Abstract
Background.
The 7th AJCC T-stage system for adrenocortical carcinoma (ACC), based on size and extra-adrenal invasion, does not adequately stratify patients by survival. Lymphovascular invasion (LVI) is a known poor prognostic factor. We propose a novel T-stage system that incorporates LVI to better risk-stratify patients undergoing resection for ACC.
Method.
Patients undergoing curative-intent resections for ACC from 1993 to 2014 at 13 institutions comprising the US ACC Group were included. Primary outcome was disease-specific survival (DSS).
Results.
Of the 265 patients with ACC, 149 were included for analysis. The current T-stage system failed to differentiate patients with T2 versus T3 disease (p = 0.10). Presence of LVI was associated with worse DSS versus no LVI (36 mo vs. 168 mo; p = 0.001). After accounting for the individual components of the current T-stage system (size, extra-adrenal invasion), LVI remained a poor prognostic factor on multivariable analysis (hazard ratio 2.14, 95% confidence interval 1.05–4.38, p = 0.04). LVI positivity further stratified patients with T2 and T3 disease (T2: 37 mo vs. median not reached; T3: 36 mo vs. 96 mo; p = 0.03) but did not influence survival in patients with T1 or T4 disease. By incorporating LVI, a new T-stage classification system was created: [T1: ≤ 5 cm, (–)local invasion, (+/−)LVI; T2: > 5 cm, (−)local invasion, (−)LVI OR any size, (+)local invasion, (−)LVI; T3: > 5 cm, (−)local invasion, (+)LVI OR any size, (+)local invasion, (+)LVI; T4: any size, (+)adjacent organ invasion, (+/−)LVI]. Each progressive new T-stage group was associated with worse median DSS (T1: 167 mo; T2: 96 mo; T3: 37 mo; T4: 15 mo; p < 0.001).
Conclusions.
Compared with the current T-stage system, the proposed T-stage system, which incorporates LVI, better differentiates T2 and T3 disease and accurately stratifies patients by disease-specific survival. If externally validated, this T-stage classification should be considered for future AJCC staging systems.
Adrenocortical carcinoma (ACC) is rare and aggressive, with 5-year overall survival (OS) ranging from 39 to 55% after curative-intent surgical resection.1–5 The majority of patients are diagnosed at an advanced stage, and approximately half have metastatic disease at diagnosis.1,6 There has been limited success with systemic therapy, and surgery is the only potentially curative treatment option for ACC.4
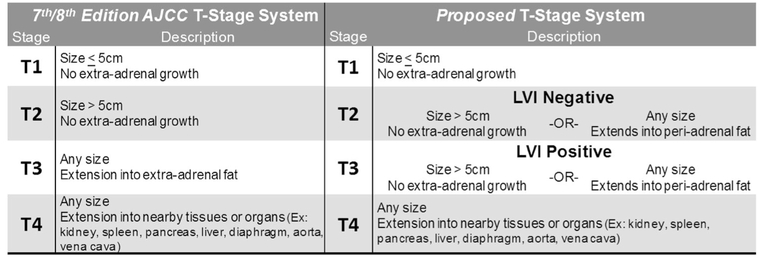
Given these poor outcomes, determining individual prognosis is vital to patient counseling and treatment planning. Two separate validation studies of the AJCC 7th edition revealed that stage II and III did not differ significantly in terms of disease-specific survival.7,8 The T-stage system for ACC differentiates T1 and T2 based on the size of the tumor, whereas advanced T-stages are differentiated by degree of local invasion (T3: into extra-adrenal fat, T4: into nearby structures and organs; Fig. 1). Although the T-stage system remained unchanged in the recent AJCC 8th edition, adjustments to the overall staging system attempted to address its deficiencies, such that stage III is now largely defined by lymph node status, and includes patients who are T1–2 and node-positive.9 However, during surgical resection of ACC, there is often very low to no lymph node yield, with only 8% of patients undergoing lymphadenectomy in large series.1 Thus, although the AJCC 8th edition appears to better predict survival in stage II versus stage III disease, the stage III categorization for early-stage patients is based on lymph node data that is often clinically unavailable after ACC resection. In this study, we sought to redefine the T-stage system, largely because lymph node status is often not available to better predict survival from ACC.
FIG. 1.
