Abstract
Pharmacogenetic studies indicate that a variable response to anti-vascular endothelial growth factor (VEGF) therapy in patients with neovascular form of AMD (nAMD) may be due to polymorphisms in the complement factor H gene (CFH). This study is the first to investigate the association between CFH Y402H polymorphism and the response to ranibizumab therapy in Malaysian patients with nAMD. We included 134 patients with nAMD, examined between September 2014 and February 2016. The diagnosis of nAMD was confirmed by ophthalmologic examination, before ranibizumab therapy was started. Each patient received an intravitreal injection of 0.5 mg/0.05 ml ranibizumab following a treat-and-extend (TE) regimen. Best-corrected visual acuity (BCVA) and central retinal thickness (CRT) were recorded after 3 and 6 months following the first injection and compared with the baseline values. Genotyping of Y402H (rs1061170) polymorphism was performed using PCR-RFLP and the amplified product was digested with MluCI restriction enzyme. Association between the Y402H genotypes and response to treatment was determined by a logistic regression analysis of responder (n = 49) and non-responder (n = 84) group. Significantly worse mean BCVA was observed for the CC genotype compared to the TT + CT genotype in the total sample after 6-month follow-up (p = 0.018). Comparing the baseline and 6-month point measurements, improved mean BCVA was observed in responder group, while worse mean BCVA was recorded for non-responder group. However, our regression analysis, adjusted for confounding factors, showed no significant association between the Y402H genotypes and response to treatment in nAMD patients under the recessive model (p > 0.05). Overall, our results suggest that factors other than Y402H polymorphism may be involved in the progression of nAMD after treatment with anti-VEGF agents, in Malaysian population.
Keywords: Age-related macular degeneration, AMD, complement factor H, CFH, ranibizumab, Y402H, SNP, treatment response
INTRODUCTION
In developed countries, especially in the West, age-related macular degeneration (AMD) is the major cause of visual impairment in older adults [1-3]. Today, a similar trend is observed in a large number of Asian countries, where pharmacogenetic studies on AMD have shown unique epidemio logical, genetic, and clinical features of AMD in Asian populations, as well as a different response to treatment, compared to the populations in the West. This may be related to several factors, such as a higher number of different racial and ethnic groups in Asia, the use of a different classification system for AMD, and dietary and lifestyle factors [4]. The neovascular form of AMD (nAMD), associated with choroidal neovascularization (CNV), is a vasculopathy resulting in serous exudation and hemorrhages [5]. Angiogenesis and vascular permeability during the progression of CNV are regulated by vascular endothelial growth factor (VEGF), among other factors. Bevacizumab [6] and ranibizumab [7] are anti-VEGF drugs commonly used to improve best-corrected visual acuity (BCVA) in AMD patients [8], and clinical studies in different populations demonstrated the efficacy of ranibizumab in treating nAMD [6,7,9].
Both environmental and genetic factors play a role in the development of nAMD [10]. The complement system of plasma proteins, important in the immune and inflammatory response of the body, is also involved in AMD pathogenesis. Complement factor H (CFH) is a regulator of the alternative pathway of the complement system; it has anti-inflammatory effects and ensures that host tissue is not damaged. Genetic variation in the CFH gene, which is located on 1q31.3 region of chromosome 1, has been associated with an increased risk of inflammatory diseases [11]. The T1277C polymorphism (rs1061170) of the CFH gene, involving a T to C transition at the 1277 position in the exon 9, and resulting in a substitution of tyrosine with histidine at the 402 position (Y402H) in the amino acid sequence, has been extensively studied in relation to the development of nAMD [12-16]. A number of pharmacogenetic studies in different countries, focused on the response of nAMD patients to anti-VEGF therapy [6-9,11,17-21], and indicated that a variable response to ranibizumab or bevacizumab therapy in nAMD patients may be due to polymorphisms in the CFH gene, among other factors. For example, the CC genotype of CFH Y402H polymorphism has been associated with a poor response while the TT genotype with a good response to intravitreal injection of ranibizumab or bevacizumab [11,17-20].
To the best of our knowledge, such results have not been reported for the Malaysian population. In this study, we investigated the association between CFH Y402H polymorphism and the response to ranibizumab therapy in Malaysian patients with nAMD.
