test or Mann-Whitney U test or chi-square tests, as appropriate. Receiver–operating characteristic (ROC) analysis was used to detect the cutoff value of RDW in prediction of LAT/LASEC in patients with non-valvular AF. Multivariate logistic regression analysis was performed to identify the independent predictors of LAT/LASEC. P < 0.05 was considered significant. All statistical analysis was carried out using SPSS 19.0 for Windows (SPSS Inc. Chicago, Illinois).
3. Results
3.1. Baseline characteristics of the LAT/LASEC and Non-LAT/LASEC groups
A total of 692 patients with non-valvular AF (67.9% males; mean age of 54.5 ± 12.23 years) were included into the study. Eighty-four patients were identified with LAT/LASEC during TEE examination. The clinical baseline, laboratory and electrocardiography characteristics were summarized on Table 1. The CHADS2 (1.60 ± 1.62 vs. 0.44 ± 0.88, P < 0.001) and CHA2DS2-VASc score (2.42 ± 2.05 vs. 0.82 ± 1.16, P < 0.001) were higher in patients with LAT/LASEC. Significant difference was observed between the two groups in term of RDW (14.34% ± 1.34% vs. 13.59% ± 1.07%, P < 0.001). However, HGB, PLT, WBC, RBC, HCT, MCV, MCH and MCHC levels were not markedly different between the two groups. The mean RDW level in patient with previous stroke was significantly higher than those without previous stroke (14.29% ± 1.25% vs. 13.66% ± 1.12%, P = 0.018). Furthermore, the RDW level was weakly correlated with CHADS2 score (r = 0.148, P < 0.001) and CHA2DS2-VASc score (r = 0.164, P < 0.001).
Table 1. Baseline characteristics of patients with or without LAT/LASEC during TEE examination.
| Non-LAT/LASEC group (n = 608) | LAT/LASEC group (n = 84) | P | |
| Clinical characteristics | |||
| Male | 422 (69.4%) | 48 (57.1%) | 0.024 |
| Age, yrs | 53.73 ± 12.18 | 60.32 ± 11.01 | 0.072 |
| CHF | 57 (9.4%) | 23 (27.4%) | < 0.001 |
| Hypertension | 54 (8.9%) | 39 (46.4%) | < 0.001 |
| Diabetes mellitus | 16 (2.6%) | 15 (17.9%) | < 0.001 |
| Vascular disease | 29 (4.8%) | 22 (26.2%) | <0.001 |
| Previous stroke | 12 (2.0%) | 7 (8.3%) | < 0.001 |
| Warfarin | 135 (22.2%) | 18 (21.4%) | 0.814 |
| CHADS2 score | 0.44 ± 0.88 | 1.60 ± 1.62 | < 0.001 |
| CHA2DS2-VASc score | 0.82 ± 1.16 | 2.42 ± 2.05 | < 0.001 |
| Laboratory examinations | |||
| RDW | 13.59% ± 1.07% | 14.34% ± 1.34% | < 0.001 |
| HGB, g/L | 139.60 ± 14.63 | 140.72 ± 13.67 | 0.507 |
| PLT, 109/L | 201.65 ± 51.46 | 197.79 ± 60.39 | 0.529 |
| WBC, 109/L | 6.75 ± 1.91 | 6.59 ± 1.62 | 0.461 |
| RBC, 109/L | 4.62 ± 0.62 | 4.64 ± 0.46 | 0.667 |
| HCT, % | 41.30% ± 4.11% | 41.68% ± 3.89% | 0.426 |
| MCV, fl | 90.62 ± 7.31 | 90.02 ± 7.98 | 0.486 |
| MCH, pg | 30.71 ± 2.80 | 30.45 ± 3.10 | 0.433 |
| MCHC, g/L | 338.56 ± 11.87 | 337.83 ± 12.21 | 0.599 |
| Echocardiogram parameters | |||
| LAD, mm | 35.46 ± 5.86 | 41.57 ± 5.64 | < 0.001 |
| LVEF | 65.56% ± 7.04% | 64.26% ± 5.94% | 0.107 |
Data are presented as mean ± SD or n (%). CHF: chronic heart failure; HCT: hematocrit; HGB: hemoglobin; LAD: left atrial diameter; LAT/LASEC: left atrial thrombus or left atrial spontaneous echo contrast; LVEF: Left ventricular ejection fraction; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; MCV: mean corpuscular volume; PLT: platelet; TEE: transesophageal echocardiography; RBC: red blood cell counts; RDW: red cell distribution width; WBC: White blood cell counts.