AJCC 7th/8th edition and proposed T-stage classification systems
One pathologic variable that has been cited as a risk factor for worse overall and disease-free survival in several different cancers is lymphovascular invasion (LVI).11–18 Our group recently proposed a novel staging system for distal cholangiocarcinoma based on tumor size and LVI status, which better differentiated patient survival compared with the current AJCC T-stage system. Although lymph node involvement is a known poor prognostic factor, its prognostic value is limited in some malignancies due to low lymph node yields during resection.12,13 LVI, which is routinely assessed in the primary tumor, has proved an independent negative prognostic factor in diseases, such as intrahepatic and hilar cholangiocarcinomas.
The purpose of this study was to investigate the prognostic value of LVI in ACC, and, given the infrequent availability of lymph node status, to determine whether incorporation of LVI data into the current AJCC T-stage system could better predict outcomes for patients with ACC.
METHODS
Study Population
The United States Adrenocortical Carcinoma Group (USACCG) represents a collaboration of 13 academic institutions across the United States: Emory University, Stanford University, The Johns Hopkins University, Medical College of Wisconsin, New York University, The Ohio State University, Washington University in St. Louis, University of Wisconsin, University of California San Diego, University of Texas Southwestern, University of California San Francisco, Vanderbilt University, and Wake Forest University. All patients who underwent resection of ACC from 1993 to 2014 were evaluated. Only patients who underwent curative-intent resection of ACC, defined as resection without gross residual disease, were analyzed. Patients with nondistant-metastatic T1, T2, and T3 disease with available LVI data were included in all analyses. Patients with T4 disease, regardless of LVI data or distant metastases, also were included in analyses. Patients who died within 30 days of surgery or with less than 30-day follow-up were excluded.
Data were gathered by chart review for demographic, radiographic, pathologic, and clinical data. Pathologic overall stage was based on the seventh American Joint Committee on Cancer (AJCC) guidelines, because this version was the most current at the time of data collection. Pathologic T-stage was based on the AJCC 7th and 8th editions, because the T-stage definitions are identical in the new AJCC edition.19 LVI was defined as the presence or absence of lymphatic and/or vascular invasion. Survival data and cause-of-death were collected by chart review and date of death was verified using the Social Security Death Index database. The Institutional Review Boards at all participating centers approved this study, and research was conducted in accordance with the Health Insurance Portability and Accountability Act of 1996.
Statistical Analyses
All statistical analyses were conducted using SPSS version 23.0 (IBM Corp., Armonk, NY). Chi-square analyses, Fisher’s exact tests, and two-tailed Student’s t tests were used for comparison of categorical and continuous variables, as appropriate, between patients with and without LVI. Kaplan-Meier log-rank tests were performed to assess the relationship of LVI with disease-specific survival (DSS), as well as to evaluate DSS for both the current and the proposed T-stage system. Univariable and multivariable cox regression analyses were used to assess the individual variables that contributed to the staging system and to determine the efficacy of the proposed new staging system compared with the current AJCC T-stage system. A p value < 0.05 was considered statistically significant for all analyses.
RESULTS
Study Population
The USACCG includes 265 total patients who underwent resection of ACC. Patients with inadequate pathologic data, R2 resections or missing margin status, 30-day mortality or less than 30-day follow-up, and tumor stages T1, T2, and T3 disease with distant metastases were excluded from analysis. A total of 149 (56%) patients were included. Of these, 9 patients had stage T4 disease without LVI data available, leaving a population of 140 for baseline LVI comparisons. Baseline patient demographic, pathologic, and clinical data are summarized in Table 1. The mean age was 51 years, and 97 patients (65%) were female. Average tumor size was 12 cm (SD = 6 cm). A quarter of patients (n = 37) had positive resection margins (R1); the rest had R0 resections (n = 112, 75%). Most patients had T2 (n = 58, 39%) or T3 disease (n = 53, 36%). Only 48 patients (32%) had lymph nodes resected, and 35% of these were node-positive (n = 17). Eighty-seven patients (61%) had recurrence of disease, of which 31% had isolated locoregional and 69% had distant recurrences.
TABLE 1.