MATERIALS AND METHODS
The study was approved by the Universiti Putra Malaysia (UPM), Universiti Kebangsaan Malaysia (UKM) Ethics Committee and Medical Research Ethics Committee (MREC), as well as by the Ministry of Health (MOH) in Malaysia, and registered in the National Medical Research Registry with a registration number NMRR-14-1176-21475.
Data collection
This prospective cohort study was conducted from September 2014 to February 2016 at the ophthalmology clinics of two tertiary centers, i.e., Hospital Selayang and Universiti Kebangsaan Malaysia Medical Centre (UKMMC). Out of 158 patients with nAMD that were invited to participate in the study, 134 patients were included. Informed consent was obtained from all participants before initiating the study.
Patient’s criteria
Before therapy with ranibizumab was started, comprehensive ophthalmologic examination was performed in all patients and the diagnosis of nAMD was made based on BCVA test and Snellen chart, slit-lamp examination, dilated fundus examination, fluorescein angiography (FA), measurement of central retinal thickness (CRT) using optical coherence tomography (OCT) and indocyanine green angiography (ICG). Active neovascularization was confirmed with FA and OCT at baseline, before treatment. None of the patients were previously treated for nAMD.
All patients aged above 50 years, with the presence of exudative or hemorrhagic features involving macula, with no previous intravitreal anti-VEGF treatment, and with minimum follow-up of 6 months after the first injection of ranibizumab (Lucentis; Novartis Pharma AG, Basel, Switzerland and Genentech Inc., South San Francisco, California, USA) were eligible for this study.
Patients who were followed up for less than 6 months, had a CNV secondary to other causes such as trauma, hereditary diseases, angioid streaks, inflammatory disease, pathologic myopia, had undergone a posterior vitrectomy, or had retinal diseases other than AMD were excluded from this study.
Measures of response to treatment
Each patient received an intravitreal injection of 0.5 mg/0.05 ml ranibizumab for the first 3 months. After the initial 3 months, if leakage was still present, the patients were receiving a monthly injection until the leakage stopped. Once the leakage was under control, a treat-and-extend (TE) regimen was applied by extending the treatment interval for a few weeks, based on the clinical examination and presence of subretinal fluid on OCT or leakage on FA. If the disease remained stable at each visit, the treatment interval was extended for another 1 to 2 weeks, however, if there was recurrence of leakage, the treatment with a monthly injection was continued until the leakage stopped. The patient’s medical history, including cigarette smoking, presence of diabetes and hypertension and symptoms of nAMD, was recorded. All patients were scheduled for follow-up, and BCVA and CRT were recorded after 3 and 6 months following the first injection of ranibizumab and compared with the baseline values. Based on the changes in BCVA and CRT, the patients were classified as either responders or non-responders. The responder group was defined as patients having an improvement of three lines or higher in the Snellen chart and/or having a decrease of more than 100 µM in CRT after 6 months following the first ranibizumab injection, compared to the baseline measurement. The non-responder group was defined as patients having no changes in BCVA and CRT or having a reduction of three lines or more in the Snellen chart and/or an increase of 100 µM in CRT after 6 months of first injection, compared to the baseline. A 2-ml blood sample was collected from each patient and transferred to blood collection tubes containing EDTA, for genotype analysis.
Genotyping
The genomic DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) and stored at –20ºC for further analysis. The genotyping of CFH Y402H (rs1061170) polymorphism was performed using a polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The PCR amplification was performed in a thermocycler (Thermo Fisher Scientific, Waltham, MA USA) with the following conditions: 95°C for 5 minutes as initial denaturation, followed by 35 cycles (denaturation at 95°C for 15 seconds, annealing of forward primer [5’-TCATTGTTATGGTCCTTAGGAAA-3’] and reverse primer [5’-GGAGTAGGAGACCAGCCATT-3’] at 57°C for 15 seconds, and extension at 72°C for 45 seconds). The final cycle was extension at 72°C for 5 minutes.
