Abstract
Disease-free infection in HIV-infected adults is associated with HLA-mediated suppression of viremia, whereas in the sooty mangabey and other healthy natural hosts of SIV, viral replication continues unabated. To better understand factors preventing HIV disease, we here investigated pediatric infection, where AIDS typically develops more rapidly than in adults. Among 170 non-progressing anti-retroviral therapy-naïve children aged >5yrs maintaining normal-for-age CD4 T-cell counts, immune activation levels were low despite high viremia (median 26,000 copies/ml). Potent, broadly neutralizing antibody responses in the majority of subjects and strong virus-specific T-cell activity were present but did not drive pediatric non-progression. However, reduced CCR5 expression and low HIV infection in long-lived central memory CD4 T-cells were observed in pediatric non-progressors. These children therefore express two cardinal immunological features of non-pathogenic SIV infection in sooty mangabeys - low immune activation despite high viremia and low CCR5 expression on long-lived central memory CD4 T-cells – suggesting closer similarities with non-pathogenetic mechanisms evolved over thousands of years in natural SIV hosts than those operating in HIV-infected adults.
INTRODUCTION
Without antiretroviral therapy (ART), HIV infection in >99% of cases inevitably results in the development of AIDS. However, despite undetectable viral loads in a small subset of ART-naïve adults (elite controllers), or in ART-treated individuals, systemic immune activation levels remain higher than in uninfected individuals (1, 2). This gives rise to an increased risk of non-AIDS mortality and morbidities normally linked with ageing, including cardiovascular disease, malignancy and cognitive dysfunction (3, 4). Even in viremic individuals, it has long been recognized that viral replication is not the major cause of HIV disease, but rather the high levels of immune activation that typically result from infection (5, 6).
The central role of immune activation rather than viral replication in HIV pathogenesis has been highlighted by studies of the natural hosts of SIV infection, such as the sooty mangabey and African green monkey (7). In these, and some 40 species of non-human primates naturally infected with SIV (8, 9), high levels of viral replication are observed, typically with viral setpoints of ~105 copies/ml, and yet these animals suffer no disease as a consequence. In adult HIV infection immune activation is linked to viral load, whereas in the natural SIV hosts immune activation remains low despite persistent high viremia. Understanding the mechanisms by which low systemic immune activation might arise in natural HIV infection, independent of viral replication, therefore, is of major importance both for vaccine development and also to address the growing burden of non-AIDS HIV-associated disease in individuals receiving long-term ART.
In pediatric HIV infection, disease progression in the absence of ART is typically more rapid than in adults, with the median time to AIDS being 1 year, compared to 10 years in untreated adult infection (10). It has long been recognized that progression in pediatric infection is biphasic (11, 12), with 60% mortality by 2.5 years (12), and thereafter progression to disease is much slower. A subset of ART-naïve, HIV-infected children exist, who are clinically healthy and maintain normal-for-age CD4 T-cell counts throughout childhood (11, 13, 14). Previous reports of pediatric non-progressors (PNP) have not been plentiful, but it is clear that PNP are much more common than their adult viremic non-progressor (AVNP) counterparts (15, 16). Although no consistent CD4 and age criteria have been used to define pediatric non-progressors (11, 14, 17), approximately 10% of ART-naïve infected children reach mid-childhood (ages 6–8yrs) without disease and maintaining normal for age absolute CD4 T-cell counts. Since, with very few exceptions, the PNP subjects described in the current study were only identified incidentally some years after birth, the precise percentage of these children becoming PNP is unknown, but a figure of 5–10% would be consistent with our own longitudinal studies in Durban (13). PNP have been understudied because the pediatric HIV epidemic is so heavily concentrated outside of North America and Europe. However, large non-progressor pediatric cohorts similar to those presented here have been described from Uganda and Thailand (17, 18).
The mechanisms of non-progression in children are not defined but differ from those in elite controller adults. Whereas ‘protective’ HLA class I alleles such as HLA-B*27 or HLA-B*57 are expressed in >50% of ‘elite controller’ adults (19), by contrast HLA class I variation does not influence progression rates significantly in pediatric infection (20). Furthermore, the high CD4 T-cell counts observed in elite controller adults are associated with low viral loads, beneath the level of detection, whilst in non-progressor children, as described previously (17, 18) and in the current study, are typically associated with persistent high viral loads. To identify potential mechanisms of HIV non-pathogenesis, we first investigated whether non-progression in pediatric infection is dependent on strong virus specific immune responses, as in adult HIV elite controllers (21), or is independent of them, as in the natural hosts of SIV infection (22). We then tested the hypothesis that, as in sooty mangabeys (23), reduced infection of the long-lived central memory (Tcm) and stem cell memory (Tscm) CD4 T-cells may contribute to the maintenance of high CD4 T-cell counts despite persistent viremia in these non-progressor children.
RESULTS
Normal CD4 T-cell counts, low immune activation, high viremia in pediatric no-nprogressors
Based on normal absolute CD4 T-cell counts in HIV-uninfected children (24, 25), we here defined pediatric non-progressors (PNP) as ART-naïve, HIV-infected children with CD4 T-cell counts of >750 cells/mm3 at age ≥5yrs, who have not met the CD4 or clinical criteria for ART initiation. An example of one such PNP followed from birth is shown in Fig. 1a–c.
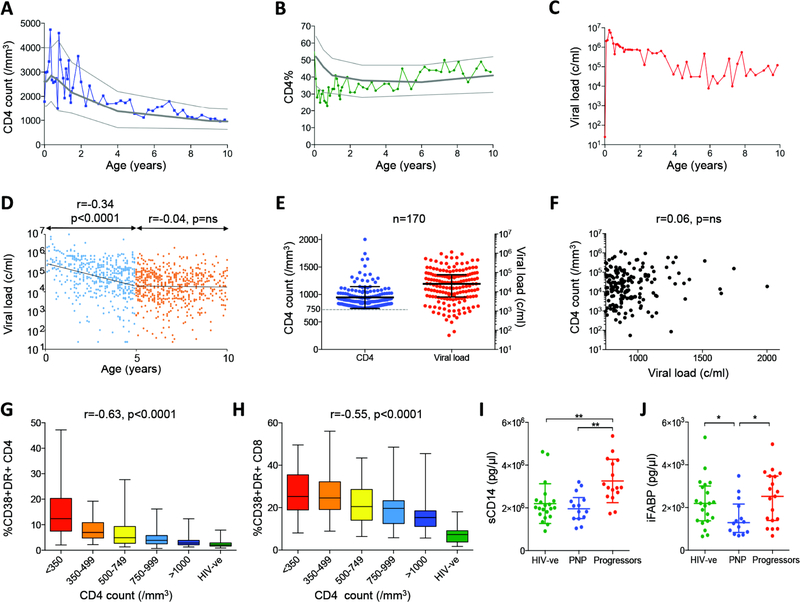
Fig. 1. Normal CD4 T-cell counts for age and low immune activation despite high viral loads in pediatric non-progressors, in association with evidence of low levels of microbial translocation.
a–c. Absolute CD4 T-cell count, CD4% (percentage of peripheral blood lymphocytes expressing CD4) and viral load in ART-naïve pediatric subject 517-C over the first 10 years of life. 10th, 50th and 90th centile of absolute CD4 T-cell counts and CD4% are shown in panels A-B for uninfected children over the first 10 years of life18–19. d. Longitudinal viral load data from 170 ART-naïve pediatric non-progressors. Viral load declines with age over the first 5 years (r=−0.34, p<0.0001) but then plateaus thereafter. e. Current absolute CD4 T-cell counts and viral loads in 170 pediatric non-progressors. f. Lack of correlation between CD4 T-cell count and viral load in 170 pediatric non-progressors. G–h. Immune activation (CD38/HLA-DR expression) on CD4+ T-cells (panel g) and CD8+ T-cells (panel h) is inversely correlated with absolute CD4 T-cell count in ART-naïve children aged>5yrs (n=163 HIV-infected children and n=21 HIV-uninfected children). i. Levels of soluble CD14 are significantly lower in pediatric non-progressors (absolute CD4 T-cell count >750 cells/mm3, n=14) than in progressors (absolute CD4 T-cell count <500 cells/mm3, n=16) and similar to HIV-uninfected children (n=21). j. Levels of intestinal fatty acid binding protein are lower in pediatric non-progressors (n=14) and HIV-uninfected children (n=21) compared to progressors (n=19). Comparisons between groups were calculated by Mann-Whitney tests (*p<0.05; **p<0.01). P- and r-values for bivariate associations were calculated by Spearman rank correlation tests.
Characteristically, although absolute CD4 T-cell count and CD4 T-cell percentage (CD4%, that is, percentage of peripheral blood lymphocytes expressing CD4) are close to the 50th centile for age-matched HIV-uninfected children, the viral loads reach a quasi setpoint of 104-105 copies/ml plasma. Pooling all the available longitudinal data from the 170 PNPs in the cohort of African children indicates a decrease in viral load over the first 5 years of life, to a viral setpoint of 20,000–30,000 copies/ml (Fig. 1d–e). Viral loads of <100c/ml among PNP are exceptionally rare (10). Furthermore, also in contrast with ART-naïve non-progressor adults, in whom sustained high CD4 T-cell counts are typically found in association with low viral loads (26), in PNP there is no association between absolute CD4 T-cell count and viral load (Fig. 1f, r=0.06, p=ns).