Baseline clinicopathologic features of all patients stratified by lymphovascular invasion status
| Variable | All patients (n = 149)# | LVI-negative (n = 56) | LVI-positive (n = 84) | p Value* |
|---|---|---|---|---|
| Age (yr), mean ± SD | 51 ± 15 | 51 ± 15 | 51 ± 15 | 0.96 |
| Male gender, n (%) | 52 (35) | 16 (29) | 35 (42) | 0.16 |
| Race, n (%) | 0.46 | |||
| White | 116 (81) | 45 (83) | 62 (76) | |
| Other | 28 (19) | 9 (17) | 19 (23) | |
| BMI (kg/m2), mean ± SD | 29 ± 9 | 29 ± 10 | 30 ± 9 | 0.69 |
| Comorbidities, n (%) | 0.79 | |||
| 0 | 102 (69) | 40 (71) | 55 (66) | |
| 1 | 36 (24) | 12 (21) | 22 (27) | |
| ≥2 | 10 (7) | 4 (7) | 6 (7) | |
| Hormonal hypersecretion, n (%) | 57 (41) | 16 (31) | 38 (47) | 0.09 |
| Symptomatic, n (%) | ||||
| Pain | 71 (50) | 22 (43) | 42 (52) | 0.43 |
| Weight loss | 20 (13) | 9 (17) | 9 (11) | 0.43 |
| Edema | 23 (16) | 5 (10) | 15 (19) | 0.29 |
| Right-sided tumor, n (%) | 70 (48) | 19 (34) | 46 (55) | 0.03 |
| Minimally invasive resection, n (%) | 29 (20) | 15 (27) | 14 (17) | 0.25 |
| Other organs resected, n (%) | 71 (49) | 24 (44) | 40 (48) | 0.80 |
| Length of stay (d), median (range) | 6 (1-50) | 5 (1-28) | 6 (1-50) | 0.08 |
| Major complications, n (%) | 16 (13) | 4 (9) | 10 (14) | 0.62 |
| Tumor size (cm), mean ± SD | 12 ± 6 | 10 ± 5 | 13 ± 6 | 0.03 |
| Margin status, n (%) | 0.006 | |||
| R0 | 112 (75) | 49 (87) | 55 (65) | |
| R1 | 37 (25) | 7 (12) | 29 (34) | |
| 7th AJCC stage, n (%) | <0.001 | |||
| I | 12 (8) | 6 (11) | 6 (7) | |
| II | 53 (36) | 32 (58) | 21 (25) | |
| III | 50 (34) | 13 (24) | 36 (43) | |
| IV | 34 (23) | 4 (7) | 21 (25) | |
| 7th/8th AJCC T stage, n (%) | <0.001 | |||
| T1 | 12 (8) | 6 (11) | 6 (7) | |
| T2 | 58 (39) | 34 (61) | 24 (29) | |
| T3 | 53 (36) | 15 (27) | 38 (45) | |
| T4 | 26 (17) | 1 (2) | 16 (19) | |
| Lymph nodes harvested, n (%) | 48 (32) | 20 (36) | 25 (30) | 0.58 |
| Lymph node positive, n (%) | 17 (35) | 3 (15) | 12 (48) | 0.03 |
| Metastatic disease, n (%) | 13 (9) | 1 (2) | 9 (11) | 0.09 |
| Recurrence, n (%) | 87 (61) | 31 (57) | 51 (63) | 0.64 |
| Region of recurrence, n (%) | 0.003 | |||
| Locoregional (isolated) | 26 (31) | 16 (55) | 10 (20) | |
| Distant | 42 (69) | 13 (45) | 40 (80) |
Statistically significant p values are indicated in bold
BMI body mass index, SD standard deviation, AJCC American Joint Committee on Cancer
Includes T4 patients with missing LVI data (n = 9)
p value derived from Chi-square, Fisher’s exact, independent samples t test, and Mann–Whitney U test as appropriate
AJCC 7th/8th Editions T-Stage and Survival
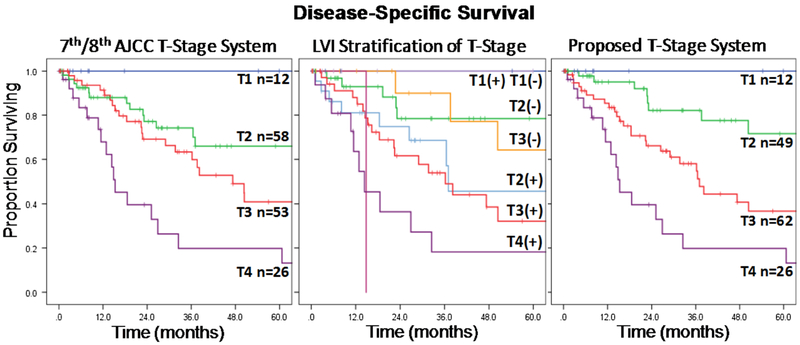
Kaplan-Meier survival analysis for the current AJCC T-stage system is shown in Fig. 2a. Overall, there was a significant difference in median DSS among T1 (167 mo, 95% confidence interval [CI] not calculated), T2 (86 mo, 95% CI 7–165 mo), T3 (47 mo, 95% CI 28–66 mo), and T4 disease (15 mo, 95% CI 10–21 mo; p < 0.001). However, on stepwise comparison, there was no difference in median DSS between T1 and T2 disease (p = 0.24) or T2 and T3 disease (p = 0.10); T3 and T4 disease were significantly different (p = 0.008).