The amplified product, a 179-bp fragment, was then digested with MluCI restriction enzyme (New England Biolabs, Beverly, MA, USA) at 65°C for 3 hours. The digested products were separated on agarose gel (2%) and visualized using a UV Alpha imager (Alpha Innotech, San Leandro, CA, USA). Random samples were used to validate the results, and identical results were obtained.
Statistical analysis
BCVA determined by the Snellen chart was converted into a logarithm of minimal angle of resolution (logMAR) for further statistical analysis. The descriptive statistics for all demographic and clinical variables were compared using the Student’s t-test and one-way analysis of variance (ANOVA) for normal continuous variables, Mann–Whitney U test for skewed continuous variables and Chi-square test for categorical variables. A logistic regression analysis was performed to determine the association between the CFH Y402H genotypes and response to ranibizumab treatment. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 21.0. (IBM Corp., Armonk, NY, USA), and p < 0.05 was considered statistically significant.
RESULTS
Patient characteristics, and mean baseline BCVA and CRT in relation to CFH Y402H genotypes
The CFH gene sequence was first amplified with PCR, the resulting 241-bp PCR product was then digested with MluCI restriction enzyme, and the following fragments for CFH Y402H were obtained: 119-bp fragment for TT wild-type genotype, 179-bp and 119-bp fragments for CT heterozygous genotype, and 179-bp fragment for CC homozygous mutant genotype. We compared the baseline characteristics of patients and the mean baseline BCVA and CRT between the CFH Y402H genotypes.
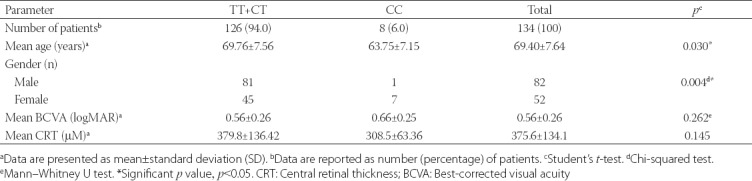
The baseline characteristics of patients are presented in Table 1. Out of 134 patients with nAMD, 126 (94.0%) had TT or CT genotype of CFH Y402H, while 8 (6.0%) had CC genotype. A significant difference was observed in the distribution of CFH Y402H genotypes in relation to the gender (p = 0.004); i.e. a higher number of males had TT+CT genotypes (n = 81 for males, n = 45 for females) while a higher number females had CC genotype (n = 7 for females, n = 1 for males). The distribution of the CFH Y402H genotypes was also significantly different with regard to the mean age; patients with the TT+CT genotypes had a higher mean age (69.76 ± 7.56 years) compared to those with the CC genotype (63.75 ± 7.15) [p = 0.030]. There was no significant difference in the mean baseline BCVA and CRT between the CFH Y402H genotypes (p > 0.05).
TABLE 1.
Baseline characteristics of study population
Changes in BCVA and CRT after ranibizumab treatment in relation to CFH Y402H genotypes
The mean BCVA and CRT obtained at baseline and after 3 and 6 months of first intravitreal injection of ranibizumab were compared between the CFH Y402H genotypes. In addition, changes in BCVA and CRT observed for 3- and 6-month time points and in relation to the baseline measurements were determined for each CFH Y402H genotype (Table 2).
TABLE 2.
Visual acuity and central retinal thickness in relation to the CFH Y402H genotypes in patients with nAMD
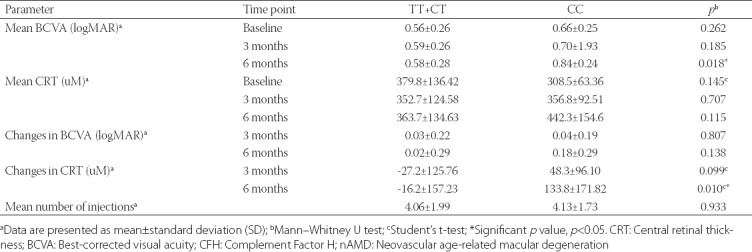
A significant difference in the mean BCVA was observed between the CFH Y402H genotypes after 6 months, with worse BCVA observed for the CC genotype (0.84 ± 0.24 logMAR) compared to the TT+CT genotypes (0.58 ± 0.28 logMAR) [p = 0.018]. The mean CRT between the CFH Y402H genotypes was not significantly different after 6 months, however, the changes in CRT after 6 months compared to the baseline measurement were significantly different (p = 0.010). The largest change in the mean CRT was observed for the CC genotype, with an increase of 133.8 ± 171.82 µM after 6 months. On the contrary, no significant difference was observed in the changes in BCVA and mean number of injections between the CFH Y402H genotypes (p > 0.05).