3.2. Predictive value of RDW on LAT/LASEC in Patients with non-valvular AF
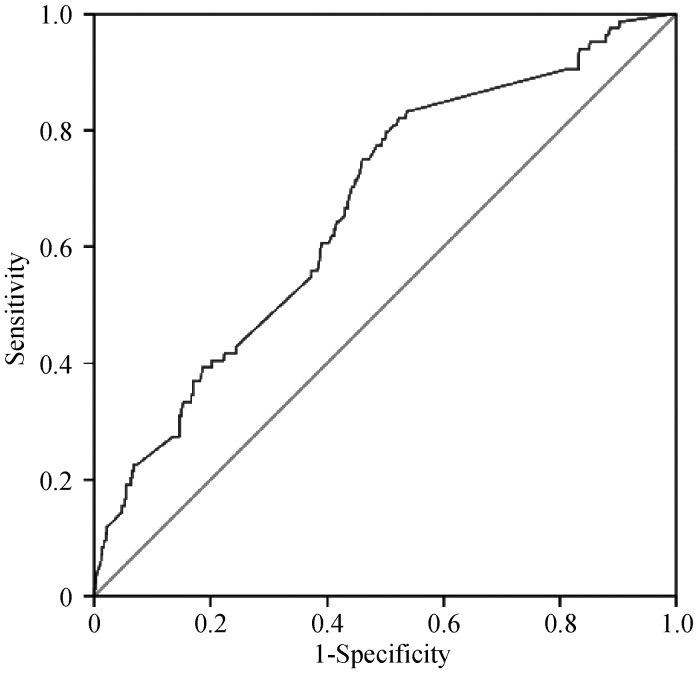
ROC curve was used to determine the best cutoff value of RDW for predicting LAT/LASEC. The RDW with 13.16 % predicted LAT/LASEC was identified as the best cutoff point with a sensitivity of 82.1% and a specificity of 52.3% in the study population (Figure 1). After adjustment for age, gender, chronic heart failure, hypertension, diabetes, previous stroke and vascular disease, multivariate logistic regression analysis showed that RDW>13.16% (OR = 2.967, P = 0.001) was the independent predictor for LAT/LASEC. Additionally, after adjustment for CHA2DS2-VASc score, RDW > 13.16% (OR = 2.911, P = 0.001) still was the independent predictor (Table 2).
Figure 1. The ROC curve analysis for RDW in predicting LAT/LASEC in patients with non-valvular AF.
Area under the curve = 0.666, P < 0.001, 95% CI: 0.626–0.726. The RDW with 13.16 % predicted LAT/LASEC was identified as the best cutoff point with 82.1% sensitivity and 52.3% specificity. AF: atrial fibrillation; LASEC: left atrial spontaneous echo contrast; LAT: left atrial thrombus; RDW: red cell distribution width; ROC: receiver-operating characteristic.
Table 2. Multivariate logistic regression analysis for LAT/LASEC in patients with non-valvular AF.
| Variables | Odds ratio | 95% CI | P-value |
| Model 1 | |||
| RDW > 13.16% | 2.967 | 1.603–5.489 | 0.001 |
| CHF | 2.770 | 1.472–5.212 | 0.002 |
| Hypertension | 4.703 | 2.475–8.938 | < 0.001 |
| Diabetes mellitus | 2.422 | 0.972–6.037 | 0.058 |
| Age ≥ 65 yrs | 1.128 | 0.610–2.086 | 0.702 |
| Vascular disease | 3.071 | 1.384–6.814 | 0.006 |
| Previous stroke | 0.620 | 0.182–2.111 | 0.444 |
| Age ≥ 75 yrs | 1.361 | 0.352–5.265 | 0.656 |
| Female | 1.595 | 0.935–2.721 | 0.087 |
| Model 2 | |||
| RDW > 13.16% | 2.911 | 1.612–5.258 | 0.001 |
| CHA2DS2-VASc (per score) | 1.745 | 1.486–2.049 | < 0.001 |
RDW: red cell distribution width; Model 1: chronic heart failure, hypertension, diabetes mellitus, age, previous stroke, vascular disease, gender and RDW>13.16%; Model 2: CHAS2DS2-VASc scoring system and RDW > 13.16%. AF: atrial fibrillation; CHF: chronic heart failure; LAT/LASEC: left atrial thrombus or left atrial spontaneous echo contrast; RDW: red cell distribution width.