FIG. 2.
AJCC 7th/8th T-stage system, LVI stratification of 7th/8th AJCC T-stage, and proposed T-stage system
Lymphovascular Invasion
Of 140 patients with LVI data, LVI was present in 84 patients (60%). There was no difference between LVI-positive patients and LVI-negative patients with regards to age, gender, race, or baseline comorbidities (Table 1). LVI-positive tumors were more often right-sided (55% vs. 34%, p = 0.03) and were larger (13 cm vs. 10 cm, p = 0.03) at baseline.
Patients with LVI were more likely to have T3 (45% vs. 27%) and T4 (19% vs. 1%) disease than patients without LVI (p < 0.001). There was a higher proportion of lymph-node positivity in LVI-positive versus LVI-negative patients (48% vs. 15%, p = 0.03), although the incidence of lymphadenectomy was low in both groups (n = 20, 36% and n = 25, 30%). LVI-positive disease was associated with more positive margins compared with LVI-negative disease (34% vs. 12%; p = 0.006). There was no difference in recurrence between LVI-positive and LVI-negative tumors (63% vs. 57%, p = 0.064), although LVI-positivity was associated with less locoregional recurrence (20% vs. 55%) and more distant recurrence (80% vs. 45%) compared with LVI-negativity (p = 0.003).
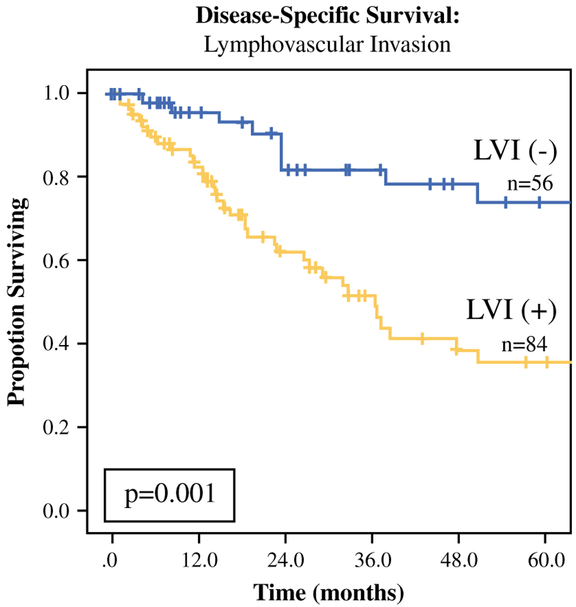
On Kaplan-Meier analysis and univariable Cox regression analysis, presence of LVI was associated with worse DSS compared with no LVI (median survival: 36 mo vs. 168 mo; p = 0.001, Fig. 3; hazard ratio [HR] 2.81, 95% CI 1.46–5.41, p = 0.002). When accounting for the individual components of the current T-stage system (tumor size > 5 cm, invasion into periadrenal fat or surrounding structures), LVI persisted as a poor prognostic factor on multivariable Cox regression (HR 2.14, 95% CI 1.05–4.38, p = 0.04; Supplemental Table 1).
FIG. 3.