Association between response to ranibizumab treatment and CFH Y402H genotypes
According to their response to ranibizumab treatment, 134 patients with nAMD were classified into responder (n = 49) and non-responder group (n = 84), as shown in Figure 1. The mean CRT was not significantly different between the two groups (p > 0.05), but the mean BCVA at baseline (p = 0.001) and after 6 months (p = 0.005) was significantly different between the two groups. At baseline, worse BCVA was observed for responder (0.66 ± 0.26 logMAR) compared to non-responder group (0.51 ± 0.25 logMAR). However, after 6 months of first injection, improved mean BCVA was observed in responder group (0.51 ± 0.26 logMAR) compared to the corresponding baseline measurement, while the mean BCVA after 6 months was worse in non-responder group (0.65 ± 0.29 logMAR) [p = 0.005].
FIGURE 1.
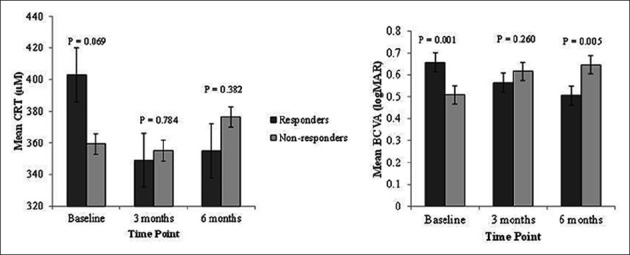
Patients with nAMD (N = 134) were classified into responder (n = 49) and non-responder group (n = 84). The mean CRT was not significantly different between the two groups (p > 0.05), but the mean BCVA at baseline (p = 0.001) and after 6 months of the first injection (p = 0.005) was significantly different between the two groups. At baseline, worse BCVA was observed for responder (0.66 ± 0.26 logMAR) compared to non-responder group (0.51 ± 0.25 logMAR). However, after 6 months of first injection, improved mean BCVA was observed in responder group (0.51 ± 0.26 logMAR) compared to the corresponding baseline measurement, while the mean BCVA in non-responder group was worse after 6 months (0.65 ± 0.29 logMAR) [p = 0.005]. nAMD: Neovascular age-related macular degeneration; CRT: Central retinal thickness; BCVA: Best-corrected visual acuity.
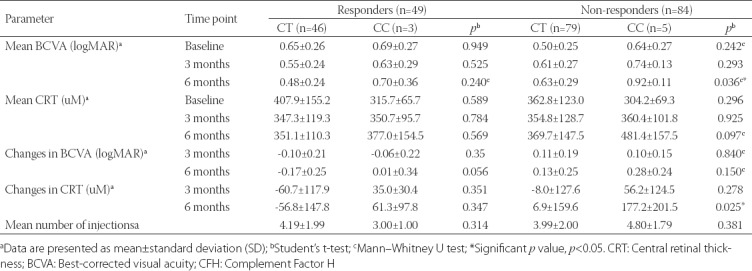
Next, we evaluated the mean BCVA and CRT in relation to the CFH Y402H genotypes in each group, as well as changes in BCVA and CRT observed for 3- and 6-month time points and in relation to the baseline measurements for each genotype in each of the two groups (Table 3). No significant difference was observed in the mean BCVA and CRT between the CFH Y402H genotypes in responder group (p > 0.05). However, in non-responder group, similar to the previous findings (Table 2), a significant difference was observed in the mean BCVA between the CT and CC genotypes after 6 months (p = 0.036), and in the changes of CRT after 6 months compared to the baseline measurement (p = 0.025). The mean BCVA was worse for the CC genotype (0.92 ± 0.11 logMAR) compared to the CT genotype (0.63 ± 0.29 logMAR). Similarly, an increase in CRT of 177.2 ± 201.5 µM was observed for the CC genotype compared to the CT genotype for which an increase of 6.9 ± 159.6 µm was observed. No significant difference was observed in the mean number of injections between the genotypes in both groups (p > 0.05).