These data suggest a similarity between PNP and the natural hosts of SIV infection, such as sooty mangabeys and African green monkeys, in whom persistent high viral loads are also associated with lack of disease progression and CD4 T-cell preservation, and levels of immune activation are low(7). We observed in the PNPs levels of immune activation (CD38/HLA-DR co-expression on CD4+ and CD8+ T-cells) that were strikingly low especially on CD4+ T-cells, and across ART-naïve HIV-infected children a strong negative correlation between levels of immune activation and absolute CD4 T-cell count or CD4% (Fig. 1g–h). Of note, CD4 T-cell activation was more strongly associated than CD8 T-cell immune activation with CD4 T-cell count among these infected children, in contrast with the stronger associations between immune activation of CD8 T-cells and CD4 T-cell count observed in adult infection (6). Furthermore, levels of soluble CD14 (sCD14), that are characteristically raised in pathogenic SIV or HIV infection, partly as a consequence of microbial translocation and chronic stimulation of monocytes/macrophages by LPS (27, 28), were similar in PNPs and uninfected children, and significantly lower than in age-matched progressor children (Fig. 1i). Pediatric ‘progressors’ are defined here as ART-naïve children aged >5yrs who met the prevailing CD4 T-cell criterion for ART initiation, that is an absolute CD4 T-cell count of <500 cells/mm3. Levels of intestinal fatty acid binding protein (iFABP), a biomarker of intestinal villous damage predictive of HIV-associated disease (29), were significantly lower in PNP compared to progressors and also compared to pediatric uninfected controls (Fig. 1j). These data are consistent with a picture similar to that in the natural hosts of SIV infection, in which low immune activation in the face of persistent high viral loads is observed, associated with evidence of low-level microbial translocation (27).
Low-level CD4 T-cell differentiation and maintained function in non-progressing children
In sooty mangabeys, non-pathogenesis despite high viremia is independent of SIV-specific cellular immunity (22). To determine whether virus-specific immune responses may play a role in non-progressing pediatric HIV infection, we first evaluated CD4 T-cell differentiation and function in PNPs. To compare CD4 T-cell responses with age-matched uninfected children as well as with progressor children, we initially measured intracellular production by CD4+ T-cells of Th1 cytokines IFN-γ, IL-2 and TNF-α in response to staphylococcal enterotoxin B (SEB). IL-2 production in PNP did not differ from uninfected controls, but was significantly higher than in progressors (Fig. 2ab). IFN-γ production was, conversely, significantly higher in progressors than in the other two groups. Similarly, in response to a pool of overlapping Gag peptides, intracellular IFN-γ production was lower and IL-2 responses in CD4 T-cells were significantly higher in PNP compared to progressors (p=0.01; Fig. 2c). The same broad patterns were observed in CD4 T-cell responses to the pools of Pol and Nef HIV proteins also tested (Fig. S1ab).
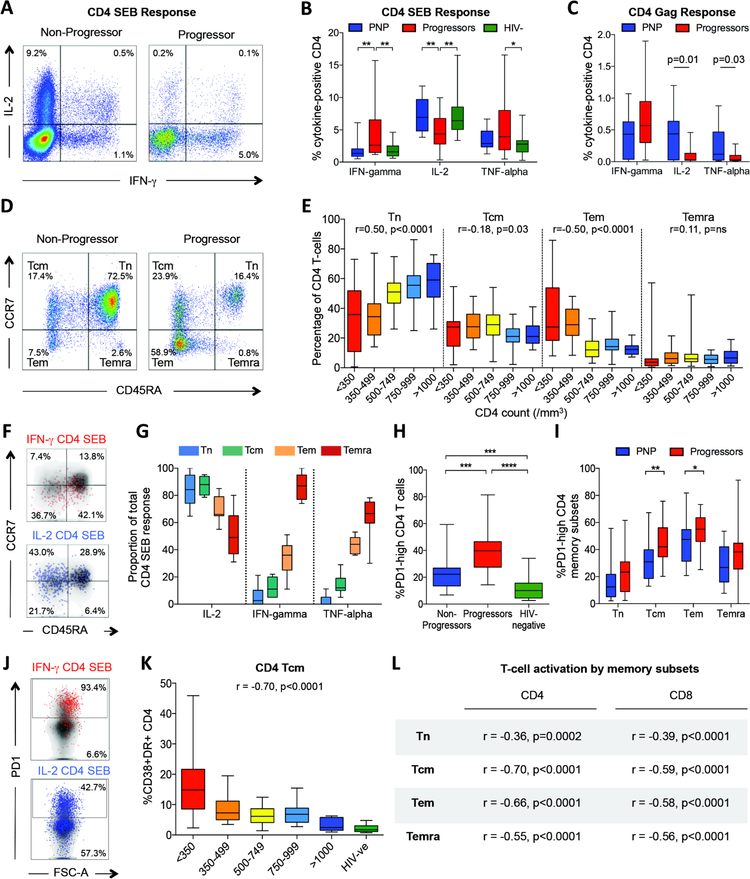
Fig. 2. CD4 T-cell differentiation and function in non-progressing pediatric infection.
a–c: IFN-γ, IL-2 and TNF-α intracellular cytokine staining (ICS) response to SEB and to Gag in pediatric non-progressors. a. Representative FACS staining in a progressor and non-progressor. b. ICS responses to SEB in non-progressors (n=14), progressors (n=32) and uninfected pediatric controls (n=19), gating on bulk CD4+ T-cells. c. ICS responses to a Gag peptide pool in non-progressors (n=26) and progressors (n=11), gating on bulk CD4+ T-cells. D–e: CD4 T-cell differentiation in pediatric progressors and non-progressors (n=161). d. Representative FACS staining in a progressor and non-progressor. e. Percentage of CD4 T-cells by absolute CD4 T-cell count defined as naïve, central memory, effector memory or terminally differentiated effector memory, by CCR7 and CD45RA expression as shown in panel d. Correlation between absolute CD4 T-cell count and % CD4 T-cells in each subset shown as Spearman rank correlation coefficient and p value. F–g: Functional differences between distinct CD4 T-cell subsets. f. Representative FACS staining in response to SEB showing IFN-γ producing cells are predominantly effector memory, and IL-2 producing cells are predominantly naïve and central memory in phenotype. g. Contribution of IL-2, IFN-γ and TNF-α to the total CD4 T-cell SEB response. The proportion of cells producing each cytokine to the total CD4 T-cell SEB response was determined; data from 11 non-progressors. H–j: PD-1 expression on CD4 T-cell subsets in pediatric progressors, non-progressors and uninfected controls h. Increased PD-1 expression on CD4+ T-cells in pediatric progressors (n=20) compared to non-progressors (n=32) and uninfected pediatric controls (n=22). i. Increased PD-1 expression on CD4+ T-cell subsets in pediatric progressors (n=20) compared to non-progressors (n=30). j. Representative FACS plots to show differential cytokine staining in relation to PD-1 expression. K–l: T-cell activation by memory subsets. Immune activation (CD38/HLA-DR expression) on CD4+ central memory T-cells (Tcm) (panel k) and other CD4+ and CD8+ T-cell memory subsets (panel l) is inversely correlated with absolute CD4 T-cell count in ART-naïve children aged>5yrs (n=97). Comparisons between groups were calculated by Mann-Whitney tests (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001). P- and r-values for bivariate associations were determined by Spearman rank correlation tests.
These functional differences reflect the distinct patterns of CD4 T-cell differentiation among PNP and progressor children. The levels of naïve CD4 T-cells in PNP are similar to those in age-matched uninfected children (24, 25) (median 58%, 10th–90th centiles 42%–74%; Fig. 2de). In pediatric HIV infection, analyzing PNP and progressors together, the percentage of naïve CD4 T-cells is strongly associated with high absolute CD4 T-cell count, whereas an increasing percentage of effector memory CD4 T-cells is associated with decreasing absolute CD4 T-cell count (Fig. 2de). Functionally, in response to SEB, IL-2 is principally produced by naïve and central memory CD4 T-cells, whilst IFN-γ and TNF-α are mainly produced by effector memory CD4 T-cells (Fig. 2fg). Expression of exhaustion markers such as PD1 and 2B4 are also higher on all CD4 T-cell subsets in progressors compared to PNP (Fig. 2hi, Fig. S1c). Together, these findings of increased CD4 T-cell differentiation, exhaustion and dysfunction in the pediatric progressors compared to PNP are consistent with previous observations of the hierarchical exhaustion of the CD4 T-cell response characteristic of chronic viral infection and persistent high viremia (30). In contrast, these features are largely absent in the pediatric non-progressors, despite persistent high viral loads.
Of note, the high proportion of naive T-cells observed here among the pediatric non-progressors (Fig. 2e) did not explain the low immune activation levels described above (Fig. 1gh) in these children. Comparing cells of the same differentiation phenotype by absolute CD4 T-cell count, in each case, immune activation levels were correlated with absolute CD4 T-cell count (Fig. 2k,l).