Lymphovascular invasion
Proposed T-Stage and Survival
When examining the influence of LVI on the current AJCC T-stage system, LVI further stratified patients with T2 (LVI negative vs. positive: median not reached (MNR) vs. 37 mo) and T3 disease (LVI negative vs. positive: 96 mo vs. 36 mo; p = 0.03) but did not appear to influence DSS in patients with T1 and T4 disease (Fig. 2b). Furthermore, DSS was similar among patients with T2/LVI-negative and T3/LVI-negative tumors (p = 0.50), as well as among T2/LVI-positive and T3/LVI-positive tumors (p = 0.58). Based on these results, a novel system was devised that incorporated LVI to better differentiate DSS for T2 and T3 disease. In the proposed system, patients with traditional T2 disease and LVI-positive pathology (n = 24) were upstaged and combined with T3/LVI-positive patients, whereas patients with traditional T3 disease and LVI-negative pathology (n = 15) were downstaged and combined with T2/LVI-negative patients (Fig. 1). Each progressive new T-stage group was associated with worse median DSS (T1: 167 mo, 95% CI not calculated; T2: 96 mo, 95% CI 43–149 mo; T3: 37 mo, 95% CI 29–45 mo; T4: 15 mo, 95% CI 10–21 mo; p < 0.001; Fig. 2c). Univariable Cox regression was performed on all relevant demographic, clinical, and pathologic variables (Supplemental Table 2), and multivariable models were built to assess the efficacy of the proposed staging system. The multivariable models revealed that, even when accounting for other adverse factors, the proposed T-stage system demonstrated better discrimination of DSS for T3 versus T2 disease (HR 2.19, 95% CI 1.02–4.69, p = 0.04) compared with the current AJCC T-stage system (HR 1.34, 95% CI 0.66–2.71, p = 0.42; Table 2).
TABLE 2.
Cox regression analyses
| Multivariable Cox regression models for disease-specific survival: comparing 7th/8th AJCC T-stage versus proposed T-stage | |||
|---|---|---|---|
| Variable | HR | 95% CI | p value |
| 7th/8th AJCC T-stage system | |||
| T2 (Ref T1) | 2.40 | 0.31–18.18 | 0.40 |
| T3 (Ref T2) | 1.34 | 0.66–2.71 | 0.42 |
| T4 (Ref T3) | 2.51 | 1.27–4.95 | 0.008 |
| Hormone hypersecretion | 1.73 | 0.99–3.01 | 0.05 |
| R1 Resection | 2.36 | 1.30–4.28 | 0.005 |
| Proposed T-stage system | |||
| T2 (Ref T1) | 1.64 | 0.20–13.16 | 0.64 |
| T3 (Ref T2) | 2.19 | 1.02–4.69 | 0.04 |
| T4 (Ref T3) | 2.18 | 1.13–4.22 | 0.02 |
| Hormone hypersecretion | 1.69 | 0.97–2.95 | 0.07 |
| R1 resection | 2.35 | 1.31–4.21 | 0.004 |
Statistically significant p values are indicated in bold
DISCUSSION
The 7th edition of the AJCC staging system for ACC does not reliably predict survival outcomes for individual patients7,8,20,21. In particular, two separate validation studies revealed that stage II and III do not differ significantly in terms of disease-specific survival (stage II: T2N0M0 and stage III: T2N1M0, T3N0M0).7,8 This led the European Network for the Study of Adrenal Tumors (ENSAT) to propose a new staging system, which was adapted into the recently released AJCC 8th edition. In this revised version, all patients who have T3–4 disease without metastasis are now stage III, and the stage IV designation is reserved for metastatic disease.9 The difference between stage II and III for patients with T1–2 disease is based on the presence or absence of nodal disease.9 It is important to note, however, that there is typically a low rate of lym-phadenectomy during surgical resection of ACC, which is generally limited to large, locally advanced tumors and metastasis.10 In fact, only 8% of patients underwent lym-phadenectomy in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database.10 Thus, although the AJCC 8th edition improved the discrimination between stage II and III disease, the underlying limitations of T-stage discrimination of outcomes and reliance on lymph node status for overall stage persisted.