TABLE 3.
Visual acuity and central retinal thickness in relation to the CFH Y402H genotypes in responder and non-responder group
The C risk allele was more frequent (53.0%) among the patients with nAMD compared to the low risk T allele [47.0%] (Table 4). However, in the logistic regression analysis adjusted for the confounding factors, no significant association (p > 0.05) was observed under the recessive model (the model with the least p-value) between the CFH Y402H genotypes and response to treatment with ranibizumab.
TABLE 4.
Regression analysis of the CFH Y402H genotypes and response to ranibizumab treatment in patients with nAMD

DISCUSSION
In this study we investigated CFH Y402H polymorphism in patients with nAMD treated with ranibizumab. Specifically, we analyzed the visual prognosis of patients and their response to ranibizumab treatment in relation to the presence/absence of the Y402H homozygous risk genotype. Overall, we found no significant association between the CFH polymorphism and patient response to the treatment after 6-month follow-up (p = 0.944).
With the increasing use of anti-VEGF agents in treating nAMD, a growing number of studies has been investigating the association between the patient response to treatment and specific gene variants [22,23]. Among the genes linked to the development of nAMD, CFH Y402H polymorphism has been studied most extensively, although with contradictory findings in different populations [24].
The Lucentis Genotype Study reported that a single nucleotide polymorphism (SNP) in the CFH gene was associated with less improvement in VA in patients receiving ranibizumab therapy [25], however, the mechanism underlying the effect of the CFH gene on the treatment response is still not completely understood. Nevertheless, considering the important role of CFH Y402H in the development of nAMD [14], investigating the association between CFH polymorphisms and response to treatments might provide additional insights about its function in nAMD.
In this prospective study, we investigated the association of CFH Y402H with the improvement in VA and response to treatment in nAMD, from both functional (BCVA) and structural (CRT) aspect. Our findings showed that, although not significantly different, patients with nAMD homozygous for the CFH Y402H risk allele (CC genotype) had worse VA at baseline compared to patients with CT or TT CFH Y402H genotype. Six months after first injection of ranibizumab, a significant difference in VA was observed between the CFH Y402H genotypes; the patients with the homozygous CC genotype had worse VA compared to those with the CT or TT genotype. Nevertheless, compared to the baseline measurements, worse VA was associated with all genotypes, with no significant improvement in BCVA over the 6-month follow-up period.
A similar study involving Turkish patients with CNV secondary to AMD showed that CFH Y402H CC accompanied a poor while TT genotype accompanied a good response in the patients undergoing ranibizumab therapy [11]. On the contrary, Lee et al. [26] reported no difference in VA between the CFH Y402H genotypes, after 6 and 9 months following ranibizumab treatment [26]. However, they reported that the patients with the homozygous CC genotype were more likely to require additional ranibizumab injections compared to the patients with the TT genotype. Considering the effect of the variant Y402H genotype on the regulatory role of CFH in the complement system, the authors suggested higher background levels of inflammation, as well as more rapid recurrence of neovascularization, as a possible pathophysiological mechanism in those patients. This could also explain why patients with the risk genotype respond differently to the treatment and require additional ranibizumab injections [26]. Veloso et al. [20] also reported that patients with nAMD carrying the CC genotype had poorer long-term functional response to intravitreal ranibizumab and required a higher mean number of injections [20].
One of the limitations of pharmacogenetic studies in AMD is the lack of uniform definition of good and poor response to the treatment [27]. In the present study, we observed a significant difference between the responder and non-responder group in the mean BCVA at baseline as well as at 6-month post-treatment time point. Furthermore, analyzing the CFH Y402H genotypes in relation to BCVA and CRT in each of the two groups, we showed a significant difference in the mean BCVA and in the changes of CRT between the baseline and 6-month post-treatment time point. In the non-responder group, the homozygous CC genotype was associated with worse mean BCVA and mean CRT after treatment compared to the heterozygous CT genotype, suggesting that the homozygous risk genotype might play a role in the treatment outcomes. This was also supported by the findings of Kloeckener-Gruissem et al. [18] and Dikmetas et al. [11], where AMD patients with CFH Y402H CC genotype had worse response to ranibizumab treatment compared to those with the CT and TT genotypes.