CD4 T-cell immune activation is the major driver of progression in pediatric infection
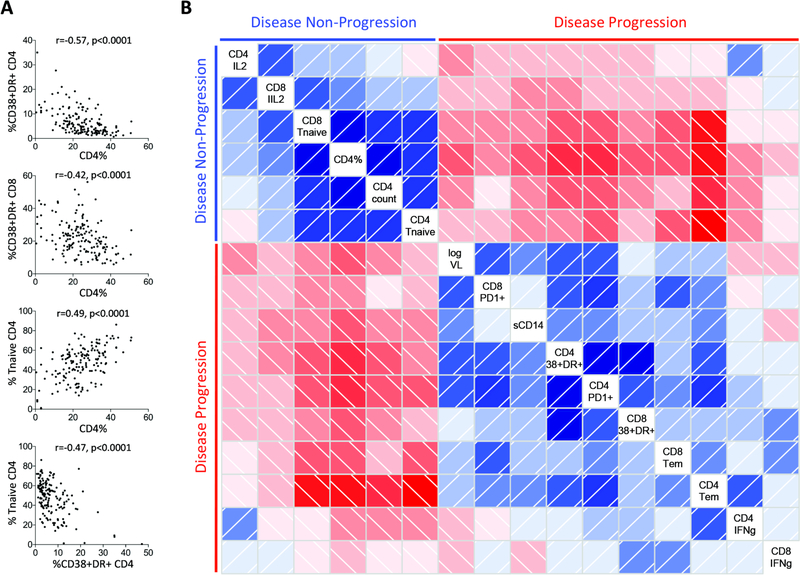
Identification of multiple variables differentiating progressing versus non-progressing pediatric infection, including level of immune activation, markers of microbial translocation, T-cell differentiation, CD4+ and CD8+ T-cell subset function, absolute CD4 T-cell count and viral load, prompts the question: what is the primary driver of progression in pediatric infection? To help visualize the nature of the associations between these parameters, a correlation matrix was constructed using data from a subset of 45 ART-naïve children in whom measurement of all parameters had been undertaken at the same study visit (Fig. 3). Variables were grouped based on principal component analysis using the R package ‘Corrgram’ (31). A clear dichotomy emerges between factors associated with disease non-progression (upper left quadrant) and with progression (lower right quadrant).
Fig. 3. Associations between immunological and clinical variables.
a: Selected bivariate associations between immunological and clinical measurements in HIV-infected ART-naïve children aged>5yrs (n=163). Spearman rank correlation tests. b: Correlation matrix in a subset of n=45 with available data for all regarded parameters from the same study visit. Positive correlations are indicated in blue and inverse correlations in red. Darker color shades indicate higher r-values based on Spearman rank correlation tests. Clustering of variables is based on principal component analysis using the R package ‘Corrgram’ and reveals two well-differentiated groups of parameters, one associated with disease non-progression (upper left quadrant) and the other with disease progression (lower right quadrant).
To better determine the major driver of these effects that are linked by multicollinearity, we assessed the influence of each parameter using a generalized linear model, applying the Least Absolute Shrinkage and Selection Operator (LASSO) principle on scaled covariates (32, 33). All three of the LASSO fitting routines in R provide very similar results (34, 35), with a consensus of 6 of the 17 covariates selected by all of the methods as making an independent contribution to CD4 T-cell count in pediatric HIV infection (Table 1). Of note, viral load made no independent contribution to pediatric progression according to these analyses, consistent with the lack of relationship between absolute CD4 T-cell count and viral load in the non-progressor children described above (Fig. 1f).
TABLE 1: Association of clinical and immunological parameters with CD4 T-cell count.
Left panel: Selected regression parameter estimates with the three LASSO approaches implemented in the R packages grplasso, penalised and glmnet. Right panel: Standardized regression parameter estimates for the selected set of covariates by an un-regularized conventional generalized linear model using the R-function glm.
| LASSO | Generalized linear model | |||||
|---|---|---|---|---|---|---|
| Variable | grplasso | penalized | glmnet | Variable | Beta-coefficient | p-value |
| CD4 CD38+DR+ | −0.1701 | −0.1754 | −0.1718 | CD4 CD38+DR+ | −0.3123 | p<10−10 |
| CD4 Tem | −0.0549 | −0.0603 | −0.0567 | CD4Tem | −0.1630 | p<10−10 |
| Age at visit | −0.0632 | −0.0674 | −0.0646 | Age at visit | −0.1582 | p<10−10 |
| CD8 TnaTve | 0.1000 | 0.1008 | 0.1003 | CD8 TnaTve | 0.1105 | p<10−10 |
| sCD14 | −0.0472 | −0.0498 | −0.0481 | sCD14 | −0.1098 | p<10−10 |
| CD4 TnaTve | 0.1322 | 0.1274 | 0.1305 | CD4 TnaTve | 0.0475 | p=0.0147 |
| iFABP | 0 | 0 | 0 | - | - | - |
| CD8 CD38+DR+ | 0 | 0 | 0 | - | - | - |
| CD8 Tem | 0 | 0 | 0 | - | - | - |
| Sex | 0 | 0 | 0 | - | - | - |
| Viral load | 0 | 0 | 0 | - | - | - |
| CD4 PD1 | 0 | 0 | 0 | - | - | - |
| CD8 PD1 | 0 | 0 | 0 | - | - | - |
| Gag CD4 IFNg | 0 | 0 | 0 | - | - | - |
| Gag CD4 IL2 | 0 | 0 | 0 | - | - | - |
| Gag CD8 IFNg | 0 | 0 | 0 | - | - | - |
| Gag CD8 IL2 | 0 | 0 | 0 | - | - | - |
Finally, an unregularized, generalized linear model analysis (36) was then run on the selected set of 6 covariates to determine statistical significance and the β-coefficient estimates (Table 1). The covariates most strongly linked to non-progression were low immune activation of CD4 T-cells, followed by low percentage of pro-inflammatory effector memory CD4 T-cells. This analysis confirms the significance of all of these 6 influences on non-progressing pediatric infection, driven principally by low immune activation on CD4 T-cells.
Potent, broadly neutralizing antibodies in pediatric compared with adult infection
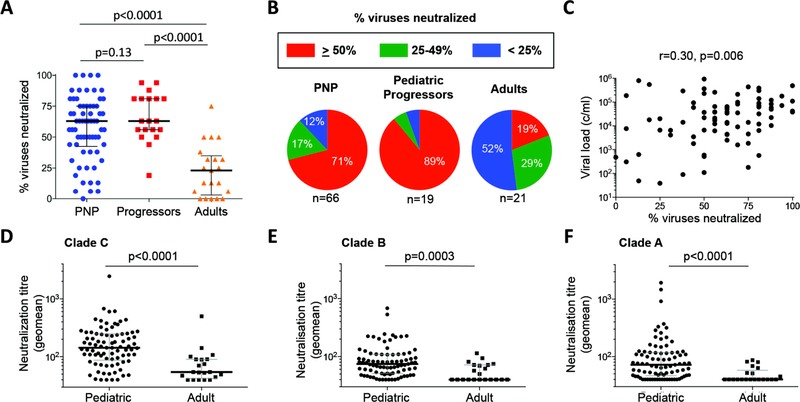
Although high titer, broadly neutralizing antibodies are not a feature of AIDS resistance in sooty mangabeys (37–39), persistent high viremia in the context of healthy CD4 T-cell activity might be expected to provide the optimal setting for the generation of broadly neutralizing antibody responses in non-progressing HIV infected children (40–42). We initially compared neutralization of a panel of 16 tier-2 and −3 clade A, B and C viruses by plasma from pediatric non-progressors and progressors with plasma from a South African cohort of adults (41) 5 years post-infection (viral load median 31,200 copies/ml (IQR 3,104–73,347 c/ml), absolute CD4 T-cell count median 449 cells/mm3 (IQR 308–568 cells/mm3)). Overall, 75% (64 of 85) of the pediatric subjects studied were able to neutralize ≥50% of the panel of 16 viruses, compared to 19% of the adults (p<0.0001, Fig. 4a). The ability of non-progressing children (median: age 6.6yrs, absolute CD4 T-cell count 1,050 cells/mm3, viral load 14,000 copies/ml) to neutralize this panel of viruses was somewhat less than that of the progressing children (median: age 8.2yrs, absolute CD4 T-cell count 225 cells/mm3, viral load 71,803 copies/ml; Fig. 4b). Although these differences between the pediatric groups approached statistical significance (p=0.13, Fig. 4a), this was likely influenced by the fact that progressors were older and had higher viral loads than the non-progressors. As in previous studies (40–43), viral load in the pediatric subjects was associated with neutralization breadth (r=0.30, p=0.006 Fig. 4c). In addition to the greater breadth of virus neutralization observed in the pediatric samples, the magnitude of these responses was also higher (Fig. 4d–f). For example, comparing neutralizing antibody activity against all 16 viruses in the panel, the frequency of responses with neutralization titers of more than 1:1000 was 5-fold higher in the pediatric than adult samples (p=0.001, Fisher’s Exact test) (Fig. S2).
Fig. 4. Neutralization of a panel of 16 tier-2 and −3 subtype C, B and A viruses by pediatric and adult plasma samples.
a: Neutralization breadth in pediatric non-progressors (n=66) and progressors (n=19) compared with adults (n=21). b. The frequency of bnAbs among pediatric non-progressors (median age 6.6yrs, absolute CD4 T-cell count 1,050 cells/mm3, viral load 14,000 copies/ml), pediatric progressors (median age 8.2yrs, absolute CD4 T-cell count 225 cells/mm3, viral load 71,803 copies/ml) and adults (five years after infection, median absolute CD4 T-cell count 449 cells/mm3, viral load 31,200 copies/ml). c. Correlation between viral load and % viruses neutralized among pediatric subjects. P- and r-values were calculated by Spearman rank correlation tests. D–f. Geometric means of neutralization titers for pediatric and adult samples against subtype C (n=6), B (n=6) and A (n=4) viruses. Comparisons between groups were calculated by Mann-Whitney tests.