This study assessed outcomes for patients undergoing curative intent resection for ACC using one of the largest multi-institutional databases available in the United States. In this cohort, as in prior studies, the current AJCC staging system was inadequate for predicting outcomes for the majority of patients, i.e., those categorized as having T2 or T3 disease. Presence of LVI was associated with more advanced stage, positive margins, and larger tumor size, as well as reduced disease-specific survival. The presence of LVI discriminated patients with T2 and T3 disease with regard to DSS, but it did not appear to influence outcomes in patients with T1 or T4 disease. A new T-staging system was devised, which redefined T2 and T3 disease based on LVI status (T2: LVI neg; T3: LVI pos). This proposed system demonstrated better discrimination of DSS compared with the current AJCC T-stage system, even after accounting for other adverse clinicopathologic factors. To the authors’ knowledge, this is the first study that has proposed a novel, more predictive pathologic T-stage system for ACC.
LVI is a known risk factor for worse overall and disease-free survival in several cancers: breast cancer, cholangio-carcinoma, gastric adenocarcinoma, incidental gallbladder cancer, cervical cancer, and colorectal cancer.11–18,23 In addition, LVI has been associated with other poor prognostic factors, such as poorly differentiated pathology, increased preoperative serum carcinoembryonic antigen levels, and lymph node metastasis.11,16,24 Several studies have proposed using LVI as an independent risk factor, in lieu of lymph node involvement, to help guide prognosis and adjuvant treatment decisions, especially for diseases where lymph node status often cannot be deter-mined.12,13,24 For ACC, lymph node harvest often is omitted, as only 32% of the current cohort and 8% of the SEER database had lymph nodes resected.10,11 Thus, the fact that the revisions of the AJCC 8th edition staging system are largely based on lymph node status poses a problem.
Analysis of the current cohort revealed a significant difference in survival between patients with LVI-positive compared with LVI-negative tumors (HR 2.81, p = 0.002). When stratifying the AJCC T-stage system by LVI, patients with T3 disease who were LVI-negative had a similar median survival to patients with T2 disease who were LVI-negative. The converse was true for patients with T2 and T3 disease who were LVI-positive. Thus, the proposed T-stage system utilized LVI status, a pathologic characteristic that can be assessed in every resected specimen, unlike lymph node status, to redefine T2 and T3 disease. The proposed new T-staging system demonstrated better discrimination of DSS for T3 vs. T2 disease (HR 2.19, p = 0.04) compared with the current AJCC T-stage system (HR 1.34, p = 0.42) even when accounting for other adverse pathologic factors (Table 2).
The results of this study are limited by the retrospective nature of its design, which precludes us from establishing a causal relationship between LVI and oncologic outcomes. However, the formation of the USACCG database comprised of 13 institutions across the United States represents the largest American collaborative database and a unique opportunity to investigate prognostic factors and oncologic outcomes for ACC. The multi-institutional nature of this collaboration limits single-institution bias and better represents geographic diversity, making it uniquely qualified to examine ACC staging. The data for this database were collected over a two-decade period, so there is additional concern for variation in institutional preoperative management and treatment protocols. However, a 2008 study by Bilimoria et al. concluded that there has been little change in ACC management strategies or outcomes over this time period.5 Additionally, there was no pathologic rereview because of the significant logistic difficulties this would pose; each participating institution, however, has pathologists that are expert in rare malignancies. Our study focused only on T-stage and did not attempt to propose a new comprehensive staging system, largely due to limited data on lymph node status. This limitation reflects the clinical reality that few patients undergoing resection of ACC actually have lymph nodes resected with the specimen and, thus, underscores the importance of accurate T-staging for this disease. Finally, this proposed T-stage system needs to be externally validated in a large dataset before incorporation into the next staging system.
CONCLUSIONS
The revised AJCC 8th edition staging system for adrenocortical carcinoma merely redistributed the existing T and N classification systems into different stages. The fact that a minority of patients have lymph node status available after resection, however, underscores the importance of having a T-staging system that accurately stratifies patients with respect to survival. The proposed T-stage classification system incorporates lymphovascular invasion status—a pathologic characteristic that is routinely available in each specimen—into the existing system, which is based on size and extent of local invasion. This novel T-stage system better differentiates patients by their survival outcomes after resection, and if validated, this new T-stage classification system could be considered for incorporation into the next AJCC staging system for adrenocortical carcinoma.
Supplementary Material
Footnotes
DISCLOSURE No authors have any disclosures relevant to this manuscript.