In the present study, in addition to BCVA, we investigated structural changes in the retina by measuring CRT before and after treatment. Our CRT analysis confirmed the possible effect of CFH Y402H CC genotype on a worse response to treatment, as patients with the CC genotype had a greater change in CRT after 6 months of the first injection compared to the TT and CT genotypes. Some studies [19,28,29] focused only on VA as the main outcome measure of treatment, while other studies reported conflicting results for CRT in evaluating patient response to treatment [19,20,30,31]. Nevertheless, with the advancement in OCT, CRT in combination with other measures, is becoming a more important tool in evaluating the progress of patients with AMD [32].
Even though we observed significant differences in the BCVA and CRT between the CFH Y402H genotypes, the regression analysis of the responder and non-responder groups showed no association between the CFH Y402H genotypes and response to ranibizumab treatment.
The results of pharmacogenetic studies can be used to improve the design of clinical trials and drug development. However, studies investigating the association between specific genetic variants and patient response to treatment often use different designs, sample sizes, age and ethnic groups, and follow-up periods, which leads to inconsistent results. In the case of AMD, studies including larger samples of elderly patients and longer post-treatment follow-up periods reported more often that patients with the risk CFH Y402H C allele had a poorer response to treatment compared to the carriers of the low-risk T allele [11,18,19,27]. Other studies showed contradictory findings, i.e., the association between the risk CC genotype of CFH Y402H and response to treatment was not observed in different populations of AMD patients, based on VA and other clinical measures [22,23,28,33,34].
Our study has several limitations. The major limitation is the lack of data on different nAMD phenotypes and subtypes of CNV, which could have been used to further clarify the causes of variable treatment response. Moreover, we performed the analysis only on one gene associated with the development of AMD. The follow-up period of 6 months was also relatively short compared to some other studies. Finally, the small sample size of patients with nAMD in this study could have affected the results of the association analysis.
CONCLUSION
This study is the first report on the association between CFH Y402H polymorphism and response to ranibizumab treatment in Malaysian patients with nAMD. Overall, we found no association between the CFH polymorphism and patient response to the treatment after 6-month follow-up. However, we observed a significant difference in the mean BCVA and changes in CRT between the CFH Y402H genotypes at 6-month post-treatment time point, suggesting that other factors may be involved in the progression of nAMD after treatment. Future studies with a larger sample size are required to confirm the association between the risk genotypes and ranibizumab treatment response in nAMD patients, before it can be used in genetic screening or standard treatment of AMD. Polymorphisms of other candidate genes related to AMD should also be comprehensively investigated, including the VEGF gene family, age-related maculopathy susceptibility 2 (ARMS2), and HtrA serine peptidase 1 (HTRA1).