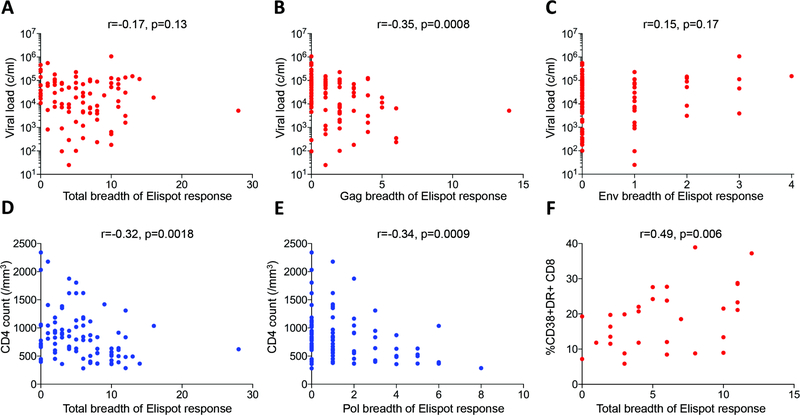
CD8+ T-cell breadth associates with lower viral load but not non-progression in children
We next investigated whether HIV-specific CD8+ T-cell responses might influence progression in pediatric infection. In sooty mangabeys, SIV-specific CD8+ T-cell breadth is unrelated to CD4 T-cell count or viral load (22), but in adult HIV infection increasing breadth of the virus-specific Gag-specific CD8+ T-cell response is related to decreasing viral load and increasing CD4 T-cell count (44). For each subject, recognition of a panel of 410 overlapping peptides spanning the HIV-1 C clade proteome was determined in elispot assays, initially using pools of 12 peptides and then confirming recognition of individual peptides in a second assay. Exactly as previously described in adults (44), there is a discordant protein-specific relationship between the breadth of the CD8+ T-cell response and viral load, with increasing Gag breadth associated with decreasing viral setpoint, and increasing Env breadth associated with increasing viral setpoint (Fig. 5a–c). However, in contrast to adults, the breadth and magnitude of the HIV-specific CD8+ T-cell response was associated with decreasing absolute CD4 T-cell count, an indicator of disease progression (Fig. 5d). The main contributions in the pediatric cohort to decreasing CD4 T-cell count in association with increasing CD8+ T-cell breadth were the Pol-specific responses (r=−0.34, p=0.0009; Fig 5e). Generalized linear modeling of data from the pediatric subjects in whom immune activation had been measured contemporaneous with the elispot assays demonstrated that CD8+ HLA-DR/CD38 expression is significantly and independently correlated with both viral load and CD8+ T-cell breadth (Fig. 5f, Table S1). These data further support the conclusion that control of viral replication is less important than avoidance of raised immune activation in the maintenance of pediatric HIV non-progression, since generation of a broad HIV-specific CD8+ T-response may actually drive disease progression through immune activation.
Fig. 5. HIV-specific CD8+ T-cell responses in pediatric infection in relation to viral load and absolute CD4 count.
a–c. Breadth of IFN-γ elispot responses correlate with viral load in a protein-specific fashion. a–b. Total breadth and Gag-specific breadth of IFN-γ elispot response (n=90). c. Env-specific breadth of IFN-γ elispot response (n=90). d–e. Total breadth and Pol-specific breadth of IFN-γ elispot response in ART-naïve pediatric subjects is inversely correlated with absolute CD4 count (n=90). f. Total breadth of IFN-γ elispot response directly correlates with CD8+ T-cell activation in pediatric infection (n=30). Spearman rank correlation tests.
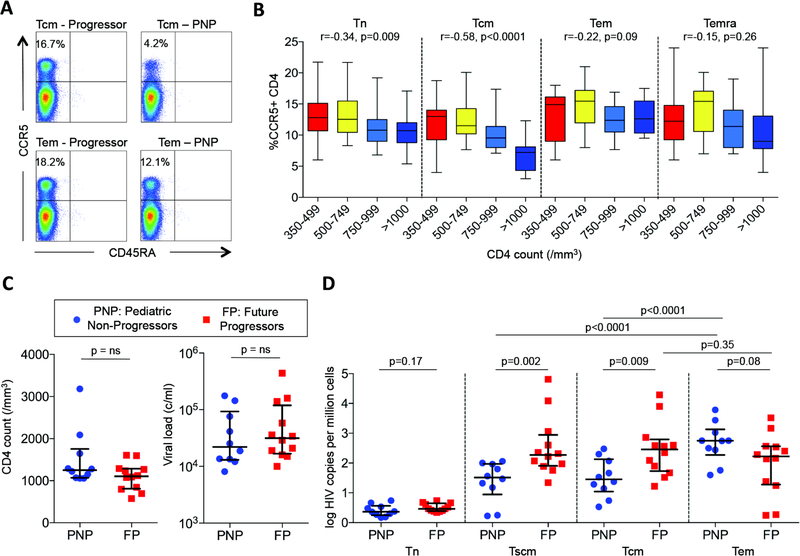
Decreased HIV infection in long-lived memory CD4 T-cells (Tcm and Tscm) in PNP
To further investigate mechanisms to explain the maintenance of normal-for-age CD4 T-cell counts in non-progressing children despite persistent high viral loads, we next addressed the hypothesis that in these children the CD4 T-cells predominantly infected with HIV are the short-lived effector memory subsets, in contrast to progressive HIV infection in which the long-lived central memory CD4 T-cells are the main T-cell subset infected with HIV. Similar observations have been made in the natural hosts of SIV - sooty mangabeys and African green monkeys – compared with pathogenic SIV infection in the rhesus macaque (23). We first determined expression of CCR5 on different CD4 T-cell subsets, and noted strikingly lower CCR5 expression on central memory (Tcm) CD4 T-cells in the non-progressor children both compared to progressor children (p<0.0001), and also compared to CCR5 expression in non-progressors on effector memory cells (Tem) (Fig. 6ab). In order to compare HIV DNA levels in the non-progressor children with control subjects in whom data were not confounded by the effects of CD4 T-cell depletion, we identified a group of progressing HIV-infected adults, termed here ‘future progressors’, from whom cryopreserved samples were available prior to progression, at a time when absolute CD4 T-cell counts (median 1106 CD4 T-cells/mm3, IQR 811–1284 cells/mm3; CD4% 36%, IQR 28%–40%) were well within the normal range for uninfected individuals. Viral loads in this control group (median 31,500, IQR 16,750–119,500) were also matched with the PNP (Fig. 6c). Consistent with the CCR5 expression data described above, in the pediatric non-progressors lower HIV DNA levels were observed in the long-lived stem cell memory (Tscm) and central memory (Tcm) CD4 T-cell subsets compared to those in effector memory cells (Tem) (p<0.0001 in each case) (Fig. 6d). As previously shown in pathogenic SIV infection in rhesus macaques (23), and also in studies of HIV-infected adult progressors (15), in the ‘future progressors’, HIV infection of Tscm and Tcm was higher than in Tem, whereas the reverse was the case in the non-progressing children (Fig. 6cd). These data therefore are consistent with the hypothesis that, in non-progressing children, reduced HIV infection of the long-lived CD4 T-cell subsets, Tscm and Tcm, in association with relatively low expression of CCR5 on these cells, enables absolute CD4 T-cell counts to be maintained despite persistent high viremia.
Fig. 6. CCR5 expression and HIV infection is lower in central memory CD4+ T-cells in pediatric non-progressors than in progressors.
a. Representative FACS data of CCR5 expression in pediatric progressors versus non-progressors. b. CCR5 expression on CD4+ T-cell subsets in ART-naïve children aged>5yrs by absolute CD4 T-cell count (n=59). P- and r-values were calculated by Spearman rank correlation tests. c. Absolute CD4 T-cell counts and viral loads in pediatric non-progressors and adult future progressors. d. HIV infection in Tn, Tscm, Tcm and Tem in pediatric non-progressors and adult future progressors, determined by qPCR of HIV DNA in sorted CD4 T-cell subsets. P-values were determined by Mann-Whitney tests
DISCUSSION
The mechanisms by which disease-free infection is achieved in HIV-infected elite controllers and in the natural hosts of SIV infection, such as the sooty mangabey and African green monkey, are diametrically opposed. Elite controllers suppress HIV replication to undetectable levels via immune responses mediated principally by protective HLA class I molecules such as HLA-B*27 and HLA-B*57. In contrast, non-pathogenic SIV infection is independent of virus-specific immunity, and viral replication continues unabated but without ill consequences. These current studies of non-progressing pediatric HIV infection demonstrate that, although – in contrast to the sooty mangabeys - strong virus-specific immune activity is present, it is not the major contributor to the maintenance of normal CD4 T-cell counts and absence of disease, consistent with observations in the natural hosts of SIV (22, 37–39). The normal-for-age CD4 T-cell counts and low immune activation despite persistent high viral loads seen in these non-progressing children are also characteristic of SIV infection in African non-human primates (7). In both cases CD4 T-cell count is unrelated to viral load. A further feature of non-progressing pediatric HIV infection in common with non-pathogenic SIV infection in the sooty mangabey natural hosts is decreased CCR5 expression on long-lived central memory CD4 T-cells and the associated low level of virus infection in these Tscm and Tcm subsets (23). Together these data suggest that the mechanisms that operate to achieve disease-free pediatric HIV infection may have closer similarities to those that have evolved over thousands of years (45) in African non-human primates as a consequence of SIV infection than to the well-described HLA-mediated immune suppression of HIV observed in adult elite controllers.