4. Discussion
As the increasing morbidity of AF in recent years, the corresponding complications were brought to the forefront. AF was an independent risk factor for stroke. LAT or LASEC were deemed to be associated with increased thrombo-embolism risk.[3],[8]–[10] Black, et al.[11] had shown that red cell aggregation, manifested as LASEC, appeared to be a precursor to thrombosis. TEE was supposed to be the best way to find LAT or LASEC out, but always accompanied with operational risk and patients' sufferings. It seemed necessary to identify some noninvasive predictor for LAT/LASEC in AF patients. RDW was an indication represented the heterogeneity of red blood cells. It could be measured routinely as a part of daily automated CBC and its formula was (SD of erythrocyte volume/mean cell volume) × 100. RDW had been found to be an independent prognostic factor for cardiovascular outcome and all-cause mortality.[12],[13] A national retrospective study analyzed by Ani, et al.[14] showed the elevated RDW was associated with stroke occurrence and strongly associated with cardiovascular and all-cause death in subjects with known stroke. Saliba, et al.[15] showed that RDW was directly associated with the risk of stroke regardless of anemia status and improved the predictive accuracy for stroke in patients with AF. Kurt, et al.[16] also showed that RDW value was highly associated with thrombo-embolism risk shown by CHA2DS2-VASc score in patients with AF.
Only few studies had assessed the link of RDW with LAT or LASEC.[17] The study investigated by Zhao, et al.[18] had similarly shown the elevated RDW was associated with LAT or LASEC in the condition of matched baseline except previous stroke. However, further multivariate logistic regression analysis showed RDW could not independently predict the presence of LAT/LASEC in patients with non-valvular AF (OR = 1.312, P = 0.452). A cross-sectional study by Providencia, et al.[19] showed RDW was predictors of LAT or LASEC in the condition of mismatched baseline, but the predictive value turned to be negative when adding clinical risk factors from CHADS2 and CHA2DS2-VASc in logistic regression models.
In our study, a total of 692 patients were reviewed to evaluate the relationship between RDW level and LAT/LASEC. Although there were some mismatched baseline characteristics due to the retrospective nature of the study, the independent predictive effect of binary RDW level had been identified via adjustment of those mismatched baseline characteristics and CHA2DS2-VASc scoring system.
The underlying mechanisms of the link between RDW and LAT/LASEC in patients with AF were still largely unclear. It had been reported the RDW level was strongly bound up with oxidative stress and inflammation.[20] Oxidative stress influenced RDW level by decreasing erythrocytes lifespan and making them vulnerable to hemolysis. Some studies showed that inflammatory cytokines, such as tumor necrosis factor or IL-6, were related to ineffective erythropoiesis which might indirectly affected the RDW level.[21] Oxidative stress and some inflammation factors also had been demonstrated as markers which were correlated with ischemic stroke or LAT.[22] Further studies would be essential to clarify the link between RDW and LAT or LASEC in patients with AF.
4.1. Limitation
The present study was a single-center retrospective study with a limited sample size and no follow-up data. As a result, potential cause-effect relationship could not be clarified. Therefore, it was hard to determine the prognostic significance. Then, although the patients with anemia were excluded in our study, some other factors such as routine iron, vitamin B12, folic acid, fibrinogen levels were not measured. Further large-scale and prospective studies would be needed to demonstrate the role of RDW with regard to prediction of LAT/LASEC in patients with non-valvular AF.
4.2. Conclusions
Elevated RDW level is associated with the presence of LAT/LASEC in patients with non-valvular AF and could be with moderate predictive value for LAT/LASEC. Comparing with the factors of CHA2DS2-VASc scores, RDW level >13.16% could be an independent risk factor for LAT/LASEC in patients with non-valvular AF.
Acknowledgments
We appreciated Xuan Jiang for the statistical analysis. This work was supported by National Nature Science Foundation of China (No.81370295), Science and Technology Program of Guangdong Province, China (No. 2017A020215054), Science and Technology Planning of Guangzhou City, China (No.2014B070705005). The authors declared no potential conflicts of interest with respect to the research, authorship or publication of this article.