ACKNOWLEDGMENTS
This study was supported by Putra Grant (Grant No: 9409800 and 9432700).We thank all the staff at Hospital Selayang and UKMMC involved in this study for their continuous support. We also thank all the patients who participated in the study.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–72. doi: 10.1001/archopht.122.4.564. https://doi.org/10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137(3):486–95. doi: 10.1016/j.ajo.2003.11.069. https://doi.org/10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: Current estimates. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF5–ORSF13. doi: 10.1167/iovs.13-12789. http://dx.doi.org/10.1167/iovs.13-12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan W-M, Lai TYY. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asia. Hong Kong Journal of Ophthalmology. 2016;14(1):14–9. [Google Scholar]
- 5.Laude A, Cackett PD, Vithana EN, Yeo IY, Wong D, Koh AH, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: Same or different disease? Prog Retin Eye Res. 2010;29(1):19–29. doi: 10.1016/j.preteyeres.2009.10.001. https://doi.org/10.1016/j.preteyeres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 6.CATT Research Group. Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–908. doi: 10.1056/NEJMoa1102673. https://doi.org/10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31. doi: 10.1056/NEJMoa054481. https://doi.org/10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 8.Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;(8):CD005139. doi: 10.1002/14651858.CD005139.pub3. https://doi.org/10.1002/14651858.CD005139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65.e55. doi: 10.1016/j.ophtha.2008.10.018. https://doi.org/10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107(12):2224–32. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dikmetas O, Kadayifcilar S, Eldem B. The effect of CFH polymorphisms on the response to the treatment of age-related macular degeneration (AMD) with intravitreal ranibizumab. Mol Vis. 2013;19:2571–8. eCollection 2013. [PMC free article] [PubMed] [Google Scholar]
- 12.Baird PN, Islam FM, Richardson AJ, Cain M, Hunt N, Guymer R. Analysis of the Y402H variant of the complement factor H gene in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(10):4194–8. doi: 10.1167/iovs.05-1285. https://doi.org/10.1167/iovs.05-1285. [DOI] [PubMed] [Google Scholar]
- 13.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–21. doi: 10.1126/science.1110359. https://doi.org/10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 14.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–9. doi: 10.1126/science.1109557. https://doi.org/10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau LI, Chen SJ, Cheng CY, Yen MY, Lee FL, Lin MW, et al. Association of the Y402H polymorphism in complement factor H gene and neovascular age-related macular degeneration in Chinese patients. Invest Ophthalmol Vis Sci. 2006;47(8):3242–6. doi: 10.1167/iovs.05-1532. https://doi.org/10.1167/iovs.05-1532. [DOI] [PubMed] [Google Scholar]
- 16.Hao XF, Xie LK, Tang YZ, Xie WK, Zhang ZF, Qi YX, et al. Association of complement factor H gene polymorphisms with age-related macular degeneration susceptibility. Int J Clin Exp Pathol. 2015;8(3):3186–91. eCollection 2015. [PMC free article] [PubMed] [Google Scholar]
- 17.Brantley MA, Jr, Fang AM, King JM, Tewari A, Kymes SM, Shiels A. Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to intravitreal bevacizumab. Ophthalmology. 2007;114(12):2168–73. doi: 10.1016/j.ophtha.2007.09.008. https://doi.org/10.1016/j.ophtha.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Kloeckener-Gruissem B, Barthelmes D, Labs S, Schindler C, Kurz-Levin M, Michels S, et al. Genetic association with response to intravitreal ranibizumab in patients with neovascular AMD. Invest Ophthalmol Vis Sci. 2011;52(7):4694–702. doi: 10.1167/iovs.10-6080. https://doi.org/10.1167/iovs.10-6080. [DOI] [PubMed] [Google Scholar]
- 19.Nischler C, Oberkofler H, Ortner C, Paikl D, Riha W, Lang N, et al. Complement factor H Y402H gene polymorphism and response to intravitreal bevacizumab in exudative age-related macular degeneration. Acta Ophthalmol. 2011;89(4):e344–9. doi: 10.1111/j.1755-3768.2010.02080.x. https://doi.org/10.1111/j.1755-3768.2010.02080.x. [DOI] [PubMed] [Google Scholar]
- 20.Veloso CE, Almeida LN, Nehemy MB. CFH Y402H polymorphism and response to intravitreal ranibizumab in Brazilian patients with neovascular age-related macular degeneration. Rev Col Bras Cir. 2014;41(6):386–92. doi: 10.1590/0100-69912014006002. https://doi.org/10.1590/0100-69912014006002. [DOI] [PubMed] [Google Scholar]