Clear differences, however, are apparent between non-pathogenic SIV infection and non-progressing pediatric HIV infection, particularly in relation to virus-specific immune responses observed. SIV-specific CD4+ T-cell responses in sooty mangabeys are relatively low in magnitude and breadth (22) whereas the same cytokine responses - IFN-γ, IL-2 and TNF-α - measured here in response to HIV peptides in the non-progressing HIV-infected children, were of relatively high magnitude and breadth. Similarly, neutralizing antibody responses in sooty mangabeys are detectable but relatively low-level (37–39), whereas remarkably potent and broadly neutralizing antibody responses are observed in the pediatric non-progressors, albeit somewhat lower than in the progressors. Nonetheless, in common with the sooty mangabey, the virus-specific cellular and humoral responses detected in the non-progressing children appear not to be primarily responsible for lack of immunodeficiency virus disease, as evidenced by the generalized linear modeling and the observation of equivalent or potentially broader nAb responses in the progressor children compared to the non-progressors.
The second of the two key immunological features of non-pathogenic SIV infection shared by pediatric non-progressors, in addition to low immune activation despite high viral load, is low CCR5 expression on long-lived central memory CD4 T-cells. This finding would help explain how normal absolute CD4 T-cell counts can be maintained despite high viral loads, since if most infected cells are Tem CD4 T-cells that are in any case short-lived, replenishment of the modest loss of long-lived Tscm and Tcm memory could be achieved via a moderate increase in thymic output whilst maintaining normal naïve CD4 T-cell numbers. Low CCR5 expression has also been proposed to play a role in the surprisingly low mother-to-child transmission rates (<7%) observed in sooty mangabeys, given their high viral loads (46), compared to HIV-infected humans (~40% in breast-fed infants) (10).
Comparable studies of cellular subset localization of virus have been undertaken in the rare group of HIV-infected adults – so-called adult viremic non-progressors (AVNP) - who express a similar phenotype to the pediatric non-progressor children of normal CD4 T-cell counts despite persistent high viral loads (15). In a comparison of cellular localization of HIV infection in AVNP with putative progressor adults who were matched for CD4 T-cell count, viral load and immune activation levels but studied prior to progression in order to minimize possible confounding effects of CD4 T-cell decline, reduced HIV infection in Tscm and Tcm was observed in the AVNP in association with increased numbers of these long-lived memory cells (15). The AVNP showed increased proliferation of CD4+ memory T-cells compared to the putative progressors, as do SIV-infected sooty mangabeys compared either to uninfected sooty mangabeys or to SIV-infected rhesus macaques with progressive disease (47) consistent with rapid turnover of Tem being maintained by modestly increased thymic output in non-pathogenic infection. In progressive HIV and SIV infections, the viral reservoir is principally localized in the central memory CD4+ T-cells (23, 48) and preferential infection of Tscm is a feature of progressive HIV infections (49).
Although the cellular distribution of HIV in PNP is substantially lower in long-lived central memory versus effector memory CD4 T-cells, and therefore is similar to the cellular localization of virus observed in SIV infected sooty mangabeys, the precise contribution of low CCR5 expression, independent of immune activation, remains to be fully evaluated in these non-progressor children. In the measurements of CCR5 expression undertaken here, resting and activated cells were not differentiated as immune activation markers were in a separate antibody panel. In part, therefore, CCR5 expression differences between PNP and progressors could theoretically have resulted from differences in immune activation. However, the current data can accurately be used to compare different subsets within the same patient where levels of activation will be consistent. Importantly, these data show that in PNP there are substantially lower levels of CCR5 in central memory cells compared to effector memory cells. In the progressors, however, levels of CCR5 in central and effector memory cells are similar. These data indicate that substantially lower levels of CCR5 in longer-lived subsets only exist in PNP and not progressors, consistent with lower levels of CCR5 in longer-lived subsets as a mechanism explaining the PNP phenotype.
A further point to note with respect to the measurements of viral DNA in different T-cell subsets presented both here and in the sooty mangabey and AVNP studies described above (15, 23) is the fact that total viral DNA is being measured, as distinct from replication competent virus (50). Unfortunately there is no assay currently available that accurately quantifies replication competent virus. PCR approaches substantially over-estimate, and viral outgrowth assays substantially underestimate, true levels of replication competent virus (51). It has however been proposed that total HIV DNA measured here is the most meaningful of all the assays available, since this measure correlates with time to viral rebound after coming off ART (52). Additionally, total HIV DNA has been recently shown to correlate with viral outgrowth measures (53).
The contrast between non-pathogenic HIV infection achieved via suppression of viral replication in adult elite controllers and via mechanisms independent of HIV-specific immune responses in non-progressing children deserves further comment. Previous studies have shown that, whereas more than 50% of adult elite controllers express one of the main ‘protective’ HLA class I alleles, HLA-B*27 or HLA-B*57 in Caucasians (19), or HLA-B*57/58:01/81:01 in African populations (54), HLA class I differences do not significantly influence disease progression in pediatric infection (20). Whereas suppression of viremia as a result of strong CD8+ T-cell responses presented by these protective class I molecules can interrupt and terminate the vicious cycle of immune activation and CD4 T-cell decline in adult infection, in pediatric infection, reduction in viremia via increasing potency of the HIV-specific CD8+ T-cell response coincides with increased immune activation and therefore comes at the cost of CD4 T-cell decline (Fig 5f). The fact that no elite controllers of HIV infection have been described in ART-naïve pediatric infection is itself evidence of the dangers of an immune strategy designed to suppress viremia if at the same time this is associated with increased immune activation. Consistent with this are the reports of a subset of ART-naïve elite controller adults who succeed in suppressing viral replication to undetectable levels but have progressed to AIDS because of increased immune activation (2).
The potency and high frequency of the bnAb responses in non-progressing children is an additional feature that is striking. As well-described previously, bnAbs typically do not neutralize contemporaneous autologous virus (55) and therefore do not protect against disease progression (56); and indeed are characteristically seen in HIV-infected individuals with high viral loads progressing to AIDS (41). Similarly, the bnAbs observed in the PNPs do not appear to protect against progression, since levels are, if anything, higher in the progressors. Nonetheless, bnAbs, defined by neutralization of ≥50% of a panel of tier-2 and tier-3 clade A, B and C viruses, were observed in 75% of ART-naïve pediatric subjects studied, compared to only 19% of adults infected with same HIV subtype (clade C). This figure of approximately one in five HIV-infected adults having bnAbs is highly consistent with other studies (40, 41, 56). In part this high frequency of bnAbs in the pediatric subjects is driven by persistent high viremia, although the effect here was not strong. However, high viral loads in typical adult infection lead to progression, whereas in the pediatric non-progressors it does not. Thus exposure to high viral loads for a longer duration is more common in pediatric infection. Previous studies have also shown early generation of bnAbs in HIV-infected children in the first 2 years of life (42). Additional factors contributing to this observation therefore may include the Th2 bias of the immune response in early life, that potentiates humoral immunity and therefore might be expected to favor bnAb development (57); and the hypothetical possibility that a proportion of the mothers of PNP may share the same phenotype, and therefore transmit a virus already molded by bnAbs in the mother. Passive immunization of bnAbs in macaques has been shown to result in enhanced autologous neutralizing responses in infants (58).
Irrespective of the apparent lack of a role for the potent bnAb responses in maintaining disease non-progression in these infected children, nonetheless access to pediatric cohorts such as those described here represents a precious resource from which there is the potential to isolate novel monoclonal bnAbs that have a key role in the combination bnAb treatment and cure strategies that are being developed against HIV (59). It will be important also in future work to determine whether the bnAbs generated by HIV-infected children differ in specificity from those generated in adult infection, as has been suggested (42).
The study of pediatric non-progressors described here has focused primarily on HIV-specific cellular immunity, limited to CD8+ T-cell and Th1 CD4 T-cell activity, and the neutralizing antibody response. Further work is necessary to investigate the potential role of other CD4 T-cell subsets, including Th2, Th17, and regulatory CD4+ T-cells in contributing to non-progression in these children. The observation of potent bnAb responses among PNP, although not contributing to slow progression, prompts the question of the mechanism by which these responses are generated, and the hypothesis that aspects of follicular helper CD4 T cell activity (60) specific to early life may be key. If sufficient study subject numbers can be recruited to make genetic studies feasible, it would be important to determine the genetic determinants of the immune strategy adopted by PNP, and the impact of such an immune strategy on responses to vaccines and pathogens other than HIV. Previous studies have addressed both the role of HLA class I variation of replicative capacity of the transmitted virus in pediatric non-progression (20). Finally, the ability of the innate immune system to sense HIV-1, and the resulting activation of innate immune pathways, clearly differs across distinct patient groups (61). Immune activation is a strong independent predictor for the speed of HIV-1 disease progression, and low levels of innate immune activation might contribute to the PNP phenotype.
Further studies are therefore needed to determine the specific mechanisms underlying low immune activation and non-progression in HIV-infected children, as well as in the abovementioned adult viremic non-progressors (AVNP). AVNP, in contrast to their pediatric viremic non-progressor counterparts described here, appear to be extremely rare. Of the four studies published to date, the largest cohort of AVNP has comprised only 9 subjects (15). Although their rarity has restricted the analyses possible, there are indications in AVNP also of mechanisms in common with the natural SIV hosts, including localization of viral infection predominantly in Tem CD4+ T-cell subsets as described above (15), and also of high expression of certain regulatory genes such as SOCS1 and EEF1D (16). Of note, even among the natural hosts of SIV infection, notably the sooty mangabeys (23) and African green monkeys (62), distinct specific mechanisms have evolved to arrive at the similar disease-free phenotype characterized by high SIV viral loads, low immune activation and preservation of long-lived memory CD4 T-cells. Whilst Darwinian selection has had sufficient time in the natural hosts of SIV to eliminate the less well-adapted animals, HIV in humans is a very recent infection in the evolutionary timescale, and as a result there is the opportunity here to study both the well-adapted non-progressors and the ill-adapted progressors. Further defining these mechanisms is relevant both to reaching a better understanding of HIV pathogenesis and therefore the development of new immunotherapeutic approaches to prevent HIV disease, but also to the HIV Cure field, where blocking infection of long-lived memory CD4 T-cell subsets such as Tscm may be a pre-requisite to elimination of HIV infection (48).