References
- 1.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 2.Gunawardene MA, Dickow J, Schaeffer BN, et al. Risk stratification of patients with left atrial appendage thrombus prior to catheter ablation of atrial fibrillation: an approach towards an individualized use of transesophageal echocardiography. J Cardiovasc Electrophysiol. 2017;28:1127–1136. doi: 10.1111/jce.13279. [DOI] [PubMed] [Google Scholar]
- 3.Scherr D, Dalal D, Chilukuri K, et al. Incidence and predictors of left atrial thrombus prior to catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:379–384. doi: 10.1111/j.1540-8167.2008.01336.x. [DOI] [PubMed] [Google Scholar]
- 4.Luo S H, Jia Y J, Nie S P, et al. Increased red cell distribution width in patients with slow coronary flow syndrome. Clinics (Sao Paulo) 2013;68:732–737. doi: 10.6061/clinics/2013(06)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karabulut A, Uyarel H, Uzunlar B, et al. Elevated red cell distribution width level predicts worse postinterventional thrombolysis in myocardial infarction flow reflecting abnormal reperfusion in acute myocardial infarction treated with a primary coronary intervention. Coron Artery Dis. 2012;23:68–72. doi: 10.1097/MCA.0b013e32834f1188. [DOI] [PubMed] [Google Scholar]
- 6.Dogdu O, Koc F, Kalay N, et al. Assessment of red cell distribution width (RDW) in patients with coronary artery ectasia. Clin Appl Thromb Hemost. 2012;18:211–214. doi: 10.1177/1076029611418964. [DOI] [PubMed] [Google Scholar]
- 7.Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23:961–969. doi: 10.1016/0735-1097(94)90644-0. [DOI] [PubMed] [Google Scholar]
- 8.Liao HT, Liu FZ, Xue YM, et al. Predictive value of serum uric acid on left atrial spontaneous echo contrast in non-valvular atrial fibrillation patients. J Geriatr Cardiol. 2015;12:641–646. doi: 10.11909/j.issn.1671-5411.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu FZ, Liao HT, Lin WD, et al. Predictive effect of hyperuricemia on left atrial stasis in non-valvular atrial fibrillation patients. Int J Cardiol. 2018;258:103–108. doi: 10.1016/j.ijcard.2018.01.080. [DOI] [PubMed] [Google Scholar]
- 10.Kuang R R, Liu F Z, Li Y P, et al. Hemoglobin A1c and risk of left atrial thrombus and spontaneous echo contrast in non-valvular atrial fibrillation patients. Eur J Med Res. 2017;22:15. doi: 10.1186/s40001-017-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black IW. Spontaneous echo contrast: where there's smoke there's fire. Echocardiography. 2000;17:373–382. doi: 10.1111/j.1540-8175.2000.tb01153.x. [DOI] [PubMed] [Google Scholar]
- 12.Li XL, Hong LF, Jia YJ, et al. Significance of red cell distribution width measurement for the patients with isolated coronary artery ectasia. J Transl Med. 2014;12:62. doi: 10.1186/1479-5876-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montagnana M, Cervellin G, Meschi T, et al. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med. 2011;50:635–641. doi: 10.1515/cclm.2011.831. [DOI] [PubMed] [Google Scholar]
- 14.Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277:103–108. doi: 10.1016/j.jns.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Saliba W, Barnett-Griness O, Elias M, et al. The association between red cell distribution width and stroke in patients with atrial fibrillation. Am J Med. 2015;128:111–192. doi: 10.1016/j.amjmed.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Kurt M, Tanboga IH, Buyukkaya E, et al. Relation of red cell distribution width with CHA2DS2-VASc score in patients with nonvalvular atrial fibrillation. Clin Appl Thromb Hemost. 2014;20:687–692. doi: 10.1177/1076029613478157. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, Shao Q, Korantzopoulos P, et al. Relation of red blood cell distribution width with CHADS2 and CHA2DS2-VASc score in Chinese patients with non-valvular atrial fibrillation. Int J Cardiol. 2017;228:861–864. doi: 10.1016/j.ijcard.2016.11.255. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Liu T, Korantzopoulos P, et al. Red blood cell distribution width and left atrial thrombus or spontaneous echo contrast in patients with non-valvular atrial fibrillation. Int J Cardiol. 2015;180:63–65. doi: 10.1016/j.ijcard.2014.11.145. [DOI] [PubMed] [Google Scholar]
- 19.Providencia R, Ferreira M J, Goncalves L, et al. Mean corpuscular volume and red cell distribution width as predictors of left atrial stasis in patients with non-valvular atrial fibrillation. Am J Cardiovasc Dis. 2013;3:91–102. [PMC free article] [PubMed] [Google Scholar]
- 20.Patel KV, Semba RD, Ferrucci L, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2010;65:258–265. doi: 10.1093/gerona/glp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petretta M, Condorelli GL, Spinelli L, et al. Circulating levels of cytokines and their site of production in patients with mild to severe chronic heart failure. Am Heart J. 2000;140:E28. doi: 10.1067/mhj.2000.110935. [DOI] [PubMed] [Google Scholar]
- 22.Lip G Y, Patel J V, Hughes E, et al. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke. 2007;38:1229–1237. doi: 10.1161/01.STR.0000260090.90508.3e. [DOI] [PubMed] [Google Scholar]