- 21.Hata M, Tsujikawa A, Miyake M, Yamashiro K, Ooto S, Oishi A, et al. Two-year visual outcome of ranibizumab in typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(2):221–7. doi: 10.1007/s00417-014-2688-1. https://doi.org/10.1007/s00417-014-2688-1. [DOI] [PubMed] [Google Scholar]
- 22.Hagstrom SA, Ying GS, Pauer GJ, Sturgill-Short GM, Huang J, Callanan DG, et al. Pharmacogenetics for genes associated with age-related macular degeneration in the Comparison of AMD Treatments Trials (CATT) Ophthalmology. 2013;120(3):593–9. doi: 10.1016/j.ophtha.2012.11.037. https://doi.org/10.1016/j.ophtha.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitchens JW, Kassem N, Wood W, Stone TW, Isernhagen R, Wood E, et al. A pharmacogenetics study to predict outcome in patients receiving anti-VEGF therapy in age related macular degeneration. Clin Ophthalmol. 2013;7:1987–93. doi: 10.2147/OPTH.S39635. https://doi.org/10.2147/OPTH.S39635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Pharmacogenetics of complement factor H Y402H polymorphism and treatment of neovascular AMD with anti-VEGF agents: A meta-analysis. Sci Rep. 2015;5:14517. doi: 10.1038/srep14517. https://doi.org/10.1038/srep14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis PJ. The influence of genetics on response to treatment with ranibizumab (Lucentis) for age-related macular degeneration: The Lucentis Genotype Study (an American Ophthalmological Society Thesis) Trans Am Ophthalmol Soc. 2011;109:115–56. [PMC free article] [PubMed] [Google Scholar]
- 26.Lee AY, Raya AK, Kymes SM, Shiels A, Brantley MA., Jr Pharmacogenetics of complement factor H (Y402H) and treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2009;93(5):610–3. doi: 10.1136/bjo.2008.150995. https://doi.org/10.1136/bjo.2008.150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah AR, Williams S, Baumal CR, Rosner B, Duker JS, Seddon JM. Predictors of response to intravitreal anti-vascular endothelial growth factor treatment of age-related macular degeneration. Am J Ophthalmol. 2016;163:154–66.e8. doi: 10.1016/j.ajo.2015.11.033. https://doi.org/10.1016/j.ajo.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habibi I, Sfar I, Kort F, Aounallah-Skhiri H, Chebil A, Chouchene I, et al. Y402H polymorphism in complement factor H and age-related macular degeneration in the Tunisian population. Ophthalmic Res. 2013;49(4):177–84. doi: 10.1159/000345068. https://doi.org/10.1159/000345068. [DOI] [PubMed] [Google Scholar]
- 29.Yamashiro K, Tomita K, Tsujikawa A, Nakata I, Akagi-Kurashige Y, Miyake M, et al. Factors associated with the response of age-related macular degeneration to intravitreal ranibizumab treatment. Am J Ophthalmol. 2012;154(1):125–36. doi: 10.1016/j.ajo.2012.01.010. https://doi.org/10.1016/j.ajo.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Kang HK, Yoon MH, Lee DH, Chin HS. Pharmacogenetic influence of LOC387715/HTRA1 on the efficacy of bevacizumab treatment for age-related macular degeneration in a Korean population. Korean J Ophthalmol. 2012;26(6):414–22. doi: 10.3341/kjo.2012.26.6.414. https://doi.org/10.3341/kjo.2012.26.6.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang W, Noh DH, Sagong M, Kim IT. Pharmacogenetic association with early response to intravitreal ranibizumab for age-related macular degeneration in a Korean population. Mol Vis. 2013;19:702–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Sayed KM, Naito T, Nagasawa T, Katome T, Mitamura Y. Early visual impacts of optical coherence tomographic parameters in patients with age-related macular degeneration following the first versus repeated ranibizumab injection. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1449–58. doi: 10.1007/s00417-011-1672-2. https://doi.org/10.1007/s00417-011-1672-2. [DOI] [PubMed] [Google Scholar]
- 33.Yuan D, Yuan D, Liu X, Yuan S, Xie P, Liu Q. Genetic association with response to intravitreal ranibizumab for neovascular age-related macular degeneration in the Han Chinese population. Ophthalmologica. 2013;230(4):227–32. doi: 10.1159/000355068. https://doi.org/10.1159/000355068. [DOI] [PubMed] [Google Scholar]
- 34.Park UC, Shin JY, Kim SJ, Shin ES, Lee JE, McCarthy LC, et al. Genetic factors associated with response to intravitreal ranibizumab in Korean patients with neovascular age-related macular degeneration. Retina. 2014;34(2):288–97. doi: 10.1097/IAE.0b013e3182979e1e. https://doi.org/10.1097/IAE.0b013e3182979e1e. [DOI] [PubMed] [Google Scholar]