MATERIALS AND METHODS
Study design
The aim of this observational cohort study was to describe the clinical and immunological phenotype of non-progressing HIV-infected children. More detailed information on conception of individual experiments is provided in the specific sections below.
Study Subjects
A total of 275 ART-naïve HIV-1 C clade infected children from Southern Africa were studied from clinics at Kimberley Hospital, Kimberley, South Africa, Ithembalabanthu Clinic, Durban, South Africa, and Great Ormond St Hospital, London, UK. Pediatric non-progressors (PNP; n=170), were defined as ART-naïve children infected via mother-to-child transmission, aged >5.0 yrs, whose absolute CD4 T-cell count was >750 cells/mm3. PNPs had a median age 8.3 yrs (IQR 6.6–10.5 yrs), absolute CD4 T-cell count 885 cells/mm3 (IQR 815–1,019), median viral load 25,957 c/ml (IQR 5,338–75,500). With very few exceptions these PNP were not followed from birth and were identified incidentally some years later. Pediatric progressors were defined as ART-naïve children infected via mother-to-child transmission, aged >5.0 yrs, whose absolute CD4 T-cell count was <500 cells/mm3. For these progressors, the median age was 10.6 years (IQR 8.35–13.0), median CD4 T-cell count 336 cells/mm3 (IQR 197–432), and median viral load 57,500 c/ml (IQR 19,000–159,424). South African HIV-uninfected control children (n=21) were aged median 13.1 years (IQR 10.1–15.5).
For the Corrgram (Figure 3) and the LASSO model (Table 1) a subset of n=45 HIV-infected ART-naïve children with available data for all regarded parameters from the same study visit was included in the analysis. This subset comprised ART-naïve HIV-infected children and included both progressors and non-progressors: median age 11.5 years (IQR 8.9–13.9yrs), median absolute CD4 T-cell count 425 (327–717 cells/mm3); median viral load 39,815 (IQR 7,950–158,500).
The adult subjects in whom neutralization assays were undertaken were from the South African CAPRISA-002 cohort, as previously described (41). Five years after infection in these adults, median viral loads were 31,200 copies/ml (IQR 3,104–73,347 c/ml), and median absolute CD4 T-cell counts were 449 cells/mm3 (IQR 308–568 cells/mm3).
The subjects termed ‘future progressors’ (Fig 5) were infected young adults from Durban, South Africa (median: age 24yrs, absolute CD4 T-cell count 1106 cells/mm3, CD4% 36%, viral load 31,500) selected to match the pediatric non-progressors for CD4 T-cell count and viral load in order that measurements of HIV infection in distinct CD4 T-cell subsets would not be confounded by CD4 T-cell decline. These adults were termed ‘future’ progressors because these individuals were young and therefore identified relatively early in infection and likely to be progressors; in 7/12 of the ‘future progressors’, later samples with accompanying clinical data were available showing that in all 7 cases CD4 T-cell count had indeed declined subsequent to the initial time point, by a median of 347 CD4 T-cells/mm3 within a 6 month period (p=0.05, paired t-test), whilst viral loads had not altered significantly (p=0.38, Wilcoxon matched-pairs signed rank test).
Viral load measurement was undertaken using the Roche Amplicor version 1.5 assay until 2010 (range 400–750,000 c/ml). Thereafter the COBAS AmpliPrep/COBAS TaqMan HIV-1 Test version 2.0 by Roche (CAP/CTM v2.0) (range 20–10,000,000 c/ml) was used except at the Ithembalabanthu clinic where the BioMérieux NucliSens Version 2.0 Easy Q/ Easy Mag (NucliSens v2.0) assay (range 20–10,000,000 c/ml) was used from 2010.
Informed consent was obtained from all adult study participants and for children under age from their caregivers. Additionally, assent to participate in the study was given directly by children in the appropriate age groups. Studies were approved by the University of the Free State Ethics Committee, Bloemfontein; the Biomedical Research Ethics Committee, University of KwaZulu-Natal, Durban; and the Research Ethics Committee, University of Oxford.
Flow cytometry and intracellular cytokine staining (ICS) assays
Isolated PBMCs were used for staining with fluorescent monoclonal antibodies against markers of immune activation (HLA-DR and CD38), immune exhaustion (PD1 and 2B4), memory differentiation (CD45RA and CCR7) and CCR5 expression. Briefly, cells were rested in R10 media for 1h at 37°C in 5% CO2, then washed with PBS and stained in the dark for 20minutes on a 96-well plate in a volume of 50ul staining solution with titrated concentrations of fluorochrome conjugated monoclonal antibodies against cell surface markers and Near-IR Dead Cell Stain (ThermoFisher Scientific) as viability marker. The following antibodies were used: CD3/BV605 (SK7; BD Biosciences), CD4/V450 (RPA-T4; BD Biosciences), CD8/V500 (RPA-T8; BD Biosciences), PD-1/APC (MIH4, eBioscience), CCR7/PE (150503, R…D Systems), CD45RA/AlexaFluor700 (HI100, BioLegend), HLA-DR/FITC (L243, BD Biosciences), CD38/PECy (HIT2, BD Biosciences), CCR5/APC (2D7, BD Biosciences). Cells were then washed twice in PBS and fixed in 2% paraformaldehyde to be acquired on a flow cytometer.
ICS assays to measure IFN-γ, IL-2 and TNF-α production following stimulation with SEB at 1ug/ml or with pools of overlapping 18mer HIV peptides (Gag, Pol, or Nef) (44) of purified PBMC or of T-cells in a whole blood assay, respectively, were performed as previously described (63, 64). In brief, whole blood was incubated within 2hrs of collection with peptide pools (at 2ug/ml for each peptide) in the presence of anti-CD28 and anti-CD49 at 1ug/ml (BD Bioscience). After 5hrs incubation at 37°C, 5ug/ml Brefeldin A (Sigma) was added and the cells incubated for a further 5hrs, before 2mM EDTA was added for 15min at room temp and FACS lysing solution (BD Biosciences) for 10min at room temperature. Cells were then washed twice and cryopreserved at −80°C. Subsequently cells were thawed and stained with surface antibodies against CD3/AlexaFluor700 (UCHT1, BD Pharmingen), CD4/Qdot605 (S3.5, Life Technologies) and CD8/Pacific Blue (RPA-T8, BD Biosciences). Following 20min incubation at room temperature in the dark, cells were washed twice and resuspended in Fix/Perm solution (BD Biosciences) for 20 minutes at 4°C. Cells were then washed twice and resuspended in the ICS antibody mix with fluorescence-conjugated antibodies against IFNγ/PECy7 (4S.B3, eBioscience), IL-2/PE (PN IM2718U, Beckman Coulter) and TNFα/Alexa Flour 647 (MAb11, Biolegend) for 30 mins at room temperature in the dark. Cells were again washed twice and the pellet suspended in 2% paraformaldehyde in PBS. Flow cytometric acquisition was performed on an LSR II (BD) flow cytometer within 12 hours of staining and analyzed using FlowJo version 8.8.6.
Soluble CD14 and iFABP assays
Plasma levels of sCD14 were quantified using a commercially available Luminex kit (R…D systems). Plasma samples were used at a 100-fold dilution in duplicate as per manufacturers recommendations. Plasma levels of iFABP were quantified using a commercially available ELISA kit (R…D systems) at a 1:10 in duplicate. Results are expressed in pg/μl.
Corrgram
A correlation matrix (31) was used to visualize the Spearman correlations between selected variables comprising absolute CD4 T-cell count, CD4%, viral load, phenotypic frequencies of naïve (Tn) and effector memory T-cells (Tem), activated (CD38+HLADR+) and PD1high (PD1+) CD4+ and CD8+ T-cells, plasma levels of sCD14 and functional CD4+ and CD8+ T-cell responses (IFN-γ and IL-2 production) upon SEB-stimulation. A dataset of n=45 vertically HIV-infected ART-naïve children above 5 years of age for whom all measurements were available from the same study visit was used for analysis. Variables are grouped based on principal component analysis using the R package ‘corrgram’.
Virus neutralization assays
The ability of plasma from infected children and adults to neutralize HIV was measured against a panel of 16 clade A, clade B and clade C tier-2 and tier-3 viruses (listed in Table S2) (41) as a reduction in luciferase gene expression after a single round of infection of JC53bl-13 cells, also known as TZM-bl cells (NIH AIDS Research and Reference Reagent Program), with Envpseudotyped viruses (65). Titer was calculated as the reciprocal plasma/serum dilution causing a 50% reduction of relative light units (ID50).
Cell sorting and cell associated virus quantification
CD4+ T cells were sorted on a MoFlo XDP (Beckman Coulter) into T-cell subsets: Tn (naïve), Tscm (stem cell memory), Tcm (central memory) and Tem (effector memory), based on expression of CD27, CD45RO, CCR7 and CD95, as previously described (15). DNA was extracted (Qiagen) and used as input DNA for PCR. Cell copy number and Total HIV-1 DNA levels were quantified as previously described (52, 66). Briefly, cell copy number was quantified in triplicate at two dilutions using an albumin qPCR assay (52, 66). PCR amplification of the 5’LTR region of HIV was performed in a single round of 40 cycles. 8E5 cells containing one integrated copy of HIV-1 per cell were used in duplicate as standards with cell and HIV copy numbers ranging in serial 10-fold dilutions from 1 × 105 to 1 DNA copy/reaction. Template DNA samples were diluted to contain 106, 5×105, and 2.5 × 105 cells per well for analysis in triplicate wells of a 96 well plate. Template copies were calculated using ABI software.