Electronic supplementary material The online version of this article (http://doi.org/10.1245/s10434-017-6236-1) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30:872–78. 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 2.Ayala-Ramirez M, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol. 2013;169:891–99. 10.1530/EJE-13-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6:719–26. [DOI] [PubMed] [Google Scholar]
- 4.Postlewait LM et al. Outcomes of adjuvant mitotane after resection of adrenocortical carcinoma: a 13-institution study by the US Adrenocortical Carcinoma Group. J Am Coll Surg. 2015. 10.1016/j.jamcollsurg.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilimoria KY, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–6. 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 6.Livhits M, Li N, Yeh MW, Harari A. Surgery is associated with improved survival for adrenocortical cancer, even in metastatic disease. Surgery. 2014;156:1531–40; discussion 1540–1. 10.1016/j.surg.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lughezzani G, et al. The European network for the study of adrenal tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. Eur J Cancer. 2010;46:713–9. 10.1016/j.ejca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Fassnacht M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer. 2009:115:243–50. 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 9.Amin MB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–9. 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 10.Nilubol N, Patel D, Kebebew E. Does lymphadenectomy improve survival in patients with adrenocortical carcinoma? A population-based study. World J Surg. 2016;40:697–705. 10.1007/s00268-015-3283-2. [DOI] [PubMed] [Google Scholar]
- 11.Schoppmann SF, et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004;240:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SH, Kooby DA, Staley CA 3rd, Sarmiento JM, Maithel SK. The prognostic importance of lymphovascular invasion in cholangiocarcinoma above the cystic duct: a new selection criterion for adjuvant therapy? HPB (Oxford). 2011;13:605–11. 10.1111/j.1477-2574.2011.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher SB, et al. Lymphovascular and perineural invasion as selection criteria for adjuvant therapy in intrahepatic cholangio-carcinoma: a multi-institution analysis. HPB (Oxford). 2012;14: 514–22. 10.1111/j.1477-2574.2012.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postlewait LM, et al. Proposal for a new T-stage classification system for distal cholangiocarcinoma: a 10-institution study from the US Extrahepatic Biliary Malignancy Consortium. HPB (Oxford). 2016;18:793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin LX, et al. Factors associated with recurrence and survival in lymph node-negative gastric adenocarcinoma: a 7-institution study of the US Gastric Cancer Collaborative. Ann Surg. 2015;262:999–1005. https://doi.org/10.1097/SLA.000000000000 1084. [DOI] [PubMed] [Google Scholar]
- 16.Lim SB, et al. Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis Colon Rectum. 2010;53: 377–84. 10.1007/DCR.0b013e3181cf8ae5. [DOI] [PubMed] [Google Scholar]
- 17.Cardona K, et al. Detailed pathologic characteristics of the primary colorectal tumor independently predict outcome after hepatectomy for metastases. Ann Surg Oncol 2013;20:148–54. 10.1245/s10434-012-2540-y. [DOI] [PubMed] [Google Scholar]
- 18.Ethun CG, et al. A novel pathology-based preoperative risk score to predict locoregional residual and distant disease and survival for incidental gallbladder cancer: a 10-institution study from the US Extrahepatic Biliary Malignancy Consortium. Ann Surg Oncol. 2016. 10.1245/s10434-016-5637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–74. 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 20.Asare EA, et al. A novel staging system for adrenocortical carcinoma better predicts survival in patients with stage I/II disease. Surgery. 2014;156:1378–85. https://doi.org/10.1016/j.surg.2014.08.018;discussion 1385-6. [DOI] [PubMed] [Google Scholar]
- 21.Libe R Adrenocortical carcinoma (ACC): diagnosis, prognosis, and treatment. Front Cell Dev Biol. 2015;3:45 10.3389/fcell.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller BS, Gauger PG, Hammer GD, Giordano TJ, Doherty GM. Proposal for modification of the ENSAT staging system for adrenocortical carcinoma using tumor grade. Langenbecks Arch Surg. 2010;395:955–61. 10.1007/s00423-010-0698-y. [DOI] [PubMed] [Google Scholar]
- 23.Eifel PJ, Burke TW, Delclos L, Wharton JT, Oswald MJ. Early-stage I adenocarcinoma of the uterine cervix: treatment results in patients with tumors less than or equal to 4 cm in diameter. Gynecol Oncol. 1991;41:199–205. [DOI] [PubMed] [Google Scholar]
- 24.Eguchi T, et al. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Mod Pathol. 2006;19:475–80. 10.1038/modpathol.3800557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.