IFN-γ elispot assays
Responses were determined to a set of 410 overlapping peptides, whose sequence was based on the 2001 C-clade consensus, arranged in a matrix system with 11–12 peptides in each pool. Responses to matrix pools were deconvoluted by subsequent testing with the individual 18mer peptides within each pool, and the identity of the individual 18mers recognized were thus confirmed, as previously described (54).
Statistical Analysis
The influence of several predictor variables on the absolute number of CD4 T-cell counts are assessed by a generalized linear model (GLM) (32). In a GLM, the linear predictor has the form ηi= β0+ xi1β1+ …+ xipβp and is connected to the (conditional) mean of the response variable, i.e. μi ≔ E[ yi∣ xi]= h(ηi) or ηi = g(μi), respectively, where g(•) and h(•) denote suitable link and response functions, respectively, xi ≔ (xil, ‥xp1)T , i= 1,…, n, is the vector of covariates and β ≔ (β0, β1, …, βp)T represents the vector of covariate effects. Additionally, given xi, the responses yi are assumed to be (conditionally) independent observations from a simple exponential family. In the present situation a Poisson regression model with log-link function is chosen. In the following approaches to variable selection are used by applying the so-called Least Absolute Shrinkage and Selection Operator (LASSO) principle on scaled covariates (33). All computations have been carried out in the statistical software R. For GLMs, there exist three different LASSO fitting routines in R that allow to fit the underlying model, the glmnet (34), the penalized (35) and the grplasso (36) packages. For all three methods, the optimal tuning parameter λ, which reflects the amount of penalization and, hence, controls variable selection, is determined via 10-fold cross-validation based on the model’s deviance. A final un-regularized conventional GLM is fitted on the selected set of covariates using the R-function glm.
Other statistical analysis was undertaken using Prism Graphpad software version 6.0. For exploration of bivariate associations Spearman rank correlation test was used. For comparisons between groups p-values were calculated using Mann-Whitney test. All p-values are two-sided, and a p-value of less than 0.05 was considered significant. In scatter plots median values and interquartile ranges are indicated.
Supplementary Material
Fig. S1. Decreased CD4 T-cell function and increased expression of exhaustion markers in pediatric progressors.
Fig. S2. Neutralizing antibody potency in pediatric and adult subjects.
Table S1. CD8+ T-cell activation is independently associated with total breadth of HIV-specific CTL responses and viral load.
Table S2. List of pseudoviruses tested for neutralizing sera activity by HIV-1 subtype.
Source data for Fig 1i, 1j, 2b, 2c, 2g, 6c, 6d
ACKNOWLEDGEMENTS
We thank Dr Zanele Ditse and Dr Mashudu Madzivhandila for technical support with the neutralizing antibody measurements.
Funding
This work was funded by the Wellcome Trust (WT104748MA, P.J.R.G.) and by the National Institutes of Health (RO1 AI46995, P.J.R.G). L.M. and P.L.M. acknowledge funding from the South African Medical Research Council (MRC) SHIP program. P.L.M. is supported by the SARChI Initiative of the SA DST and National Research Foundation of South Africa (Grant No 98341). A.J.P. is funded by the Wellcome Trust (093768/Z/10/Z).
Footnotes
Competing interests: The authors declare that they have no competing interests.
References
- 1.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG, T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. The Journal of infectious diseases 187, 1534 (May 15, 2003). [DOI] [PubMed] [Google Scholar]
- 2.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, Hsue P, Emu B, Krone M, Lampiris H, Douek D, Martin JN, Deeks SG, Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. The Journal of infectious diseases 197, 126 (January 1, 2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK, Arterial inflammation in patients with HIV. Jama 308, 379 (July 25, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW, Residual immune dysregulation syndrome in treated HIV infection. Advances in immunology 119, 51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A, Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nature medicine 1, 129 (February, 1995). [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM, Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104, 942 (August 15, 2004). [DOI] [PubMed] [Google Scholar]
- 7.Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, Staprans SI, Feinberg MB, Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18, 441 (March, 2003). [DOI] [PubMed] [Google Scholar]
- 8.VandeWoude S, Apetrei C, Going wild: lessons from naturally occurring T-lymphotropic lentiviruses. Clinical microbiology reviews 19, 728 (October, 2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G, Natural SIV hosts: showing AIDS the door. Science 335, 1188 (March 9, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goulder PJ, Lewin SR, Leitman EM, Paediatric HIV infection: the potential for cure. Nature reviews. Immunology 16, 259 (April, 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanche S, Newell ML, Mayaux MJ, Dunn DT, Teglas JP, Rouzioux C, Peckham CS, Morbidity and mortality in European children vertically infected by HIV-1. The French Pediatric HIV Infection Study Group and European Collaborative Study. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association 14, 442 (April 15, 1997). [DOI] [PubMed] [Google Scholar]
- 12.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F, A. S. W. G. o. H. I. V. I. i. W. Ghent International, Children, Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 364, 1236 (October 2–8, 2004). [DOI] [PubMed] [Google Scholar]
- 13.Mphatswe W, Blanckenberg N, Tudor-Williams G, Prendergast A, Thobakgale C, Mkhwanazi N, McCarthy N, Walker BD, Kiepiela P, Goulder P, High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. Aids 21, 1253 (June 19, 2007). [DOI] [PubMed] [Google Scholar]
- 14.Paul ME, Mao C, Charurat M, Serchuck L, Foca M, Hayani K, Handelsman EL, Diaz C, McIntosh K, Shearer WT, Women S Infants Transmission, Predictors of immunologic long-term nonprogression in HIV-infected children: implications for initiating therapy. The Journal of allergy and clinical immunology 115, 848 (April, 2005). [DOI] [PubMed] [Google Scholar]
- 15.Klatt NR, Bosinger SE, Peck M, Richert-Spuhler LE, Heigele A, Gile JP, Patel N, Taaffe J, Julg B, Camerini D, Torti C, Martin JN, Deeks SG, Sinclair E, Hecht FM, Lederman MM, Paiardini M, Kirchhoff F, Brenchley JM, Hunt PW, Silvestri G, Limited HIV infection of central memory and stem cell memory CD4+ T cells is associated with lack of progression in viremic individuals. PLoS pathogens 10, e1004345 (August, 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotger M, Dalmau J, Rauch A, McLaren P, Bosinger SE, Martinez R, Sandler NG, Roque A, Liebner J, Battegay M, Bernasconi E, Descombes P, Erkizia I, Fellay J, Hirschel B, Miro JM, Palou E, Hoffmann M, Massanella M, Blanco J, Woods M, Gunthard HF, de Bakker P, Douek DC, Silvestri G, Martinez-Picado J, Telenti A, Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. The Journal of clinical investigation 121, 2391 (June, 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ananworanich J, Apornpong T, Kosalaraksa P, Jaimulwong T, Hansudewechakul R, Pancharoen C, Bunupuradah T, Chandara M, Puthanakit T, Ngampiyasakul C, Wongsawat J, Kanjanavanit S, Luesomboon W, Klangsinsirikul P, Ngo-Giang-Huong N, Kerr SJ, Ubolyam S, Mengthaisong T, Gelman RS, Pattanapanyasat K, Saphonn V, Ruxrungtham K, Shearer WT, Group PS, Characteristics of lymphocyte subsets in HIV-infected, long-term nonprogressor, and healthy Asian children through 12 years of age. The Journal of allergy and clinical immunology 126, 1294 (December, 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ssewanyana I, Elrefaei M, Dorsey G, Ruel T, Jones NG, Gasasira A, Kamya M, Nakiwala J, Achan J, Charlebois E, Havlir D, Cao H, Profile of T cell immune responses in HIV-infected children from Uganda. The Journal of infectious diseases 196, 1667 (December 1, 2007). [DOI] [PubMed] [Google Scholar]
- 19.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, Baker B, Trocha A, Rosenberg R, Mackey E, Ueda P, Lu Z, Cohen D, Wrin T, Petropoulos CJ, Rosenberg ES, Walker BD, Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. The Journal of infectious diseases 197, 563 (February 15, 2008). [DOI] [PubMed] [Google Scholar]
- 20.Adland E, Paioni P, Thobakgale C, Laker L, Mori L, Muenchhoff M, Csala A, Clapson M, Flynn J, Novelli V, Hurst J, Naidoo V, Shapiro R, Huang KH, Frater J, Prendergast A, Prado JG, Ndung’u T, Walker BD, Carrington M, Jooste P, Goulder PJ, Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection. PLoS pathogens 11, e1004954 (June, 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goulder PJ, Walker BD, HIV and HLA class I: an evolving relationship. Immunity 37, 426 (September 21, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunham R, Pagliardini P, Gordon S, Sumpter B, Engram J, Moanna A, Paiardini M, Mandl JN, Lawson B, Garg S, McClure HM, Xu YX, Ibegbu C, Easley K, Katz N, Pandrea I, Apetrei C, Sodora DL, Staprans SI, Feinberg MB, Silvestri G, The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood 108, 209 (July 1, 2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, Vinton C, Gordon SN, Bosinger SE, Francella N, Hallberg PL, Cramer E, Schlub T, Chan ML, Riddick NE, Collman RG, Apetrei C, Pandrea I, Else J, Munch J, Kirchhoff F, Davenport MP, Brenchley JM, Silvestri G, Low levels of SIV infection in sooty mangabey central memory CD(4)(+) T cells are associated with limited CCR5 expression. Nature medicine 17, 830 (July, 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, Wara DW, Douglas SD, Luzuriaga K, McFarland EJ, Yogev R, Rathore MH, Levy W, Graham BL, Spector SA, Pediatric ACTG, Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. The Journal of allergy and clinical immunology 112, 973 (November, 2003). [DOI] [PubMed] [Google Scholar]
- 25.Lugada ES, Mermin J, Kaharuza F, Ulvestad E, Were W, Langeland N, Asjo B, Malamba S, Downing R, Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clinical and diagnostic laboratory immunology 11, 29 (January, 2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR Jr., Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of internal medicine 126, 946 (June 15, 1997). [DOI] [PubMed] [Google Scholar]
- 27.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC, Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine 12, 1365 (December, 2006). [DOI] [PubMed] [Google Scholar]
- 28.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC, Group ISS, Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases 203, 780 (March 15, 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM, Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. The Journal of infectious diseases 210, 1228 (October 15, 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry EJ, Kurachi M, Molecular and cellular insights into T cell exhaustion. Nature reviews. Immunology 15, 486 (August, 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friendly M, Corrgrams : Exploratory displays for correlation matrices. The American Statistician 56, 316 (1991). [Google Scholar]
- 32.McCullagh P, Nelder JA, Generalized Linear Models (2nd ed.) (New York: Chapman … Hall, 1989). [Google Scholar]
- 33.Tibshirani R, Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society 58, 267 (1996). [Google Scholar]
- 34.Friedman J, Hastie T, Tibshirani R, Regularization Paths for Generalized Linear Models via Coordinate Descent. Journal of Statistical Software 33, 1 (2010). [PMC free article] [PubMed] [Google Scholar]
- 35.Goeman JJ, L1 penalized estimation in the Cox proportional hazards model. Biometrical Journal 52, 70 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Meier L, Geer S. v. d., Bühlmann P, The Group Lasso for Logistic Regression. Journal of the Royal Statistical Society 70, 53 (2008). [Google Scholar]
- 37.Li B, Stefano-Cole K, Kuhrt DM, Gordon SN, Else JG, Mulenga J, Allen S, Sodora DL, Silvestri G, Derdeyn CA, Nonpathogenic simian immunodeficiency virus infection of sooty mangabeys is not associated with high levels of autologous neutralizing antibodies. Journal of virology 84, 6248 (June, 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer W, Apetrei C, Santiago ML, Li Y, Gautam R, Pandrea I, Shaw GM, Hahn BH, Letvin NL, Nabel GJ, Korber BT, Distinct evolutionary pressures underlie diversity in simian immunodeficiency virus and human immunodeficiency virus lineages. Journal of virology 86, 13217 (December, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilgore KM, Murphy MK, Burton SL, Wetzel KS, Smith SA, Xiao P, Reddy S, Francella N, Sodora DL, Silvestri G, Cole KS, Villinger F, Robinson JE, Pulendran B, Hunter E, Collman RG, Amara RR, Derdeyn CA, Characterization and Implementation of a Diverse Simian Immunodeficiency Virus SIVsm Envelope Panel in the Assessment of Neutralizing Antibody Breadth Elicited in Rhesus Macaques by Multimodal Vaccines Expressing the SIVmac239 Envelope. Journal of virology 89, 8130 (August, 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L, Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. Journal of virology 83, 757 (January, 2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L, Team CS, The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. Journal of virology 85, 4828 (May, 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goo L, Chohan V, Nduati R, Overbaugh J, Early development of broadly neutralizing antibodies in HIV-1-infected infants. Nature medicine 20, 655 (June, 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derdeyn CA, Moore PL, Morris L, Development of broadly neutralizing antibodies from autologous neutralizing antibody responses in HIV infection. Current opinion in HIV and AIDS 9, 210 (May, 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P, CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nature medicine 13, 46 (January, 2007). [DOI] [PubMed] [Google Scholar]
- 45.Ma D, Jasinska A, Kristoff J, Grobler JP, Turner T, Jung Y, Schmitt C, Raehtz K, Feyertag F, Martinez Sosa N, Wijewardana V, Burke DS, Robertson DL, Tracy R, Pandrea I, Freimer N, Apetrei C, C. International Vervet Research, SIVagm infection in wild African green monkeys from South Africa: epidemiology, natural history, and evolutionary considerations. PLoS pathogens 9, e1003011 (January, 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chahroudi A, Cartwright E, Lee ST, Mavigner M, Carnathan DG, Lawson B, Carnathan PM, Hashempoor T, Murphy MK, Meeker T, Ehnert S, Souder C, Else JG, Cohen J, Collman RG, Vanderford TH, Permar SR, Derdeyn CA, Villinger F, Silvestri G, Target cell availability, rather than breast milk factors, dictates mother-to-infant transmission of SIV in sooty mangabeys and rhesus macaques. PLoS pathogens 10, e1003958 (March, 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGary CS, Cervasi B, Chahroudi A, Micci L, Taaffe J, Meeker T, Silvestri G, Davenport MP, Paiardini M, Increased stability and limited proliferation of CD4+ central memory T cells differentiate nonprogressive simian immunodeficiency virus (SIV) infection of sooty mangabeys from progressive SIV infection of rhesus macaques. Journal of virology 88, 4533 (April, 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP, HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nature medicine 15, 893 (August, 2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M, HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nature medicine 20, 139 (February, 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, Abdel-Mohsen M, Hoh R, Hecht F, Hunt P, Somsouk M, Wong J, Johnston R, Siliciano RF, Richman DD, O’Doherty U, Palmer S, Deeks SG, Siliciano JD, Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS pathogens 9, e1003174 (February, 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF, Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540 (October 24, 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams JP, Hurst J, Stohr W, Robinson N, Brown H, Fisher M, Kinloch S, Cooper D, Schechter M, Tambussi G, Fidler S, Carrington M, Babiker A, Weber J, Koelsch KK, Kelleher AD, Phillips RE, Frater J, Investigators SP, HIV-1 DNA predicts disease progression and post-treatment virological control. eLife 3, e03821 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiselinova M, De Spiegelaere W, Buzon MJ, Malatinkova E, Lichterfeld M, Vandekerckhove L, Integrated and Total HIV-1 DNA Predict Ex Vivo Viral Outgrowth. PLoS pathogens 12, e1005472 (March, 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, Rathnavalu P, Moore C, Pfafferott KJ, Hilton L, Zimbwa P, Moore S, Allen T, Brander C, Addo MM, Altfeld M, James I, Mallal S, Bunce M, Barber LD, Szinger J, Day C, Klenerman P, Mullins J, Korber B, Coovadia HM, Walker BD, Goulder PJ, Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432, 769 (December 9, 2004). [DOI] [PubMed] [Google Scholar]
- 55.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM, Antibody neutralization and escape by HIV-1. Nature 422, 307 (March 20, 2003). [DOI] [PubMed] [Google Scholar]
- 56.Euler Z, van Gils MJ, Bunnik EM, Phung P, Schweighardt B, Wrin T, Schuitemaker H, Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. The Journal of infectious diseases 201, 1045 (April 1, 2010). [DOI] [PubMed] [Google Scholar]
- 57.Prendergast AJ, Klenerman P, Goulder PJ, The impact of differential antiviral immunity in children and adults. Nature reviews. Immunology 12, 636 (September, 2012). [DOI] [PubMed] [Google Scholar]
- 58.Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L, Anderson D, Landucci G, Richardson BA, Burton DR, Forthal DN, Haigwood NL, Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nature medicine 16, 1117 (October, 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hessell AJ, Jaworski JP, Epson E, Matsuda K, Pandey S, Kahl C, Reed J, Sutton WF, Hammond KB, Cheever TA, Barnette PT, Legasse AW, Planer S, Stanton JJ, Pegu A, Chen X, Wang K, Siess D, Burke D, Park BS, Axthelm MK, Lewis A, Hirsch VM, Graham BS, Mascola JR, Sacha JB, Haigwood NL, Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nature medicine 22, 362 (April, 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crotty S, Follicular helper CD4 T cells (TFH). Annual review of immunology 29, 621 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M, Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nature medicine 15, 955 (August, 2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM, CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nature medicine 15, 879 (August, 2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ, HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nature medicine 5, 518 (May, 1999). [DOI] [PubMed] [Google Scholar]
- 64.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, Murray RA, Hawkridge A, Haslett PA, Ress S, Hussey GD, Kaplan G, Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. Journal of immunological methods 291, 185 (August, 2004). [DOI] [PubMed] [Google Scholar]
- 65.Montefiori DC, Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays Current protocols in immunology / edited by Coligan John E. … [et al. ] Chapter 12, Unit 12 11 (January, 2005). [DOI] [PubMed] [Google Scholar]
- 66.Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA, T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. Journal of virology 78, 1160 (February, 2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Decreased CD4 T-cell function and increased expression of exhaustion markers in pediatric progressors.
Fig. S2. Neutralizing antibody potency in pediatric and adult subjects.
Table S1. CD8+ T-cell activation is independently associated with total breadth of HIV-specific CTL responses and viral load.
Table S2. List of pseudoviruses tested for neutralizing sera activity by HIV-1 subtype.
Source data for Fig 1i, 1j, 2b, 2c, 2g, 6c, 6d